Recent Progress in Nanotechnology for COVID-19 Prevention, Diagnostics and Treatment
Abstract
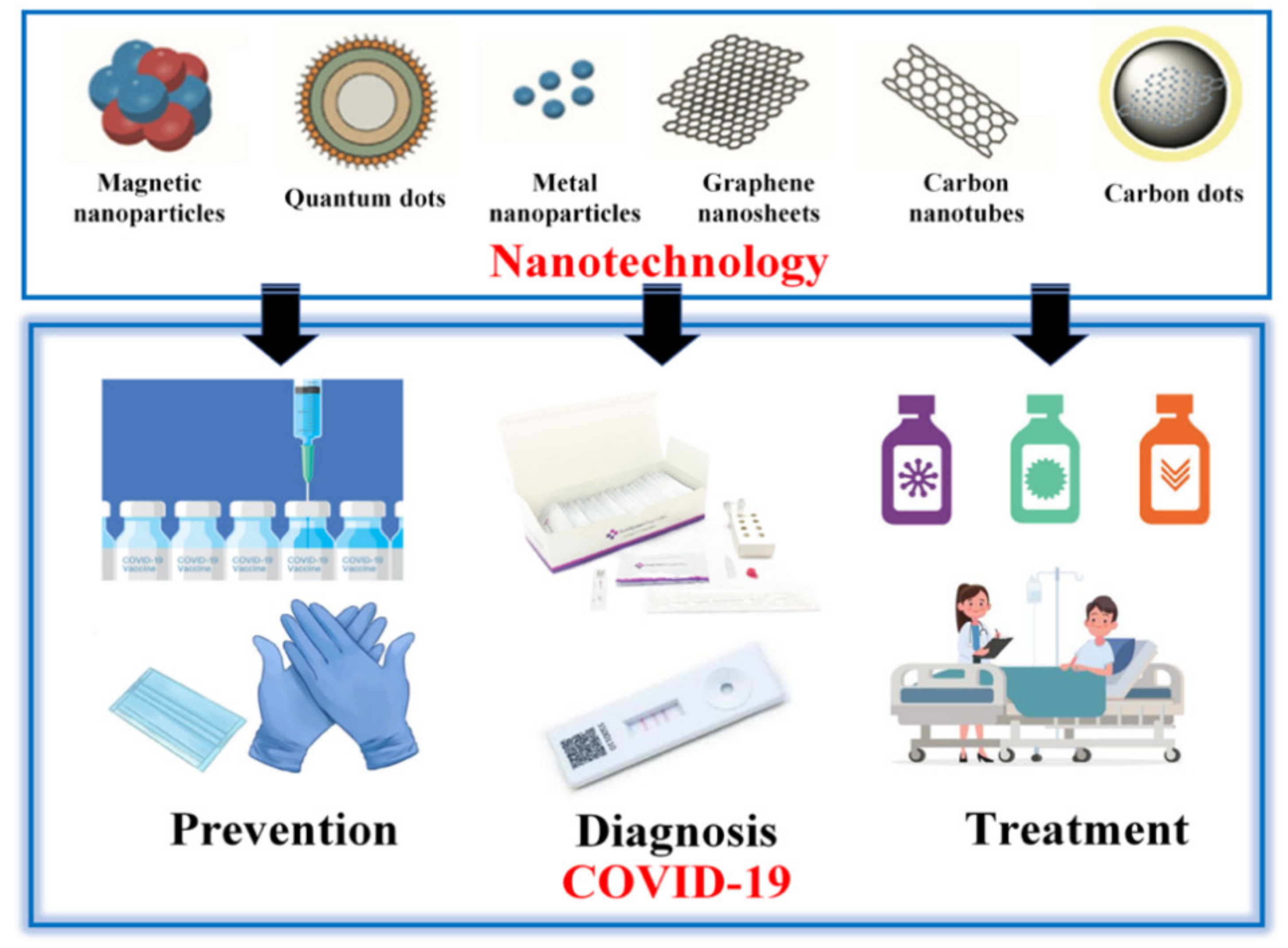
:1. Introduction
2. Prevention
2.1. Nanomaterials in Masks
2.2. Nanomaterials in Gloves
2.3. Nanomaterials in Disinfectants
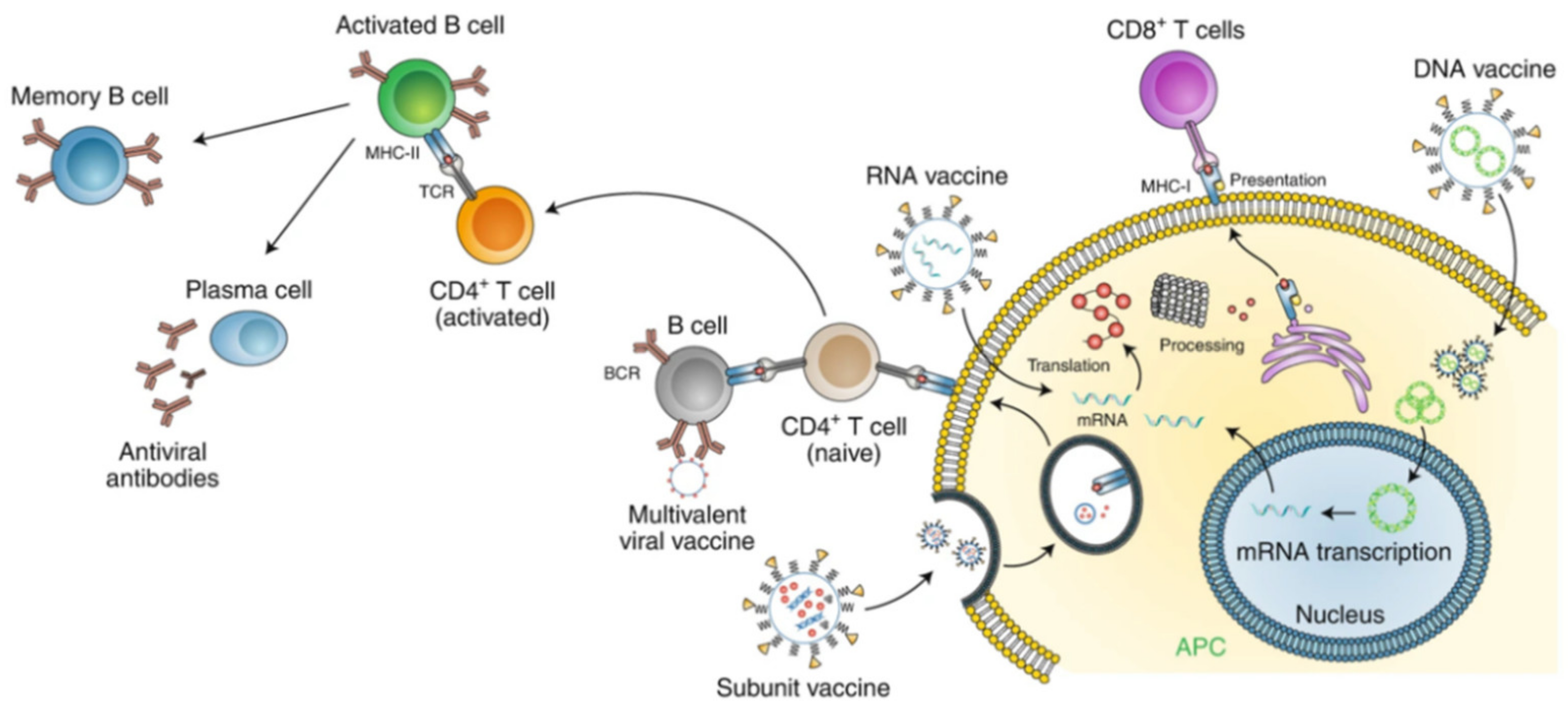
2.4. Nanomaterials in Vaccines
2.4.1. Gold Nanoparticles
2.4.2. Ferritin-Based Nanoparticles
2.4.3. Spike Protein Nanoparticles
2.4.4. Hollow Polymeric Nanoparticles
2.4.5. Lipid Nanoparticles
2.4.6. Protein Nanoparticles
3. Diagnostics
3.1. Gold Nanoparticles
3.2. Magnetic NPs (MNPs)
3.3. Quantum Dots
3.4. Carbon-Based Nanomaterials
3.5. Nanozymes
4. Treatment
4.1. Exosomes
4.2. Metal Nanoparticles
4.3. Metal Oxide Nanoparticles (MONPs)
4.4. Carbon-Based Nanomaterials
4.5. Quantum Dots (QDs)
4.6. Drugs and Chemical Compounds
4.6.1. Peptide Inhibitors
4.6.2. Curcumin
4.6.3. Dexamethasone
4.6.4. Nanoceria
5. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Ting, D.S.W.; Carin, L.; Dzau, V.; Wong, T.Y. Digital Technology and COVID-19. Nat. Med. 2020, 26, 459–461. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Tang, K. Combating COVID-19: Health Equity Matters. Nat. Med. 2020, 26, 458. [Google Scholar] [CrossRef] [PubMed]
- Menni, C.; Valdes, A.M.; Freidin, M.B.; Sudre, C.H.; Nguyen, L.H.; Drew, D.A.; Ganesh, S.; Varsavsky, T.; Cardoso, M.J.; Moustafa, J.S.E.-S. Real-Time Tracking of Self-Reported Symptoms to Predict Potential COVID-19. Nat. Med. 2020, 26, 1037–1040. [Google Scholar] [CrossRef] [PubMed]
- GeurtsvanKessel, C.H.; Okba, N.M.; Igloi, Z.; Bogers, S.; Embregts, C.W.; Laksono, B.M.; Leijten, L.; Rokx, C.; Rijnders, B.; Rahamat-Langendoen, J. An Evaluation of COVID-19 Serological Assays Informs Future Diagnostics and Exposure Assessment. Nat. Commun. 2020, 11, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Mei, X.; Lee, H.-C.; Diao, K.; Huang, M.; Lin, B.; Liu, C.; Xie, Z.; Ma, Y.; Robson, P.M.; Chung, M.; et al. Artificial Intelligence–Enabled Rapid Diagnosis of Patients with COVID-19. Nat. Med. 2020, 26, 1224–1228. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.R. Development of Point-of-Care Biosensors for COVID-19. Front. Chem. 2020, 8. [Google Scholar] [CrossRef] [PubMed]
- Sivasankarapillai, V.S.; Pillai, A.M.; Rahdar, A.; Sobha, A.P.; Das, S.S.; Mitropoulos, A.C.; Mokarrar, M.H.; Kyzas, G.Z. On Facing the SARS-CoV-2 (COVID-19) with Combination of Nanomaterials and Medicine: Possible Strategies and First Challenges. Nanomaterials 2020, 10, 852. [Google Scholar] [CrossRef]
- Carvalho, A.P.A.; Conte-Junior, C.A. Recent Advances on Nanomaterials to COVID-19 Management: A Systematic Review on Antiviral/Virucidal Agents and Mechanisms of SARS-CoV-2 Inhibition/Inactivation. Glob. Chall. 2021, 5, 2000115. [Google Scholar] [CrossRef]
- Ruiz-Hitzky, E.; Darder, M.; Wicklein, B.; Ruiz-Garcia, C.; Martín-Sampedro, R.; Del Real, G.; Aranda, P. Nanotechnology Responses to COVID-19. Adv. Healthc. Mater. 2020, 9, 2000979. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Zhang, X.; Shu, Y.; Guo, M.; Zhang, H.; Tao, W. Insights from Nanotechnology in COVID-19 Treatment. Nano Today 2021, 36, 101019. [Google Scholar] [CrossRef]
- Abdelhamid, H.N.; Badr, G. Nanobiotechnology as a Platform for the Diagnosis of COVID-19: A Review. Nanotechnol. Environ. Eng. 2021, 6, 19. [Google Scholar] [CrossRef]
- Bhalla, N.; Pan, Y.; Yang, Z.; Payam, A.F. Opportunities and Challenges for Biosensors and Nanoscale Analytical Tools for Pandemics: COVID-19. ACS Nano 2020, 14, 7783–7807. [Google Scholar] [CrossRef]
- Srivastava, M.; Srivastava, N.; Mishra, P.K.; Malhotra, B.D. Prospects of Nanomaterials-Enabled Biosensors for COVID-19 Detection. Sci. Total Environ. 2021, 754, 142363. [Google Scholar] [CrossRef]
- Campos, E.V.; Pereira, A.E.; de Oliveira, J.L.; Carvalho, L.B.; Guilger-Casagrande, M.; de Lima, R.; Fraceto, L.F. How Can Nanotechnology Help to Combat COVID-19? Opportunities and Urgent Need. J. Nanobiotechnol. 2020, 18, 1–23. [Google Scholar] [CrossRef]
- Callaway, E. The Race for Coronavirus Vaccines: A Graphical Guide. Nature 2020, 580, 576–577. [Google Scholar] [CrossRef] [PubMed]
- Gong, P.; He, X.; Wang, K.; Tan, W.; Xie, W.; Wu, P.; Li, H. Combination of Functionalized Nanoparticles and Polymerase Chain Reaction-Based Method for SARS-CoV Gene Detection. J. Nanosci. Nanotechnol. 2008, 8, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Loczechin, A.; Séron, K.; Barras, A.; Giovanelli, E.; Belouzard, S.; Chen, Y.-T.; Metzler-Nolte, N.; Boukherroub, R.; Dubuisson, J.; Szunerits, S. Functional Carbon Quantum Dots as Medical Countermeasures to Human Coronavirus (HCoV). ACS Appl. Mater. Interfaces 2019, 11, 42964–42974. [Google Scholar] [CrossRef]
- Antoine, T.E.; Mishra, Y.K.; Trigilio, J.; Tiwari, V.; Adelung, R.; Shukla, D. Prophylactic, Therapeutic and Neutralizing Effects of Zinc Oxide Tetrapod Structures against Herpes Simplex Virus Type-2 Infection. Antivir. Res. 2012, 96, 363–375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Y.; Ren, J.; Qu, X. Nanozymes: Classification, Catalytic Mechanisms, Activity Regulation, and Applications. Chem. Rev. 2019, 119, 4357–4412. [Google Scholar] [CrossRef] [PubMed]
- Hassanpour, M.; Rezaie, J.; Nouri, M.; Panahi, Y. The Role of Extracellular Vesicles in COVID-19 Virus Infection. Infect. Genet. Evol. 2020, 85, 104422. [Google Scholar] [CrossRef] [PubMed]
- O’Dowd, K.; Nair, K.M.; Forouzandeh, P.; Mathew, S.; Grant, J.; Moran, R.; Bartlett, J.; Bird, J.; Pillai, S.C. Face Masks and Respirators in the Fight against the COVID-19 Pandemic: A Review of Current Materials, Advances and Future Perspectives. Materials 2020, 13, 3363. [Google Scholar] [CrossRef]
- Brienen, N.C.J.; Timen, A.; Wallinga, J.; van Steenbergen, J.E.; Teunis, P.F.M. The Effect of Mask Use on the Spread of Influenza during a Pandemic. Risk Anal. 2010, 30, 1210–1218. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.-W.; Zhou, M.-Y.; Ji, G.-H.; Ye, L.; Cheng, Y.-R.; Feng, Z.-H.; Chen, J. Mask Crisis during the COVID-19 Outbreak. Eur. Rev. Med. Pharm. Sci. 2020, 24, 3397–3399. [Google Scholar] [CrossRef]
- Li, Y.; Leung, P.; Yao, L.; Song, Q.W.; Newton, E. Antimicrobial Effect of Surgical Masks Coated with Nanoparticles. J. Hosp. Infect. 2006, 62, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Borkow, G.; Zhou, S.S.; Page, T.; Gabbay, J. A Novel Anti-Influenza Copper Oxide Containing Respiratory Face Mask. PLoS ONE 2010, 5, e11295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pini, M.; Cedillo González, E.; Neri, P.; Siligardi, C.; Ferrari, A. Assessment of Environmental Performance of TiO2 Nanoparticles Coated Self-Cleaning Float Glass. Coatings 2017, 7, 8. [Google Scholar] [CrossRef] [Green Version]
- Akduman, C.; Kumbasar, E.P.A. Nanofibers in Face Masks and Respirators to Provide Better Protection. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Istanbul, Turkey, 20–22 June 2018. [Google Scholar]
- Thavasi, V.; Singh, G.; Ramakrishna, S. Electrospun Nanofibers in Energy and Environmental Applications. Energy Environ. Sci. 2008, 1, 205–221. [Google Scholar] [CrossRef]
- Ramaseshan, R.; Sundarrajan, S.; Liu, Y.; Barhate, R.S.; Lala, N.L.; Ramakrishna, S. Functionalized Polymer Nanofibre Membranes for Protection from Chemical Warfare Stimulants. Nanotechnology 2006, 17, 2947. [Google Scholar] [CrossRef]
- Zhu, M.; Han, J.; Wang, F.; Shao, W.; Xiong, R.; Zhang, Q.; Pan, H.; Yang, Y.; Samal, S.K.; Zhang, F.; et al. Electrospun Nanofibers Membranes for Effective Air Filtration. Macromol. Mater. Eng. 2017, 302. [Google Scholar] [CrossRef]
- Tebyetekerwa, M.; Xu, Z.; Yang, S.; Ramakrishna, S. Electrospun Nanofibers-Based Face Masks. Adv. Fiber Mater. 2020, 2, 161–166. [Google Scholar] [CrossRef]
- Skaria, S.D.; Smaldone, G.C. Respiratory Source Control Using Surgical Masks With Nanofiber Media. Ann. Occup. Hyg. 2014, 58, 771–781. [Google Scholar]
- Suen, L.K.P.; Guo, Y.P.; Ho, S.S.K.; Au-Yeung, C.H.; Lam, S.C. Comparing Mask Fit and Usability of Traditional and Nanofibre N95 Filtering Facepiece Respirators before and after Nursing Procedures. J. Hosp. Infect. 2020, 104, 336–343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tong, H.W.; Kwok, S.K.C.; Kwok, H.C. Protective Masks with Coating Comprising Different Electrospun Fibers Interweaved with Each Other, Formulations Forming the Same, and Method of Producing Thereof 2016. U.S. Patent 10,201,198, 12 February 2019. [Google Scholar]
- Aydemir, D.; Ulusu, N.N. Correspondence: Angiotensin-Converting Enzyme 2 Coated Nanoparticles Containing Respiratory Masks, Chewing Gums and Nasal Filters May Be Used for Protection against COVID-19 Infection. Travel Med. Infect. Dis. 2020, 37, 101697. [Google Scholar] [CrossRef] [PubMed]
- Imai, Y.; Kuba, K.; Rao, S.; Huan, Y.; Guo, F.; Guan, B.; Yang, P.; Sarao, R.; Wada, T.; Leong-Poi, H.; et al. Angiotensin-Converting Enzyme 2 Protects from Severe Acute Lung Failure. Nature 2005, 436, 112–116. [Google Scholar] [CrossRef]
- Aydemir, D.; Gecili, F.; Özdemir, N.; Nuray Ulusu, N. Synthesis and Characterization of a Triple Enzyme-Inorganic Hybrid Nanoflower (TrpE@ihNF) as a Combination of Three Pancreatic Digestive Enzymes Amylase, Protease and Lipase. J. Biosci. Bioeng. 2020, 129, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Dyshlyuk, L.; Babich, O.; Ivanova, S.; Vasilchenco, N.; Prosekov, A.; Sukhikh, S. Suspensions of Metal Nanoparticles as a Basis for Protection of Internal Surfaces of Building Structures from Biodegradation. Case Stud. Constr. Mater. 2020, 12, e00319. [Google Scholar] [CrossRef]
- Morones, J.R.; Elechiguerra, J.L.; Camacho, A.; Holt, K.; Kouri, J.B.; Ramírez, J.T.; Yacaman, M.J. The Bactericidal Effect of Silver Nanoparticles. Nanotechnology 2005, 16, 2346. [Google Scholar] [CrossRef] [Green Version]
- Vaze, N.; Pyrgiotakis, G.; McDevitt, J.; Mena, L.; Melo, A.; Bedugnis, A.; Kobzik, L.; Eleftheriadou, M.; Demokritou, P. Inactivation of Common Hospital Acquired Pathogens on Surfaces and in Air Utilizing Engineered Water Nanostructures (EWNS) Based Nano-Sanitizers. Nanomed. Nanotechnol. Biol. Med. 2019, 18, 234–242. [Google Scholar] [CrossRef]
- Gelperina, S.; Kisich, K.; Iseman, M.D.; Heifets, L. The Potential Advantages of Nanoparticle Drug Delivery Systems in Chemotherapy of Tuberculosis. Am. J. Respir. Crit. Care Med. 2005, 172. [Google Scholar] [CrossRef] [Green Version]
- Tacken, P.J.; de Vries, I.J.M.; Torensma, R.; Figdor, C.G. Dendritic-Cell Immunotherapy: From Ex Vivo Loading to in Vivo Targeting. Nat. Rev. Immunol. 2007, 7, 790–802. [Google Scholar] [CrossRef]
- Ahmad, S.; Zamry, A.A.; Tan, H.-T.T.; Wong, K.K.; Lim, J.; Mohamud, R. Targeting Dendritic Cells through Gold Nanoparticles: A Review on the Cellular Uptake and Subsequent Immunological Properties. Mol. Immunol. 2017, 91, 123–133. [Google Scholar] [CrossRef]
- Inner-View of Nanomaterial Incited Protein Conformational Changes: Insights into Designable Interaction. Available online: https://spj.sciencemag.org/journals/research/2018/9712832 (accessed on 19 October 2020).
- Hofmann, H.; Hattermann, K.; Marzi, A.; Gramberg, T.; Geier, M.; Krumbiegel, M.; Kuate, S.; Uberla, K.; Niedrig, M.; Pöhlmann, S. S Protein of Severe Acute Respiratory Syndrome-Associated Coronavirus Mediates Entry into Hepatoma Cell Lines and Is Targeted by Neutralizing Antibodies in Infected Patients. J. Virol. 2004, 78, 6134–6142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sekimukai, H.; Iwata-Yoshikawa, N.; Fukushi, S.; Tani, H.; Kataoka, M.; Suzuki, T.; Hasegawa, H.; Niikura, K.; Arai, K.; Nagata, N. Gold Nanoparticle-adjuvanted S Protein Induces a Strong Antigen-specific IgG Response against Severe Acute Respiratory Syndrome-related Coronavirus Infection, but Fails to Induce Protective Antibodies and Limit Eosinophilic Infiltration in Lungs. Microbiol. Immunol. 2020, 64, 33–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, W.; Guo, W.; Chang, J.; Zhang, B. Protein/Peptide-Templated Biomimetic Synthesis of Inorganic Nanoparticles for Biomedical Applications. J. Mater. Chem. B 2017, 5, 401–417. [Google Scholar] [CrossRef]
- Kim, Y.-S.; Son, A.; Kim, J.; Kwon, S.B.; Kim, M.H.; Kim, P.; Kim, J.; Byun, Y.H.; Sung, J.; Lee, J.; et al. Chaperna-Mediated Assembly of Ferritin-Based Middle East Respiratory Syndrome-Coronavirus Nanoparticles. Front. Immunol. 2018, 9. [Google Scholar] [CrossRef] [Green Version]
- Weiss, C.; Carriere, M.; Fusco, L.; Capua, I.; Regla-Nava, J.A.; Pasquali, M.; Scott, J.A.; Vitale, F.; Unal, M.A.; Mattevi, C.; et al. Toward Nanotechnology-Enabled Approaches against the COVID-19 Pandemic. ACS Nano 2020, 14, 6383–6406. [Google Scholar] [CrossRef]
- Pillaiyar, T.; Manickam, M.; Namasivayam, V.; Hayashi, Y.; Jung, S.-H. An Overview of Severe Acute Respiratory Syndrome–Coronavirus (SARS-CoV) 3CL Protease Inhibitors: Peptidomimetics and Small Molecule Chemotherapy. J. Med. Chem. 2016, 59, 6595–6628. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.-Y.; Kang, K.W.; Lee, E.-Y.; Seo, D.-W.; Kim, H.-L.; Kim, H.; Kwon, T.; Park, H.-L.; Kim, H.; Lee, S.-M.; et al. Heterologous Prime-Boost Vaccination with Adenoviral Vector and Protein Nanoparticles Induces Both Th1 and Th2 Responses against Middle East Respiratory Syndrome Coronavirus. Vaccine 2018, 36, 3468–3476. [Google Scholar] [CrossRef]
- Lin, L.C.W.; Huang, C.-Y.; Yao, B.-Y.; Lin, J.-C.; Agrawal, A.; Algaissi, A.; Peng, B.-H.; Liu, Y.-H.; Huang, P.-H.; Juang, R.-H.; et al. Viromimetic STING Agonist-Loaded Hollow Polymeric Nanoparticles for Safe and Effective Vaccination against Middle East Respiratory Syndrome Coronavirus. Adv. Funct. Mater. 2019, 29, 1807616. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Petrovsky, N. Molecular Mechanisms for Enhanced DNA Vaccine Immunogenicity. Expert Rev. Vaccines 2016, 15, 313–329. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Gascon, A.; del Pozo-Rodriguez, A.; Solinis, M.A. Development of Nucleic Acid Vaccines: Use of Self-Amplifying RNA in Lipid Nanoparticles. Int. J. Nanomed. 2014, 9, 1833–1843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dolgin, E. Business: The Billion-Dollar Biotech. Nature 2015, 522, 26–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kranz, L.M.; Diken, M.; Haas, H.; Kreiter, S.; Loquai, C.; Reuter, K.C.; Meng, M.; Fritz, D.; Vascotto, F.; Hefesha, H.; et al. Systemic RNA Delivery to Dendritic Cells Exploits Antiviral Defence for Cancer Immunotherapy. Nature 2016, 534, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Safety and Immunogenicity Study of 2019-NCoV Vaccine (MRNA-1273) for Prophylaxis of SARS-CoV-2 Infection (COVID-19)—Full Text View—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT04283461?term=NCT04283461&draw=2&rank=1 (accessed on 2 March 2021).
- Zhao, Y.; Huang, L. Lipid Nanoparticles for Gene Delivery. In Advances in Genetics; Elsevier: Amsterdam, The Netherlands, 2014; Volume 88, pp. 13–36. ISBN 978-0-12-800148-6. [Google Scholar]
- Shin, M.D.; Shukla, S.; Chung, Y.H.; Beiss, V.; Chan, S.K.; Ortega-Rivera, O.A.; Wirth, D.M.; Chen, A.; Sack, M.; Pokorski, J.K.; et al. COVID-19 Vaccine Development and a Potential Nanomaterial Path Forward. Nat. Nanotechnol. 2020, 15, 646–655. [Google Scholar] [CrossRef]
- Scheerlinck, J.-P.Y.; Greenwood, D.L.V. Virus-Sized Vaccine Delivery Systems. Drug Discov. Today 2008, 13, 882–887. [Google Scholar] [CrossRef]
- Hervé, P.-L.; Deloizy, C.; Descamps, D.; Rameix-Welti, M.-A.; Fix, J.; McLellan, J.S.; Eléouët, J.-F.; Riffault, S. RSV N-Nanorings Fused to Palivizumab-Targeted Neutralizing Epitope as a Nanoparticle RSV Vaccine. Nanomedicine 2017, 13, 411–420. [Google Scholar] [CrossRef] [Green Version]
- Zottig, X.; Côté-Cyr, M.; Arpin, D.; Archambault, D.; Bourgault, S. Protein Supramolecular Structures: From Self-Assembly to Nanovaccine Design. Nanomaterials 2020, 10, 1008. [Google Scholar] [CrossRef]
- Lee, Y.-T.; Ko, E.-J.; Lee, Y.; Kim, K.-H.; Kim, M.-C.; Lee, Y.-N.; Kang, S.-M. Intranasal Vaccination with M2e5x Virus-like Particles Induces Humoral and Cellular Immune Responses Conferring Cross-Protection against Heterosubtypic Influenza Viruses. PLoS ONE 2018, 13, e0190868. [Google Scholar] [CrossRef] [Green Version]
- Lang, R.; Winter, G.; Vogt, L.; Zurcher, A.; Dorigo, B.; Schimmele, B. Rational Design of a Stable, Freeze-Dried Virus-like Particle-Based Vaccine Formulation. Drug Dev. Ind. Pharm. 2009, 35, 83–97. [Google Scholar] [CrossRef]
- Kumar, R.; Nagpal, S.; Kaushik, S.; Mendiratta, S. COVID-19 Diagnostic Approaches: Different Roads to the Same Destination. VirusDisease 2020, 31, 1–9. [Google Scholar] [CrossRef]
- Choi, J.R.; Hu, J.; Wang, S.; Yang, H.; Wan Abas, W.A.B.; Pingguan-Murphy, B.; Xu, F. Paper-Based Point-of-Care Testing for Diagnosis of Dengue Infections. Crit. Rev. Biotechnol. 2017, 37, 100–111. [Google Scholar] [CrossRef]
- Gong, Y.; Hu, J.; Choi, J.R.; You, M.; Zheng, Y.; Xu, B.; Wen, T.; Xu, F. Improved LFIAs for Highly Sensitive Detection of BNP at Point-of-Care. Int. J. Nanomed. 2017, 12, 4455–4466. [Google Scholar] [CrossRef] [Green Version]
- Udugama, B.; Kadhiresan, P.; Kozlowski, H.N.; Malekjahani, A.; Osborne, M.; Li, V.Y.; Chen, H.; Mubareka, S.; Gubbay, J.B.; Chan, W.C. Diagnosing COVID-19: The Disease and Tools for Detection. ACS Nano 2020, 14, 3822–3835. [Google Scholar] [CrossRef] [Green Version]
- Tuaillon, E.; Bolloré, K.; Pisoni, A.; Debiesse, S.; Renault, C.; Marie, S.; Groc, S.; Niels, C.; Pansu, N.; Dupuy, A.M.; et al. Detection of SARS-CoV-2 Antibodies Using Commercial Assays and Seroconversion Patterns in Hospitalized Patients. J. Infect. 2020, 81, e39–e45. [Google Scholar] [CrossRef]
- Perera, R.A.; Mok, C.K.; Tsang, O.T.; Lv, H.; Ko, R.L.; Wu, N.C.; Yuan, M.; Leung, W.S.; Chan, J.M.; Chik, T.S.; et al. Serological Assays for Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), March 2020. Eurosurveillance 2020, 25, 2000421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halfpenny, K.C.; Wright, D.W. Nanoparticle Detection of Respiratory Infection. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2010, 2, 277–290. [Google Scholar] [CrossRef] [PubMed]
- Draz, M.S.; Shafiee, H. Applications of Gold Nanoparticles in Virus Detection. Theranostics 2018, 8, 1985. [Google Scholar] [CrossRef]
- Choi, J.R.; Nilghaz, A.; Chen, L.; Chou, K.C.; Lu, X. Modification of Thread-Based Microfluidic Device with Polysiloxanes for the Development of a Sensitive and Selective Immunoassay. Sens. Actuators B Chem. 2018, 260, 1043–1051. [Google Scholar] [CrossRef]
- Yew, C.H.T.; Azari, P.; Choi, J.R.; Muhamad, F.; Pingguan-Murphy, B. Electrospun Polycaprolactone Nanofibers as a Reaction Membrane for Lateral Flow Assay. Polymers 2018, 10, 1387. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Rothberg, L. Colorimetric Detection of DNA Sequences Based on Electrostatic Interactions with Unmodified Gold Nanoparticles. Proc. Natl. Acad. Sci. USA 2004, 101, 14036–14039. [Google Scholar] [CrossRef] [Green Version]
- Park, T.J.; Lee, S.Y.; Lee, S.J.; Park, J.P.; Yang, K.S.; Lee, K.-B.; Ko, S.; Park, J.B.; Kim, T.; Kim, S.K. Protein Nanopatterns and Biosensors Using Gold Binding Polypeptide as a Fusion Partner. Anal. Chem. 2006, 78, 7197–7205. [Google Scholar] [CrossRef] [PubMed]
- Bian, H.; Xu, F.; Jia, Y.; Wang, L.; Deng, S.; Jia, A.; Tang, Y. A New Immunochromatographic Assay for On-Site Detection of Porcine Epidemic Diarrhea Virus Based on Monoclonal Antibodies Prepared by Using Cell Surface Fluorescence Immunosorbent Assay. BMC Vet. Res. 2019, 15, 32. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Park, M.; Hwang, J.; Kim, J.H.; Chung, D.-R.; Lee, K.; Kang, M. Development of Label-Free Colorimetric Assay for MERS-CoV Using Gold Nanoparticles. ACS Sens. 2019, 4, 1306–1312. [Google Scholar] [CrossRef]
- Martínez-Paredes, G.; González-García, M.B.; Costa-García, A. Genosensor for SARS Virus Detection Based on Gold Nanostructured Screen-printed Carbon Electrodes. Electroanal. Int. J. Devoted Fundam. Pract. Asp. Electroanal. 2009, 21, 379–385. [Google Scholar] [CrossRef]
- Ahmed, S.R.; Nagy, É.; Neethirajan, S. Self-Assembled Star-Shaped Chiroplasmonic Gold Nanoparticles for an Ultrasensitive Chiro-Immunosensor for Viruses. RSC Adv. 2017, 7, 40849–40857. [Google Scholar] [CrossRef] [Green Version]
- Layqah, L.A.; Eissa, S. An Electrochemical Immunosensor for the Corona Virus Associated with the Middle East Respiratory Syndrome Using an Array of Gold Nanoparticle-Modified Carbon Electrodes. Microchimica Acta 2019, 186, 224. [Google Scholar] [CrossRef] [Green Version]
- Moitra, P.; Alafeef, M.; Dighe, K.; Frieman, M.B.; Pan, D. Selective Naked-Eye Detection of SARS-CoV-2 Mediated by N Gene Targeted Antisense Oligonucleotide Capped Plasmonic Nanoparticles. ACS Nano 2020, 14, 7617–7627. [Google Scholar] [CrossRef]
- Huang, C.; Wen, T.; Shi, F.J.; Zeng, X.Y.; Jiao, Y.J. Rapid Detection of IgM Antibodies against the SARS-CoV-2 Virus via Colloidal Gold Nanoparticle-Based Lateral-Flow Assay. ACS Omega 2020, 5, 12550–12556. [Google Scholar] [CrossRef]
- Zhao, Z.; Cui, H.; Song, W.; Ru, X.; Zhou, W.; Yu, X. A Simple Magnetic Nanoparticles-Based Viral RNA Extraction Method for Efficient Detection of SARS-CoV-2. bioRxiv 2020. [Google Scholar] [CrossRef]
- Giovannini, G.; Haick, H.; Garoli, D. Detecting COVID-19 from Breath: A Game Changer for a Big Challenge. ACS Sens. 2021, 6, 1408–1417. [Google Scholar] [CrossRef]
- Shan, B.; Broza, Y.Y.; Li, W.; Wang, Y.; Wu, S.; Liu, Z.; Wang, J.; Gui, S.; Wang, L.; Zhang, Z.; et al. Multiplexed Nanomaterial-Based Sensor Array for Detection of COVID-19 in Exhaled Breath. ACS Nano 2020, 14, 12125–12132. [Google Scholar] [CrossRef] [PubMed]
- Abd Ellah, N.H.; Gad, S.F.; Muhammad, K.; Batiha, G.E.; Hetta, H.F. Nanomedicine as a Promising Approach for Diagnosis, Treatment and Prophylaxis against COVID-19. Nanomedicine 2020, 15, 2085–2102. [Google Scholar] [CrossRef] [PubMed]
- Somvanshi, S.; Kharat, B.P.; Saraf, S.T.; Somwanshi, S.; Shejul, S.; Jadhav, K. Multifunctional Nano-Magnetic Particles Assisted Viral RNA-Extraction Protocol for Potential Detection of COVID-19. Mater. Res. Innov. 2020, 25, 169–174. [Google Scholar] [CrossRef]
- Pinals, R.L.; Ledesma, F.; Yang, D.; Navarro, N.; Jeong, S.; Pak, J.E.; Kuo, L.; Chuang, Y.C.; Cheng, Y.W.; Sun, H.Y.; et al. Rapid SARS-CoV-2 Spike Protein Detection by Carbon Nanotube-Based Near-Infrared Nanosensors. Nano Lett. 2021, 21, 2272–2280. [Google Scholar] [CrossRef]
- Torrente-Rodríguez, R.M.; Lukas, H.; Tu, J.; Min, J.; Yang, Y.; Xu, C.; Rossiter, H.B.; Gao, W. SARS-CoV-2 RapidPlex: A Graphene-Based Multiplexed Telemedicine Platform for Rapid and Low-Cost COVID-19 Diagnosis and Monitoring. Matter 2020, 3, 1981–1998. [Google Scholar] [CrossRef]
- Liu, D.; Ju, C.; Han, C.; Shi, R.; Chen, X.; Duan, D.; Yan, J.; Yan, X. Nanozyme Chemiluminescence Paper Test for Rapid and Sensitive Detection of SARS-CoV-2 Antigen. Biosens. Bioelectron. 2021, 173, 112817. [Google Scholar] [CrossRef]
- Peer, D.; Karp, J.M.; Hong, S.; Farokhzad, O.C.; Margalit, R.; Langer, R. Nanocarriers as an Emerging Platform for Cancer Therapy. Nat. Nanotechnol. 2007, 2, 751–760. [Google Scholar] [CrossRef]
- Nikazar, S.; Sivasankarapillai, V.S.; Rahdar, A.; Gasmi, S.; Anumol, P.S.; Shanavas, M.S. Revisiting the Cytotoxicity of Quantum Dots: An in-Depth Overview. Biophys. Rev. 2020, 12, 703–718. [Google Scholar] [CrossRef]
- Manivannan, S.; Ponnuchamy, K. Quantum Dots as a Promising Agent to Combat COVID-19. Appl. Organomet. Chem. 2020, 34, e5887. [Google Scholar] [CrossRef]
- Ashiba, H.; Sugiyama, Y.; Wang, X.; Shirato, H.; Higo-Moriguchi, K.; Taniguchi, K.; Ohki, Y.; Fujimaki, M. Detection of Norovirus Virus-like Particles Using a Surface Plasmon Resonance-Assisted Fluoroimmunosensor Optimized for Quantum Dot Fluorescent Labels. Biosens. Bioelectron. 2017, 93, 260–266. [Google Scholar] [CrossRef] [Green Version]
- Roh, C.; Jo, S.K. Quantitative and Sensitive Detection of SARS Coronavirus Nucleocapsid Protein Using Quantum Dots-Conjugated RNA Aptamer on Chip. J. Chem. Technol. Biotechnol. 2011, 86, 1475–1479. [Google Scholar] [CrossRef]
- Liu, S.L.; Wang, Z.G.; Xie, H.Y.; Liu, A.A.; Lamb, D.C.; Pang, D.W. Single-Virus Tracking: From Imaging Methodologies to Virological Applications. Chem. Rev. 2020, 120, 1936–1979. [Google Scholar] [CrossRef] [Green Version]
- Georgakilas, V.; Perman, J.A.; Tucek, J.; Zboril, R. Broad Family of Carbon Nanoallotropes: Classification, Chemistry, and Applications of Fullerenes, Carbon Dots, Nanotubes, Graphene, Nanodiamonds, and Combined Superstructures. Chem. Rev. 2015, 115, 4744–4822. [Google Scholar] [CrossRef]
- Xu, X.; Ray, R.; Gu, Y.; Ploehn, H.J.; Gearheart, L.; Raker, K.; Scrivens, W.A. Electrophoretic Analysis and Purification of Fluorescent Single-Walled Carbon Nanotube Fragments. J. Am. Chem. Soc. 2004, 126, 12736–12737. [Google Scholar] [CrossRef]
- Hola, K.; Zhang, Y.; Wang, Y.; Giannelis, E.P.; Zboril, R.; Rogach, A.L. Carbon Dots—Emerging Light Emitters for Bioimaging, Cancer Therapy and Optoelectronics. Nano Today 2014, 9, 590–603. [Google Scholar] [CrossRef]
- Lim, S.Y.; Shen, W.; Gao, Z. Carbon Quantum Dots and Their Applications. Chem. Soc. Rev. 2015, 44, 362–381. [Google Scholar] [CrossRef]
- Huang, Q.; Chen, Y.; Liu, L.; Tao, D.; Li, X. On Combining Biclustering Mining and AdaBoost for Breast Tumor Classification. IEEE Trans. Knowl. Data Eng. 2019, 32, 728–738. [Google Scholar] [CrossRef]
- Yeh, Y.-T.; Tang, Y.; Sebastian, A.; Dasgupta, A.; Perea-Lopez, N.; Albert, I.; Lu, H.; Terrones, M.; Zheng, S.-Y. Tunable and Label-Free Virus Enrichment for Ultrasensitive Virus Detection Using Carbon Nanotube Arrays. Sci. Adv. 2016, 2, e1601026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, M.A.; Hu, C.; Jahan, S.; Yuan, B.; Saleh, M.S.; Ju, E.; Gao, S.-J.; Panat, R. Sensing of COVID-19 Antibodies in Seconds via Aerosol Jet Nanoprinted Reduced-Graphene-Oxide-Coated 3D Electrodes. Adv. Mater. 2021, 33, 2006647. [Google Scholar] [CrossRef] [PubMed]
- Miller, B.S.; Bezinge, L.; Gliddon, H.D.; Huang, D.; Dold, G.; Gray, E.R.; Heaney, J.; Dobson, P.J.; Nastouli, E.; Morton, J.J.L.; et al. Spin-Enhanced Nanodiamond Biosensing for Ultrasensitive Diagnostics. Nature 2020, 587, 588–593. [Google Scholar] [CrossRef] [PubMed]
- Nasrollahzadeh, M.; Sajjadi, M.; Soufi, G.J.; Iravani, S.; Varma, R.S. Nanomaterials and Nanotechnology-Associated Innovations against Viral Infections with a Focus on Coronaviruses. Nanomaterials 2020, 10, 1072. [Google Scholar] [CrossRef]
- Jiang, D.; Ni, D.; Rosenkrans, Z.T.; Huang, P.; Yan, X.; Cai, W. Nanozyme: New Horizons for Responsive Biomedical Applications. Chem. Soc. Rev. 2019, 48, 3683–3704. [Google Scholar] [CrossRef]
- Wang, Q.; Wei, H.; Zhang, Z.; Wang, E.; Dong, S. Nanozyme: An Emerging Alternative to Natural Enzyme for Biosensing and Immunoassay. TrAC Trends Anal. Chem. 2018, 105, 218–224. [Google Scholar] [CrossRef]
- Wei, H.; Wang, E. Nanomaterials with Enzyme-like Characteristics (Nanozymes): Next-Generation Artificial Enzymes. Chem. Soc. Rev. 2013, 42, 6060–6093. [Google Scholar] [CrossRef]
- Flühmann, B.; Ntai, I.; Borchard, G.; Simoens, S.; Mühlebach, S. Nanomedicines: The Magic Bullets Reaching Their Target? Eur. J. Pharm. Sci. 2019, 128, 73–80. [Google Scholar] [CrossRef]
- Kerry, R.G.; Malik, S.; Redda, Y.T.; Sahoo, S.; Patra, J.K.; Majhi, S. Nano-Based Approach to Combat Emerging Viral (NIPAH Virus) Infection. Nanomed. Nanotechnol. Biol. Med. 2019, 18, 196–220. [Google Scholar] [CrossRef]
- Milovanovic, M.; Arsenijevic, A.; Milovanovic, J.; Kanjevac, T.; Arsenijevic, N. Nanoparticles in antiviral therapy. In Antimicrobial Nanoarchitectonics; Elsevier: Amsterdam, The Netherlands, 2017; pp. 383–410. [Google Scholar]
- Hang, X.; Peng, H.; Song, H.; Qi, Z.; Miao, X.; Xu, W. Antiviral Activity of Cuprous Oxide Nanoparticles against Hepatitis C Virus in Vitro. J. Virol. Methods 2015, 222, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-N.; Hsueh, Y.-H.; Hsieh, C.-T.; Tzou, D.-Y.; Chang, P.-L. Antiviral Activity of Graphene–Silver Nanocomposites against Non-Enveloped and Enveloped Viruses. Int. J. Environ. Res. Public Health 2016, 13, 430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gartner, T.E., III; Jayaraman, A. Modeling and Simulations of Polymers: A Roadmap. Macromolecules 2019, 52, 755–786. [Google Scholar] [CrossRef] [Green Version]
- McNeil, S.E. Unique benefits of nanotechnology to drug delivery and diagnostics. In Characterization of Nanoparticles Intended for Drug Delivery; Springer: Cham, Switzerland, 2011; pp. 3–8. [Google Scholar]
- Petros, R.A.; DeSimone, J.M. Strategies in the Design of Nanoparticles for Therapeutic Applications. Nat. Rev. Drug Discov. 2010, 9, 615–627. [Google Scholar] [CrossRef] [PubMed]
- Galdiero, S.; Falanga, A.; Vitiello, M.; Cantisani, M.; Marra, V.; Galdiero, M. Silver Nanoparticles as Potential Antiviral Agents. Molecules 2011, 16, 8894–8918. [Google Scholar] [CrossRef] [Green Version]
- Gupta, S.; Krishnakumar, V.; Sharma, Y.; Dinda, A.K.; Mohanty, S. Mesenchymal Stem Cell Derived Exosomes: A Nano Platform for Therapeutics and Drug Delivery in Combating COVID-19. Stem Cell Rev. Rep. 2020, 17, 1–11. [Google Scholar]
- Akbari, A.; Rezaie, J. Potential Therapeutic Application of Mesenchymal Stem Cell-Derived Exosomes in SARS-CoV-2 Pneumonia. Stem Cell Res. Ther. 2020, 11, 1–10. [Google Scholar] [CrossRef]
- Alzahrani, F.A.; Saadeldin, I.M.; Ahmad, A.; Kumar, D.; Azhar, E.I.; Siddiqui, A.J.; Kurdi, B.; Sajini, A.; Alrefaei, A.F.; Jahan, S. The Potential Use of Mesenchymal Stem Cells and Their Derived Exosomes as Immunomodulatory Agents for COVID-19 Patients. Stem Cells Int. 2020, 2020, e8835986. [Google Scholar] [CrossRef]
- Vader, P.; Mol, E.A.; Pasterkamp, G.; Schiffelers, R.M. Extracellular Vesicles for Drug Delivery. Adv. Drug Deliv. Rev. 2016, 106, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Bunggulawa, E.J.; Wang, W.; Yin, T.; Wang, N.; Durkan, C.; Wang, Y.; Wang, G. Recent Advancements in the Use of Exosomes as Drug Delivery Systems. J. Nanobiotechnol. 2018, 16, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Pascucci, L.; Coccè, V.; Bonomi, A.; Ami, D.; Ceccarelli, P.; Ciusani, E.; Viganò, L.; Locatelli, A.; Sisto, F.; Doglia, S.M. Paclitaxel Is Incorporated by Mesenchymal Stromal Cells and Released in Exosomes That Inhibit in Vitro Tumor Growth: A New Approach for Drug Delivery. J. Control. Release 2014, 192, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Lakhal, S.; Wood, M.J. Exosome Nanotechnology: An Emerging Paradigm Shift in Drug Delivery: Exploitation of Exosome Nanovesicles for Systemic in Vivo Delivery of RNAi Heralds New Horizons for Drug Delivery across Biological Barriers. Bioessays 2011, 33, 737–741. [Google Scholar] [CrossRef] [PubMed]
- Gattinoni, L.; Coppola, S.; Cressoni, M.; Busana, M.; Rossi, S.; Chiumello, D. COVID-19 Does Not Lead to a “Typical” Acute Respiratory Distress Syndrome. Am. J. Respir. Crit. Care Med. 2020, 201, 1299–1300. [Google Scholar] [CrossRef] [Green Version]
- Bhavana, V.; Thakor, P.; Singh, S.B.; Mehra, N.K. COVID-19: Pathophysiology, Treatment Options, Nanotechnology Approaches, and Research Agenda to Combating the SARS-CoV2 Pandemic. Life Sci. 2020, 261, 118336. [Google Scholar] [CrossRef]
- Sarkar, S. Silver Nanoparticles with Bronchodilators Through Nebulisation to Treat COVID 19 Patients. J. Curr. Med. Res. Opin. 2020, 3, 449–450. [Google Scholar] [CrossRef] [Green Version]
- Murugan, K.; Wei, J.; Alsalhi, M.S.; Nicoletti, M.; Paulpandi, M.; Samidoss, C.M.; Dinesh, D.; Chandramohan, B.; Paneerselvam, C.; Subramaniam, J. Magnetic Nanoparticles Are Highly Toxic to Chloroquine-Resistant Plasmodium Falciparum, Dengue Virus (DEN-2), and Their Mosquito Vectors. Parasitol. Res. 2017, 116, 495–502. [Google Scholar] [CrossRef]
- Jeremiah, S.S.; Miyakawa, K.; Morita, T.; Yamaoka, Y.; Ryo, A. Potent Antiviral Effect of Silver Nanoparticles on SARS-CoV-2. Biochem. Biophys. Res. Commun. 2020, 533, 195–200. [Google Scholar] [CrossRef]
- Behbudi, G. Effect of Silver Nanoparticles Disinfectant on COVID-19. Adv. Appl. NanoBioTechnol. 2021, 2, 63–67. [Google Scholar]
- Williams, K.; Milner, J.; Boudreau, M.D.; Gokulan, K.; Cerniglia, C.E.; Khare, S. Effects of Subchronic Exposure of Silver Nanoparticles on Intestinal Microbiota and Gut-Associated Immune Responses in the Ileum of Sprague-Dawley Rats. Nanotoxicology 2015, 9, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Marques Neto, L.M.; Kipnis, A.; Junqueira-Kipnis, A.P. Role of Metallic Nanoparticles in Vaccinology: Implications for Infectious Disease Vaccine Development. Front. Immunol. 2017, 8, 239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Itani, R.; Tobaiqy, M.; Al Faraj, A. Optimizing Use of Theranostic Nanoparticles as a Life-Saving Strategy for Treating COVID-19 Patients. Theranostics 2020, 10, 5932. [Google Scholar] [CrossRef] [PubMed]
- Arias, L.S.; Pessan, J.P.; Vieira, A.P.M.; Lima, T.M.T.; de Delbem, A.C.B.; Monteiro, D.R. Iron Oxide Nanoparticles for Biomedical Applications: A Perspective on Synthesis, Drugs, Antimicrobial Activity, and Toxicity. Antibiotics 2018, 7, 46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abo-zeid, Y.; Williams, G.R. The Potential Anti-infective Applications of Metal Oxide Nanoparticles: A Systematic Review. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2020, 12, e1592. [Google Scholar] [CrossRef]
- Raghunath, A.; Perumal, E. Metal Oxide Nanoparticles as Antimicrobial Agents: A Promise for the Future. Int. J. Antimicrob. Agents 2017, 49, 137–152. [Google Scholar] [CrossRef]
- Kumar, R.; Nayak, M.; Sahoo, G.C.; Pandey, K.; Sarkar, M.C.; Ansari, Y.; Das, V.; Topno, R.; Madhukar, M.; Das, P. Iron Oxide Nanoparticles Based Antiviral Activity of H1N1 Influenza A Virus. J. Infect. Chemother. 2019, 25, 325–329. [Google Scholar] [CrossRef]
- Gutierrez, L.; Li, X.; Wang, J.; Nangmenyi, G.; Economy, J.; Kuhlenschmidt, T.B.; Kuhlenschmidt, M.S.; Nguyen, T.H. Adsorption of Rotavirus and Bacteriophage MS2 Using Glass Fiber Coated with Hematite Nanoparticles. Water Res. 2009, 43, 5198–5208. [Google Scholar] [CrossRef]
- Coyne, D.W. Ferumoxytol for Treatment of Iron Deficiency Anemia in Patients with Chronic Kidney Disease. Expert Opin. Pharmacother. 2009, 10, 2563–2568. [Google Scholar] [CrossRef]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on Zinc Oxide Nanoparticles: Antibacterial Activity and Toxicity Mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef] [Green Version]
- Fouad, G.I. A Proposed Insight into the Anti-Viral Potential of Metallic Nanoparticles against Novel Coronavirus Disease-19 (COVID-19). Bull. Natl. Res. Cent. 2021, 45, 1–22. [Google Scholar]
- Ghaffari, H.; Tavakoli, A.; Moradi, A.; Tabarraei, A.; Bokharaei-Salim, F.; Zahmatkeshan, M.; Farahmand, M.; Javanmard, D.; Kiani, S.J.; Esghaei, M. Inhibition of H1N1 Influenza Virus Infection by Zinc Oxide Nanoparticles: Another Emerging Application of Nanomedicine. J. Biomed. Sci. 2019, 26, 70. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Hu, Z.; Zabihi, F.; Chen, Z.; Zhu, M. Progress and Perspective of Antiviral Protective Material. Adv. Fiber Mater. 2020, 2, 123–139. [Google Scholar] [CrossRef]
- Yadavalli, T.; Shukla, D. Role of Metal and Metal Oxide Nanoparticles as Diagnostic and Therapeutic Tools for Highly Prevalent Viral Infections. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 219–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Jamal, W.; Kostarelos, K. Liposome–Nanoparticle Hybrids for Multimodal Diagnostic and Therapeutic Applications. Nanomedicine 2007, 2, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Rafiei, S.; Rezatofighi, S.E.; Ardakani, M.R.; Rastegarzadeh, S. Gold Nanoparticles Impair Foot-and-Mouth Disease Virus Replication. IEEE Trans. Nanobioscience 2015, 15, 34–40. [Google Scholar] [CrossRef]
- Lysenko, V.; Lozovski, V.; Lokshyn, M.; Gomeniuk, Y.V.; Dorovskih, A.; Rusinchuk, N.; Pankivska, Y.; Povnitsa, O.; Zagorodnya, S.; Tertykh, V. Nanoparticles as Antiviral Agents against Adenoviruses. Adv. Nat. Sci. Nanosci. Nanotechnol. 2018, 9, 025021. [Google Scholar] [CrossRef]
- Ye, S.; Shao, K.; Li, Z.; Guo, N.; Zuo, Y.; Li, Q.; Lu, Z.; Chen, L.; He, Q.; Han, H. Antiviral Activity of Graphene Oxide: How Sharp Edged Structure and Charge Matter. ACS Appl. Mater. Interfaces 2015, 7, 21571–21579. [Google Scholar] [CrossRef] [PubMed]
- Stein, A.Z.; Wang, G.; Fierke, M.A. Functionalization of Porous Carbon Materials with Designed Pore Architecture. Adv. Mater 2009, 21, 265. [Google Scholar] [CrossRef]
- Matsushita, T.; Suzuki, H.; Shirasaki, N.; Matsui, Y.; Ohno, K. Adsorptive Virus Removal with Super-Powdered Activated Carbon. Sep. Purif. Technol. 2013, 107, 79–84. [Google Scholar] [CrossRef]
- Serrano-Aroca, Á.; Takayama, K.; Tuñón-Molina, A.; Seyran, M.; Hassan, S.S.; Choudhury, P.P.; Uversky, V.N.; Lundstrom, K.; Adadi, P.; Palù, G. Carbon-Based Nanomaterials: Promising Antiviral Agents to Combat COVID-19 in the Microbial Resistant Era. ACS Nano 2021, 15, 8069–8086. [Google Scholar] [CrossRef]
- Zhu, S.; Li, J.; Huang, A.-G.; Huang, J.-Q.; Huang, Y.-Q.; Wang, G.-X. Anti-Betanodavirus Activity of Isoprinosine and Improved Efficacy Using Carbon Nanotubes Based Drug Delivery System. Aquaculture 2019, 512, 734377. [Google Scholar] [CrossRef]
- Zhu, B.; Liu, G.-L.; Ling, F.; Wang, G.-X. Carbon Nanotube-Based Nanocarrier Loaded with Ribavirin against Grass Carp Reovirus. Antivir. Res. 2015, 118, 29–38. [Google Scholar] [CrossRef]
- Hassanzadeh, P.; Arbabi, E.; Atyabi, F.; Dinarvand, R. Application of Carbon Nanotubes as the Carriers of the Cannabinoid, 2-Arachidonoylglycerol: Towards a Novel Treatment Strategy in Colitis. Life Sci. 2017, 179, 66–72. [Google Scholar] [CrossRef]
- Hassanzadeh, P.; Arbabi, E.; Atyabi, F.; Dinarvand, R. Nerve Growth Factor-Carbon Nanotube Complex Exerts Prolonged Protective Effects in an in Vitro Model of Ischemic Stroke. Life Sci. 2017, 179, 15–22. [Google Scholar] [CrossRef]
- Hassanzadeh, P.; Arbabi, E.; Atyabi, F.; Dinarvand, R. Carbon Nanotubes Provide Longer Lasting Gastroprotective Effects for Anandamide in Stress-Induced Gastric Ulcer in Rat. Physiol. Pharmacol. 2018, 22, 38–47. [Google Scholar]
- Hassanzadeh, P.; Atyabi, F.; Dinarvand, R. Application of Carbon Nanotubes for Controlled Release of Growth Factors or Endocannabinoids: A Breakthrough in Biomedicine. Biomed. Rev. 2017, 27, 41–50. [Google Scholar] [CrossRef] [Green Version]
- Hassanzadeh, P.; Arbabi, E.; Rostami, F.; Atyabi, F.; Dinarvand, R. Carbon Nanotubes Prolong the Regulatory Action of Nerve Growth Factor on the Endocannabinoid Signaling. Physiol. Pharmacol. 2015, 19, 167–176. [Google Scholar]
- Elechiguerra, J.L.; Burt, J.L.; Morones, J.R.; Camacho-Bragado, A.; Gao, X.; Lara, H.H.; Yacaman, M.J. Interaction of Silver Nanoparticles with HIV-1. J. Nanobiotechnol. 2005, 3, 6. [Google Scholar] [CrossRef] [Green Version]
- Ting, D.; Dong, N.; Fang, L.; Lu, J.; Bi, J.; Xiao, S.; Han, H. Multisite Inhibitors for Enteric Coronavirus: Antiviral Cationic Carbon Dots Based on Curcumin. ACS Appl. Nano Mater. 2018, 1, 5451–5459. [Google Scholar] [CrossRef]
- Garg, P.; Sangam, S.; Kochhar, D.; Pahari, S.; Kar, C.; Mukherjee, M. Exploring the Role of Triazole Functionalized Heteroatom Co-Doped Carbon Quantum Dots against Human Coronaviruses. Nano Today 2020, 35, 101001. [Google Scholar] [CrossRef] [PubMed]
- Smelcerovic, A.; Kocic, G.; Gajic, M.; Tomovic, K.; Djordjevic, V.; Stankovic-Djordjevic, D.; Anderluh, M. DPP-4 Inhibitors in the Prevention/Treatment of Pulmonary Fibrosis, Heart and Kidney Injury Caused by COVID-19—A Therapeutic Approach of Choice in Type 2 Diabetic Patients? Front. Pharmacol. 2020, 11, 1185. [Google Scholar] [CrossRef] [PubMed]
- Gholami, M.F.; Lauster, D.; Ludwig, K.; Storm, J.; Ziem, B.; Severin, N.; Böttcher, C.; Rabe, J.P.; Herrmann, A.; Adeli, M. Functionalized Graphene as Extracellular Matrix Mimics: Toward Well-Defined 2D Nanomaterials for Multivalent Virus Interactions. Adv. Funct. Mater. 2017, 27, 1606477. [Google Scholar] [CrossRef]
- Tong, T.; Hu, H.; Zhou, J.; Deng, S.; Zhang, X.; Tang, W.; Fang, L.; Xiao, S.; Liang, J. Glycyrrhizic-acid-based Carbon Dots with High Antiviral Activity by Multisite Inhibition Mechanisms. Small 2020, 16, 1906206. [Google Scholar] [CrossRef] [Green Version]
- Wiehe, A.; O’Brien, J.M.; Senge, M.O. Trends and Targets in Antiviral Phototherapy. Photochem. Photobiol. Sci. 2019, 18, 2565–2612. [Google Scholar] [CrossRef]
- Rodríguez-González, V.; Obregón, S.; Patrón-Soberano, O.A.; Terashima, C.; Fujishima, A. An Approach to the Photocatalytic Mechanism in the TiO2-Nanomaterials Microorganism Interface for the Control of Infectious Processes. Appl. Catal. B Environ. 2020, 270, 118853. [Google Scholar] [CrossRef]
- Loutfy, S.A.; Elberry, M.H.; Farroh, K.Y.; Mohamed, H.T.; Mohamed, A.A.; Mohamed, E.B.; Faraag, A.H.I.; Mousa, S.A. Antiviral Activity of Chitosan Nanoparticles Encapsulating Curcumin Against Hepatitis C Virus Genotype 4a in Human Hepatoma Cell Lines. Int. J. Nanomed. 2020, 15, 2699. [Google Scholar] [CrossRef] [Green Version]
- Zahedipour, F.; Hosseini, S.A.; Sathyapalan, T.; Majeed, M.; Jamialahmadi, T.; Al-Rasadi, K.; Banach, M.; Sahebkar, A. Potential Effects of Curcumin in the Treatment of COVID-19 Infection. Phytother. Res. 2020, 34, 2911–2920. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X. Clinical Features of Patients Infected with 2019 Novel Coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Salabei, J.K.; Conklin, D.J. Cardiovascular Autophagy: Crossroads of Pathology, Pharmacology and Toxicology. Cardiovasc. Toxicol. 2013, 13, 220–229. [Google Scholar] [CrossRef]
- Sahebkar, A.; Henrotin, Y. Analgesic Efficacy and Safety of Curcuminoids in Clinical Practice: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Pain Med. 2016, 17, 1192–1202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Fang, Q.; Tian, X.; Wang, X.; Ao, Q.; Hou, W.; Tong, H.; Fan, J.; Bai, S. Curcumin Attenuates the Development of Thoracic Aortic Aneurysm by Inhibiting VEGF Expression and Inflammation. Mol. Med. Rep. 2017, 16, 4455–4462. [Google Scholar] [CrossRef] [PubMed]
- Thimmulappa, R.K.; Kumar, M.N.K.; Shivamallu, C.; Subramaniam, K.T.; Radhakrishnan, A.; Suresh, B.; Kuppusamy, G. Antiviral and Immunomodulatory Activity of Curcumin: A Case for Prophylactic Therapy for COVID-19. Heliyon 2021, 7, e06350. [Google Scholar] [CrossRef]
- Lammers, T.; Sofias, A.M.; van der Meel, R.; Schiffelers, R.; Storm, G.; Tacke, F.; Koschmieder, S.; Brümmendorf, T.H.; Kiessling, F.; Metselaar, J.M. Dexamethasone Nanomedicines for COVID-19. Nat. Nanotechnol. 2020, 15, 622–624. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, A.; Fisch, A.; Seuwen, K.; Baumgarten, B.; Ruffner, H.; Aebi, A.; Rausch, M.; Kiessling, F.; Bartneck, M.; Weiskirchen, R. Glucocorticoid-Loaded Liposomes Induce a pro-Resolution Phenotype in Human Primary Macrophages to Support Chronic Wound Healing. Biomaterials 2018, 178, 481–495. [Google Scholar] [CrossRef]
- Quan, L.; Zhang, Y.; Crielaard, B.J.; Dusad, A.; Lele, S.M.; Rijcken, C.J.; Metselaar, J.M.; Kostková, H.; Etrych, T.; Ulbrich, K. Nanomedicines for Inflammatory Arthritis: Head-to-Head Comparison of Glucocorticoid-Containing Polymers, Micelles, and Liposomes. ACS Nano 2014, 8, 458–466. [Google Scholar] [CrossRef] [Green Version]
- Metselaar, J.M.; Wauben, M.H.; Wagenaar-Hilbers, J.P.; Boerman, O.C.; Storm, G. Complete Remission of Experimental Arthritis by Joint Targeting of Glucocorticoids with Long-circulating Liposomes. Arthritis Rheum. Off. J. Am. Coll. Rheumatol. 2003, 48, 2059–2066. [Google Scholar] [CrossRef]
- George, P.M.; Wells, A.U.; Jenkins, R.G. Pulmonary Fibrosis and COVID-19: The Potential Role for Antifibrotic Therapy. Lancet Respir. Med. 2020, 8, 807–815. [Google Scholar] [CrossRef]
- Johnson, R.M.; Vinetz, J.M. Dexamethasone in the Management of Covid-19. BMJ 2020, 370. [Google Scholar] [CrossRef] [PubMed]
- Lester, M.; Sahin, A.; Pasyar, A. The Use of Dexamethasone in the Treatment of COVID-19. Ann. Med. Surg. 2020, 56, 218. [Google Scholar] [CrossRef] [PubMed]
- Horby, P.; Landrain, M. Low-Cost Dexamethasone Reduces Death by up to One Third in Hospitalised Patients with Severe Respiratory Complications of COVID-19. RECOVERY Trial Press Release 2020, 5, 2020. [Google Scholar]
- Selvaraj, V.; Manne, N.D.; Arvapalli, R.; Rice, K.M.; Nandyala, G.; Fankenhanel, E.; Blough, E.R. Effect of Cerium Oxide Nanoparticles on Sepsis Induced Mortality and NF-κB Signaling in Cultured Macrophages. Nanomedicine 2015, 10, 1275–1288. [Google Scholar] [CrossRef] [PubMed]
- Selvaraj, V.; Nepal, N.; Rogers, S.; Manne, N.D.; Arvapalli, R.; Rice, K.M.; Asano, S.; Fankhanel, E.; Ma, J.J.; Shokuhfar, T. Inhibition of MAP Kinase/NF-KB Mediated Signaling and Attenuation of Lipopolysaccharide Induced Severe Sepsis by Cerium Oxide Nanoparticles. Biomaterials 2015, 59, 160–171. [Google Scholar] [CrossRef] [Green Version]
- Manne, N.D.; Arvapalli, R.; Nepal, N.; Thulluri, S.; Selvaraj, V.; Shokuhfar, T.; He, K.; Rice, K.M.; Asano, S.; Maheshwari, M. Therapeutic Potential of Cerium Oxide Nanoparticles for the Treatment of Peritonitis Induced by Polymicrobial Insult in Sprague-Dawley Rats. Crit. Care Med. 2015, 43, e477–e489. [Google Scholar] [CrossRef] [PubMed]
- Khurana, A.; Tekula, S.; Godugu, C. Nanoceria Suppresses Multiple Low Doses of Streptozotocin-Induced Type 1 Diabetes by Inhibition of Nrf2/NF-κB Pathway and Reduction of Apoptosis. Nanomedicine 2018, 13, 1905–1922. [Google Scholar] [CrossRef]
- Allawadhi, P.; Khurana, A.; Allwadhi, S.; Joshi, K.; Packirisamy, G.; Bharani, K.K. Nanoceria as a Possible Agent for the Management of COVID-19. Nano Today 2020, 35, 100982. [Google Scholar] [CrossRef]
- Rzigalinski, B.A.; Seal, S.; Bailey, D.; Patil, S. Cerium Oxide Nanoparticles and Use in Enhancing Cell Survivability. U.S. Patent 7,534,453, 19 May 2009. [Google Scholar]
- Singh, N.; Cohen, C.A.; Rzigalinski, B.A. Treatment of Neurodegenerative Disorders with Radical Nanomedicine. Ann. N. Y. Acad. Sci. 2007, 1122, 219–230. [Google Scholar] [CrossRef]
- Halliwell, B. Oxidative Stress and Neurodegeneration: Where Are We Now? J. Neurochem. 2006, 97, 1634–1658. [Google Scholar] [CrossRef] [PubMed]
- Arya, A.; Sethy, N.K.; Singh, S.K.; Das, M.; Bhargava, K. Cerium Oxide Nanoparticles Protect Rodent Lungs from Hypobaric Hypoxia-Induced Oxidative Stress and Inflammation. Int. J. Nanomed. 2013, 8, 4507. [Google Scholar]
- Hassanzadeh, P. Nanotheranostics against COVID-19: From Multivalent to Immune-Targeted Materials. J. Control. Release 2020, 328, 112–126. [Google Scholar] [CrossRef] [PubMed]


| Nanomaterial | Function | Main Role for COVID-19 | Refs. |
|---|---|---|---|
| Metal nanoparticle |
| prevention, diagnostics and treatment | [14] |
| Lipid nanoparticle |
| prevention | [15] |
| Magnetic nanoparticle |
| diagnostics | [16] |
| Quantum dot |
| virus inactivation, prevention, diagnostics and treatment | [17] |
| Carbon-based nanoparticle |
| virus inactivation, prevention, diagnostics and treatment | [8] |
| Nanodrug |
| treatment | [18] |
| Nanozyme |
| diagnostics | [19] |
| Exosome |
| treatment | [20] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rasmi, Y.; Saloua, K.S.; Nemati, M.; Choi, J.R. Recent Progress in Nanotechnology for COVID-19 Prevention, Diagnostics and Treatment. Nanomaterials 2021, 11, 1788. https://doi.org/10.3390/nano11071788
Rasmi Y, Saloua KS, Nemati M, Choi JR. Recent Progress in Nanotechnology for COVID-19 Prevention, Diagnostics and Treatment. Nanomaterials. 2021; 11(7):1788. https://doi.org/10.3390/nano11071788
Chicago/Turabian StyleRasmi, Yousef, Kouass Sahbani Saloua, Mahdieh Nemati, and Jane Ru Choi. 2021. "Recent Progress in Nanotechnology for COVID-19 Prevention, Diagnostics and Treatment" Nanomaterials 11, no. 7: 1788. https://doi.org/10.3390/nano11071788
APA StyleRasmi, Y., Saloua, K. S., Nemati, M., & Choi, J. R. (2021). Recent Progress in Nanotechnology for COVID-19 Prevention, Diagnostics and Treatment. Nanomaterials, 11(7), 1788. https://doi.org/10.3390/nano11071788






