Electrically Conductive Networks from Hybrids of Carbon Nanotubes and Graphene Created by Laser Radiation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Method for Modeling of Contacts Formation between CNTs and Graphene Sheets
2.2. Method of Creating Homogeneous Dispersed Media from Carbon Nanomaterials
2.3. Method of Thin Layers Application
2.4. Method of Laser Formation of Carbon Nanomaterials Films
2.5. Materials Characterization
2.5.1. Scanning Electron Microscopy
2.5.2. Raman Spectroscopy
2.5.3. Hardness Measurement
2.5.4. Conductivity Measurement
3. Results and Discussion
3.1. Modeling the Process of Forming SWCNTs and Graphene Sheets Hybrids
3.2. Structural Features of the Created Carbon Nanomaterials
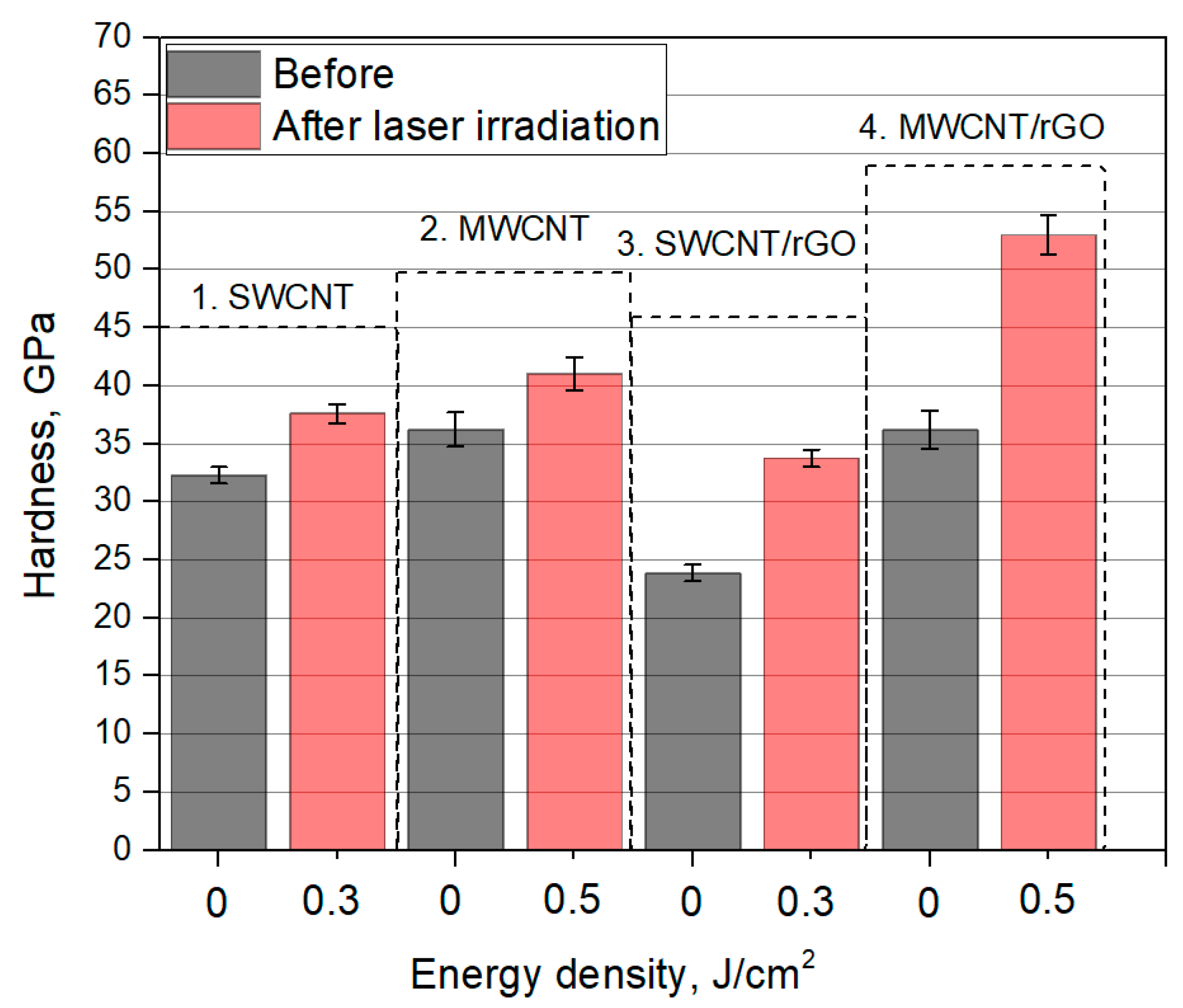
3.3. Hardness of Nanomaterials
3.4. Electrical Conductivity of Nanomaterials
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ramalingame, R.; Lakshmanan, A.; Müller, F.; Thomas, U.; Kanoun, O. Highly sensitive capacitive pressure sensors for robotic applications based on carbon nanotubes and PDMS polymer nanocomposite. J. Sens. Sens. Syst. 2019, 8, 87–94. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Tran, V.-T.; Du, H.; Wang, J.; Chen, C. A Direct-Writing Approach for Fabrication of CNT/Paper-Based Piezoresistive Pressure Sensors for Airflow Sensing. Micromachines 2021, 12, 504. [Google Scholar] [CrossRef]
- Wang, G.; Liu, J.; Chen, K.; Pathak, R.; Gurung, A.; Qiao, Q. High-performance carbon electrode-based CsPbI2Br inorganic perovskite solar cell based on poly(3-hexylthiophene)-carbon nanotubes composite hole-transporting layer. J. Colloid Interface Sci. 2019, 555, 180–186. [Google Scholar] [CrossRef]
- Jeon, I.; Matsuo, Y.; Maruyama, S. Single-Walled Carbon Nanotubes in Solar Cells. Top. Curr. Chem. 2018, 376, 4. [Google Scholar] [CrossRef]
- Wu, X.; Xie, L.; Lin, K.; Lu, J.; Wang, K.; Feng, W.; Fan, B.; Yin, P.; Wei, Z. Efficient and stable carbon-based perovskite solar cells enabled by the inorganic interface of CuSCN and carbon nanotubes. J. Mater. Chem. A 2019, 7, 12236–12243. [Google Scholar] [CrossRef]
- Putra, N.; Rawi, S.; Amin, M.; Kusrini, E.; Kosasih, E.A.; Indra Mahlia, T.M. Preparation of beeswax/multi-walled carbon nanotubes as novel shape-stable nanocomposite phase-change material for thermal energy storage. J. Energy Storage 2019, 21, 32–39. [Google Scholar] [CrossRef]
- Yan, H.; Xue, X.; Fu, Y.; Wu, X.; Dong, J. Three-dimensional carbon nanotubes-encapsulated Li2FeSiO4 microspheres as advanced positive materials for lithium energy storage. Ceram. Int. 2020, 46, 9729–9733. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, H.; Lv, H.; Chen, Z.; Zhang, J.; Yan, X.; Lee, L.; Wang, Z.M.; Chueh, Y.-L. Highly stable three-dimensional nickel–cobalt hydroxide hierarchical heterostructures hybridized with carbon nanotubes for high-performance energy storage devices. ACS Nano 2019, 13, 11235–11248. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Wang, X.; Wang, Y.; Zhang, Q. Roles of carbon nanotubes in novel energy storage devices. Carbon N. Y. 2017, 122, 462–474. [Google Scholar] [CrossRef]
- Li, M.; Zhou, X.; Ma, X.; Chen, L.; Zhang, D.; Xu, S.; Duan, D.; Chen, C.; Yuan, Q.; Liu, S. Development of sulfonated-carbon nanotubes/graphene three-dimensional conductive spongy framework with ion-selective effect as cathode in high-performance lithium-sulfur batteries. Chem. Eng. J. 2021, 409, 128164. [Google Scholar] [CrossRef]
- Yu, J.; Ma, T.; Liu, S. Enhanced photocatalytic activity of mesoporous TiO2 aggregates by embedding carbon nanotubes as electron-transfer channel. Phys. Chem. Chem. Phys. 2011, 13, 3491–3501. [Google Scholar] [CrossRef]
- Fuhrer, M.S. Crossed Nanotube Junctions. Science 2000, 288, 494–497. [Google Scholar] [CrossRef] [Green Version]
- Close, G.F.; Yasuda, S.; Paul, B.; Fujita, S.; Wong, H.-S.P. A 1 GHz Integrated circuit with carbon nanotube interconnects and silicon transistors. Nano Lett. 2008, 8, 706–709. [Google Scholar] [CrossRef]
- Park, W.J.; Choi, K.J.; Kim, M.H.; Koo, B.H.; Lee, J.-L.; Baik, J.M. Self-assembled and highly selective sensors based on air-bridge-structured nanowire junction arrays. ACS Appl. Mater. Interfaces 2013, 5, 6802–6807. [Google Scholar] [CrossRef]
- Malapanis, A.; Perebeinos, V.; Sinha, D.P.; Comfort, E.; Lee, J.U. Quantum efficiency and capture cross section of first and second excitonic transitions of single-walled carbon nanotubes measured through photoconductivity. Nano Lett. 2013, 13, 3531–3538. [Google Scholar] [CrossRef]
- Franklin, A.D.; Koswatta, S.O.; Farmer, D.B.; Smith, J.T.; Gignac, L.; Breslin, C.M.; Han, S.-J.; Tulevski, G.S.; Miyazoe, H.; Haensch, W.; et al. Carbon nanotube complementary wrap-gate transistors. Nano Lett. 2013, 13, 2490–2495. [Google Scholar] [CrossRef]
- Cretu, O.; Botello-Mendez, A.R.; Janowska, I.; Pham-Huu, C.; Charlier, J.-C.; Banhart, F. Electrical transport measured in atomic carbon chains. Nano Lett. 2013, 13, 3487–3493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, H.; Moon, K.; Wong, C.P. Recent advances of nanolead-free solder material for low processing temperature interconnect applications. Microelectron. Reliab. 2013, 53, 1968–1978. [Google Scholar] [CrossRef]
- Eder, D. Carbon nanotube−inorganic hybrids. Chem. Rev. 2010, 110, 1348–1385. [Google Scholar] [CrossRef] [PubMed]
- Balandin, A.A. Thermal properties of graphene and nanostructured carbon materials. Nat. Mater. 2011, 10, 569–581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franklin, A.D.; Luisier, M.; Han, S.-J.; Tulevski, G.; Breslin, C.M.; Gignac, L.; Lundstrom, M.S.; Haensch, W. Sub-10 nm carbon nanotube transistor. Nano Lett. 2012, 12, 758–762. [Google Scholar] [CrossRef]
- Shulaker, M.M.; Hills, G.; Patil, N.; Wei, H.; Chen, H.-Y.; Wong, H.-S.P.; Mitra, S. Carbon nanotube computer. Nature 2013, 501, 526–530. [Google Scholar] [CrossRef]
- Shulaker, M.M.; Van Rethy, J.; Wu, T.F.; Suriyasena Liyanage, L.; Wei, H.; Li, Z.; Pop, E.; Gielen, G.; Wong, H.-S.P.; Mitra, S. Carbon nanotube circuit integration up to sub-20 nm channel lengths. ACS Nano 2014, 8, 3434–3443. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Celis, A.; Nair, M.N.; Taleb-Ibrahimi, A.; Conrad, E.H.; Berger, C.; de Heer, W.A.; Tejeda, A. Graphene nanoribbons: Fabrication, properties and devices. J. Phys. D. Appl. Phys. 2016, 49, 143001. [Google Scholar] [CrossRef]
- Miller, J.R.; Outlaw, R.A.; Holloway, B.C. Graphene double-layer capacitor with ac line-filtering performance. Science 2010, 329, 1637–1639. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Park, S.-J.; Kim, S. Synthesis and electrochemical characterization of nanostructured Ni-Co-MOF/graphene oxide composites as capacitor electrodes. Electrochim. Acta 2019, 311, 62–71. [Google Scholar] [CrossRef]
- Hao, Y.; Wang, S.; Shao, Y.; Wu, Y.; Miao, S. High-energy density Li-ion capacitor with Layered SnS2 /reduced graphene oxide anode and BCN nanosheet cathode. Adv. Energy Mater. 2020, 10, 1902836. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, X.; Fu, Y.; Wu, X.; Wang, Q.; Zhang, W.; Luo, C. Enhanced microwave absorption performances of polyaniline/graphene aerogel by covalent bonding. Compos. Part B Eng. 2019, 169, 221–228. [Google Scholar] [CrossRef]
- Yang, X.; Fan, S.; Li, Y.; Guo, Y.; Li, Y.; Ruan, K.; Zhang, S.; Zhang, J.; Kong, J.; Gu, J. Synchronously improved electromagnetic interference shielding and thermal conductivity for epoxy nanocomposites by constructing 3D copper nanowires/thermally annealed graphene aerogel framework. Compos. Part A Appl. Sci. Manuf. 2020, 128, 105670. [Google Scholar] [CrossRef]
- Farmani, A.; Mir, A. Graphene sensor based on surface plasmon resonance for optical scanning. IEEE Photonics Technol. Lett. 2019, 31, 643–646. [Google Scholar] [CrossRef]
- Patil, P.O.; Pandey, G.R.; Patil, A.G.; Borse, V.B.; Deshmukh, P.K.; Patil, D.R.; Tade, R.S.; Nangare, S.N.; Khan, Z.G.; Patil, A.M.; et al. Graphene-based nanocomposites for sensitivity enhancement of surface plasmon resonance sensor for biological and chemical sensing: A review. Biosens. Bioelectron. 2019, 139, 111324. [Google Scholar] [CrossRef] [PubMed]
- Tahernejad-Javazmi, F.; Shabani-Nooshabadi, M.; Karimi-Maleh, H. 3D reduced graphene oxide/FeNi3-ionic liquid nanocomposite modified sensor; an electrical synergic effect for development of tert-butylhydroquinone and folic acid sensor. Compos. Part B Eng. 2019, 172, 666–670. [Google Scholar] [CrossRef]
- Chu, K.; Wang, J.; Liu, Y.; Li, Y.; Jia, C.; Zhang, H. Creating defects on graphene basal-plane toward interface optimization of graphene/CuCr composites. Carbon N. Y. 2019, 143, 85–96. [Google Scholar] [CrossRef]
- Liu, L.; Qing, M.; Wang, Y.; Chen, S. Defects in graphene: Generation, healing, and their effects on the properties of graphene: A review. J. Mater. Sci. Technol. 2015, 31, 599–606. [Google Scholar] [CrossRef]
- Vicarelli, L.; Heerema, S.J.; Dekker, C.; Zandbergen, H.W. Controlling defects in graphene for optimizing the electrical properties of graphene nanodevices. ACS Nano 2015, 9, 3428–3435. [Google Scholar] [CrossRef] [PubMed]
- López-Polín, G.; Gómez-Navarro, C.; Parente, V.; Guinea, F.; Katsnelson, M.I.; Pérez-Murano, F.; Gómez-Herrero, J. Increasing the elastic modulus of graphene by controlled defect creation. Nat. Phys. 2015, 11, 26–31. [Google Scholar] [CrossRef]
- Mazánek, V.; Luxa, J.; Matějková, S.; Kučera, J.; Sedmidubský, D.; Pumera, M.; Sofer, Z. Ultrapure Graphene Is a poor electrocatalyst: Definitive proof of the key role of metallic impurities in graphene-based electrocatalysis. ACS Nano 2019, 13, 1574–1582. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, M.; Markevich, A.; Böttger, R.; Facsko, S.; Besley, E.; Kotakoski, J.; Susi, T. Implanting germanium into graphene. ACS Nano 2018, 12, 4641–4647. [Google Scholar] [CrossRef] [Green Version]
- Chua, C.K.; Sofer, Z.; Khezri, B.; Webster, R.D.; Pumera, M. Ball-milled sulfur-doped graphene materials contain metallic impurities originating from ball-milling apparatus: Their influence on the catalytic properties. Phys. Chem. Chem. Phys. 2016, 18, 17875–17880. [Google Scholar] [CrossRef]
- Ovsianytskyi, O.; Nam, Y.-S.; Tsymbalenko, O.; Lan, P.-T.; Moon, M.-W.; Lee, K.-B. Highly sensitive chemiresistive H2S gas sensor based on graphene decorated with Ag nanoparticles and charged impurities. Sens. Actuators B Chem. 2018, 257, 278–285. [Google Scholar] [CrossRef]
- Atanasov, V.; Saxena, A. Tuning the electronic properties of corrugated graphene: Confinement, curvature, and band-gap opening. Phys. Rev. B 2010, 81, 205409. [Google Scholar] [CrossRef] [Green Version]
- Pereira, V.M.; Castro Neto, A.H.; Peres, N.M.R. Tight-binding approach to uniaxial strain in graphene. Phys. Rev. B 2009, 80, 045401. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Reddy, C.D.; Jiang, J.; Baimova, J.A.; Dmitriev, S.V.; Nazarov, A.A.; Zhou, K. Morphology and in-plane thermal conductivity of hybrid graphene sheets. Appl. Phys. Lett. 2012, 101, 211909. [Google Scholar] [CrossRef]
- Withers, F.; Russo, S.; Dubois, M.; Craciun, M.F. Tuning the electronic transport properties of grapheme through functionalisation with fluorine. Nanoscale Res. Lett. 2011, 6, 526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, Y.; Wu, D.; Zhou, M.; Zheng, Y.; Wang, T.; Lu, C.; Zhang, L.; Liu, H.; Liu, C. Wearable strain sensors based on a porous polydimethylsiloxane hybrid with carbon nanotubes and graphene. ACS Appl. Mater. Interfaces 2021, 13, 15572–15583. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Wei, A.; Ma, J.; Shao, L.; Jiang, H.; Dong, D.; Ji, Z.; Wang, Q.; Kang, S. Lightweight, compressible and electrically conductive polyurethane sponges coated with synergistic multiwalled carbon nanotubes and graphene for piezoresistive sensors. Nanoscale 2018, 10, 7116–7126. [Google Scholar] [CrossRef]
- Li, L.; Hong, S.K.; Jo, Y.; Tian, M.; Woo, C.Y.; Kim, S.H.; Kim, J.-M.; Lee, H.W. Transparent, flexible heater based on hybrid 2D platform of graphene and dry-spun carbon nanotubes. ACS Appl. Mater. Interfaces 2019, 11, 16223–16232. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Yan, J.; Zhi, L.; Zhang, Q.; Wei, T.; Feng, J.; Zhang, M.; Qian, W.; Wei, F. A Three-dimensional carbon nanotube/graphene sandwich and its application as electrode in supercapacitors. Adv. Mater. 2010, 22, 3723–3728. [Google Scholar] [CrossRef] [PubMed]
- Dimitrakakis, G.K.; Tylianakis, E.; Froudakis, G.E. Pillared graphene: A new 3-d network nanostructure for enhanced hydrogen storage. Nano Lett. 2008, 8, 3166–3170. [Google Scholar] [CrossRef]
- Gao, M.; Huang, Z.L.; Zeng, B.; Pan, T.S.; Zhang, Y.; Peng, H.B.; Lin, Y. Carbon nanotube-graphene junctions studied by impedance spectra. Appl. Phys. Lett. 2015, 106, 051601. [Google Scholar] [CrossRef]
- Du, F.; Yu, D.; Dai, L.; Ganguli, S.; Varshney, V.; Roy, A.K. Preparation of tunable 3D pillared carbon nanotube–graphene networks for high-performance capacitance. Chem. Mater. 2011, 23, 4810–4816. [Google Scholar] [CrossRef]
- Chen, J.; Walther, J.H.; Koumoutsakos, P. Covalently bonded graphene-carbon nanotube hybrid for high-performance thermal interfaces. Adv. Funct. Mater. 2015, 25, 7539–7545. [Google Scholar] [CrossRef] [Green Version]
- Varshney, V.; Patnaik, S.S.; Roy, A.K.; Froudakis, G.; Farmer, B.L. Modeling of thermal transport in pillared-graphene architectures. ACS Nano 2010, 4, 1153–1161. [Google Scholar] [CrossRef] [PubMed]
- Mirri, F.; Ma, A.W.K.; Hsu, T.T.; Behabtu, N.; Eichmann, S.L.; Young, C.C.; Tsentalovich, D.E.; Pasquali, M. High-performance carbon nanotube transparent conductive films by scalable dip coating. ACS Nano 2012, 6, 9737–9744. [Google Scholar] [CrossRef]
- Lu, Z.; Foroughi, J.; Wang, C.; Long, H.; Wallace, G.G. Superelastic hybrid CNT/graphene fibers for wearable energy storage. Adv. Energy Mater. 2018, 8, 1702047. [Google Scholar] [CrossRef]
- Kumar, R.; Singh, R.K.; Dubey, P.K.; Singh, D.P.; Yadav, R.M. Self-assembled hierarchical formation of conjugated 3D cobalt oxide nanobead–CNT–graphene nanostructure using microwaves for high-performance supercapacitor electrode. ACS Appl. Mater. Interfaces 2015, 7, 15042–15051. [Google Scholar] [CrossRef]
- Kim, Y.-S.; Kumar, K.; Fisher, F.T.; Yang, E.-H. Out-of-plane growth of CNTs on graphene for supercapacitor applications. Nanotechnology 2012, 23, 015301. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Ahmed, Z.; Wang, Q.; Yu, C.; Feng, Z.; Li, G.; Zhang, M.; Zhou, C.; Senegor, R.; Yang, C.Y. Structures, properties, and applications of CNT-graphene heterostructures. 2D Mater. 2019, 6, 042005. [Google Scholar] [CrossRef]
- Yan, Z.; Ma, L.; Zhu, Y.; Lahiri, I.; Hahm, M.G.; Liu, Z.; Yang, S.; Xiang, C.; Lu, W.; Peng, Z.; et al. Three-dimensional metal–Graphene–Nanotube multifunctional hybrid materials. ACS Nano 2013, 7, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Kim, J.E.; Han, T.H.; Hwang, J.W.; Jeon, S.; Choi, S.-Y.; Hong, S.H.; Lee, W.J.; Ruoff, R.S.; Kim, S.O. Versatile carbon hybrid films composed of vertical carbon nanotubes grown on mechanically compliant graphene films. Adv. Mater. 2010, 22, 1247–1252. [Google Scholar] [CrossRef]
- Lee, S.H.; Lee, D.H.; Lee, W.J.; Kim, S.O. Tailored assembly of carbon nanotubes and graphene. Adv. Funct. Mater. 2011, 21, 1338–1354. [Google Scholar] [CrossRef]
- Gerasimenko, A.Y.; Kitsyuk, E.P.; Kuksin, A.V.; Ryazanov, R.M.; Savitskiy, A.I.; Savelyev, M.S.; Pavlov, A.A. Influence of laser structuring and barium nitrate treatment on morphology and electrophysical characteristics of vertically aligned carbon nanotube arrays. Diam. Relat. Mater. 2019, 96, 104–111. [Google Scholar] [CrossRef]
- Qian, D.; Dickey, E.C.; Andrews, R.; Rantell, T. Load transfer and deformation mechanisms in carbon nanotube-polystyrene composites. Appl. Phys. Lett. 2000, 76, 2868–2870. [Google Scholar] [CrossRef] [Green Version]
- Kuzumaki, T.; Miyazawa, K.; Ichinose, H.; Ito, K. Processing of carbon nanotube reinforced aluminum composite. J. Mater. Res. 1998, 13, 2445–2449. [Google Scholar] [CrossRef]
- Lin Hwang, G.; Chu Hwang, K. Carbon nanotube reinforced ceramics. J. Mater. Chem. 2001, 11, 1722–1725. [Google Scholar] [CrossRef]
- Markov, A.; Wördenweber, R.; Ichkitidze, L.; Gerasimenko, A.; Kurilova, U.; Suetina, I.; Mezentseva, M.; Offenhäusser, A.; Telyshev, D. Biocompatible SWCNT conductive composites for biomedical applications. Nanomaterials 2020, 10, 2492. [Google Scholar] [CrossRef]
- Gerasimenko, A.Y.; Kurilova, U.E.; Savelyev, M.S.; Murashko, D.T.; Glukhova, O.E. Laser fabrication of composite layers from biopolymers with branched 3D networks of single-walled carbon nanotubes for cardiovascular implants. Compos. Struct. 2021, 260, 113517. [Google Scholar] [CrossRef]
- Demidenko, N.; Kuksin, A.; Murashko, D.; Cherepanova, N.; Semak, A.; Bychkov, V.; Komarchev, A.; Eganova, E.; Dudin, A.; Pavlov, A.; et al. Laser Formation of Electrically Conductive Nanocomposites for Bioelectronic Applications. In Proceedings of the 3D Printed Optics and Additive Photonic Manufacturing II, Strasbourg, France, 6–10 April 2020; von Freymann, G., Herkommer, A.M., Flury, M., Eds.; SPIE: Strasbourg, France, 2020; p. 32. [Google Scholar]
- Kuksin, A.V.; Polokhin, A.A.; Ryazanov, R.M.; Kitsyuk, E.P.; Gerasimenko, A.Y. Chemical and laser structuring of carbon nanotubes arrays for use in biomedical devices. In Proceedings of the 2019 IEEE Conference of Russian Young Researchers in Electrical and Electronic Engineering, ElConRus 2019, St. Petersburg, Russia, 28–31 January 2019; IEEE: Piscataway, NJ, USA, 2019; pp. 2299–2303. [Google Scholar]
- Yao, Y.; Jiang, F.; Yang, C.; Fu, K.K.; Hayden, J.; Lin, C.-F.; Xie, H.; Jiao, M.; Yang, C.; Wang, Y.; et al. Epitaxial welding of carbon nanotube networks for aqueous battery current collectors. ACS Nano 2018, 12, 5266–5273. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Hou, P.-X.; Chen, M.-L.; Wang, B.-W.; Sun, D.-M.; Tang, D.-M.; Jin, Q.; Guo, Q.-X.; Zhang, D.-D.; Du, J.-H.; et al. Ultrahigh-performance transparent conductive films of carbon-welded isolated single-wall carbon nanotubes. Sci. Adv. 2018, 4, eaap9264. [Google Scholar] [CrossRef] [Green Version]
- Saito, T.; Ohshima, S.; Okazaki, T.; Ohmori, S.; Yumura, M.; Iijima, S. Selective diameter control of single-walled carbon nanotubes in the gas-phase synthesis. J. Nanosci. Nanotechnol. 2008, 8, 6153–6157. [Google Scholar] [CrossRef]
- Jaeger, H.; Behrsing, T. The dual nature of vapour-grown carbon fibres. Compos. Sci. Technol. 1994, 51, 231–242. [Google Scholar] [CrossRef]
- Tereshchenko, S.A.; Savelyev, M.S.; Podgaetsky, V.M.; Gerasimenko, A.Y.; Selishchev, S.V. Nonlinear threshold effect in the Z-scan method of characterizing limiters for high-intensity laser light. J. Appl. Phys. 2016, 120, 093109. [Google Scholar] [CrossRef]
- Kichambare, P.D.; Chen, L.C.; Wang, C.T.; Ma, K.J.; Wu, C.T.; Chen, K.H. Laser irradiation of carbon nanotubes. Mater. Chem. Phys. 2001, 72, 218–222. [Google Scholar] [CrossRef]
- Yuan, Y.; Chen, J. Morphology adjustments of multi-walled carbon nanotubes by laser irradiation. Laser Phys. Lett. 2016, 13, 066001. [Google Scholar] [CrossRef]
- Yuan, Y.; Chen, J. Nano-welding of multi-walled carbon nanotubes on silicon and silica surface by laser irradiation. Nanomaterials 2016, 6, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Z.; Yuan, Y.; Shang, Y.; Han, W. Structural changes and electrical properties of nanowelded multiwalled carbon nanotube junctions. Appl. Opt. 2018, 57, 7435. [Google Scholar] [CrossRef]
- Yuan, Y.; Liu, Z.; Zhang, K.; Han, W.; Chen, J. Nanoscale welding of multi-walled carbon nanotubes by 1064 nm fiber laser. Opt. Laser Technol. 2018, 103, 327–329. [Google Scholar] [CrossRef]
- Terrones, M.; Banhart, F.; Grobert, N.; Charlier, J.-C.; Terrones, H.; Ajayan, P.M. Molecular junctions by joining single-walled carbon nanotubes. Phys. Rev. Lett. 2002, 89, 075505. [Google Scholar] [CrossRef] [PubMed]
- Krasheninnikov, A.V.; Banhart, F. Engineering of nanostructured carbon materials with electron or ion beams. Nat. Mater. 2007, 6, 723–733. [Google Scholar] [CrossRef]
- Banhart, F. The formation of a connection between carbon nanotubes in an electron beam. Nano Lett. 2001, 1, 329–332. [Google Scholar] [CrossRef]
- Gerasimenko, A.Y.; Glukhova, O.E.; Savostyanov, G.V.; Podgaetsky, V.M. Laser structuring of carbon nanotubes in the albumin matrix for the creation of composite biostructures. J. Biomed. Opt. 2017, 22, 065003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerasimenko, A.Y.; Zhurbina, N.N.; Cherepanova, N.G.; Semak, A.E.; Zar, V.V.; Fedorova, Y.O.; Eganova, E.M.; Pavlov, A.A.; Telyshev, D.V.; Selishchev, S.V.; et al. Frame coating of single-walled carbon nanotubes in collagen on pet fibers for artificial joint ligaments. Int. J. Mol. Sci. 2020, 21, 6163. [Google Scholar] [CrossRef] [PubMed]
- Bonafé, F.P.; Aradi, B.; Hourahine, B.; Medrano, C.R.; Hernández, F.J.; Frauenheim, T.; Sánchez, C.G. A real-time time-dependent density functional tight-binding implementation for semiclassical excited state electron–nuclear dynamics and pump–probe spectroscopy simulations. J. Chem. Theory Comput. 2020, 16, 4454–4469. [Google Scholar] [CrossRef]
- DFTB+. Available online: https://dftbplus.org (accessed on 20 May 2021).
- Elstner, M.; Porezag, D.; Jungnickel, G.; Elsner, J.; Haugk, M.; Frauenheim, T.; Suhai, S.; Seifert, G. Self-consistent-charge density-functional tight-binding method for simulations of complex materials properties. Phys. Rev. B 1998, 58, 7260–7268. [Google Scholar] [CrossRef]
- Kataura, H.; Kumazawa, Y.; Maniwa, Y.; Umezu, I.; Suzuki, S.; Ohtsuka, Y.; Achiba, Y. Optical properties of single-wall carbon nanotubes. Synth. Met. 1999, 103, 2555–2558. [Google Scholar] [CrossRef]
- Telg, H.; Maultzsch, J.; Reich, S.; Hennrich, F.; Thomsen, C. Chirality distribution and transition energies of carbon nanotubes. Phys. Rev. Lett. 2004, 93, 177401. [Google Scholar] [CrossRef] [Green Version]
- Tian, Y.; Jiang, H.; Laiho, P.; Kauppinen, E.I. Validity of measuring metallic and semiconducting single-walled carbon nanotube fractions by quantitative raman spectroscopy. Anal. Chem. 2018, 90, 2517–2525. [Google Scholar] [CrossRef] [Green Version]
- Labunov, V.A.; Tabulina, L.V.; Komissarov, I.V.; Grapov, D.V.; Prudnikova, E.L.; Shaman, Y.P.; Basaev, S.A.; Pavlov, A.A. Features of the reduction of graphene from graphene oxide. Russ. J. Phys. Chem. A 2017, 91, 1088–1092. [Google Scholar] [CrossRef]
- Henrie, J.; Kellis, S.; Schultz, S.M.; Hawkins, A. Electronic color charts for dielectric films on silicon. Opt. Express 2004, 12, 1464. [Google Scholar] [CrossRef] [Green Version]
- Savelyev, M.S.; Gerasimenko, A.Y.; Podgaetskii, V.M.; Tereshchenko, S.A.; Selishchev, S.V.; Tolbin, A.Y. Conjugates of thermally stable phthalocyanine J-type dimers with single-walled carbon nanotubes for enhanced optical limiting applications. Opt. Laser Technol. 2019, 117, 272–279. [Google Scholar] [CrossRef]
- Kalbac, M.; Hsieh, Y.-P.; Farhat, H.; Kavan, L.; Hofmann, M.; Kong, J.; Dresselhaus, M.S. Defects in individual semiconducting single wall carbon nanotubes: Raman spectroscopic and in situ raman spectroelectrochemical study. Nano Lett. 2010, 10, 4619–4626. [Google Scholar] [CrossRef]
- Fomin, A. Theoretical dependence of hardness on the structural parameters for hard and superhard ceramics. Int. J. Refract. Met. Hard Mater. 2020, 88, 105201. [Google Scholar] [CrossRef]
- Li, M.; Liu, X.; Zhao, X.; Yang, F.; Wang, X.; Li, Y. Metallic catalysts for structure-controlled growth of single-walled carbon nanotubes. Top. Curr. Chem. 2017, 375, 29. [Google Scholar] [CrossRef]
- Kleshch, V.I.; Eremina, V.A.; Serbun, P.; Orekhov, A.S.; Lützenkirchen-Hecht, D.; Obraztsova, E.D.; Obraztsov, A.N. A comparative study of field emission from semiconducting and metallic single-walled carbon nanotube planar emitters. Phys. Status Solidi 2018, 255, 1700268. [Google Scholar] [CrossRef]
- Bobrinetsky, I.I.; Gerasimenko, A.Y.; Podgaetsky, V.M.; Saveliev, M.S. Spectral characteristics of materials based on carbon nanotubes. Biomed. Eng. 2015, 48, 318–323. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Dresselhaus, G.; Saito, R.; Jorio, A. Raman spectroscopy of carbon nanotubes. Phys. Rep. 2005, 409, 47–99. [Google Scholar] [CrossRef]
- Corio, P.; Santos, P.; Pimenta, M.; Dresselhaus, M. Evolution of the molecular structure of metallic and semiconducting carbon nanotubes under laser irradiation. Chem. Phys. Lett. 2002, 360, 557–564. [Google Scholar] [CrossRef]
- Mei, H.; Cheng, Y.; Yin, H.; Wei, F.; Fang, X. Femtosecond pulsed laser-induced interconnection of single-walled carbon nanotubes. Ferroelectrics 2020, 563, 21–30. [Google Scholar] [CrossRef]
- Marković, Z.; Kepić, D.; Holclajtner Antunović, I.; Nikolić, M.; Dramićanin, M.; Marinović Cincović, M.; Todorović Marković, B. Raman study of single wall carbon nanotube thin films treated by laser irradiation and dynamic and isothermal oxidation. J. Raman Spectrosc. 2012, 43, 1413–1422. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Jorio, A.; Souza Filho, A.G.; Saito, R. Defect characterization in graphene and carbon nanotubes using Raman spectroscopy. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2010, 368, 5355–5377. [Google Scholar] [CrossRef] [PubMed]
- Tian, G.-L.; Zhao, M.-Q.; Yu, D.; Kong, X.-Y.; Huang, J.-Q.; Zhang, Q.; Wei, F. Nitrogen-doped graphene/carbon nanotube hybrids: In Situ formation on bifunctional catalysts and their superior electrocatalytic activity for oxygen evolution/reduction reaction. Small 2014, 10, 2251–2259. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; He, Z.; Chen, J.; Zhao, J.; Feng, X.; Ma, Y.; Fan, Q.; Wang, L.; Huang, W. Highly conductive three-dimensional MnO2–carbon nanotube–graphene–Ni hybrid foam as a binder-free supercapacitor electrode. Nanoscale 2014, 6, 1079–1085. [Google Scholar] [CrossRef]








| Dispersion Number | Dispersion Composition |
|---|---|
| 1 | SWCNT |
| 2 | MWCNT |
| 3 | SWCNT/rGO |
| 4 | MWCNT/rGO |
| Sample | Energy Density, J/cm2 | Maximum RBM, cm−1 | G, cm−1 | D, cm−1 | ID/IG | 2D, cm−1 |
|---|---|---|---|---|---|---|
| 1. SWCNT | 0.0 | 154, 178, 183, 186 | 1571 (G−), 1592 (G+) | 1343 | 0.029 | 2681 |
| 0.3 | 151, 179, 182, 187 | 1571 (G−), 1592 (G+) | 1344 | 0.037 | 2685 | |
| 2. MWCNT | 0.0 | – | 1587 | 1350 | 1.152 | 2704 |
| 0.5 | – | 1586 | 1352 | 1.057 | 2705 | |
| 3. SWCNT/rGO | 0.0 | 150, 152, 178, 184, 187 | 1570(G−), 1591 (G+) | 1344 | 0.023 | 2682 |
| 0.3 | 150, 177, 185 | 1573 (G−), 1593 (G+) | 1349 | 0.102 | 2683 | |
| 4. MWCNT/rGO | 0.0 | – | 1591 | 1353 | 1.176 | 2707 |
| 0.5 | – | 1588 | 1352 | 1.207 | 2709 |
| Sample | Energy Density, J/cm2 | Sheet Resistance, kΩ/Square | Conductivity, kS/m |
|---|---|---|---|
| 1. SWCNT | 0.00 | 0.56 | 3.61 |
| 0.14 | 0.38 | 5.23 | |
| 0.30 | 0.17 | 11.51 | |
| 0.50 | 0.21 | 9.44 | |
| 0.80 | 0.65 | 3.09 | |
| 2. MWCNT | 0.00 | 0.14 | 14.32 |
| 0.14 | 0.13 | 15.11 | |
| 0.30 | 0.12 | 16.58 | |
| 0.50 | 0.11 | 18.43 | |
| 0.80 | 0.40 | 5.03 | |
| 4. SWCNT/rGO | 0.00 | 0.34 | 5.91 |
| 0.14 | 0.25 | 7.87 | |
| 0.30 | 0.19 | 10.34 | |
| 0.50 | 0.91 | 2.23 | |
| 0.80 | 5.00 | 0.41 | |
| 5. MWCNT/rGO | 0.00 | 0.12 | 16.32 |
| 0.14 | 0.11 | 17.54 | |
| 0.30 | 0.10 | 19.42 | |
| 0.50 | 0.09 | 22.60 | |
| 0.80 | 2.00 | 1.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gerasimenko, A.Y.; Kuksin, A.V.; Shaman, Y.P.; Kitsyuk, E.P.; Fedorova, Y.O.; Sysa, A.V.; Pavlov, A.A.; Glukhova, O.E. Electrically Conductive Networks from Hybrids of Carbon Nanotubes and Graphene Created by Laser Radiation. Nanomaterials 2021, 11, 1875. https://doi.org/10.3390/nano11081875
Gerasimenko AY, Kuksin AV, Shaman YP, Kitsyuk EP, Fedorova YO, Sysa AV, Pavlov AA, Glukhova OE. Electrically Conductive Networks from Hybrids of Carbon Nanotubes and Graphene Created by Laser Radiation. Nanomaterials. 2021; 11(8):1875. https://doi.org/10.3390/nano11081875
Chicago/Turabian StyleGerasimenko, Alexander Yu., Artem V. Kuksin, Yury P. Shaman, Evgeny P. Kitsyuk, Yulia O. Fedorova, Artem V. Sysa, Alexander A. Pavlov, and Olga E. Glukhova. 2021. "Electrically Conductive Networks from Hybrids of Carbon Nanotubes and Graphene Created by Laser Radiation" Nanomaterials 11, no. 8: 1875. https://doi.org/10.3390/nano11081875
APA StyleGerasimenko, A. Y., Kuksin, A. V., Shaman, Y. P., Kitsyuk, E. P., Fedorova, Y. O., Sysa, A. V., Pavlov, A. A., & Glukhova, O. E. (2021). Electrically Conductive Networks from Hybrids of Carbon Nanotubes and Graphene Created by Laser Radiation. Nanomaterials, 11(8), 1875. https://doi.org/10.3390/nano11081875