Short- and Long-Term Dentin Bond Strength of Bioactive Glass-Modified Dental Adhesives
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material Preparation
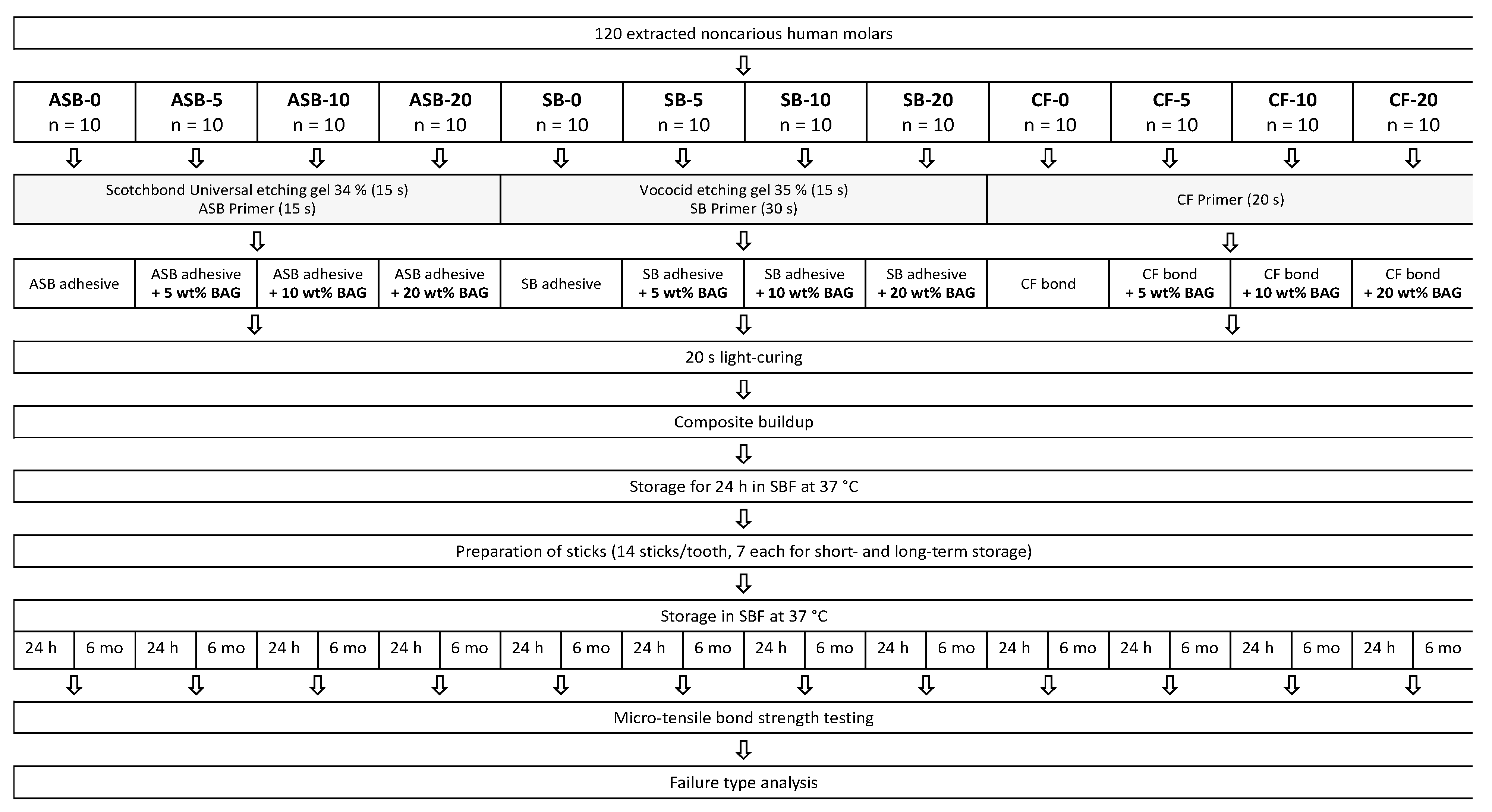
2.2. Specimen Preparation
2.3. Restoration
2.4. Micro-Tensile Bond Strength Test
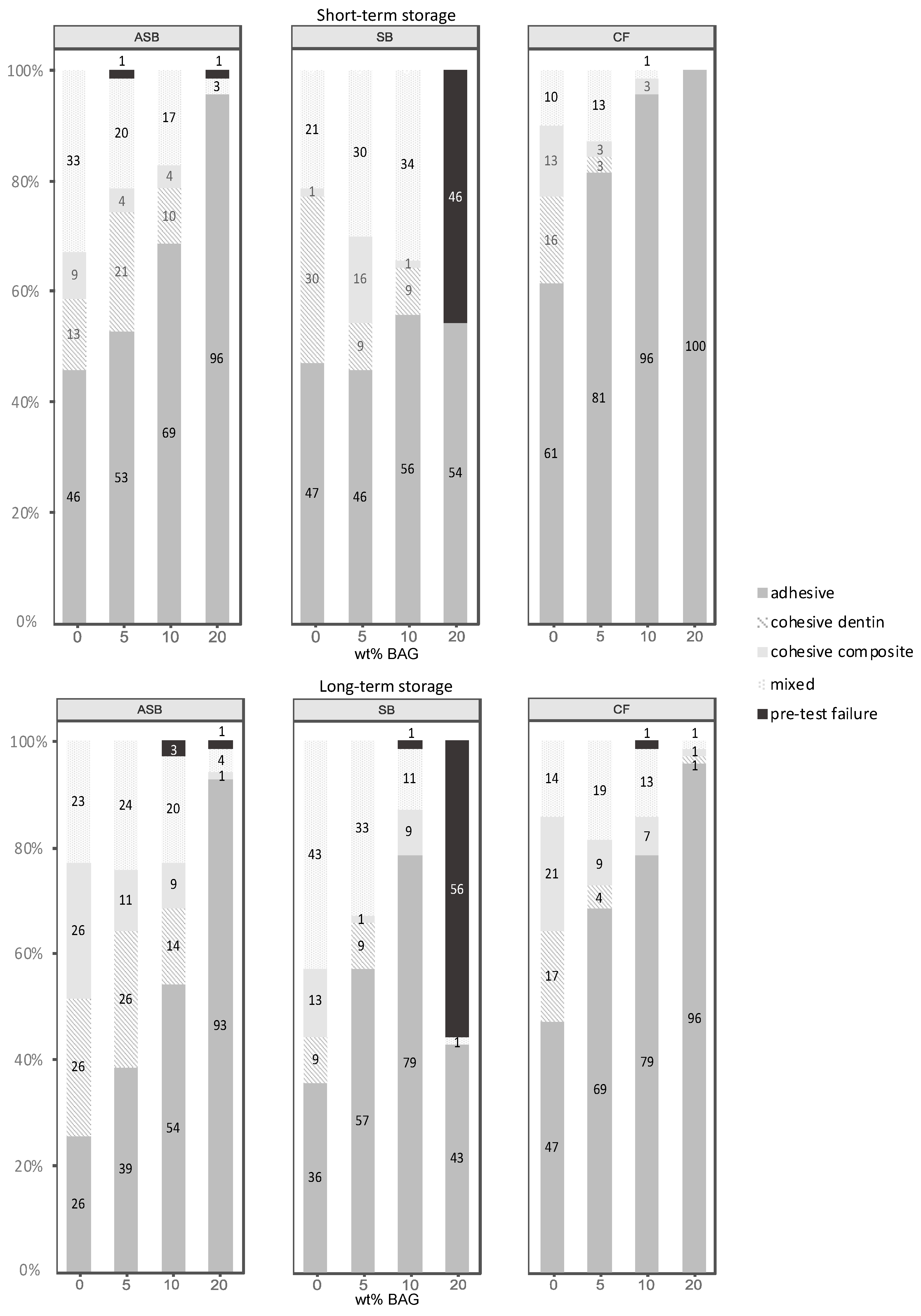
2.5. Failure Analysis
2.6. Statistical Analysis
3. Results
3.1. Micro-Tensile Bond Strength
3.2. Failure Analysis
4. Discussion
5. Conclusions
- The etch-and-rinse adhesives could be functionalized with 5 wt% (Solobond Plus) or up to 10 wt% (Adper Scotchbond Multi-Purpose) of nano-sized bioactive glass with no negative effect on their dentin bond strength.
- Although addition of bioactive glass to the self-etch adhesive (Clearfil SE Bond) significantly diminished its performance for all bioactive glass concentrations, a beneficial effect was identified in terms of maintaining stable dentin bond strength over the 6 months aging period.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ferracane, J.L. Resin composite—State of the art. Dent. Mater. 2011, 27, 29–38. [Google Scholar] [CrossRef]
- Roeters, F.J.M.; Opdam, N.J.M.; Loomans, B.A.C. The amalgam-free dental school. J. Dent. 2004, 32, 371–377. [Google Scholar] [CrossRef]
- Wiegand, A.; Credé, A.; Tschammler, C.; Attin, T.; Tauböck, T.T. Enamel wear by antagonistic restorative materials under erosive conditions. Clin. Oral Investig. 2017, 21, 2689–2693. [Google Scholar] [CrossRef]
- McLean, J.W. Dentinal bonding agents versus glass-ionomer cements. Quintessence Int. 1996, 27, 659–667. [Google Scholar] [PubMed]
- Spencer, P.; Ye, Q.; Park, J.; Topp, E.M.; Misra, A.; Marangos, O.; Wang, Y.; Bohaty, B.S.; Singh, V.; Sene, F.; et al. Adhesive/Dentin interface: The weak link in the composite restoration. Ann. Biomed. Eng. 2010, 38, 1989–2003. [Google Scholar] [CrossRef] [Green Version]
- Sarrett, D.C. Clinical challenges and the relevance of materials testing for posterior composite restorations. Dent. Mater. 2005, 21, 9–20. [Google Scholar] [CrossRef]
- Mjör, I.A.; Shen, C.; Eliasson, S.T.; Richter, S. Placement and replacement of restorations in general dental practice in Iceland. Oper. Dent. 2002, 27, 117–123. [Google Scholar]
- Mazzoni, A.; Tjäderhane, L.; Checchi, V.; Di Lenarda, R.; Salo, T.; Tay, F.R.; Pashley, D.H.; Breschi, L. Role of dentin MMPs in caries progression and bond stability. J. Dent. Res. 2015, 94, 241–251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pashley, D.H.; Tay, F.R.; Breschi, L.; Tjäderhane, L.; Carvalho, R.M.; Carrilho, M.; Tezvergil-Mutluay, A. State of the art etch-and-rinse adhesives. Dent. Mater. 2011, 27, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carrilho, M.R.; Geraldeli, S.; Tay, F.; de Goes, M.F.; Carvalho, R.M.; Tjäderhane, L.; Reis, A.F.; Hebling, J.; Mazzoni, A.; Breschi, L.; et al. In vivo preservation of the hybrid layer by chlorhexidine. J. Dent. Res. 2007, 86, 529–533. [Google Scholar] [CrossRef]
- Osorio, R.; Yamauti, M.; Sauro, S.; Watson, T.F.; Toledano, M. Experimental resin cements containing bioactive fillers reduce matrix metalloproteinase-mediated dentin collagen degradation. J. Endod. 2012, 38, 1227–1232. [Google Scholar] [CrossRef] [PubMed]
- Profeta, A.C.; Mannocci, F.; Foxton, R.M.; Thompson, I.; Watson, T.F.; Sauro, S. Bioactive effects of a calcium/sodium phosphosilicate on the resin-dentine interface: A microtensile bond strength, scanning electron microscopy, and confocal microscopy study. Eur. J. Oral Sci. 2012, 120, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Tezvergil-Mutluay, A.; Seseogullari-Dirihan, R.; Feitosa, V.P.; Cama, G.; Brauer, D.S.; Sauro, S. Effects of composites containing bioactive glasses on demineralized dentin. J. Dent. Res. 2017, 96, 999–1005. [Google Scholar] [CrossRef] [PubMed]
- Allan, I.; Newman, H.; Wilson, M. Antibacterial activity of particulate bioglass against supra- and subgingival bacteria. Biomaterials 2001, 22, 1683–1687. [Google Scholar] [CrossRef]
- Bauer, J.; Silva, A.S.E.; Carvalho, E.M.; Ferreira, P.V.C.; Carvalho, C.N.; Manso, A.P.; Carvalho, R.M. Dentin pretreatment with 45S5 and niobophosphate bioactive glass: Effects on pH, antibacterial, mechanical properties of the interface and microtensile bond strength. J. Mech. Behav. Biomed. Mater. 2019, 90, 374–380. [Google Scholar] [CrossRef]
- Eshghi, A.; Khoroushi, M.; Rezvani, A. Resin bonding using etch-and-rinse and self-etch adhesives to decalcified deciduous enamel after bioactive glass air abrasion. J. Contemp. Dent. Pract. 2014, 15, 595–602. [Google Scholar]
- Abuna, G.; Feitosa, V.P.; Correr, A.B.; Cama, G.; Giannini, M.; Sinhoreti, M.A.; Pashley, D.H.; Sauro, S. Bonding performance of experimental bioactive/biomimetic self-etch adhesives doped with calcium-phosphate fillers and biomimetic analogs of phosphoproteins. J. Dent. 2016, 52, 79–86. [Google Scholar] [CrossRef]
- Dressano, D.; Salvador, M.V.; Oliveira, M.T.; Marchi, G.M.; Fronza, B.M.; Hadis, M.; Palin, W.M.; Lima, A.F. Chemistry of novel and contemporary resin-based dental adhesives. J. Mech. Behav. Biomed. Mater. 2020, 110, 103875. [Google Scholar] [CrossRef]
- Profeta, A.C. Preparation and properties of calcium-silicate filled resins for dental restoration. Part I: Chemical-physical characterization and apatite-forming ability. Acta Odontol. Scand. 2014, 72, 597–606. [Google Scholar] [CrossRef]
- Sauro, S.; Osorio, R.; Watson, T.F.; Toledano, M. Therapeutic effects of novel resin bonding systems containing bioactive glasses on mineral-depleted areas within the bonded-dentine interface. J. Mater. Sci. Mater. Med. 2012, 23, 1521–1532. [Google Scholar] [CrossRef]
- Balbinot, G.S.; Collares, F.M.; Herpich, T.L.; Visioli, F.; Samuel, S.M.W.; Leitune, V.C.B. Niobium containing bioactive glasses as remineralizing filler for adhesive resins. Dent. Mater. 2020, 36, 221–228. [Google Scholar] [CrossRef]
- Rizk, M.; Hohlfeld, L.; Thanh, L.T.; Biehl, R.; Lühmann, N.; Mohn, D.; Wiegand, A. Bioactivity and properties of a dental adhesive functionalized with polyhedral oligomeric silsesquioxanes (POSS) and bioactive glass. Dent. Mater. 2017, 33, 1056–1065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tauböck, T.T.; Zehnder, M.; Schweizer, T.; Stark, W.J.; Attin, T.; Mohn, D. Functionalizing a dentin bonding resin to become bioactive. Dent. Mater. 2014, 30, 868–875. [Google Scholar] [CrossRef] [PubMed]
- Dieckmann, P.; Mohn, D.; Zehnder, M.; Attin, T.; Tauböck, T.T. Light transmittance and polymerization of bulk-fill composite materials doped with bioactive micro-fillers. Materials 2019, 12, 4087. [Google Scholar] [CrossRef] [Green Version]
- Brunner, T.J.; Grass, R.N.; Stark, W.J. Glass and bioglass nanopowders by flame synthesis. Chem. Commun. 2006, 13, 1384–1386. [Google Scholar] [CrossRef] [PubMed]
- Heid, S.; Stoessel, P.R.; Tauböck, T.T.; Stark, W.J.; Zehnder, M.; Mohn, D. Incorporation of particulate bioactive glasses into a dental root canal sealer. Biomed. Glasses 2016, 2, 29–37. [Google Scholar] [CrossRef]
- Vollenweider, M.; Brunner, T.J.; Knecht, S.; Grass, R.N.; Zehnder, M.; Imfeld, T.; Stark, W.J. Remineralization of human dentin using ultrafine bioactive glass particles. Acta Biomater. 2007, 3, 936–943. [Google Scholar] [CrossRef]
- Waltimo, T.; Brunner, T.J.; Vollenweider, M.; Stark, W.J.; Zehnder, M. Antimicrobial effect of nanometric bioactive glass 45S5. J. Dent. Res. 2007, 86, 754–757. [Google Scholar] [CrossRef]
- Bauer, J.; Carvalho, E.M.; Carvalho, C.N.; Meier, M.M.; de Souza, J.P.; de Carvalho, R.M.; Loguercio, A.D. Development of a simplified etch-and-rinse adhesive containing niobiophosphate bioactive glass. Int. J. Adhes. Adhes. 2016, 69, 110–114. [Google Scholar] [CrossRef]
- Choi, Y.; Sun, W.; Kim, Y.; Kim, I.-R.; Gong, M.-K.; Yoon, S.-Y.; Bae, M.-K.; Park, B.-S.; Park, S.-B.; Kim, Y.-I. Effects of Zn-doped mesoporous bioactive glass nanoparticles in etch-and-rinse adhesive on the microtensile bond strength. Nanomaterials 2020, 10, 1943. [Google Scholar] [CrossRef]
- Odermatt, R.; Par, M.; Mohn, D.; Wiedemeier, D.B.; Attin, T.; Tauböck, T.T. Bioactivity and physico-chemical properties of dental composites functionalized with nano- vs. micro-sized bioactive glass. J. Clin. Med. 2020, 9, 772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hild, N.; Tawakoli, P.N.; Halter, J.G.; Sauer, B.; Buchalla, W.; Stark, W.J.; Mohn, D. pH-dependent antibacterial effects on oral microorganisms through pure PLGA implants and composites with nanosized bioactive glass. Acta Biomater. 2013, 9, 9118–9125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohrs, N.H.; Schulz-Schönhagen, K.; Jenny, F.; Mohn, D.; Stark, W.J. Bioactive glass containing silicone composites for left ventricular assist device drivelines: Role of Bioglass 45S5® particle size on mechanical properties and cytocompatibility. J. Mater. Sci. 2017, 52, 9023–9038. [Google Scholar] [CrossRef] [Green Version]
- Cho, S.; Nakanishi, K.; Kokubo, T.; Soga, N.; Ohtsuki, C.; Nakamura, T.; Kitsugi, T.; Yamamuro, T. Dependence of apatite formation on silica gel on its structure: Effect of heat treatment. J. Am. Ceram. Soc. 1995, 78, 1769–1774. [Google Scholar] [CrossRef]
- Armstrong, S.; Breschi, L.; Özcan, M.; Pfefferkorn, F.; Ferrari, M.; Van Meerbeek, B. Academy of Dental Materials guidance on in vitro testing of dental composite bonding effectiveness to dentin/enamel using micro-tensile bond strength (μTBS) approach. Dent. Mater. 2017, 33, 133–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2016; Available online: https://wwwR-projectorg (accessed on 20 August 2020).
- Pohlert, T. The Pairwise Multiple Comparison of Mean Ranks Package (PMCMR). R Package 2014. Available online: https://CRANR-projectorg/package=PMCMR (accessed on 20 August 2020).
- Mangiafico, S. Rcompanion: Functions to Support Extension Education Program Evaluation. 2020. Available online: https://CRANR-projectorg/package=rcompanion (accessed on 20 August 2020).
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; Available online: https://ggplot2tidyverseorg (accessed on 20 August 2020).
- Cadenaro, M.; Maravic, T.; Comba, A.; Mazzoni, A.; Fanfoni, L.; Hilton, T.; Ferracane, J.; Breschi, L. The role of polymerization in adhesive dentistry. Dent. Mater. 2019, 35, e1–e22. [Google Scholar] [CrossRef]
- Giannini, M.; Makishi, P.; Ayres, A.P.; Vermelho, P.M.; Fronza, B.M.; Nikaido, T.; Tagami, J. Self-etch adhesive systems: A literature review. Braz. Dent. J. 2015, 26, 3–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ikemura, K.; Kadoma, Y.; Endo, T. A review of the developments of self-etching primers and adhesives—Effects of acidic adhesive monomers and polymerization initiators on bonding to ground, smear layer-covered teeth. Dent. Mater. J. 2011, 30, 769–789. [Google Scholar] [CrossRef] [Green Version]
- Van Meerbeek, B.; Yoshihara, K.; Yoshida, Y.; Mine, A.; De Munck, J.; Van Landuyt, K.L. State of the art of self-etch adhesives. Dent. Mater. 2011, 27, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Björkvik, L.; Wang, X.; Hupa, L. Dissolution of bioactive glasses in acidic solutions with the focus on lactic acid. Int. J. Appl. Glass Sci. 2016, 7, 154–163. [Google Scholar] [CrossRef]
- Hench, L.L. Bioceramics: From concept to clinic. J. Am. Ceram. Soc. 1991, 74, 1487–1510. [Google Scholar] [CrossRef]
- Forsback, A.; Areva, S.; Salonen, J. Mineralization of dentin induced by treatment with bioactive glass S53P4 in vitro. Acta Odontol. Scand. 2004, 62, 14–20. [Google Scholar] [CrossRef]
- Sauro, S.; Osorio, R.; Fulgêncio, R.; Watson, T.F.; Cama, G.; Thompson, I.; Toledano, M. Remineralisation properties of innovative light-curable resin-based dental materials containing bioactive micro-fillers. J. Mater. Chem. B 2013, 1, 2624–2638. [Google Scholar] [CrossRef]
- Liu, Y.; Tjäderhane, L.; Breschi, L.; Mazzoni, A.; Li, N.; Mao, J.; Pashley, D.H.; Tay, F.R. Limitations in bonding to dentin and experimental strategies to prevent bond degradation. J. Dent. Res. 2011, 90, 953–968. [Google Scholar] [CrossRef] [PubMed]
- Calheiros, F.C.; Kawano, Y.; Stansbury, J.W.; Braga, R.R. Influence of radiant exposure on contraction stress, degree of conversion and mechanical properties of resin composites. Dent. Mater. 2006, 22, 799–803. [Google Scholar] [CrossRef] [PubMed]
- Sirovica, S.; Skoda, M.W.A.; Podgorski, M.; Thompson, P.B.J.; Palin, W.M.; Guo, Y.; Smith, A.J.; Dewan, K.; Addison, O.; Martin, R.A. Structural evidence that the polymerization rate dictates order and intrinsic strain generation in photocured methacrylate biomedical polymers. Macromolecules 2019, 52, 5377–5388. [Google Scholar] [CrossRef] [Green Version]
- Tsenoglou, C.J.; Pavlidou, S.; Papaspyrides, C.D. Evaluation of interfacial relaxation due to water absorption in fiber–polymer composites. Compos. Sci. Technol. 2006, 66, 2855–2864. [Google Scholar] [CrossRef]
- Par, M.; Lapas-Barisic, M.; Gamulin, O.; Panduric, V.; Spanovic, N.; Tarle, Z. Long term degree of conversion of two bulk-fill composites. Acta Stomatol. Croat. 2016, 50, 292–300. [Google Scholar] [CrossRef]
- Lovell, L.G.; Berchtold, K.A.; Elliott, J.E.; Lu, H.; Bowman, C.N. Understanding the kinetics and network formation of dimethacrylate dental resins. Polym. Adv. Technol. 2001, 12, 335–345. [Google Scholar] [CrossRef]
- Nishitani, Y.; Yoshiyama, M.; Wadgaonkar, B.; Breschi, L.; Mannello, F.; Mazzoni, A.; Carvalho, R.M.; Tjäderhane, L.; Tay, F.R.; Pashley, D.H. Activation of gelatinolytic/collagenolytic activity in dentin by self-etching adhesives. Eur. J. Oral Sci. 2006, 114, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Pashley, D.H.; Tay, F.R.; Yiu, C.; Hashimoto, M.; Breschi, L.; Carvalho, R.M.; Ito, S. Collagen degradation by host-derived enzymes during aging. J. Dent. Res. 2004, 83, 216–221. [Google Scholar] [CrossRef]
- Toledano, M.; Yamauti, M.; Osorio, E.; Osorio, R. Zinc-inhibited MMP-mediated collagen degradation after different dentine demineralization procedures. Caries Res. 2012, 46, 201–207. [Google Scholar] [CrossRef]
- Hebling, J.; Pashley, D.H.; Tjäderhane, L.; Tay, F.R. Chlorhexidine arrests subclinical degradation of dentin hybrid layers in vivo. J. Dent. Res. 2005, 84, 741–746. [Google Scholar] [CrossRef]
- Tezvergil-Mutluay, A.; Seseogullari-Dirihan, R.; Feitosa, V.P.; Tay, F.R.; Watson, T.F.; Pashley, D.H.; Sauro, S. Zoledronate and ion-releasing resins impair dentin collagen degradation. J. Dent. Res. 2014, 93, 999–1004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tjäderhane, L.; Nascimento, F.D.; Breschi, L.; Mazzoni, A.; Tersariol, I.L.; Geraldeli, S.; Tezvergil-Mutluay, A.; Carrilho, M.R.; Carvalho, R.M.; Tay, F.R.; et al. Optimizing dentin bond durability: Control of collagen degradation by matrix metalloproteinases and cysteine cathepsins. Dent. Mater. 2013, 29, 116–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Spencer, P. Continuing etching of an all-in-one adhesive in wet dentin tubules. J. Dent. Res. 2005, 84, 350–354. [Google Scholar] [CrossRef] [PubMed]
- Frankenberger, R.; Lopes, M.; Perdigão, J.; Ambrose, W.W.; Rosa, B.T. The use of flowable composites as filled adhesives. Dent. Mater. 2002, 18, 227–238. [Google Scholar] [CrossRef]
- Par, M.; Tarle, Z.; Hickel, R.; Ilie, N. Polymerization kinetics of experimental bioactive composites containing bioactive glass. J. Dent. 2018, 76, 83–88. [Google Scholar] [CrossRef]
- Par, M.; Spanovic, N.; Tauböck, T.T.; Attin, T.; Tarle, Z. Degree of conversion of experimental resin composites containing bioactive glass 45S5: The effect of post-cure heating. Sci. Rep. 2019, 9, 17245. [Google Scholar] [CrossRef]
- Par, M.; Spanovic, N.; Mohn, D.; Attin, T.; Tauböck, T.T.; Tarle, Z. Curing potential of experimental resin composites filled with bioactive glass: A comparison between Bis-EMA and UDMA based resin systems. Dent. Mater. 2020, 36, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Jäger, F.; Mohn, D.; Attin, T.; Tauböck, T.T. Polymerization and shrinkage stress formation of experimental resin composites doped with nano- vs. micron-sized bioactive glasses. Dent. Mater. J. 2021, 40, 110–115. [Google Scholar] [CrossRef] [PubMed]

| Product | Manufacturer | Type | wt% | Composition | pH | LOT |
|---|---|---|---|---|---|---|
| Scotchbond Universal Etchant | 3M, St. Paul, MN, USA | Etchant: | <1 | 5572623 | ||
| 55–65 | Water | |||||
| 30–40 | Phosphoric acid | |||||
| 5–10 | Silica | |||||
| 1–5 | Polyglycol | |||||
| <2 | Aluminum oxide | |||||
| Adper Scotchbond Multi-Purpose Adhesive (ASB) | 3M, St. Paul, MN, USA | 3-step etch-and-rinse | Primer: | 2.9–4.0 | NA37642 | |
| 40–50 | Water | |||||
| 35–45 | HEMA 1 | |||||
| 10–20 | Copolymer of itaconic and acrylic acid | |||||
| Adhesive: | neutral | NA44272 | ||||
| 60–70 | Bis-GMA 2 | |||||
| 30–40 | HEMA | |||||
| <0.5 | Triphenylatimone | |||||
| <0.2 | Triphenylphosphine | |||||
| <0.05 | Hydroquinone | |||||
| Vococid® | VOCO GmbH, Cuxhaven, Germany | Etchant: | 0.8 | 1923187 | ||
| 25–50 | Phosphoric acid | |||||
| Solobond Plus (SB) | VOCO GmbH, Cuxhaven, Germany | 3-step etch-and-rinse | Primer: | 2.5 | 1926411 | |
| 10–25 | HEMA | |||||
| 10–25 | Acetone | |||||
| 10–25 | Hydroxypropyl methacrylate | |||||
| ≤2.5 | Catalyst | |||||
| Adhesive: | 4.6 | 1915395 | ||||
| 50–100 | Acetone | |||||
| 10–25 | Bis-GMA | |||||
| 10–25 | TEGDMA 3 | |||||
| 5–10 | HEMA | |||||
| ≤2.5 | Catalyst | |||||
| Clearfil SE Bond (CF) | Kuraray Noritake Dental Inc., Osaka, Japan | 2-step self-etch | 20–40 | Primer: HEMA 10-MDP 4, camphorquinone, hydrophilic dimethacrylate | 2.0 | 3R0326 |
25–45 20–40 | Bonding: Bis-GMA HEMA 10-MDP, aliphatic dimethylacrylate, dl-camphorquinone, accelerator, water, colorants | 2.3–2.4 | 2T0543 | |||
| Compounds | Amount in 1000 mL |
|---|---|
| NaCl | 7.995 g |
| (HOCH2)3CNH2 | 6.055 g |
| CaCl2 | 0.368 g |
| NaHCO3 | 0.353 g |
| MgCl2 | 0.305 g |
| KCl | 0.224 g |
| K2HOP4 | 0.174 g |
| Na2SO4 | 0.071 g |
| 1.0 M HCl | 40 mL |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oltramare, R.; Par, M.; Mohn, D.; Wiedemeier, D.B.; Attin, T.; Tauböck, T.T. Short- and Long-Term Dentin Bond Strength of Bioactive Glass-Modified Dental Adhesives. Nanomaterials 2021, 11, 1894. https://doi.org/10.3390/nano11081894
Oltramare R, Par M, Mohn D, Wiedemeier DB, Attin T, Tauböck TT. Short- and Long-Term Dentin Bond Strength of Bioactive Glass-Modified Dental Adhesives. Nanomaterials. 2021; 11(8):1894. https://doi.org/10.3390/nano11081894
Chicago/Turabian StyleOltramare, Ramona, Matej Par, Dirk Mohn, Daniel B. Wiedemeier, Thomas Attin, and Tobias T. Tauböck. 2021. "Short- and Long-Term Dentin Bond Strength of Bioactive Glass-Modified Dental Adhesives" Nanomaterials 11, no. 8: 1894. https://doi.org/10.3390/nano11081894






