Combined Spectroscopic and Computational Study of Nitrobenzene Activation on Non-Noble Metals-Based Mono- and Bimetallic Catalysts
Abstract
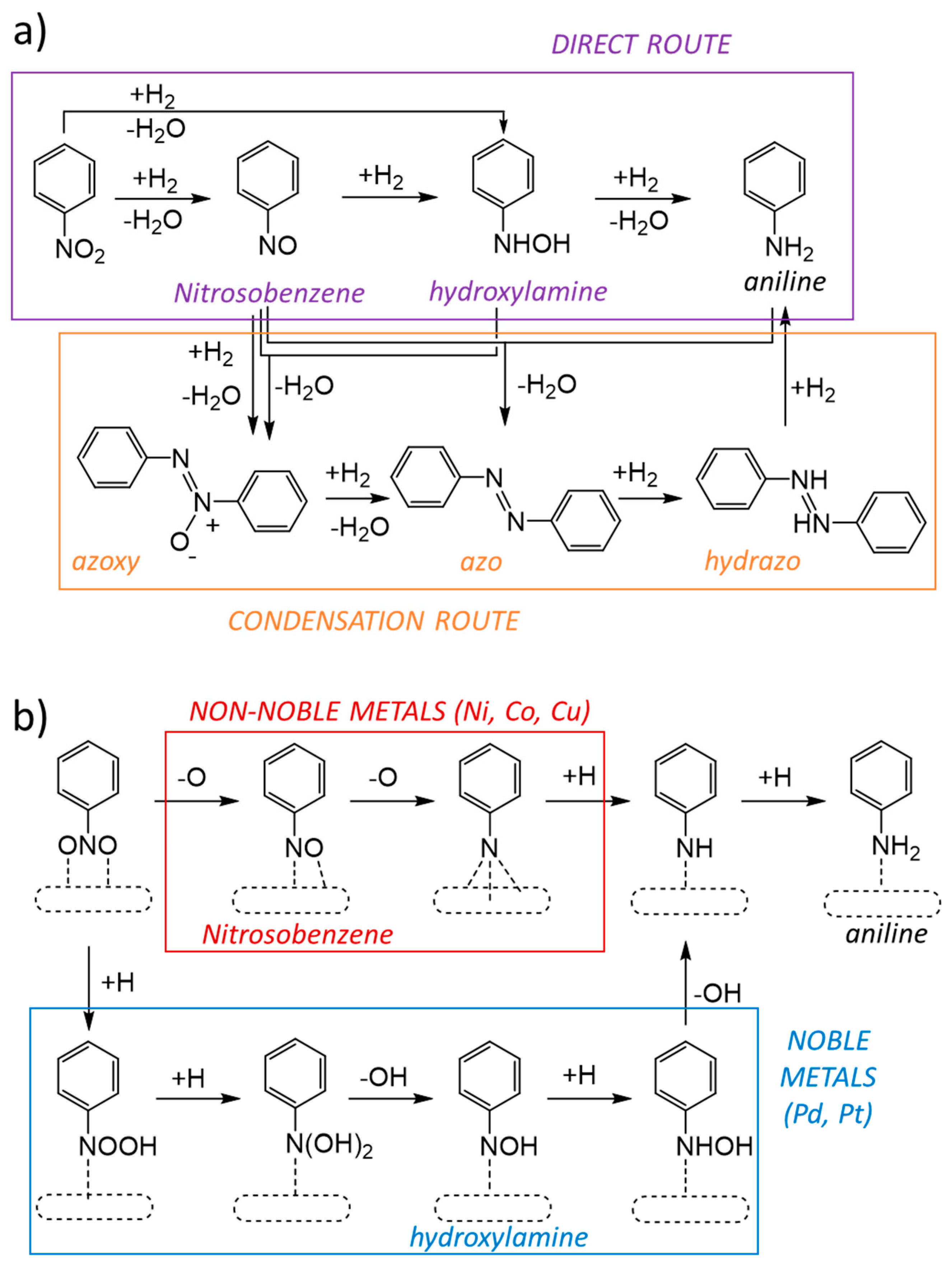
:1. Introduction
2. Materials and Methods
2.1. Catalyst Synthesis and Characterization
2.1.1. Catalyst Synthesis
2.1.2. Characterization
2.2. IR Spectroscopy
2.3. Computational Details
3. Results
3.1. Synthesis and Characterization of Monometallic Ni/SiO2, Cu/SiO2 and Pd/SiO2 Catalysts
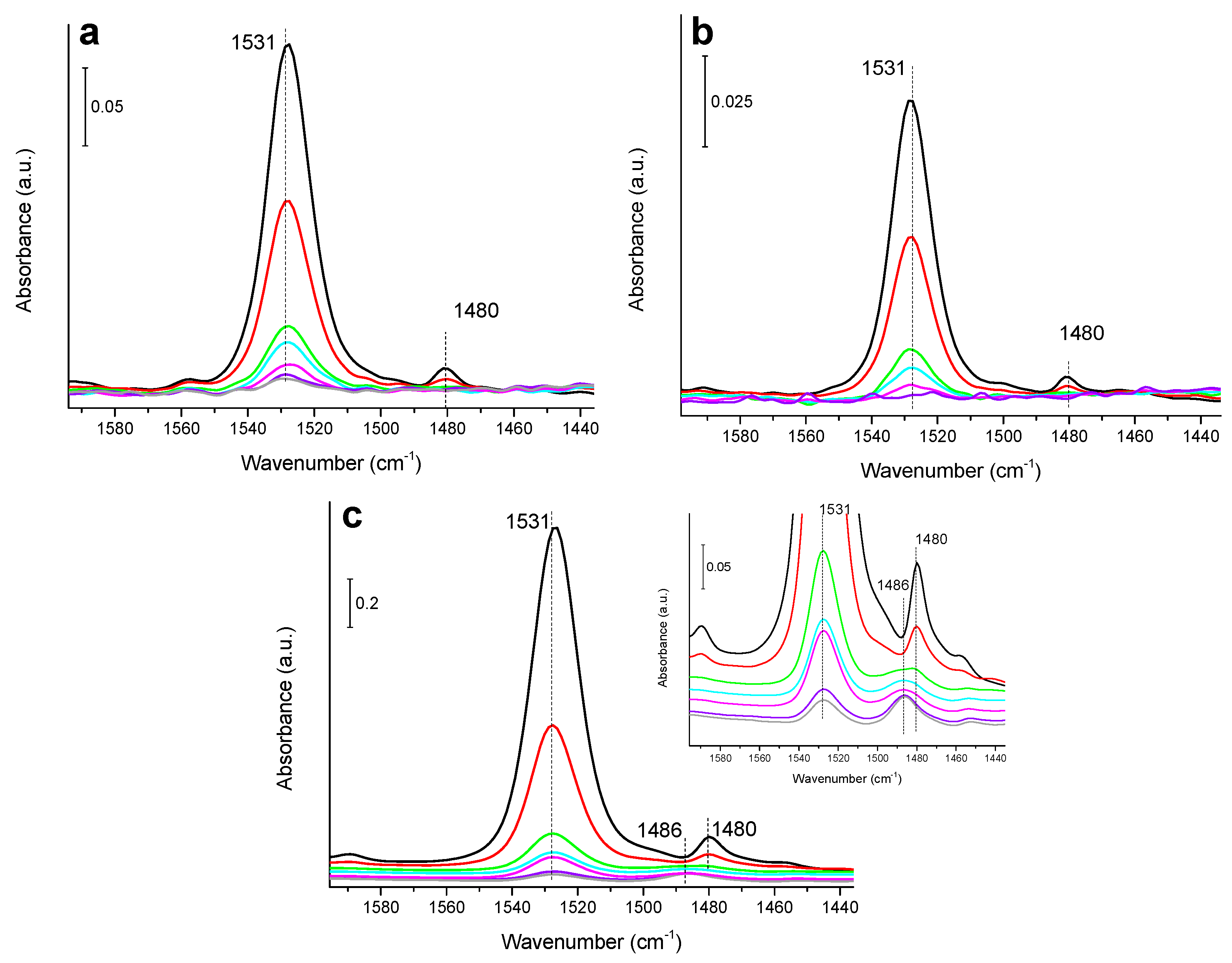
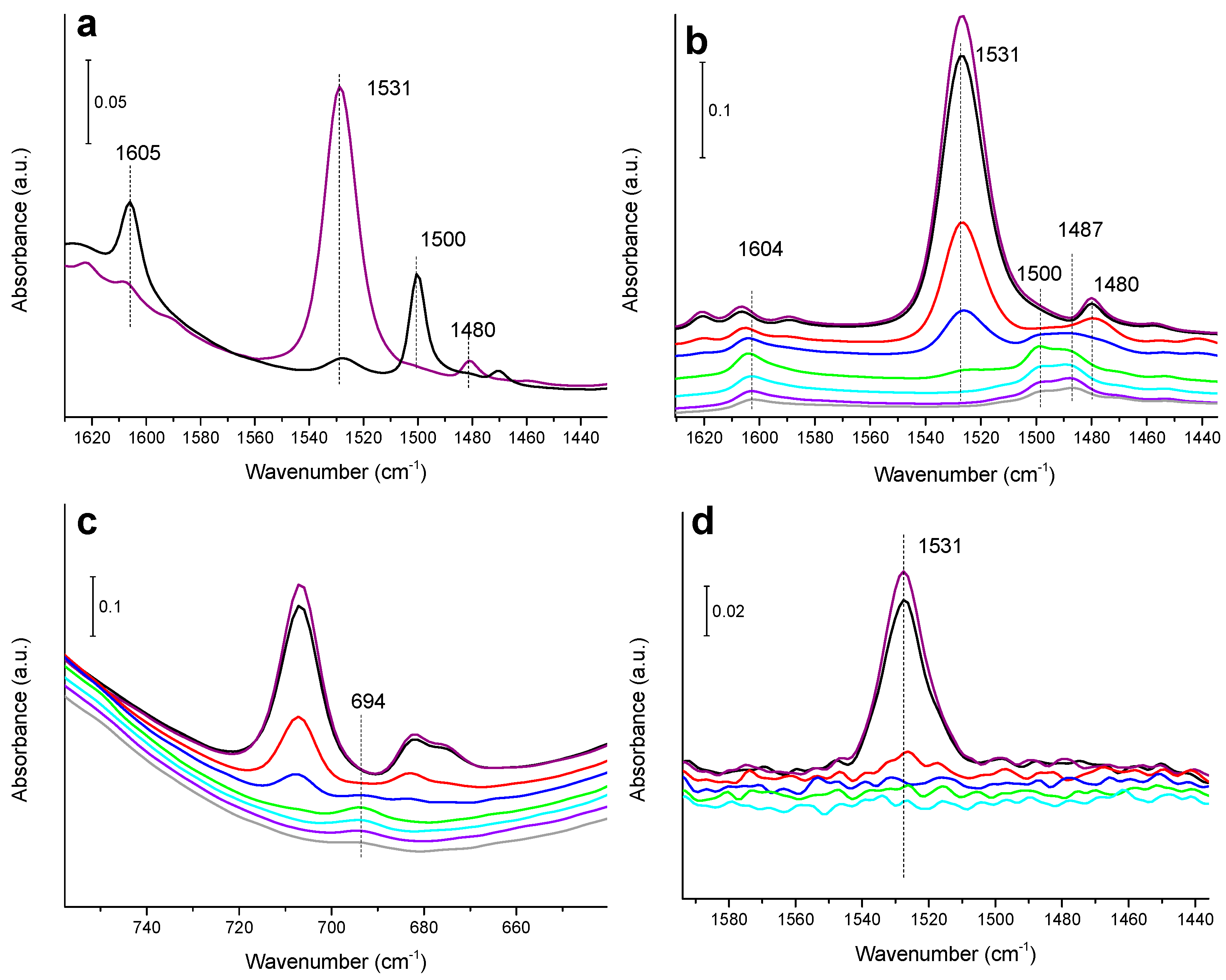
3.2. IR Study of Nitrobenzene Hydrogenation on Monometallic Ni/SiO2, Cu/SiO2 and Pd/SiO2 Catalysts
3.3. Synthesis and Characterization of Bimetallic CuNi and CuPd Catalysts
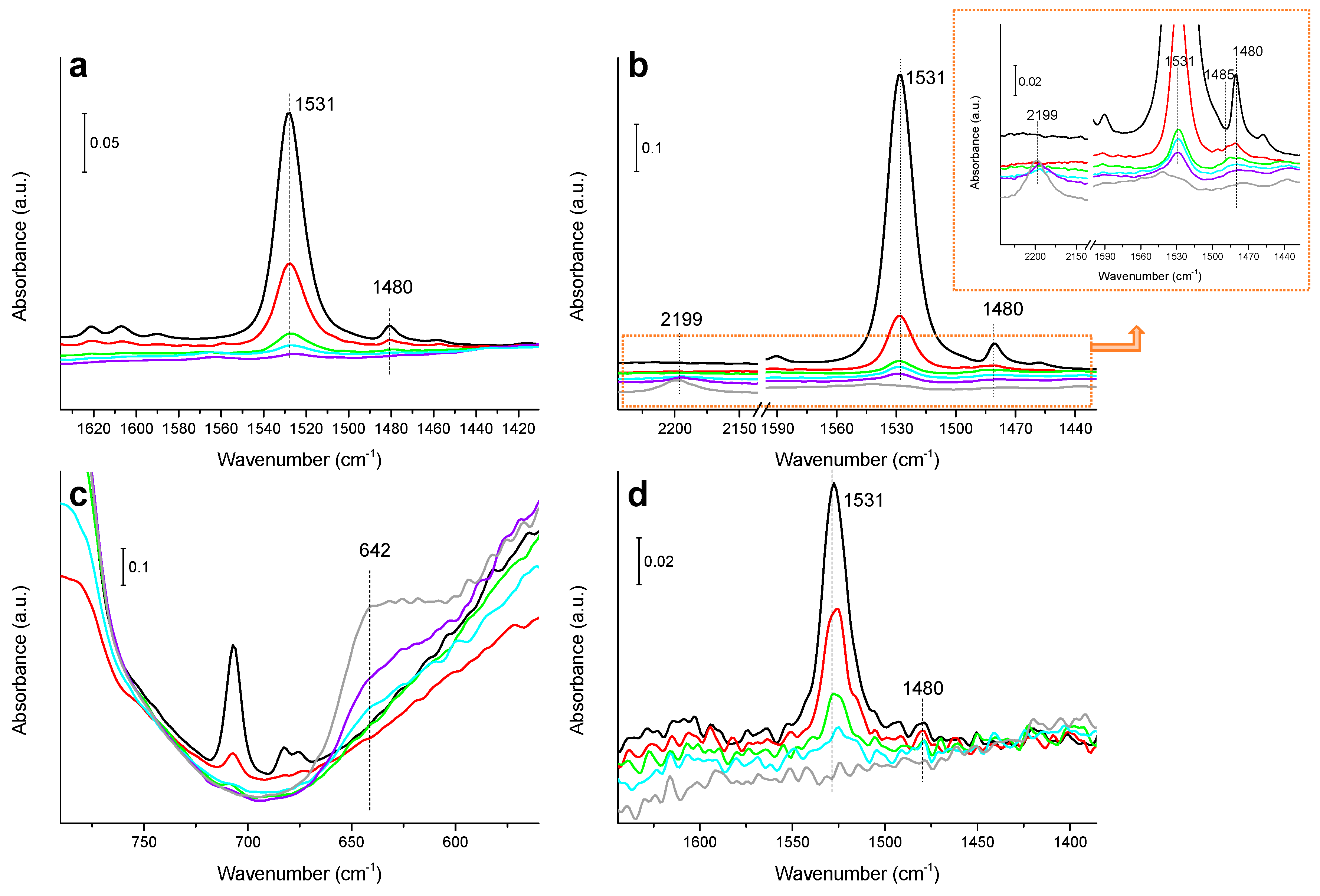
3.4. IR Study of Nitrobenzene Hydrogenation on Bimetallic CuNi and CuPd Catalysts
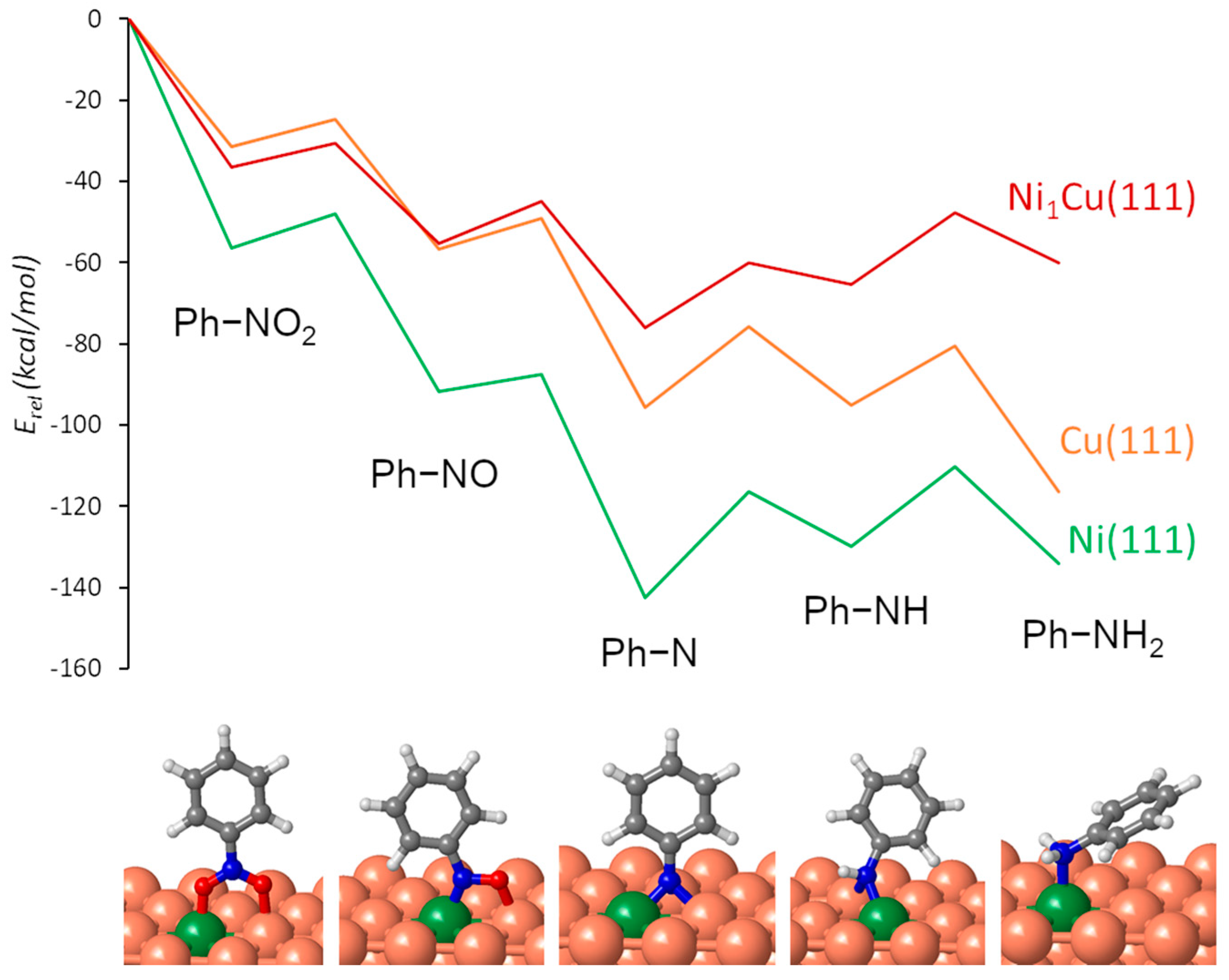
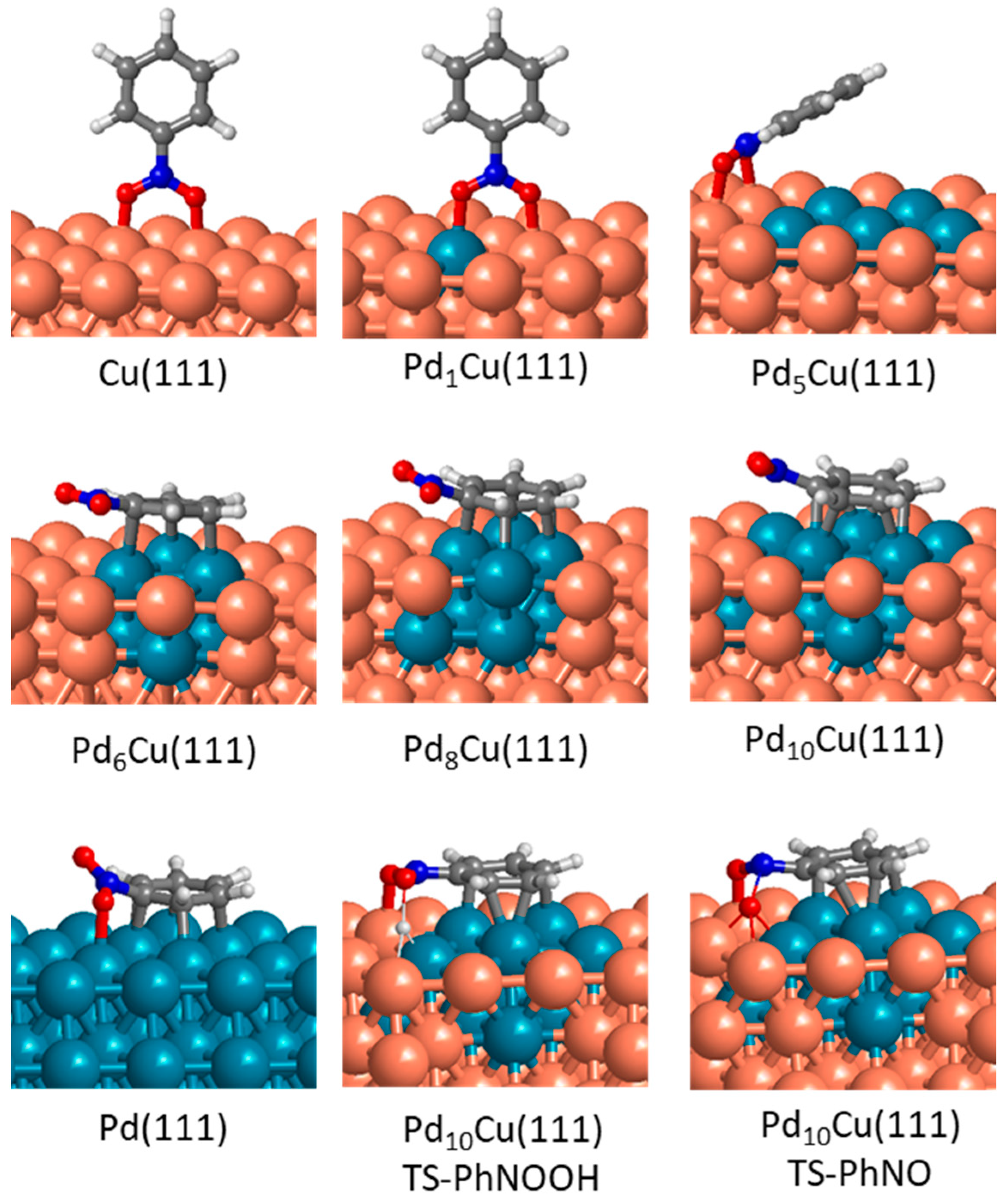
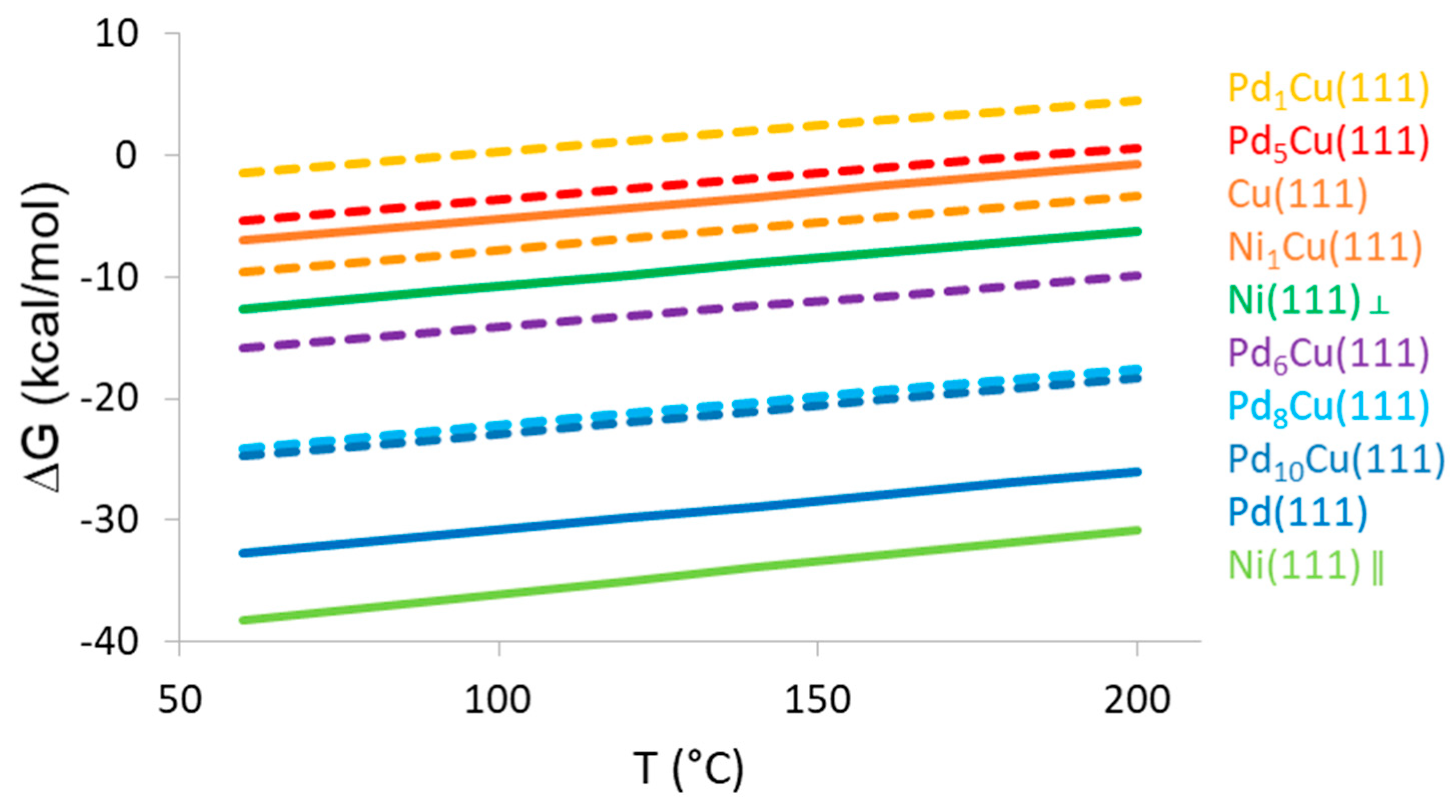
3.5. DFT Study of Nitrobenzene Adsorption and Reaction on Mono- and Bimetallic Catalysts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tafesh, A.M.; Weiguny, J. A Review of the Selective Catalytic Reduction of Aromatic Nitro Compounds into Aromatic Amines, Isocyanates, Carbamates, and Ureas Using CO. Chem. Rev. 1996, 96, 2035–2052. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.A.; van Bekkum, H. Fine Chemicals through Heterogeneous Catalysis; John Wiley & Sons: Hoboken, NJ, USA, 2008; ISBN 3527612971. [Google Scholar]
- Blaser, H.-U.; Malan, C.; Pugin, B.; Spindler, F.; Steiner, H.; Studer, M. Selective Hydrogenation for Fine Chemicals: Recent Trends and New Developments. Adv. Synth. Catal. 2003, 345, 103–151. [Google Scholar] [CrossRef]
- Blaser, H.-U.; Steiner, H.; Studer, M. Selective Catalytic Hydrogenation of Functionalized Nitroarenes: An Update. ChemCatChem 2009, 1, 210–221. [Google Scholar] [CrossRef]
- Boymans, E.; Boland, S.; Witte, P.T.; Müller, C.; Vogt, D. Chemoselective Hydrogenation of Functionalized Nitroarenes Using Supported Mo Promoted Pt Nanoparticles. ChemCatChem 2013, 5, 431–434. [Google Scholar] [CrossRef]
- Lara, P.; Philippot, K. The Hydrogenation of Nitroarenes Mediated by Platinum Nanoparticles: An Overview. Catal. Sci. Technol. 2014, 4, 2445–2465. [Google Scholar] [CrossRef]
- Pietrowski, M. Recent Developments in Heterogeneous Selective Hydrogenation of Halogenated Nitroaromatic Compounds to Halogenated Anilines. Curr. Org. Synth. 2012, 9, 470–487. [Google Scholar] [CrossRef]
- Formenti, D.; Ferretti, F.; Scharnagl, F.K.; Beller, M. Reduction of Nitro Compounds Using 3d-Non-Noble Metal Catalysts. Chem. Rev. 2019, 119, 2611–2680. [Google Scholar] [CrossRef]
- Corma, A.; Serna, P. Chemoselective Hydrogenation of Nitro Compounds with Supported Gold Catalysts. Science 2006, 313, 332. [Google Scholar] [CrossRef]
- Serna, P.; Concepción, P.; Corma, A. Design of Highly Active and Chemoselective Bimetallic Gold–Platinum Hydrogenation Catalysts through Kinetic and Isotopic Studies. J. Catal. 2009, 265, 19–25. [Google Scholar] [CrossRef]
- Wei, H.; Liu, X.; Wang, A.; Zhang, L.; Qiao, B.; Yang, X.; Huang, Y.; Miao, S.; Liu, J.; Zhang, T. FeOx-Supported Platinum Single-Atom and Pseudo-Single-Atom Catalysts for Chemoselective Hydrogenation of Functionalized Nitroarenes. Nat. Commun. 2014, 5, 5634. [Google Scholar] [CrossRef] [Green Version]
- Shimizu, K.; Miyamoto, Y.; Satsuma, A. Size- and Support-Dependent Silver Cluster Catalysis for Chemoselective Hydrogenation of Nitroaromatics. J. Catal. 2010, 270, 86–94. [Google Scholar] [CrossRef]
- Lin, L.; Yao, S.; Gao, R.; Liang, X.; Yu, Q.; Deng, Y.; Liu, J.; Peng, M.; Jiang, Z.; Li, S.; et al. A Highly CO-Tolerant Atomically Dispersed Pt Catalyst for Chemoselective Hydrogenation. Nat. Nanotechnol. 2019, 14, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Corma, A.; Serna, P.; Concepción, P.; Calvino, J.J. Transforming Nonselective into Chemoselective Metal Catalysts for the Hydrogenation of Substituted Nitroaromatics. J. Am. Chem. Soc. 2008, 130, 8748–8753. [Google Scholar] [CrossRef]
- Macino, M.; Barnes, A.J.; Althahban, S.M.; Qu, R.; Gibson, E.K.; Morgan, D.J.; Freakley, S.J.; Dimitratos, N.; Kiely, C.J.; Gao, X.; et al. Tuning of Catalytic Sites in Pt/TiO2 Catalysts for the Chemoselective Hydrogenation of 3-Nitrostyrene. Nat. Catal. 2019, 2, 873–881. [Google Scholar] [CrossRef]
- Wang, Y.; Qin, R.; Wang, Y.; Ren, J.; Zhou, W.; Li, L.; Ming, J.; Zhang, W.; Fu, G.; Zheng, N. Chemoselective Hydrogenation of Nitroaromatics at the Nanoscale Iron(III)–OH–Platinum Interface. Angew. Chem. Int. Ed. 2020, 59, 12736–12740. [Google Scholar] [CrossRef]
- Vilé, G.; Almora-Barrios, N.; López, N.; Pérez-Ramírez, J. Structure and Reactivity of Supported Hybrid Platinum Nanoparticles for the Flow Hydrogenation of Functionalized Nitroaromatics. ACS Catal. 2015, 5, 3767–3778. [Google Scholar] [CrossRef] [Green Version]
- Takasaki, M.; Motoyama, Y.; Higashi, K.; Yoon, S.-H.; Mochida, I.; Nagashima, H. Chemoselective Hydrogenation of Nitroarenes with Carbon Nanofiber-Supported Platinum and Palladium Nanoparticles. Org. Lett. 2008, 10, 1601–1604. [Google Scholar] [CrossRef]
- Boronat, M.; Concepción, P.; Corma, A.; González, S.; Illas, F.; Serna, P. A Molecular Mechanism for the Chemoselective Hydrogenation of Substituted Nitroaromatics with Nanoparticles of Gold on TiO2 Catalysts: A Cooperative Effect between Gold and the Support. J. Am. Chem. Soc. 2007, 129, 16230–16237. [Google Scholar] [CrossRef]
- Boronat, M.; Corma, A. Origin of the Different Activity and Selectivity toward Hydrogenation of Single Metal Au and Pt on TiO2 and Bimetallic Au−Pt/TiO2 Catalysts. Langmuir 2010, 26, 16607–16614. [Google Scholar] [CrossRef]
- Boronat, M.; Concepción, P. Combined Theoretical and Spectroscopic Mechanistic Studies for Improving Activity and Selectivity in Heterogeneous Catalysis. Catal. Today 2017, 285, 166–178. [Google Scholar] [CrossRef]
- Westerhaus, F.A.; Jagadeesh, R.V.; Wienhöfer, G.; Pohl, M.-M.; Radnik, J.; Surkus, A.-E.; Rabeah, J.; Junge, K.; Junge, H.; Nielsen, M.; et al. Heterogenized Cobalt Oxide Catalysts for Nitroarene Reduction by Pyrolysis of Molecularly Defined Complexes. Nat. Chem. 2013, 5, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Jagadeesh, R.V.; Surkus, A.-E.; Junge, H.; Pohl, M.-M.; Radnik, J.; Rabeah, J.; Huan, H.; Schünemann, V.; Brückner, A.; Beller, M. Nanoscale Fe2O3-Based Catalysts for Selective Hydrogenation of Nitroarenes to Anilines. Science 2013, 342, 1073. [Google Scholar] [CrossRef]
- Wei, Z.; Wang, J.; Mao, S.; Su, D.; Jin, H.; Wang, Y.; Xu, F.; Li, H.; Wang, Y. In Situ-Generated Co0-Co3O4/N-Doped Carbon Nanotubes Hybrids as Efficient and Chemoselective Catalysts for Hydrogenation of Nitroarenes. ACS Catal. 2015, 5, 4783–4789. [Google Scholar] [CrossRef]
- Liu, L.; Concepción, P.; Corma, A. Non-Noble Metal Catalysts for Hydrogenation: A Facile Method for Preparing Co Nanoparticles Covered with Thin Layered Carbon. J. Catal. 2016, 340, 1–9. [Google Scholar] [CrossRef]
- Ren, Y.; Wei, H.; Yin, G.; Zhang, L.; Wang, A.; Zhang, T. Oxygen Surface Groups of Activated Carbon Steer the Chemoselective Hydrogenation of Substituted Nitroarenes over Nickel Nanoparticles. Chem. Commun. 2017, 53, 1969–1972. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Olivos-Suarez, A.I.; Osadchii, D.; Romero, M.J.V.; Kapteijn, F.; Gascon, J. Single Cobalt Sites in Mesoporous N-Doped Carbon Matrix for Selective Catalytic Hydrogenation of Nitroarenes. J. Catal. 2018, 357, 20–28. [Google Scholar] [CrossRef]
- Yang, F.; Wang, M.; Liu, W.; Yang, B.; Wang, Y.; Luo, J.; Tang, Y.; Hou, L.; Li, Y.; Li, Z.; et al. Atomically Dispersed Ni as the Active Site towards Selective Hydrogenation of Nitroarenes. Green Chem. 2019, 21, 704–711. [Google Scholar] [CrossRef]
- Pan, H.; Peng, Y.; Lu, X.; He, J.; He, L.; Wang, C.; Yue, F.; Zhang, H.; Zhou, D.; Xia, Q. Well-Constructed Ni@CN Material Derived from Di-Ligands Ni-MOF to Catalyze Mild Hydrogenation of Nitroarenes. Mol. Catal. 2020, 485, 110838. [Google Scholar] [CrossRef]
- Tang, Q.; Yuan, Z.; Jin, S.; Yao, K.; Yang, H.; Chi, Q.; Liu, B. Biomass-Derived Carbon-Supported Ni Catalyst: An Effective Heterogeneous Non-Noble Metal Catalyst for the Hydrogenation of Nitro Compounds. React. Chem. Eng. 2020, 5, 58–65. [Google Scholar] [CrossRef]
- Petkar, D.R.; Kadu, B.S.; Chikate, R.C. Highly Efficient and Chemoselective Transfer Hydrogenation of Nitroarenes at Room Temperature over Magnetically Separable Fe–Ni Bimetallic Nanoparticles. RSC Adv. 2014, 4, 8004–8010. [Google Scholar] [CrossRef]
- Zhang, J.; Lu, G.; Cai, C. Chemoselective Transfer Hydrogenation of Nitroarenes by Highly Dispersed Ni-Co BMNPs. Catal. Commun. 2016, 84, 25–29. [Google Scholar] [CrossRef]
- Liu, L.; Gao, F.; Concepción, P.; Corma, A. A New Strategy to Transform Mono and Bimetallic Non-Noble Metal Nanoparticles into Highly Active and Chemoselective Hydrogenation Catalysts. J. Catal. 2017, 350, 218–225. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.; Tang, W.; Li, K.; Yin, H.; Zhao, S.; Zhou, S. Design of Cu-Based Intermetallic Nanocrystals for Enhancing Hydrogenation Selectivity. Chem. Eng. Sci. 2019, 196, 402–413. [Google Scholar] [CrossRef]
- Huang, L.; Lv, Y.; Wu, S.; Liu, P.; Xiong, W.; Hao, F.; Luo, H. Activated Carbon Supported Bimetallic Catalysts with Combined Catalytic Effects for Aromatic Nitro Compounds Hydrogenation under Mild Conditions. Appl. Catal. A Gen. 2019, 577, 76–85. [Google Scholar] [CrossRef]
- González-Vera, D.; Bustamante, T.M.; de León, J.N.D.; Dinamarca, R.; Morales, R.; Osorio-Vargas, P.A.; Torres, C.C.; Campos, C.H. Chemoselective Nitroarene Hydrogenation over Ni-Pd Alloy Supported on TiO2 Prepared from Ilmenite-Type PdxNi1−xTiO3. Mater. Today Commun. 2020, 24, 101091. [Google Scholar] [CrossRef]
- Haber, F. Gradual Electrolytic Reduction of Nitrobenzene with Limited Cathode Potential. Elektrochem. Angew. Phys. Chem. 1898, 22, 506–514. [Google Scholar]
- Sheng, T.; Qi, Y.-J.; Lin, X.; Hu, P.; Sun, S.-G.; Lin, W.-F. Insights into the Mechanism of Nitrobenzene Reduction to Aniline over Pt Catalyst and the Significance of the Adsorption of Phenyl Group on Kinetics. Chem. Eng. J. 2016, 293, 337–344. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Jiang, J.; Shi, W.; Xia, S.; Ni, Z.; Xiao, X. Insights into the Hydrogenation Mechanism of Nitrobenzene to Aniline on Pd3/Pt(111): A Density Functional Theory Study. RSC Adv. 2015, 5, 34319–34326. [Google Scholar] [CrossRef]
- Mahata, A.; Rai, R.K.; Choudhuri, I.; Singh, S.K.; Pathak, B. Direct vs. Indirect Pathway for Nitrobenzene Reduction Reaction on a Ni Catalyst Surface: A Density Functional Study. Phys. Chem. Chem. Phys. 2014, 16, 26365–26374. [Google Scholar] [CrossRef]
- Millán, R.; Liu, L.; Boronat, M.; Corma, A. A New Molecular Pathway Allows the Chemoselective Reduction of Nitroaromatics on Non-Noble Metal Catalysts. J. Catal. 2018, 364, 19–30. [Google Scholar] [CrossRef]
- Millán, R.; Boronat, M. Hydrogenation of Substituted Nitroaromatics on Non-Noble Metal Catalysts: Mechanistic Insights to Improve Selectivity. Faraday Discuss. 2021, 229, 297–317. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Furthmüller, J. Efficient Iterative Schemes for Ab Initio Total-Energy Calculations Using a Plane-Wave Basis Set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Fluid Vesicles in Shear Flow. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple (Vol 77, Pg 3865, 1996). Phys. Rev. Lett. 1997, 78, 1396. [Google Scholar] [CrossRef] [Green Version]
- Grimme, S. Semiempirical GGA-Type Density Functional Constructed with a Long-Range Dispersion Correction. J. Comput. Chem. 2006, 27, 1787–1799. [Google Scholar] [CrossRef]
- Blöchl, P.E.; Jepsen, O.; Andersen, O.K. Improved Tetrahedron Method for Brillouin-Zone Integrations. Phys. Rev. B 1994, 49, 16223–16233. [Google Scholar] [CrossRef]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B Condens. Matter 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Heyden, A.; Bell, A.T.; Keil, F.J. Efficient Methods for Finding Transition States in Chemical Reactions: Comparison of Improved Dimer Method and Partitioned Rational Function Optimization Method. J. Chem. Phys. 2005, 123, 224101. [Google Scholar] [CrossRef] [Green Version]
- Henkelman, G.; Jónsson, H. A Dimer Method for Finding Saddle Points on High Dimensional Potential Surfaces Using Only First Derivatives. J. Chem. Phys. 1999, 111, 7010–7022. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special Points for Brillouin-Zone Integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Richner, G.; van Bokhoven, J.A.; Neuhold, Y.-M.; Makosch, M.; Hungerbühler, K. In Situ Infrared Monitoring of the Solid/Liquid Catalyst Interface during the Three-Phase Hydrogenation of Nitrobenzene over Nanosized Au on TiO2. Phys. Chem. Chem. Phys. 2011, 13, 12463–12471. [Google Scholar] [CrossRef] [PubMed]
- Combita, D.; Concepción, P.; Corma, A. Gold Catalysts for the Synthesis of Aromatic Azocompounds from Nitroaromatics in One Step. J. Catal. 2014, 311, 339–349. [Google Scholar] [CrossRef]
- Baylet, A.; Marécot, P.; Duprez, D.; Castellazzi, P.; Groppi, G.; Forzatti, P. In Situ Raman and in SituXRD Analysis of PdO Reduction and Pd° Oxidation Supported on γ-Al2O3 Catalyst under Different Atmospheres. Phys. Chem. Chem. Phys. 2011, 13, 4607–4613. [Google Scholar] [CrossRef] [PubMed]

| Sample | ICP (%) | H2 a mmol/g | nm b | HD/H2 c |
|---|---|---|---|---|
| Ni/SiO2 | 23.0 | n.d d | 19.7 | n.d. |
| Cu/SiO2 | 24.0 | 5.39 | 35.9 | 0.058 × 10−1 |
| Pd/SiO2 | 23.8 | n.d. | 28.7 | n.d. |
| Sample | ICP Cu (%) | ICP Me(%) | H2 a mmol/g | nm b | HD/H2 c |
|---|---|---|---|---|---|
| Cu0.5Pd/SiO2 | 26.0 | 0.5 | 4.96 | 15.9 | 1.18 × 10−1 |
| Cu2Pd/SiO2 | 23.7 | 1.6 | 5.11 | 14.8 | 4.44 × 10−1 |
| Cu2Ni/SiO2 | 25.9 | 2.0 | 5.23 | 34.4 | 2.89 × 10−1 |
| Ni(111) | Cu(111) | Ni1Cu(111) | |||||
|---|---|---|---|---|---|---|---|
| Eact | ΔE | Eact | ΔE | Eact | ΔE | ||
| (10) | Ph–NO2* → Ph–NO* + O* | 8.3 | −35.6 | 6.9 | −25.2 | 5.8 | −24.7 |
| (11) | Ph–NO* → Ph–N* + O* | 4.3 | −50.2 | 7.5 | −39.1 | 10.3 | −31.1 |
| (12) | Ph–N* + H* → Ph–NH* | 26.1 | 12.4 | 19.8 | 0.8 | 15.9 | −5.1 |
| (13) | Ph–NH* + H* → Ph–NH2* | 19.6 | −4.1 | 14.3 | −21.4 | 17.6 | −12.5 |
| Ni(111) a | Cu(111) a | Pd(111) a | Pd10Cu(111) | |
|---|---|---|---|---|
| Ph-NO2* + H* → Ph-NOOH* | 25.5 | 12.9 | 15.9 | 9.0 |
| Ph-NO2* → Ph-NO* + O* | 8.3 | 6.9 | 29.4 | 19.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Millán, R.; Soriano, M.D.; Cerdá Moreno, C.; Boronat, M.; Concepción, P. Combined Spectroscopic and Computational Study of Nitrobenzene Activation on Non-Noble Metals-Based Mono- and Bimetallic Catalysts. Nanomaterials 2021, 11, 2037. https://doi.org/10.3390/nano11082037
Millán R, Soriano MD, Cerdá Moreno C, Boronat M, Concepción P. Combined Spectroscopic and Computational Study of Nitrobenzene Activation on Non-Noble Metals-Based Mono- and Bimetallic Catalysts. Nanomaterials. 2021; 11(8):2037. https://doi.org/10.3390/nano11082037
Chicago/Turabian StyleMillán, Reisel, María Dolores Soriano, Cristina Cerdá Moreno, Mercedes Boronat, and Patricia Concepción. 2021. "Combined Spectroscopic and Computational Study of Nitrobenzene Activation on Non-Noble Metals-Based Mono- and Bimetallic Catalysts" Nanomaterials 11, no. 8: 2037. https://doi.org/10.3390/nano11082037






