Synthesis of Group II-VI Semiconductor Nanocrystals via Phosphine Free Method and Their Application in Solution Processed Photovoltaic Devices
Abstract
:1. Introduction
2. Experiment Procedure
2.1. Materials
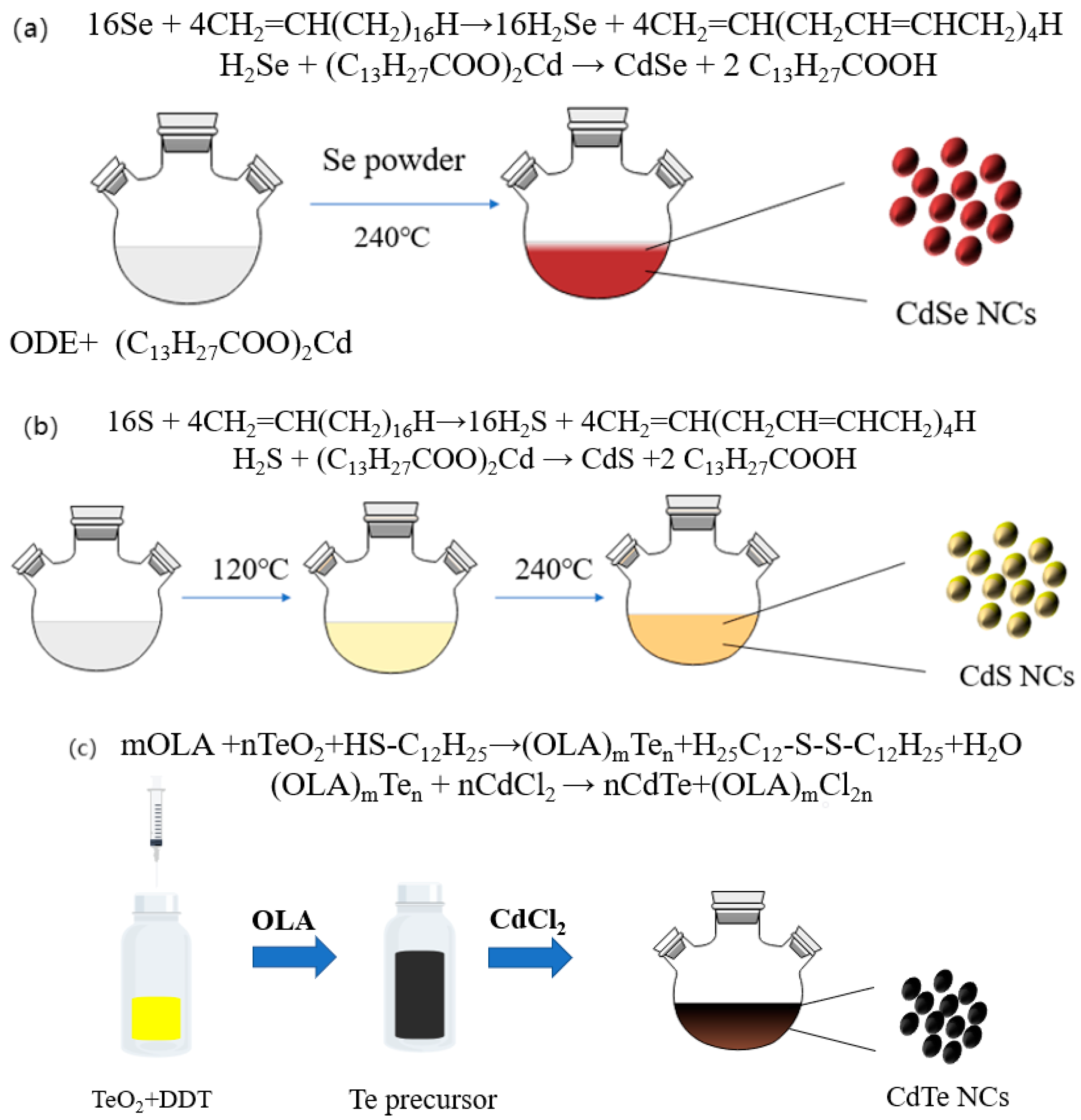
2.2. Synthesis Method of CdTe NCs
2.3. Synthesis of CdSe NCs and CdS NCs
2.4. Device Fabrication and Characterization
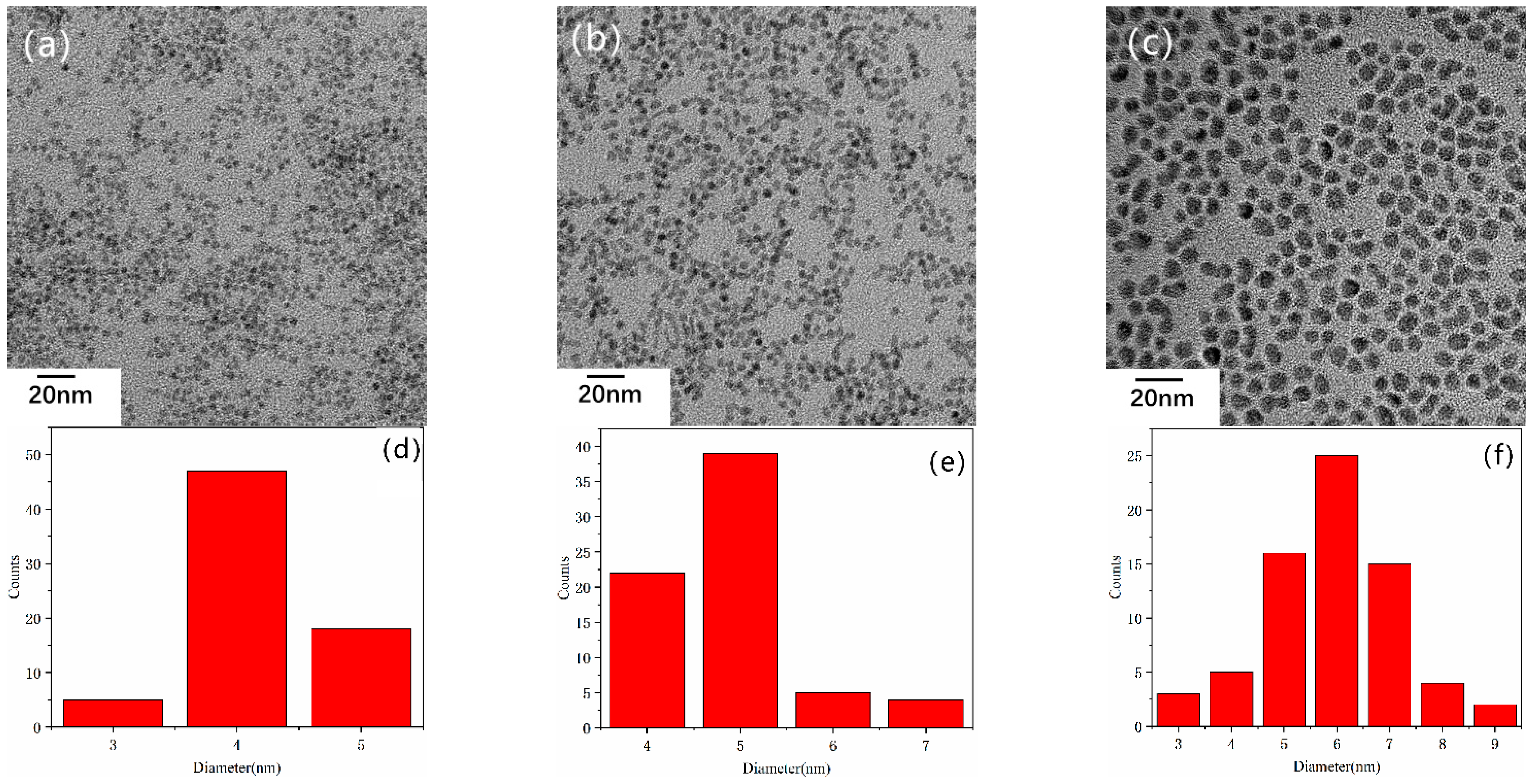
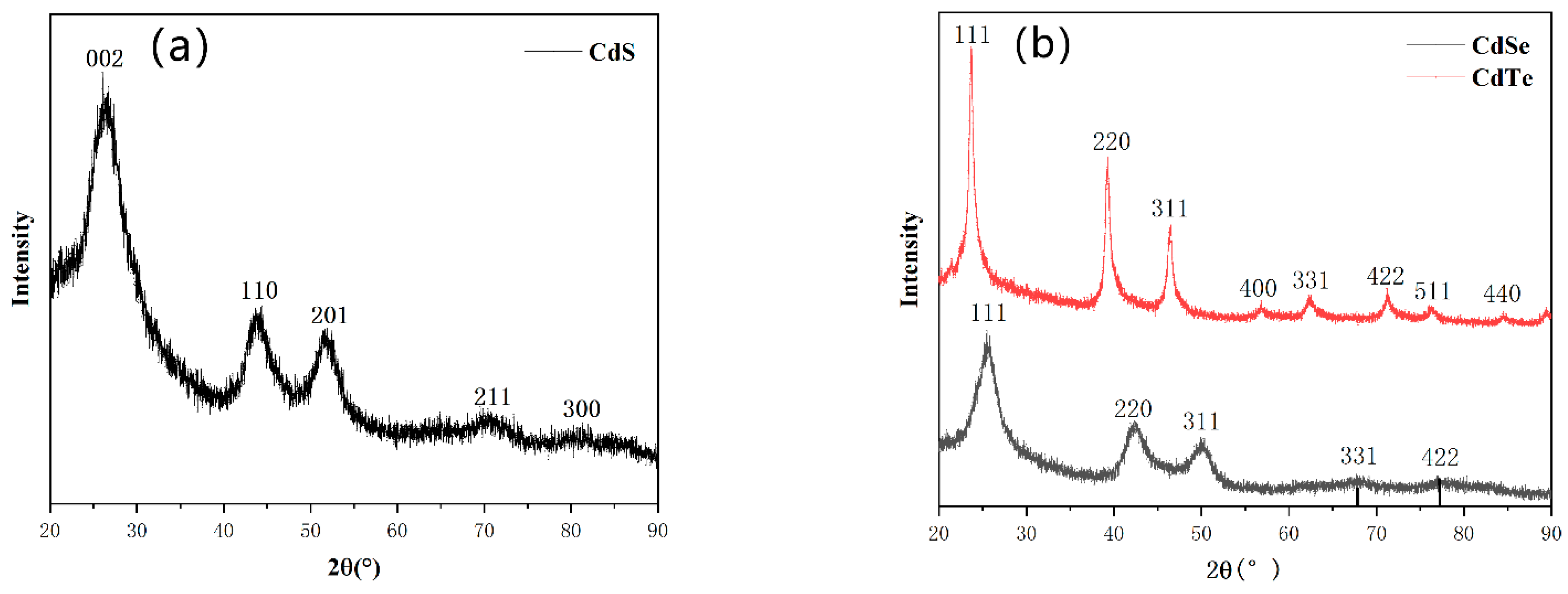
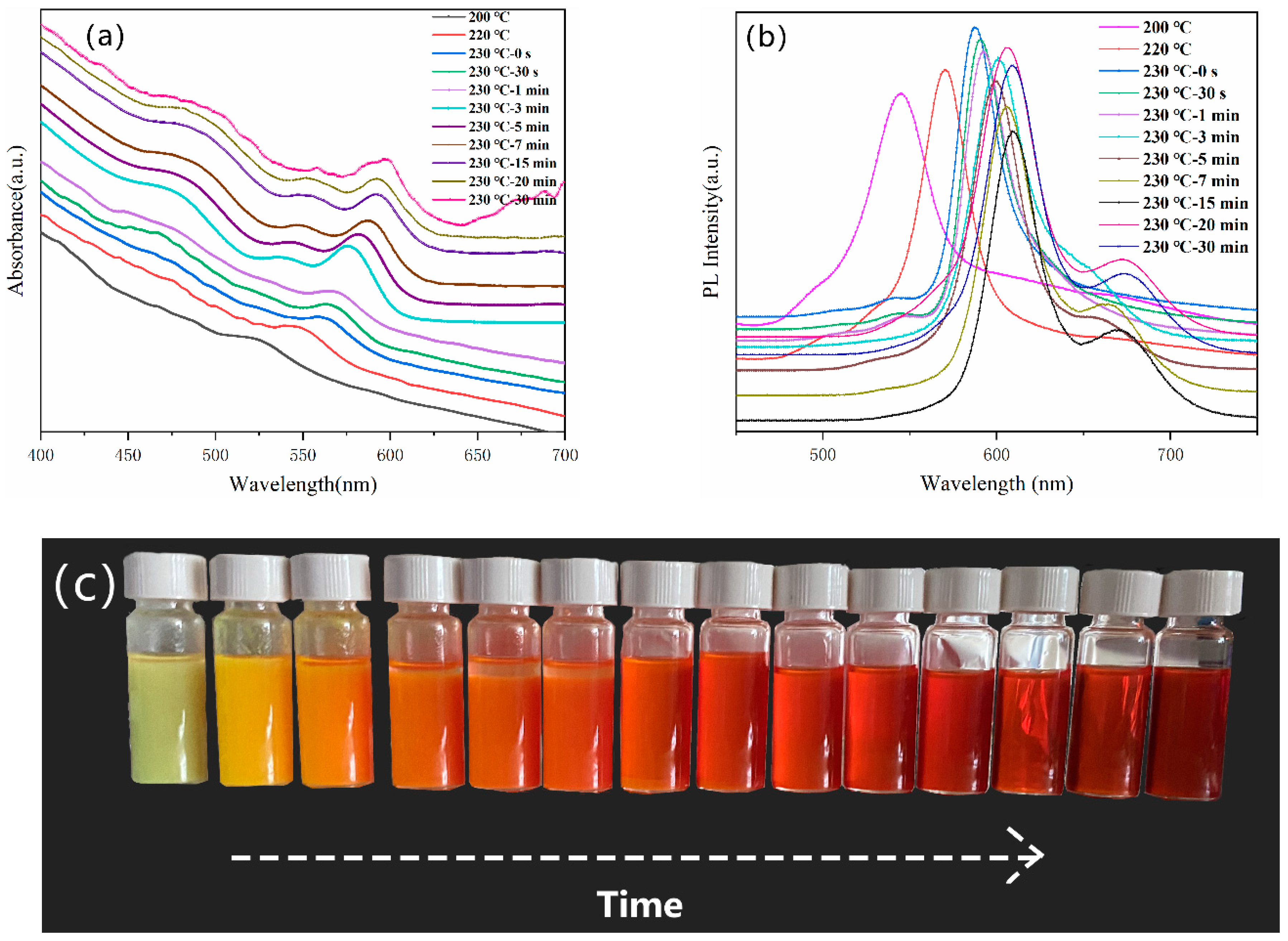
3. Result and Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Giorgi, M.; Tarì, D.; Manna, L.; Krahne, R.; Cingolani, R. Optical properties of colloidal nanocrystal spheres and tetrapods. Microelectron. J. 2005, 36, 552–554. [Google Scholar] [CrossRef]
- Dai, X.; Deng, Y.; Peng, X.; Jin, Y. Quantum-Dot Light-Emitting Diodes for Large-Area Displays: Towards the Dawn of Commercialization. Adv. Mater. 2017, 29, 1607022. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Zhang, Z.; Jin, Y.; Niu, Y.; Cao, H.; Liang, X.; Chen, L.; Wang, J.; Peng, X. Solution-processed, high-performance light-emitting diodes based on quantum dots. Nature 2014, 515, 96–99. [Google Scholar] [CrossRef]
- Pu, C.; Qin, H.; Gao, Y.; Zhou, J.; Wang, P.; Peng, X. Synthetic Control of Exciton Behavior in Colloidal Quantum Dots. J. Am. Chem. Soc. 2017, 139, 9. [Google Scholar] [CrossRef]
- Gur, I.; Fromer, N.A.; Geier, M.L.; Alivisatos, A.P. Air-Stable All-Inorganic Nanocrystal Solar Cells Processed from Solution. Science 2005, 310, 462–465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, Y.; Yang, Q.; Liu, G. Ammonium Pyrrolidine Dithiocarbamate-Modified CdTe/CdS Quantum Dots as a Turn-on Fluorescent Sensor for Detection of Trace Cadmium Ions. Sensors 2020, 20, 312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, K.; Wu, W.; Xie, S.; Jiang, Y.; Liao, S.; Qin, D. Building Solar Cells from Nanocrystal Inks. Appl. Sci. 2019, 9, 1885. [Google Scholar] [CrossRef] [Green Version]
- Capizzi, G.; Lo Sciuto, G.; Napoli, C.; Shikler, R.; Woźniak, M. Optimizing the organic solar cell manufacturing process by means of AFM measurements and neural networks. Energies 2018, 11, 1221. [Google Scholar] [CrossRef] [Green Version]
- Anderson, I.E.; Breeze, A.J.; Olson, J.D.; Yang, L.; Sahoo, Y.; Carter, S.A. All-inorganic spin-cast nanoparticlesolar cells with nonselective electrodes. Appl. Phys. Lett. 2009, 94, 063101. [Google Scholar] [CrossRef] [Green Version]
- Ju, T.; Yang, L.; Carter, S. Thickness dependence study of inorganic CdTe/CdSe solar cells fabricated fromcolloidal nanoparticle solutions. J. Appl. Phys. 2010, 10, 104311. [Google Scholar] [CrossRef]
- Olson, J.D.; Rodriguez, Y.W.; Yang, L.D.; Alers, G.B.; Carter, S.A. CdTe Schottky diodes from colloidalnanocrystals. Appl. Phys. Lett. 2010, 96, 242103. [Google Scholar] [CrossRef]
- Girard, S.; He, J.; Zhou, X.; Shoemaker, D.; Jaworski, C.; Uher, C.; Heremans, J.; Kanatzidis, M. High performance Na-doped PbTe-PbS thermoelectric materials: Electronic density of states modification and shape-controlled nanostructures. J. Am. Chem. Soc. 2011, 133, 16588–16597. [Google Scholar] [CrossRef]
- Reiss, P.; Protiere, M.; Li, L. Core/Shell semiconductor nanocrystals. Small 2009, 5, 154–168. [Google Scholar] [CrossRef]
- Murray, C.; Norris, D.; Bawendi, M. Synthesis and characterization of nearly monodisperse CdE (E = sulfur, selenium, tellurium) semiconductor nanocrystallites. J. Am. Chem. Soc. 1993, 115, 8706–8715. [Google Scholar] [CrossRef]
- Peng, X.; Wickham, J.; Alivisatos, A. Kinetics of II-VI and III-V colloidal semiconductor nanocrystal growth:“focusing” of size distributions. J. Am. Chem. Soc. 1998, 120, 5343–5344. [Google Scholar] [CrossRef]
- Qu, L.; Peng, X. Control of photoluminescence properties of CdSe nanocrystals in growth. J. Am. Chem. Soc. 2002, 124, 2049–2055. [Google Scholar] [CrossRef]
- Yu, W.W.; Qu, L.; Guo, W.; Peng, X. Experimental determination of the extinction coefficient of CdTe, CdSe, and CdS nanocrystals. Chem. Mater. 2003, 15, 2854–2860. [Google Scholar] [CrossRef]
- Yang, Y.A.; Wu, H.; Williams, K.R.; Cao, Y.C. Synthesis of CdSe and CdTe Nanocrystals without Precursor Injection. Angew. Chem. Int. Ed. 2005, 117, 6870–6873. [Google Scholar] [CrossRef]
- Yao, D.; Xin, W.; Liu, Z.; Wang, Z.; Feng, J.; Dong, C.; Liu, Y.; Yang, B.; Zhang, H. Phosphine-Free Synthesis of Metal Chalcogenide Quantum Dots by Directly Dissolving Chalcogen Dioxides in Alkylthiol as the Precursor. ACS Appl. Mater. Interfaces 2017, 9, 9840–9848. [Google Scholar] [CrossRef]
- Galanakis, I.; Mavropoulos, P. Zinc-blende compounds of transition elements with N, P, As, Sb, S, Se, and Te as half-metallic systems. Phys. Rev. B 2003, 67, 104417. [Google Scholar] [CrossRef] [Green Version]
- Zare, H.; Marandi, M.; Fardindoost, S.; Sharma, V.K.; Yeltik, A.; Akhavan, O.; Demir, H.V.; Nima Taghavinia, N. High-efficiency CdTe/CdS core/shell nanocrystals in water enabled by photo-induced colloidal hetero-epitaxy of CdS shelling at room temperature. Nano Res. 2015, 8, 2317–2328. [Google Scholar] [CrossRef]
- Kini, S.; Kulkarni, S.D.; Ganiga, V.; Nagarakshit, T.K.; Chidangil, S. Dual functionalized, stable and water dispersible CdTe Quantum Dots: Facile, one-pot aqueous synthesis, optical tuning and energy transfer applications. Mater. Res. Bull. 2018, 110, 57–66. [Google Scholar] [CrossRef]
- Li, L.; Qian, H.; Fang, N.; Ren, J. Significant enhancement of the quantum yield of CdTe nanocrystals synthesized in aqueous phase by controlling the pH and concentrations of precursor solutions. J. Lumin. 2005, 116, 59–66. [Google Scholar] [CrossRef]
- Zeng, Q.; Chen, Z.; Zhao, Y.; Du, X.; Liu, F.; Jin, G.; Dong, F.; Zhang, H.; Yang, B. Aqueous-Processed Inorganic Thin-Film Solar Cells Based on CdSe x Te1–x Nanocrystals: The Impact of Composition on Photovoltaic Performance. ACS Appl. Mater. Interfaces 2015, 7, 23223–23230. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Hu, L.; Cui, J.; Feng, T.; Du, X.; Jin, G.; Liu, F.; Ji, T.; Li, F.; Zhang, H.; et al. High-Efficiency Aqueous-Processed Polymer/CdTe Nanocrystals Planar Heterojunction Solar Cells with Optimized Band Alignment and Reduced Interfacial Charge Recombination. ACS Appl. Mater. Interfaces 2017, 9, 31345–31351. [Google Scholar] [CrossRef]
- Wen, S.; Li, M.; Yang, J.; Mei, X.; Wu, B.; Liu, X.; Heng, J.; Qin, D.; Hou, L.; Xu, W.; et al. Rationally Controlled Synthesis of CdSexTe1−x Alloy Nanocrystals and Their Application in Efficient Graded Bandgap Solar Cells. Multidiscip. Digit. Publ. Inst. 2017, 7, 380. [Google Scholar] [CrossRef] [Green Version]
- Guo, X.; Rong, Z.; Wang, L.; Liu, S.; Liu, Z.; Luo, K.; Chen, B.; Qin, D.; Ma, Y.; Wu, H.; et al. Surface passivation via acid vapor etching enables efficient and stable solution-processed CdTe nanocrystal solar cells. Sustain. Energy Fuels 2020, 4, 399–406. [Google Scholar] [CrossRef]
- Rong, Z.; Guo, X.; Lian, S.; Liu, S.; Qin, D.; Mo, Y.; Xu, W.; Wu, H.; Zhao, H.; Hou, L. Interface Engineering for Both Cathode and Anode Enables Low-Cost Highly Efficient Solution-Processed CdTe Nanocrystal Solar Cells. Adv. Funct. Mater. 2019, 29, 1904018. [Google Scholar] [CrossRef]
- Jasieniak, J.; MacDonald, B.I.; Watkins, S.E.; Mulvaney, P. Solution-processed sintered nanocrystal solar cells via layer-by-layer assembly. Nano Lett. 2011, 11, 2856–2864. [Google Scholar] [CrossRef]
- Guo, X.; Tan, Q.; Liu, S.; Qin, D.; Mo, Y.; Hou, L.; Liu, A.; Wu, H.; Ma, Y. High-efficiency solution-processed CdTe nanocrystal solar cells incorporating a novel crosslinkable conjugated polymer as the hole transport layer. Nano Energy 2018, 46, 150–157. [Google Scholar] [CrossRef]
- Panthani, M.G.; Kurley, J.M.; Crisp, R.W.; Dietz, T.C.; Ezzyat, T.; Luther, J.M.; Talapin, D.V. High efficiency solution processed sintered CdTe nanocrystal solar cells: The role of interfaces. Nano Lett. 2014, 14, 670–675. [Google Scholar] [CrossRef]
- MacDonald, B.I.; Gengenbach, T.R.; Watkins, S.E.; Mulvaney, P.; Jasieniak, J.J. Solution-processing of ultra-thin CdTe/ZnO nanocrystal solar cells. Thin Solid Film. 2014, 558, 365–373. [Google Scholar] [CrossRef]
- MacDonald, B.I.; Martucci, A.; Rubanov, S.; Watkins, S.E.; Mulvaney, P.; Jasieniak, J.J. Layer-by-layer assembly of sintered CDSE x te1–x nanocrystal solar cells. ACS Nano 2012, 6, 5995–6004. [Google Scholar] [CrossRef]
- MacDonald, B.I.; Della Gaspera, E.; Watkins, S.E.; Mulvaney, P.; Jasieniak, J.J. Enhanced photovoltaic performance of nanocrystalline CdTe/ZnO solar cells using sol-gel ZnO and positive bias treatment. J. Appl. Phys. 2014, 115, 184501. [Google Scholar] [CrossRef]
- Webber, D.H.; Brutchey, R.L. Alkahest for V2VI3 chalcogenides: Dissolution of nine bulk semiconductors in a diamine-dithiol solvent mixture. J. Am. Chem. Soc. 2013, 135, 15722–15725. [Google Scholar] [CrossRef]
- Wu, M.; Wang, Y.; Wang, H.; Wang, H.; Sui, Y.; Du, F.; Yang, X.; Zou, B. Phosphine-free engineering toward the synthesis of metal telluride nanocrystals: The role of a Te precursor coordinated at room temperature. Nanoscale 2018, 10, 21928–21935. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Qin, D.; Jiang, X.; Liu, Y.; Wang, L.; Chen, J.; Cao, Y. Synthesis of high quality zinc-blende CdSe nanocrystals and their application in hybrid solar cells. Nanotechnology 2006, 17, 4736. [Google Scholar] [CrossRef]
- Liu, S.; Liu, W.; Heng, J.; Zhou, W.; Chen, Y.; Wen, S.; Qin, D.; Hou, L.; Wang, D.; Xu, H. Solution-processed efficient nanocrystal solar cells based on CdTe and CdS nanocrystals. Coatings 2018, 8, 26. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Tao, H.; Yang, T.; Kong, L.; Qin, D.; Chen, J. A surfactant-free recipe for shape-controlled synthesis of CdSe nanocrystals. Nanotechnology 2010, 22, 045604. [Google Scholar] [CrossRef]
- Liu, H.; Tian, Y.; Zhang, Y.; Gao, K.; Lu, K.; Wu, R.; Qin, D.; Wu, H.; Peng, Z.; Hou, L.; et al. Solution processed CdTe/CdSe nanocrystal solar cells with more than 5.5% efficiency by using an inverted device structure. J. Mater. Chem. C 2015, 3, 4227–4234. [Google Scholar] [CrossRef]
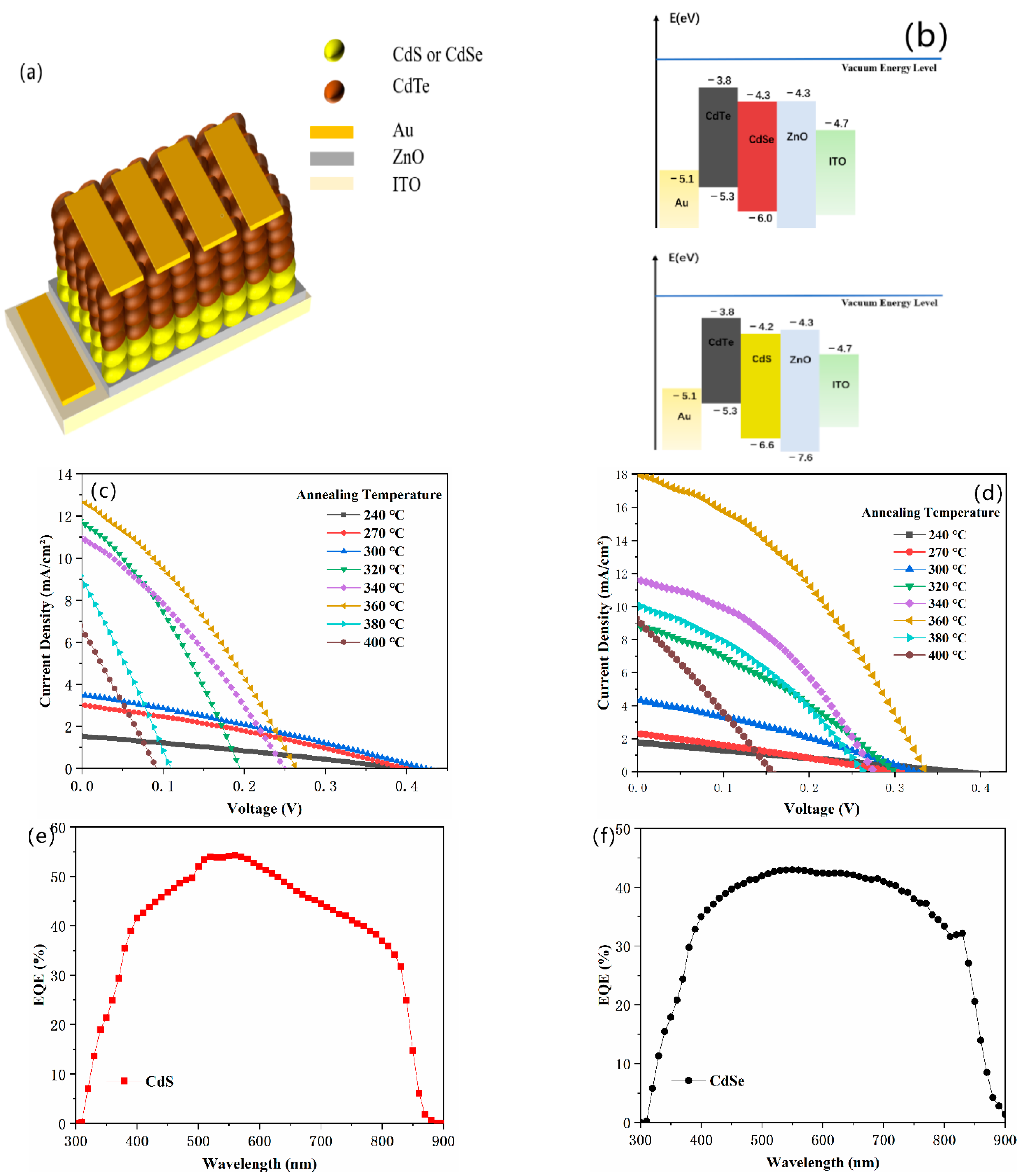
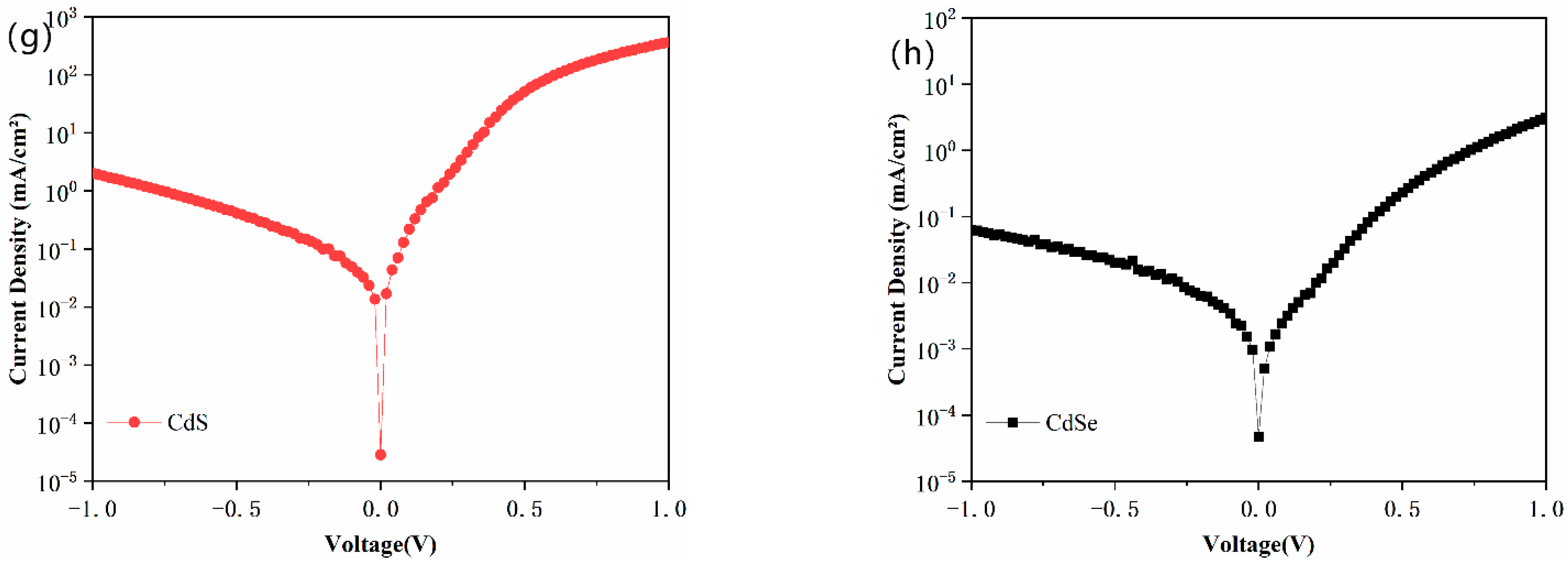

| Annealing Temperature (℃) | PCE (%) | Jsc (mA/cm2) | FF (%) | Voc (V) | Rs (Ω·cm2) | Rsh (Ω·cm2) |
|---|---|---|---|---|---|---|
| 240 | 0.17 (± 0.02) | 1.54 (± 0.2) | 27.27 (± 2) | 0.40 (± 0.02) | 1330.42 | 1671.38 |
| 270 | 0.36 (± 0.05) | 3.03 (± 0.5) | 29.68 (± 4) | 0.40 (± 0.03) | 611.66 | 1458.51 |
| 300 | 0.42 (± 0.04) | 3.52 (± 0.2) | 28.35 (± 3) | 0.42 (± 0.02) | 649.64 | 904.81 |
| 320 | 0.74 (± 0.02) | 12.04 (± 0.4) | 30.32 (± 3) | 0.20 (± 0.01) | 74.16 | 176.07 |
| 350 | 0.85 (± 0.02) | 11.01 (± 0.5) | 30.93 (± 2) | 0.25 (± 0.03) | 98.84 | 234.84 |
| 360 | 1.08 (± 0.05) | 12.74 (± 0.3) | 32.35 (± 4) | 0.26 (± 0.02) | 93.84 | 334.76 |
| 380 | 0.27 (± 0.012) | 9.16 (± 0.4) | 25.93 (± 1) | 0.11 (± 0.02) | 60.89 | 84.61 |
| 400 | 0.15 (± 0.02) | 6.80 (± 0.2) | 24.39 (± 2) | 0.09 (± 0.01) | 87.54 | 86.20 |
| Annealing Temperature (°C) | PCE (%) | Jsc (mA/cm2) | FF (%) | Voc (V) | Rs (Ω cm2) | Rsh (Ω·cm2) |
|---|---|---|---|---|---|---|
| 240 | 0.17 (± 0.02) | 1.77 (± 0.3) | 25.26 (± 2) | 0.37 (± 0.02) | 1306.76 | 1500.15 |
| 270 | 0.18 (± 0.01) | 2.32 (± 0.2) | 26.63 (± 3) | 0.30 (± 0.03) | 721.03 | 1103.55 |
| 300 | 0.44 (± 0.02) | 4.40 (± 0.5) | 30.36 (± 3) | 0.33 (± 0.02) | 337.50 | 781.55 |
| 320 | 0.87 (± 0.03) | 8.81 (± 0.3) | 33.12 (± 3) | 0.30 (± 0.03) | 145.38 | 670.95 |
| 340 | 1.26 (± 0.02) | 11.64 (± 0.6) | 39.61 (± 3) | 0.27 (± 0.01) | 77.86 | 611.93 |
| 360 | 2.28 (± 0.02) | 18.01 (± 0.6) | 37.84 (± 4) | 0.33 (± 0.02) | 70.40 | 600.15 |
| 380 | 0.93 (± 0.01) | 10.13 (± 0.3) | 34.91 (± 3) | 0.26 (± 0.02) | 97.51 | 315.32 |
| 400 | 0.38 (± 0.2) | 9.29 (± 0.2) | 26.84 (± 2) | 0.15 (± 0.03) | 105.35 | 113.92 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, M.; Zhou, Z.; Xu, A.; Xiao, K.; Li, J.; Qin, D.; Xu, W.; Hou, L. Synthesis of Group II-VI Semiconductor Nanocrystals via Phosphine Free Method and Their Application in Solution Processed Photovoltaic Devices. Nanomaterials 2021, 11, 2071. https://doi.org/10.3390/nano11082071
Hou M, Zhou Z, Xu A, Xiao K, Li J, Qin D, Xu W, Hou L. Synthesis of Group II-VI Semiconductor Nanocrystals via Phosphine Free Method and Their Application in Solution Processed Photovoltaic Devices. Nanomaterials. 2021; 11(8):2071. https://doi.org/10.3390/nano11082071
Chicago/Turabian StyleHou, Mingyue, Zhaohua Zhou, Ao Xu, Kening Xiao, Jiakun Li, Donghuan Qin, Wei Xu, and Lintao Hou. 2021. "Synthesis of Group II-VI Semiconductor Nanocrystals via Phosphine Free Method and Their Application in Solution Processed Photovoltaic Devices" Nanomaterials 11, no. 8: 2071. https://doi.org/10.3390/nano11082071






