Atomic Resolution Electron Microscopy: A Key Tool for Understanding the Activity of Nano-Oxides for Biomedical Applications
Abstract
:1. Introduction
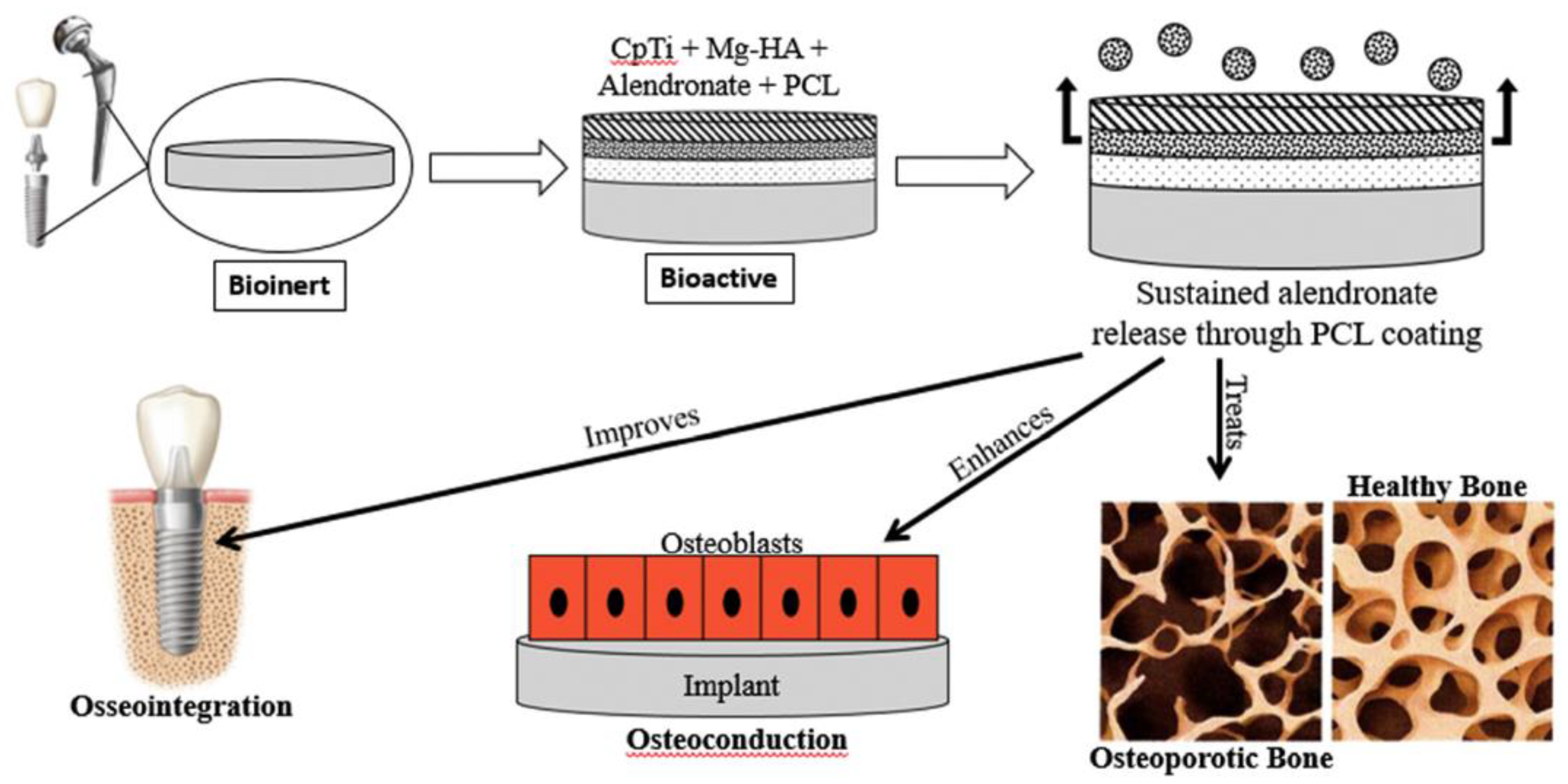
1.1. Titanium Oxide
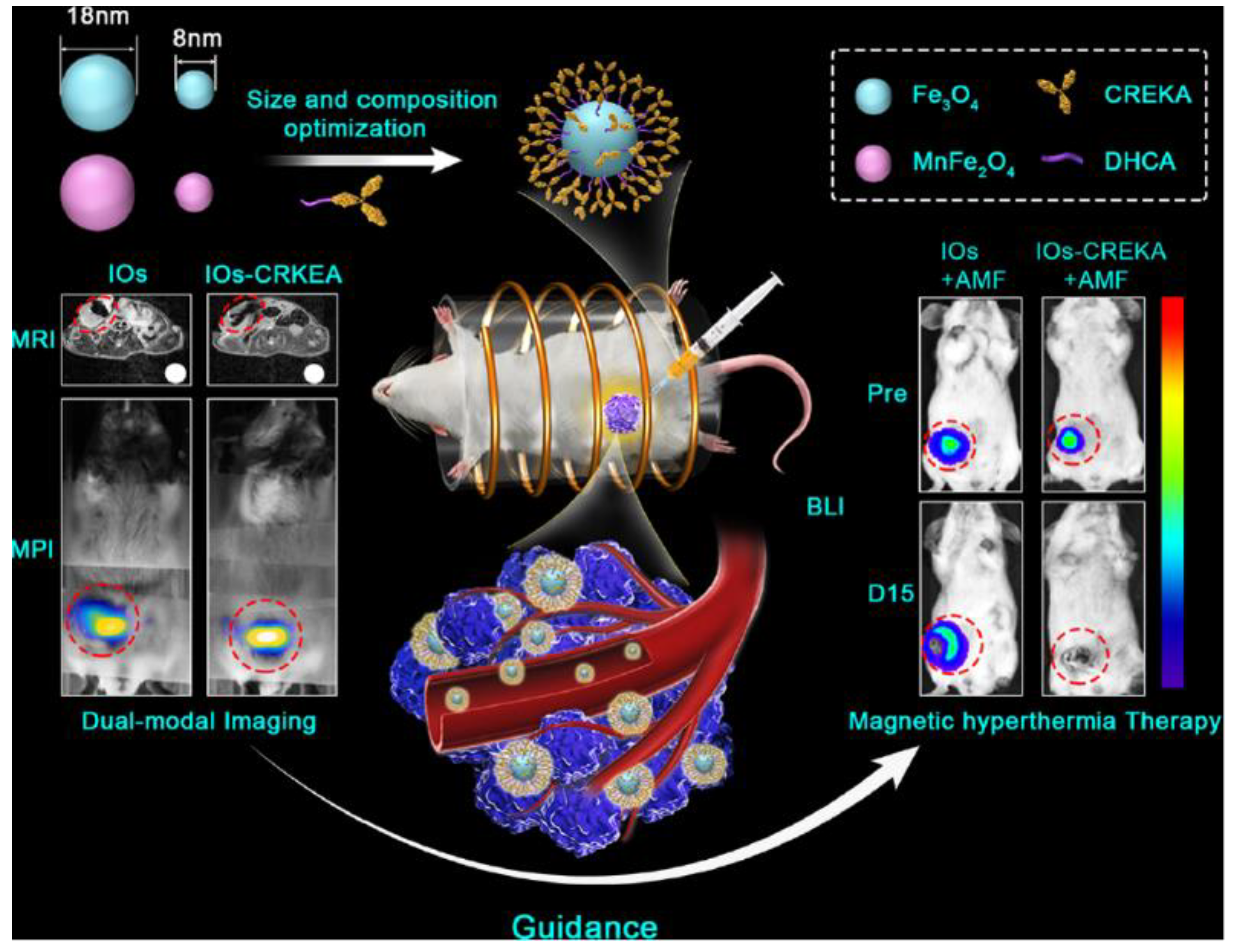
1.2. Magnetic Nanoparticles (MNPs)
1.3. Other Applications of Transition-Metal-Based NPs
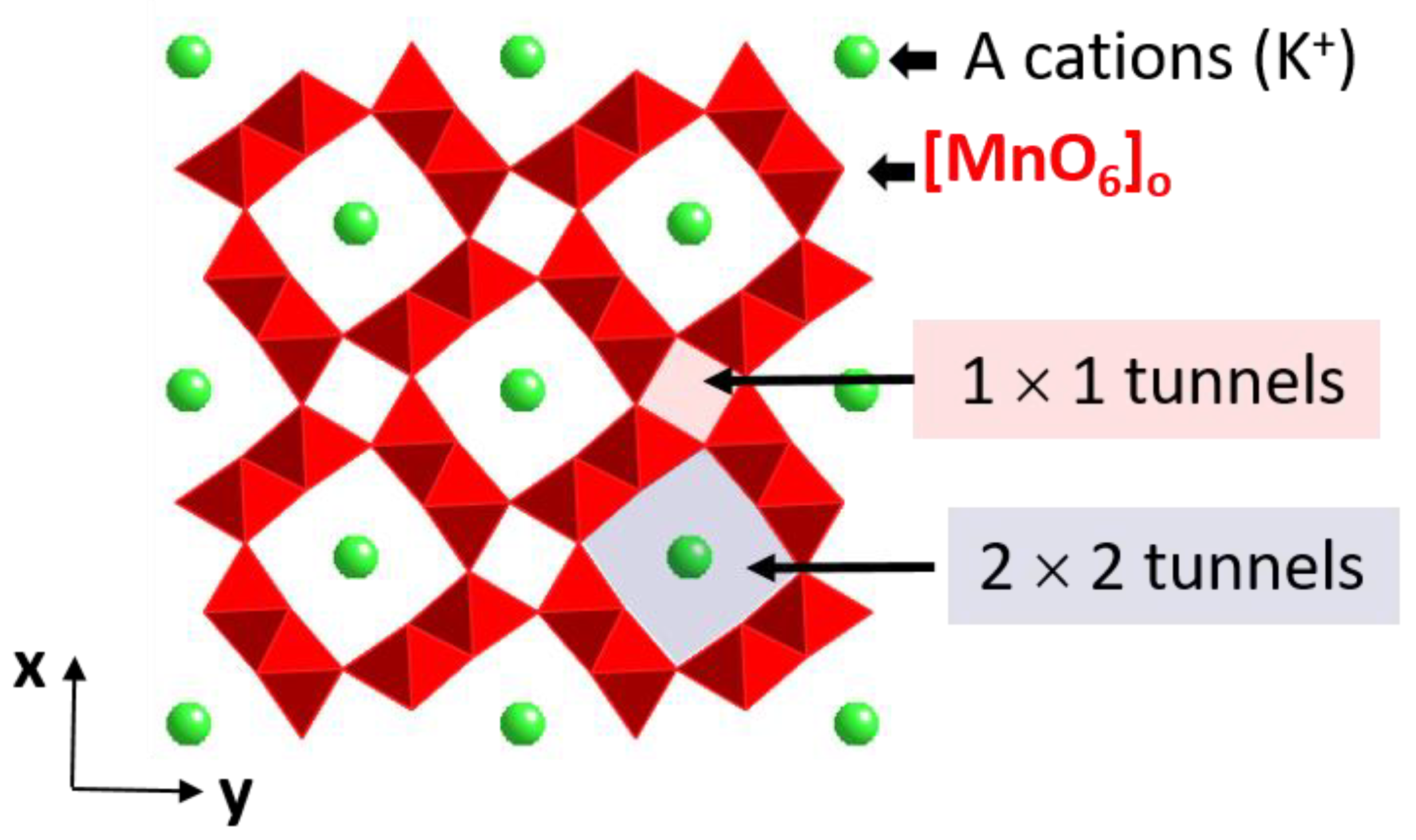
1.4. Manganese Oxides
- (i)
- Direct visualization of implant/tissue interfaces with atomic resolution in order to understand how to optimize the interactions taking place;
- (ii)
- A compositional analysis at an atomic level of biomaterials. The local distribution of some crucial bioactive dopants included in the materials would help to enlighten their functionalities.
- (iii)
- The behavior of biomedical TMOs is highly influenced by the oxidation state of the transition metal. Local analysis, mainly on the surface of the material, would allow deeper knowledge of how they will work.
2. The Role of the Electron Microscopy
2.1. Basic Microstructural Characterization of TMOs by TEM
2.2. Atomically Resolved Electron Microscopy
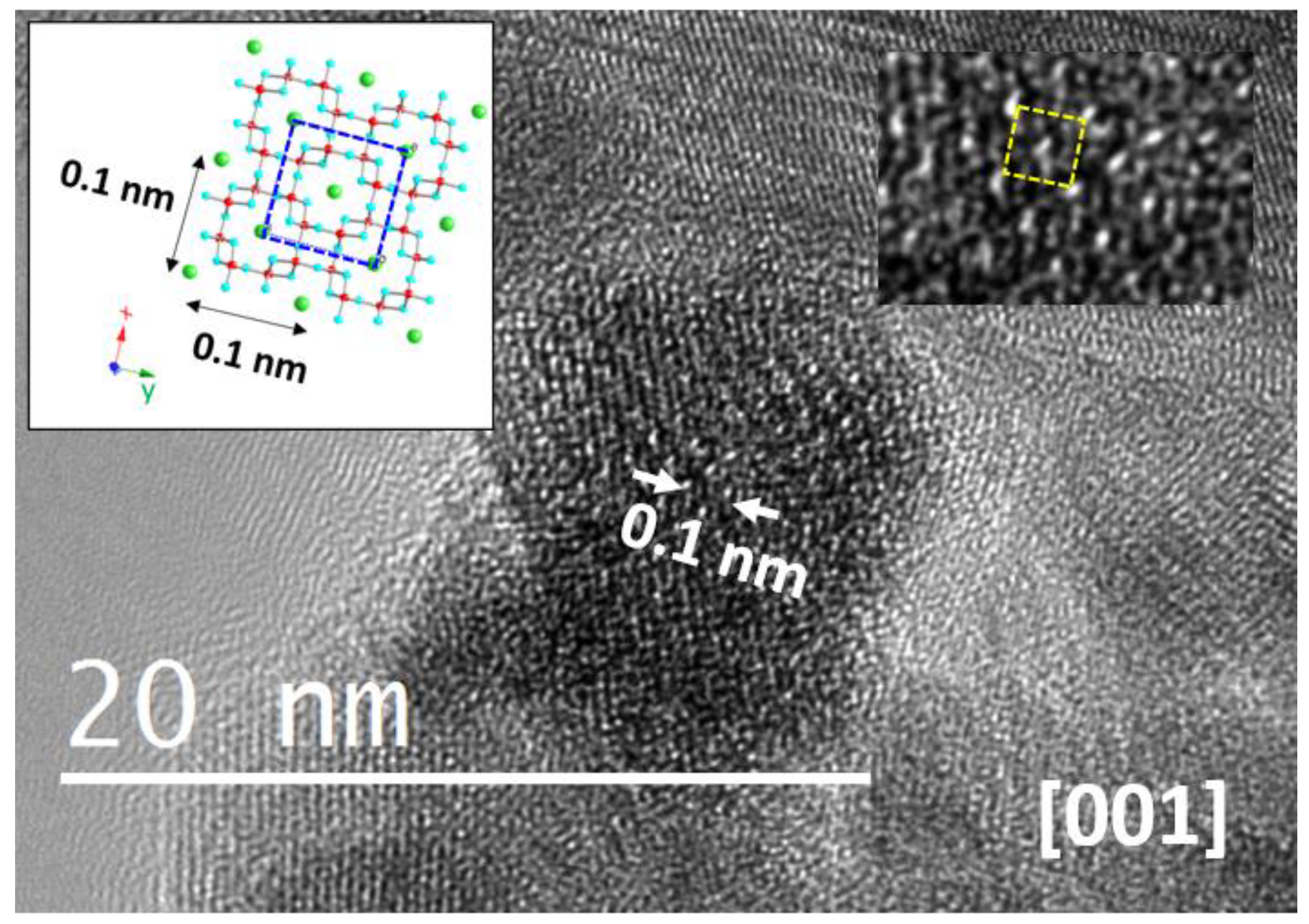
2.2.1. Structural Information
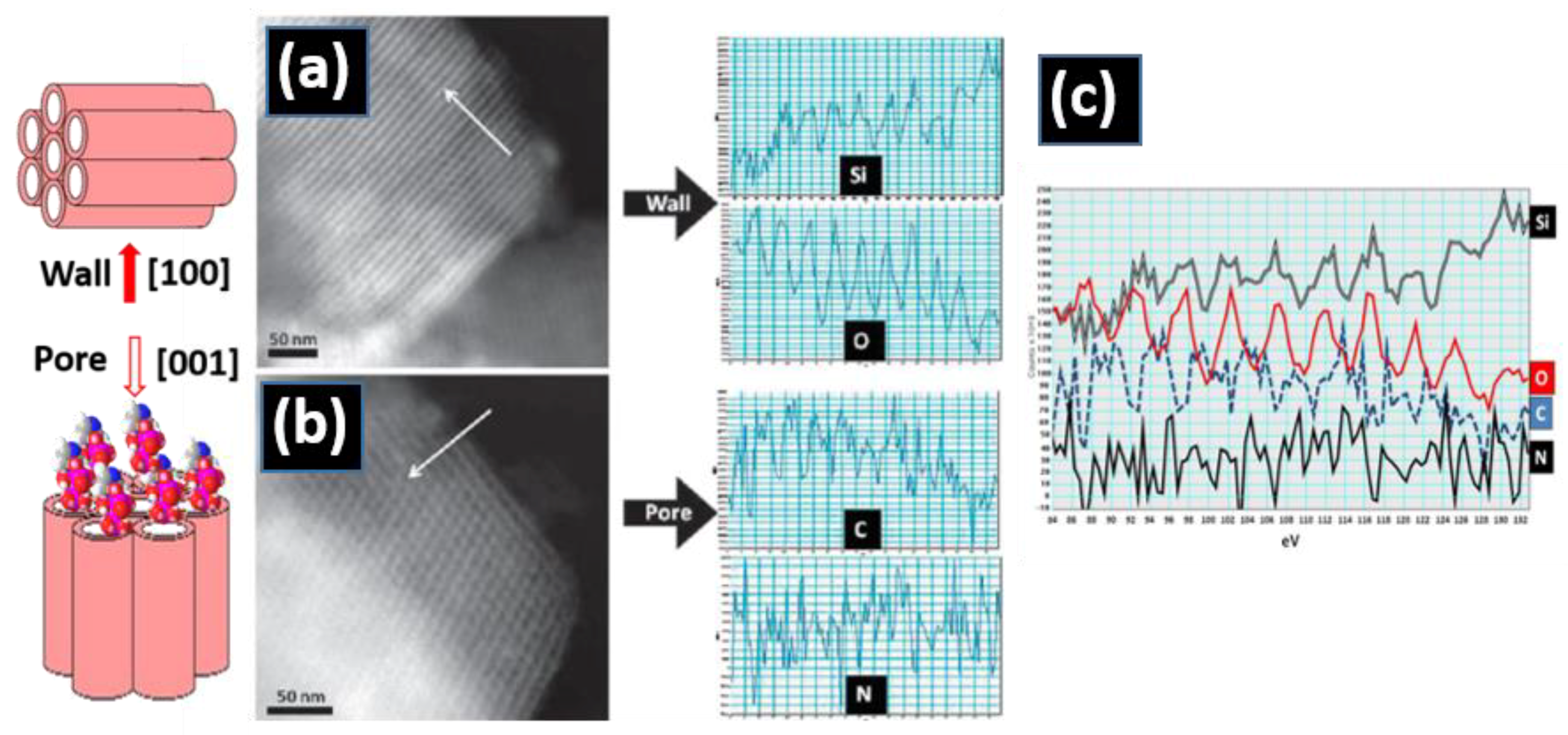
2.2.2. Compositional Information: STEM-HAADF-EELS Studies
Cation Distribution
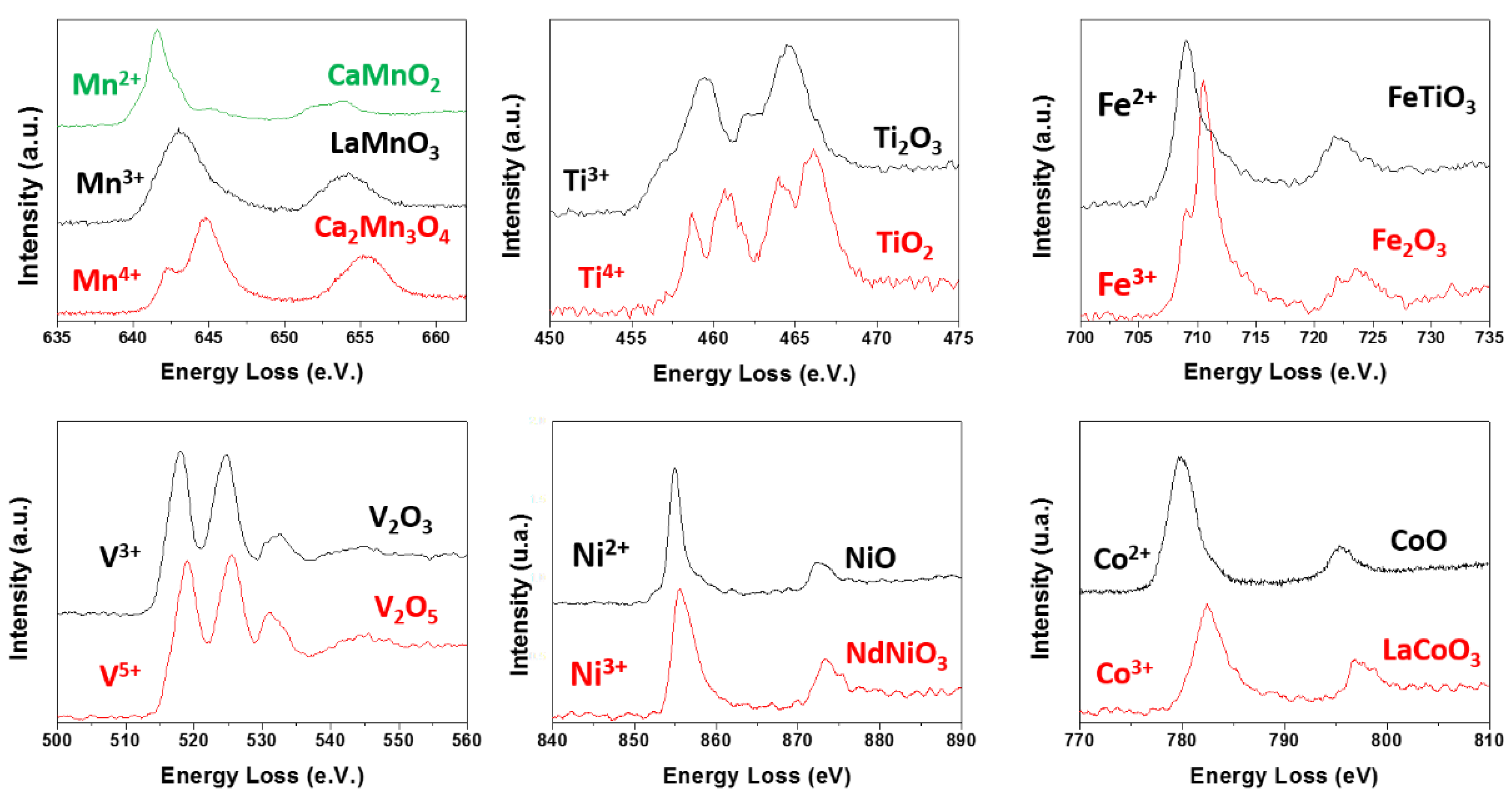
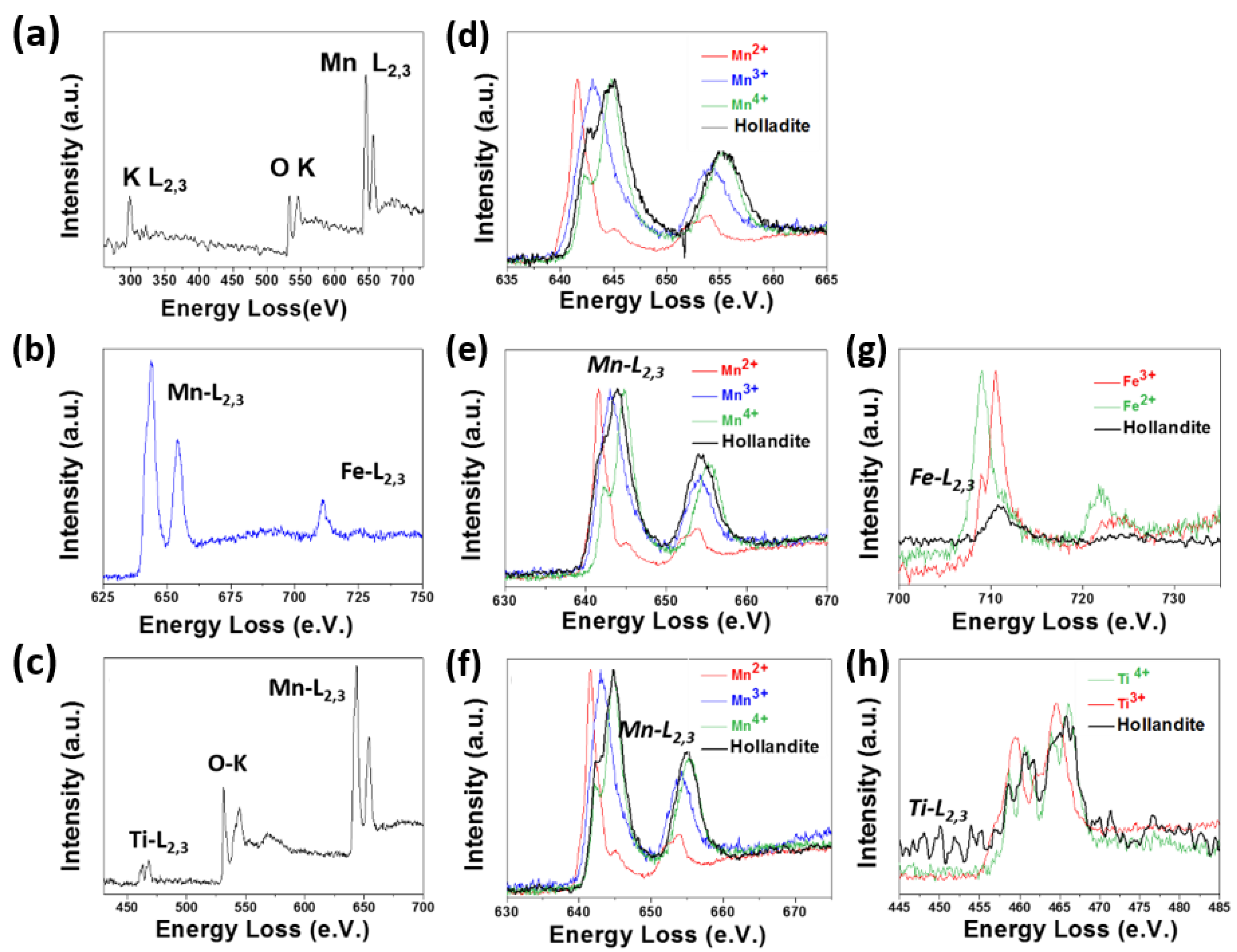
Oxidation States of TM by EELS
3. Conclusions
4. Experimental
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Azab, A.A.; Ateia, E.E.; Esmail, S.A. Comparative study on the physical properties of transition metal-doped (Co, Ni, Fe, and Mn) ZnO nanoparticles. Appl. Phys. A 2018, 124, 469. [Google Scholar] [CrossRef]
- Akbari, A.; Amini, M.; Tarassoli, A.; Eftekhari-Sis, B.; Ghasemian, N.; Jabbari, E. Transition metal oxide nanoparticles as efficient catalysts in oxidation reactions. Nano Struct. Nano Objects 2018, 14, 19–48. [Google Scholar] [CrossRef]
- Sanchez, C.; Belleville, P.; Popall, M.; Nicole, L. Applications of advanced hybrid organic–inorganic nanomaterials: From laboratory to market. Chem. Soc. Rev. 2011, 40, 696–753. [Google Scholar] [CrossRef]
- Pałka, K.; Pokrowiecki, R. Porous Titanium Implants: A Review. Adv. Eng. Mater. 2018, 20, 1700648. [Google Scholar] [CrossRef]
- Yar, A.Y.; Aschauer, U.; Bowen, P. Interaction of biologically relevant ions and organic molecules with titanium oxide (rutile) surfaces: A review on molecular dynamics studies. Colloids Surf. B Biointerfaces 2018, 161, 563–577. [Google Scholar] [CrossRef]
- Ke, D.; Vu, A.; Bandyopadhyay, A.; Bose, S. Compositionally graded doped hydroxyapatite coating on titanium using laser and plasma spray deposition for bone implants. Acta Biomater. 2018, 84, 414–423. [Google Scholar] [CrossRef]
- Trincă, L.C.; Mareci, D.; Souto, R.M.; Lozano-Gorrín, A.D.; Izquierdo, J.; Burtan, L.; Motrescu, I.; Vulpe, V.; Pavel, G.; Strungaru, S.; et al. Osseointegration evaluation of ZrTi alloys with hydroxyapatite-zirconia-silver layer in pig’s tibiae. App. Surf. Sci. 2019, 487, 127–137. [Google Scholar] [CrossRef]
- Kumari, R.; Majumdar, J.D. Wear Behavior of Plasma Spray Deposited and Post Heat-Treated Hydroxyapatite (HA)-Based Composite Coating on Titanium Alloy (Ti-6Al-4V) Substrate. Met. Mater. Trans. A 2018, 49, 3122–3132. [Google Scholar] [CrossRef]
- Nosrati, H.; Mamoory, R.S.; Le, D.Q.S.; Bünger, C.E. Preparation of reduced graphene oxide/hydroxyapatite nanocomposite and evaluation of graphene sheets/hydroxyapatite interface. Diam. Relat. Mater. 2019, 100, 107561. [Google Scholar] [CrossRef]
- Bose, S.; Vu, A.; Emshadi, K.; Bandyopadhyay, A. Effects of polycaprolactone on alendronate drug release from Mg-doped hydroxyapatite coating on titanium. Mater. Sci. Eng. C 2018, 88, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Beck, T.J.; Klust, A.; Batzill, M.; Diebold, U.; Di Valentin, C.; Tilocca, A.; Selloni, A. Mixed dissociated/molecular monolayer of water on the TiO2(011)-(2×1) surface. Surf. Sci. 2005, 591, L267–L272. [Google Scholar] [CrossRef]
- Lindan, P.J.D.; Harrison, N.M.; Gillan, M.J. Mixed Dissociative and Molecular Adsorption of Water on the Rutile (110) Surface. Phys. Rev. Lett. 1998, 80, 762–765. [Google Scholar] [CrossRef]
- Schneider, J.; Ciacchi, L.C. First principles and classical modeling of the oxidized titanium (0001) surface. Surf. Sci. 2010, 604, 1105–1115. [Google Scholar] [CrossRef]
- Schneider, J.; Ciacchi, L.C. A Classical Potential to Model the Adsorption of Biological Molecules on Oxidized Titanium Surfaces. J. Chem. Theory Comput. 2010, 7, 473–484. [Google Scholar] [CrossRef]
- Kokubo, T.; Kim, H.-M.; Kawashita, M. Novel bioactive materials with different mechanical properties. Biomaterials 2003, 24, 2161–2175. [Google Scholar] [CrossRef]
- Tas, A.C. The use of physiological solutions or media in calcium phosphate synthesis and processing. Acta Biomater. 2014, 10, 1771–1792. [Google Scholar] [CrossRef]
- Ferraris, S.; Yamaguchi, S.; Barbani, N.; Cristallini, C.; di Confiengo, G.G.; Barberi, J.; Cazzola, M.; Miola, M.; Vernè, E.; Spriano, S. The mechanical and chemical stability of the interfaces in bioactive materials: The substrate-bioactive surface layer and hydroxyapatite-bioactive surface layer interfaces. Mater. Sci. Eng. C 2020, 116, 111238. [Google Scholar] [CrossRef]
- Gossuin, Y.; Gillis, P.; Hocq, A.; Vuong, Q.L.; Roch, A. Magnetic resonance relaxation properties of superparamagnetic particles. WIRE Nanomed. Nanobiotechnol. 2009, 1, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Nahrendorf, M.; Sosnovik, D.E.; Weissleder, R. MR-optical imaging of cardiovascular molecular targets. Basic Res. Cardiol. 2008, 103, 87–94. [Google Scholar] [CrossRef] [Green Version]
- Rümenapp, C.; Gleich, B.; Haase, A. Magnetic Nanoparticles in Magnetic Resonance Imaging and Diagnostics. Pharm. Res. 2012, 29, 1165–1179. [Google Scholar] [CrossRef]
- Xie, J.; Xu, C.; Xu, Z.; Hou, Y.; Young, K.L.; Wang, S.X.; Pourmand, N.; Sun, S. Linking Hydrophilic Macromolecules to Monodisperse Magnetite (Fe3O4) Nanoparticles via Trichloro-s-triazine. Chem. Mater. 2006, 18, 5401–5403. [Google Scholar] [CrossRef] [PubMed]
- McBain, S.C.; Yiu, H.H.P.; Dobson, J. Magnetic nanoparticles for gene and drug delivery. Int. J. Nanomed. 2008, 3, 169–180. [Google Scholar]
- Vallet-Regí, M.; Ruiz-Hernández, E. Bioceramics: From Bone Regeneration to Cancer Nanomedicine. Adv. Mater. 2011, 23, 5177–5218. [Google Scholar] [CrossRef] [PubMed]
- Chomoucka, J.; Drbohlavova, J.; Huska, D.; Adam, V.; Kizek, R.; Hubalek, J. Magnetic nanoparticles and targeted drug delivering. Pharmacol. Res. 2010, 62, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Drbohlavová, J.; Hrdý, R.; Adam, V.; Kizek, R.; Schneeweiss, O.; Hubalek, J. Preparation and Properties of Various Magnetic Nanoparticles. Sensors 2009, 9, 2352–2362. [Google Scholar] [CrossRef] [Green Version]
- Tucek, J.; Zboril, R.; Petridis, D. Maghemite Nanoparticles by View of Mössbauer Spectroscopy. J. Nanosci. Nanotechnol. 2006, 6, 926–947. [Google Scholar] [CrossRef]
- Reshmi, G.; Kumar, P.M.; Malathi, M. Preparation, characterization and dielectric studies on carbonyl iron/cellulose acetate hydrogen phthalate core/shell nanoparticles for drug delivery applications. Int. J. Pharm. 2009, 365, 131–135. [Google Scholar] [CrossRef]
- Kumar, A.; Priyanka. Environmentally benign pH-responsive cytidine-5′-monophosphate molecule-mediated akaganeite (5′-CMP-β-FeOOH) soft supramolecular hydrogels induced by the puckering of ribose sugar with efficient loading/release capabilities. New J. Chem. 2019, 43, 14997–15013. [Google Scholar] [CrossRef]
- Chen, M.-L.; Shen, L.-M.; Chen, S.; Wang, X.-W.; Wang, J.-H. In situ growth of β-FeOOH nanorods on graphene oxide with ultra-high relaxivity for in vivo magnetic resonance imaging and cancer therapy. J. Mater. Chem. B 2013, 1, 2582–2589. [Google Scholar] [CrossRef]
- Zeng, L.; Ren, W.; Zheng, J.; Wu, A.; Cui, P. Synthesis of water-soluble FeOOH nanospindles and their performance for magnetic resonance imaging. Appl. Surf. Sci. 2012, 258, 2570–2575. [Google Scholar] [CrossRef]
- Bae, S.; Lee, S.W.; Hirukawa, A.; Takemura, Y.; Jo, Y.H. AC Magnetic-Field-Induced Heating and Physical Properties of Ferrite Nanoparticles for a Hyperthermia Agent in Medicine. IEEE Trans. Nanotechnol. 2008, 8, 86–94. [Google Scholar] [CrossRef]
- Guisasola, E.; Asín, L.; Beola, L.; De La Fuente, J.M.; Baeza, A.; Vallet-Regí, M. Beyond Traditional Hyperthermia: In Vivo Cancer Treatment with Magnetic-Responsive Mesoporous Silica Nanocarriers. ACS Appl. Mater. Interfaces 2017, 10, 12518–12525. [Google Scholar] [CrossRef] [Green Version]
- Darwish, M.S.A.; Kim, H.; Lee, J.Y.; Ryu, C.; Yoon, J. Synthesis of Magnetic Ferrite Nanoparticles with High Hyperthermia Performance via a Controlled Co-Precipitation Method. Nanomaterials 2019, 9, 1176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazario, E.; Menendez, N.; Herrasti, P.; Cañete, M.; Connord, V.; Carrey, J. Magnetic Hyperthermia Properties of Electrosynthesized Cobalt Ferrite Nanoparticles. J. Phys. Chem. C 2013, 117, 11405–11411. [Google Scholar] [CrossRef]
- Lee, S.W.; Bae, S.; Takemura, Y.; Shim, I.-B.; Kim, T.M.; Kim, J.; Lee, H.J.; Zurn, S.; Kim, C.S. Self-heating characteristics of cobalt ferrite nanoparticles for hyperthermia application. J. Magn. Magn. Mater. 2007, 310, 2868–2870. [Google Scholar] [CrossRef]
- Demirici Dönmez, E.; Manna, P.K.; Nickel, R.; Aktürk, S.; Van Lierop, J. Comparative Heating Efficiency of Cobalt-, Manganese-, and Nickel-Ferrite Nanoparticles for a Hyperthermia Agent in Biomedicines. ACS Appl. Mater. Interfaces 2019, 11, 6858–6866. [Google Scholar] [CrossRef] [PubMed]
- Makridis, A.; Topouridou, K.; Tziomaki, M.; Sakellari, D.; Simeonidis, K.; Angelakeris, M.; Yavropoulou, M.P.; Yovos, J.G.; Kalogirou, O. In vitro application of Mn-ferrite nanoparticles as novel magnetic hyperthermia agents. J. Mater. Chem. B 2014, 2, 8390–8398. [Google Scholar] [CrossRef]
- Dey, C.; Baishya, K.; Ghosh, A.; Goswami, M.M.; Ghosh, A.; Mandal, K. Improvement of drug delivery by hyperthermia treatment using magnetic cubic cobalt ferrite nanoparticles. J. Magn. Magn. Mater. 2017, 427, 168–174. [Google Scholar] [CrossRef]
- Du, Y.; Liu, X.; Liang, Q.; Liang, X.-J.; Tian, J. Optimization and Design of Magnetic Ferrite Nanoparticles with Uniform Tumor Distribution for Highly Sensitive MRI/MPI Performance and Improved Magnetic Hyperthermia Therapy. Nano Lett. 2019, 19, 3618–3626. [Google Scholar] [CrossRef]
- Umut, E.; Coşkun, M.; Pineider, F.; Berti, D.; Güngüneş, H. Nickel ferrite nanoparticles for simultaneous use in magnetic resonance imaging and magnetic fluid hyper-thermia. J. Colloid Interface Sci. 2019, 550, 199–209. [Google Scholar] [CrossRef]
- Huo, J.; Wei, M. Characterization and magnetic properties of nanocrystalline nickel ferrite synthesized by hydrothermal method. Mater. Lett. 2009, 63, 1183–1184. [Google Scholar] [CrossRef]
- Kang, E.; Park, J.; Hwang, Y.; Kang, M.; Park, J.-G.; Hyeon, T. Direct Synthesis of Highly Crystalline and Monodisperse Manganese Ferrite Nanocrystals. J. Phys. Chem. B 2004, 108, 13932–13935. [Google Scholar] [CrossRef]
- Šepelák, V.; Bergmann, I.; Feldhoff, A.; Heitjans, P.; Krumeich, F.; Menzel, D.; Litterst, F.J.; Campbell, S.J.; Becker, K.D. Nanocrystalline Nickel Ferrite, NiFe2O4: Mechanosynthesis, Nonequilibrium Cation Distribution, Canted Spin Arrangement, and Magnetic Behavior. J. Phys. Chem. C 2007, 111, 5026–5033. [Google Scholar] [CrossRef]
- Virden, A.; Wells, S.; O’Grady, K. Physical and magnetic properties of highly anisotropic cobalt ferrite particles. J. Magn. Magn. Mater. 2007, 316, e768–e771. [Google Scholar] [CrossRef]
- Colilla, M.; Manzano, M.; Izquierdo-Barba, I.; Vallet-Regí, M.; Boissiére, C.; Sanchez, C. Advanced Drug Delivery Vectors with Tailored Surface Properties Made of Mesoporous Binary Oxides Submicronic Spheres. Chem. Mater. 2009, 22, 1821–1830. [Google Scholar] [CrossRef]
- Abbasi, A.Z.; Prasad, P.; Cai, P.; He, C.; Foltz, W.D.; Amini, M.A.; Gordijo, C.R.; Rauth, A.M.; Wu, X.Y. Manganese oxide and docetaxel co-loaded fluorescent polymer nanoparticles for dual modal imaging and chemotherapy of breast cancer. J. Control. Release 2015, 209, 186–196. [Google Scholar] [CrossRef] [PubMed]
- Haider, M.; Uhlemann, S.; Schwan, E.; Rose, H.; Kabius, B.; Urban, K. Electron microscopy image enhanced. Nature 1998, 392, 768–769. [Google Scholar] [CrossRef]
- Krivanek, O.L.; Nellist, P.D.; Dellby, N.; Murfitt, M.F.; Szilagyi, Z. Towards sub-0.5 A electron beams. Ultramicroscopy 2003, 96, 229–237. [Google Scholar] [CrossRef]
- Vallet-Regí, M.; Manzano, M.; González-Calbet, J.M.; Okunishi, E. Evidence of drug confinement into silica mesoporous matrices by STEM spherical aberration corrected microscopy. Chem. Commun. 2010, 46, 2956–2958. [Google Scholar] [CrossRef]
- Puig, S.; Ramos-Alonso, L.; Romero, A.M.; Martínez-Pastor, M.T. The elemental role of iron in DNA synthesis and repair. Met. 2017, 9, 1483–1500. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Zhao, S.; Zhou, J.; Shen, Y.; Huang, W.; Zhang, C.; Rahaman, M.N.; Wang, D. Evaluation of borate bioactive glass scaffolds as a controlled delivery system for copper ions in stimulating osteogenesis and angiogenesis in bone healing. J. Mater. Chem. B 2014, 2, 8547–8557. [Google Scholar] [CrossRef] [PubMed]
- Hambidge, M. Human Zinc Deficiency. J. Nutr. 2000, 130, 1344S–1349S. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, H.A.; Balassa, J.J.; Tipton, I.H. Essential trace metals in man: Molybdenum. J. Chronic Dis. 1970, 23, 481–499. [Google Scholar] [CrossRef]
- Crans, D.; Trujillo, A.M.; Pharazyn, P.S.; Cohen, M.D. How environment affects drug activity: Localization, compartmentalization and reactions of a vanadium insulin-enhancing compound, dipicolinatooxovanadium(V). Coord. Chem. Rev. 2011, 255, 2178–2192. [Google Scholar] [CrossRef]
- Brokesh, A.M.; Gaharwar, A.K. Inorganic Biomaterials for Regenerative Medicine. ACS Appl. Mater. Interfaces 2020, 12, 5319–5344. [Google Scholar] [CrossRef]
- Garino, N.; Sanvitale, P.; Dumontel, B.; Laurenti, M.; Colilla, M.; Izquierdo-Barba, I.; Cauda, V.; Vallet-Regì, M. Zinc oxide nanocrystals as a nanoantibiotic and osteoinductive agent. RSC Adv. 2019, 9, 11312–11321. [Google Scholar] [CrossRef]
- Natalio, F.; Andre, R.; Hartog, A.F.; Stoll, B.; Jochum, K.P.; Wever, R.; Tremel, W. Vanadium pentoxide nanoparticles mimic vanadium haloperoxidases and thwart biofilm formation. Nat. Nanotechnol. 2012, 7, 530–535. [Google Scholar] [CrossRef]
- Qi, Y.; Qi, H.; He, Y.; Lin, W.; Yongli, Q.; Qin, L.; Hu, Y.; Chen, L.; Liu, Q.; Sun, H.; et al. Strategy of Metal–Polymer Composite Stent to Accelerate Biodegradation of Iron-Based Biomaterials. ACS Appl. Mater. Interfaces 2017, 10, 182–192. [Google Scholar] [CrossRef]
- Shi, M.; Chen, Z.; Farnaghi, S.; Friis, T.; Mao, X.; Xiao, Y.; Wu, C. Copper-doped mesoporous silica nanospheres, a promising immunomodulatory agent for inducing osteogenesis. Acta Biomater. 2016, 30, 334–344. [Google Scholar] [CrossRef]
- Prohaska, J.R. Functions of trace elements in brain metabolism. Physiol. Rev. 1987, 67, 858–901. [Google Scholar] [CrossRef]
- Bae, Y.-J.; Kim, M.-H. Manganese Supplementation Improves Mineral Density of the Spine and Femur and Serum Oste-ocalcin in Rats. Biol. Trace Elem. Res. 2008, 124, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Bose, S.; Fielding, G.; Tarafder, S.; Bandyopadhyay, A. Understanding of dopant-induced osteogenesis and angiogenesis in calcium phosphate ceramics. Trends Biotechnol. 2013, 31, 594–605. [Google Scholar] [CrossRef] [Green Version]
- Pabbruwe, M.B.; Standard, O.C.; Sorrell, C.C.; Howlett, C.R. Bone formation within alumina tubes: Effect of calcium, manganese, and chromium dopants. Biomaterials 2004, 25, 4901–4910. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Ma, S.; Sun, J.; Xia, C.; Liu, C.; Wang, Z.; Zhao, X.; Gao, F.; Gong, Q.; Song, B. Manganese ferrite nanoparticle micellar nanocomposites as MRI contrast agent for liver imaging. Biomaterials 2009, 30, 2919–2928. [Google Scholar] [CrossRef] [PubMed]
- Bae, K.H.; Lee, K.; Kim, C.; Park, T.G. Surface functionalized hollow manganese oxide nanoparticles for cancer targeted siRNA delivery and mag-netic resonance imaging. Biomaterials 2011, 32, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Prasad, P.; Gordijo, C.R.; Abbasi, A.Z.; Maeda, A.; Ip, A.; Rauth, A.M.; DaCosta, R.S. Wu, X.Y. Multifunctional Albumin–MnO2 Nanoparticles Modulate Solid Tumor Microenvironment by Attenuating Hypoxia, Acidosis, Vascular Endothelial Growth Factor and Enhance Radiation Response. ACS Nano 2014, 8, 3202–3212. [Google Scholar] [CrossRef]
- Luo, X.-L.; Xu, J.-J.; Zhao, W.; Chen, H.-Y. A novel glucose ENFET based on the special reactivity of MnO2 nanoparticles. Biosens. Bioelectron. 2004, 19, 1295–1300. [Google Scholar] [CrossRef]
- Fan, W.; Bu, W.; Shen, B.; He, Q.; Cui, Z.; Liu, Y.; Zheng, X.; Zhao, K.; Shi, J. Intelligent MnO2 Nanosheets Anchored with Upconversion Nanoprobes for Concurrent pH-/H2O2-Responsive UCL Imaging and Oxygen-Elevated Synergetic Therapy. Adv. Mater. 2015, 27, 4155–4161. [Google Scholar] [CrossRef]
- Chen, Q.; Feng, L.; Liu, J.; Zhu, W.; Dong, Z.; Wu, J.; Liu, Z. Intelligent Albumin–MnO2 Nanoparticles as pH-/H2O2-Responsive Dissociable Nanocarriers to Modulate Tumor Hypoxia for Effective Combination Therapy. Adv. Mater. 2016, 28, 7129–7136. [Google Scholar] [CrossRef]
- Yang, G.; Xu, L.; Chao, Y.; Xu, J.; Sun, X.; Wu, Y.; Peng, R.; Liu, Z. Hollow MnO2 as a tumor-microenvironment-responsive biodegradable nano-platform for combination therapy favoring antitumor immune responses. Nat. Commun. 2017, 8, 1564. [Google Scholar] [CrossRef] [Green Version]
- Wu, M.; Hou, P.; Dong, L.; Cai, L.; Chen, Z.; Zhao, M.; Li, J. Manganese dioxide nanosheets: From preparation to biomedical applications. Int. J. Nanomed. 2019, 14, 4781–4800. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Cong, H.; Shen, Y.; Yu, B. Biomedical application of manganese dioxide nanomaterials. Nanotechnology 2020, 31, 202001. [Google Scholar] [CrossRef]
- Azor-Lafarga, A.; Ruiz-González, L.; Parras, M.; Portehault, D.; Sanchez, C.; González-Calbet, J.M. Modified Synthesis Strategies for the Stabilization of low n TinO2n–1 Magnéli Phases. Chem. Record 2018, 18, 1105–1113. [Google Scholar] [CrossRef]
- Portehault, D.; Maneeratana, V.; Candolfi, C.; Oeschler, N.; Veremchuk, I.; Grin, Y.; Sanchez, C.; Antonietti, M. Facile General Route toward Tunable Magnéli Nanostructures and Their Use AS Thermoelectric Metal Oxide/Carbon Nanocomposites. ACS Nano 2011, 5, 9052–9061. [Google Scholar] [CrossRef]
- Garcia, M.A.; Merino, J.M.; Pinel, E.F.; Quesada, A.; de la Venta, J.; Gonzalez, M.L.R.; Castro, G.; Crespo, P.; Llopis, J.; Gonzalez-Calbet, J.M.; et al. Magnetic Properties of ZnO Nanoparticles. Nano Lett. 2007, 7, 1489–1494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gómez-Recio, I.; Azor-Lafarga, A.; Ruiz-González, M.L.; Hernando, M.; Parras, M.; Calvino, J.J.; Fernández-Díaz, M.T.; Portehault, D.; Sanchez, C.; González-Calbet, J.M. Unambiguous localization of titanium and iron cations in doped manganese hollandite nanowires. Chem. Commun. 2020, 56, 4812–4815. [Google Scholar] [CrossRef]
- Egerton, R.F. Electron energy-loss spectroscopy in the TEM. Rep. Prog. Phys. 2008, 72, 016502. [Google Scholar] [CrossRef]
- Colliex, C.; Manoubi, T.; Ortiz, C. Electron-energy-loss-spectroscopy near-edge fine structures in the iron-oxygen system. Phys. Rev. B 1991, 44, 11402–11411. [Google Scholar] [CrossRef]
- Garvie, L.A.J.; Craven, A.J. High-resolution parallel electron energy-loss spectroscopy of Mn L2,3-edges in inorganic manganese compounds. Phys. Chem. Miner. 1994, 21, 191–206. [Google Scholar] [CrossRef]
- Varela, M.; Oxley, M.; Luo, W.; Tao, J.; Watanabe, M.; Lupini, A.; Pantelides, S.T.; Pennycook, S.J. Atomic-resolution imaging of oxidation states in manganites. Phys. Rev. B 2009, 79, 085117. [Google Scholar] [CrossRef]
- Krivanek, O.; Disko, M.; Taftø, J.; Spence, J. Electron energy loss spectroscopy as a probe of the local atomic environment. Ultramicroscopy 1982, 9, 249–254. [Google Scholar] [CrossRef]
- Watanabe, M.; Okunishi, E.; Ishizuka, K. Analysis of spectrum-imaging datasets in atomic-resolution electron microscopy. Microsc. Anal. 2009, 135, 5. [Google Scholar]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azor-Lafarga, A.; Gómez-Recio, I.; Ruiz-González, M.L.; González-Calbet, J.M. Atomic Resolution Electron Microscopy: A Key Tool for Understanding the Activity of Nano-Oxides for Biomedical Applications. Nanomaterials 2021, 11, 2073. https://doi.org/10.3390/nano11082073
Azor-Lafarga A, Gómez-Recio I, Ruiz-González ML, González-Calbet JM. Atomic Resolution Electron Microscopy: A Key Tool for Understanding the Activity of Nano-Oxides for Biomedical Applications. Nanomaterials. 2021; 11(8):2073. https://doi.org/10.3390/nano11082073
Chicago/Turabian StyleAzor-Lafarga, Alberto, Isabel Gómez-Recio, M. Luisa Ruiz-González, and José M. González-Calbet. 2021. "Atomic Resolution Electron Microscopy: A Key Tool for Understanding the Activity of Nano-Oxides for Biomedical Applications" Nanomaterials 11, no. 8: 2073. https://doi.org/10.3390/nano11082073
APA StyleAzor-Lafarga, A., Gómez-Recio, I., Ruiz-González, M. L., & González-Calbet, J. M. (2021). Atomic Resolution Electron Microscopy: A Key Tool for Understanding the Activity of Nano-Oxides for Biomedical Applications. Nanomaterials, 11(8), 2073. https://doi.org/10.3390/nano11082073




