Synthesis of Biomimetic Melanin-Like Multifunctional Nanoparticles for pH Responsive Magnetic Resonance Imaging and Photothermal Therapy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Synthesis of Polydopamine Nanoparticles (PDANPs)
2.3. Synthesis of Amino-Fe-PDANPs
2.4. Characterization
2.5. Stability Study
2.6. MR Imaging Detection
2.7. Photothermal Effect Measurement of Amino-Fe-PDANPs
2.8. MTT Assay
3. Results and Discussion
3.1. Synthesis and Characterization of Melanin-like Multifunctional Nanoparticles
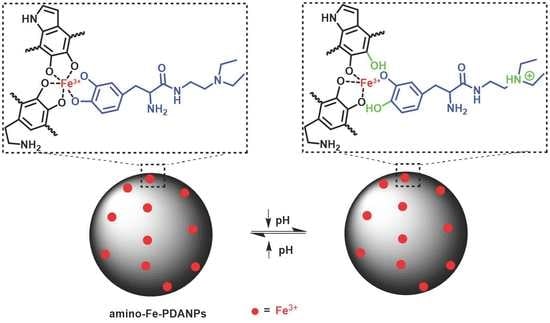
3.2. pH-Responsive Relaxivity Properties of Amino-Fe-PDANPs
3.3. Photothermal Therapy Effect
3.4. Cytotoxicity Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Watts, K.P.; Fairchild, R.G.; Slatkin, D.N.; Greenberg, D.; Packer, S.; Atkins, H.L.; Hannon, S.J. Melanin Content of Hamster Tissues, Human Tissues, and Various Melanomas. Anticancer Res. 1981, 41, 467. [Google Scholar]
- Alaluf, S.; Atkins, D.; Barrett, K.; Blount, M.; Carter, N.; Heath, A. The Impact of Epidermal Melanin on Objective Measurements of Human Skin Colour. Pigment Cell Res. 2002, 15, 119–126. [Google Scholar] [CrossRef]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-Inspired Surface Chemistry for Multifunctional Coatings. Science 2007, 318, 426. [Google Scholar] [CrossRef] [Green Version]
- Ku, S.H.; Park, C.B. Human endothelial cell growth on mussel-inspired nanofiber scaffold for vascular tissue engineering. Biomaterials 2010, 31, 9431–9437. [Google Scholar] [CrossRef]
- Fu, Y.; Li, P.; Xie, Q.; Xu, X.; Lei, L.; Chen, C.; Zou, C.; Deng, W.; Yao, S. One-Pot Preparation of Polymer-Enzyme-Metallic Nanoparticle Composite Films for High-Performance Biosensing of Glucose and Galactose. Adv. Funct. Mater. 2009, 19, 1784–1791. [Google Scholar] [CrossRef]
- Miao, Z.-H.; Wang, H.; Yang, H.; Li, Z.-L.; Zhen, L.; Xu, C.-Y. Intrinsically Mn2+-Chelated Polydopamine Nanoparticles for Simultaneous Magnetic Resonance Imaging and Photothermal Ablation of Cancer Cells. ACS Appl. Mater. Interfaces 2015, 7, 16946–16952. [Google Scholar] [CrossRef]
- Fan, Q.; Cheng, K.; Hu, X.; Ma, X.; Zhang, R.; Yang, M.; Lu, X.; Xing, L.; Huang, W.; Gambhir, S.S.; et al. Transferring Biomarker into Molecular Probe: Melanin Nanoparticle as a Naturally Active Platform for Multimodality Imaging. J. Am. Chem. Soc. 2014, 136, 15185–15194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.; Brust, T.F.; Lee, H.J.; Lee, S.C.; Watts, V.J.; Yeo, Y. Polydopamine-Based Simple and Versatile Surface Modification of Polymeric Nano Drug Carriers. ACS Nano 2014, 8, 3347–3356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bettinger, C.J.; Bruggeman, J.P.; Misra, A.; Borenstein, J.T.; Langer, R. Biocompatibility of biodegradable semiconducting melanin films for nerve tissue engineering. Biomaterials 2009, 30, 3050–3057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Ai, K.; Liu, J.; Deng, M.; He, Y.; Lu, L. Dopamine-Melanin Colloidal Nanospheres: An Efficient Near-Infrared Photothermal Therapeutic Agent for In Vivo Cancer Therapy. Adv. Mater. 2013, 25, 1353–1359. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Zhang, D.; Wu, M.; Liu, Y.; Zhang, X.; Li, L.; Li, Z.; Han, X.; Wei, X.; Liu, X. Lipid-AuNPs@PDA Nanohybrid for MRI/CT Imaging and Photothermal Therapy of Hepatocellular Carcinoma. ACS Appl. Mater. Interfaces 2014, 6, 14266–14277. [Google Scholar] [CrossRef] [PubMed]
- Pang, B.; Zhao, Y.; Luehmann, H.; Yang, X.; Detering, L.; You, M.; Zhang, C.; Zhang, L.; Li, Z.-Y.; Ren, Q.; et al. 64Cu-Doped PdCu@Au Tripods: A Multifunctional Nanomaterial for Positron Emission Tomography and Image-Guided Photothermal Cancer Treatment. ACS Nano 2016, 10, 3121–3131. [Google Scholar] [CrossRef]
- Yin, X.; Li, X.; Zhu, C.; Lin, X.; Xie, Z. Integration of fluorescence/photoacoustic imaging and targeted chemo/photothermal therapy with Ag2Se@BSA-RGD nanodots. New J. Chem. 2020, 44, 4850–4857. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, R.; Wang, D.; Sun, X.; Xu, Z. Pd nanoparticle-decorated hydroxy boron nitride nanosheets as a novel drug carrier for chemo-photothermal therapy. Colloids Surf. B 2019, 176, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Xue, X.; Lu, H.; He, Y.; Li, Y. Two-way magnetic resonance tuning and enhanced subtraction imaging for non-invasive and quantitative biological imaging. Nat. Nanotechnol. 2020, 15, 482–490. [Google Scholar] [CrossRef]
- Basser, P.J.; Pierpaoli, C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J. Magn. Reson. 2011, 213, 560–570. [Google Scholar] [CrossRef]
- Li, B.; Gong, T.; Xu, N.; Cui, F.; Yuan, B.; Yuan, Q.; Sun, H.; Wang, L.; Liu, J. Improved Stability and Photothermal Performance of Polydopamine-Modified Fe3O4 Nanocomposites for Highly Efficient Magnetic Resonance Imaging-Guided Photothermal Therapy. Small 2020, 16, 2003969. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Zhang, D.; Zeng, Y.; Wu, L.; Liu, X.; Liu, J. Nanocluster of superparamagnetic iron oxide nanoparticles coated with poly (dopamine) for magnetic field-targeting, highly sensitive MRI and photothermal cancer therapy. Nanotechnology 2015, 26, 115102. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Z.; Liu, C.; Yu, D.; Lu, Z.; Zhang, N. Gadolinium-loaded polymeric nanoparticles modified with Anti-VEGF as multifunctional MRI contrast agents for the diagnosis of liver cancer. Biomaterials 2011, 32, 5167–5176. [Google Scholar] [CrossRef]
- Cao, Y.; Liu, M.; Zhang, K.; Zu, G.; Kuang, Y.; Tong, X.; Xiong, D.; Pei, R. Poly(glycerol) Used for Constructing Mixed Polymeric Micelles as T1 MRI Contrast Agent for Tumor-Targeted Imaging. Biomacromolecules 2017, 18, 150–158. [Google Scholar] [CrossRef]
- Maddah, M.; Delavari H, H.; Mehravi, B. Preparation of Bio-Inspired Melanin Nanoplatforms Chelated with Manganese Ions as a Potential T1 MRI Contrast Agent. ChemistrySelect 2019, 4, 5860–5865. [Google Scholar] [CrossRef]
- Jing, Q.; Wang, Q.Y.; Chen, K.L.; Luo, J.B.; Zhou, Q.H.; Lin, J. Reduction/temperature/pH multi-stimuli responsive core cross-linked polypeptide hybrid micelles for triggered and intracellular drug release. Colloids Surf. B 2018, 170, 373–381. [Google Scholar]
- Yang, M.; Zhang, N.; Zhang, T.; Yin, X.; Shen, J. Fabrication of doxorubicin-gated mesoporous polydopamine nanoplatforms for multimode imaging-guided synergistic chemophotothermal therapy of tumors. Drug Delivery 2020, 27, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Peng, S.; Wang, R.; Yang, S.; Zhou, Q.; Lin, J. Stepwise pH-sensitive and biodegradable polypeptide hybrid micelles for enhanced cellular internalization and efficient nuclear drug delivery. Colloids Surf. B 2019, 181, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Senyagin, A.; Smirnova, I.; Podoprigora, I.V.; Yashina, N.Y.; Syatkin, S.P. Determination of the activity of antitumor enzyme L-lysine-a-oxidase. FEBS Open Bio 2019, 9, 369. [Google Scholar]
- Huang, X.; Huang, G.; Zhang, S.; Sagiyama, K.; Togao, O.; Ma, X.; Wang, Y.; Li, Y.; Soesbe, T.C.; Sumer, B.D.; et al. Multi-Chromatic pH-Activatable 19F-MRI Nanoprobes with Binary ON/OFF pH Transitions and Chemical-Shift Barcodes. Angew. Chem. Int. Ed. 2013, 52, 8074–8078. [Google Scholar] [CrossRef] [Green Version]
- Ge, R.; Lin, M.; Li, X.; Liu, S.; Wang, W.; Li, S.; Zhang, X.; Liu, Y.; Liu, L.; Shi, F.; et al. Cu2+-Loaded Polydopamine Nanoparticles for Magnetic Resonance Imaging-Guided pH- and Near-Infrared-Light-Stimulated Thermochemotherapy. ACS Appl. Mater. Interfaces 2017, 9, 19706–19716. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhang, S.; Kang, N.; Huang, J.; Lv, X.; Wen, K.; Ye, S.; Chen, Z.; Zhou, X.; Ren, L. Polydopamine-Coated Manganese Carbonate Nanoparticles for Amplified Magnetic Resonance Imaging-Guided Photothermal Therapy. ACS Appl. Mater. Interfaces 2017, 9, 19296–19306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grobner, T. Gadolinium-a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrol. Dial. Transplant. 2006, 21, 1104–1108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, X.; Wang, Y.; Zhao, T.; Li, Y.; Su, L.; Wang, Z.; Huang, G.; Sumer, B.D.; Gao, J. Ultra-pH-Sensitive Nanoprobe Library with Broad pH Tunability and Fluorescence Emissions. J. Am. Chem. Soc. 2014, 136, 11085–11092. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Wang, Y.; Li, Y.; Bodemann, B.; Zhao, T.; Ma, X.; Huang, G.; Hu, Z.; DeBerardinis, R.J.; White, M.A.; et al. A nanobuffer reporter library for fine-scale imaging and perturbation of endocytic organelles. Nat. Commun. 2015, 6, 8524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Zhou, K.; Huang, G.; Hensley, C.; Huang, X.; Ma, X.; Zhao, T.; Sumer, B.D.; DeBerardinis, R.J.; Gao, J. A nanoparticle-based strategy for the imaging of a broad range of tumours by nonlinear amplification of microenvironment signals. Nat. Mater. 2014, 13, 204–212. [Google Scholar] [CrossRef] [Green Version]
- Liebscher, J.; Mrówczyński, R.; Scheidt, H.A.; Filip, C.; Hădade, N.D.; Turcu, R.; Bende, A.; Beck, S. Structure of Polydopamine: A Never-Ending Story? Langmuir 2013, 29, 10539–10548. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; He, X.; Chen, H.; Zhang, J.; Zhang, H.; Wang, Z. Gram-scale synthesis of coordination polymer nanodots with renal clearance properties for cancer theranostic applications. Nat. Commun. 2015, 6, 8003. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Liu, C.; Song, L.; Cui, H.; Gao, G.; Liu, P.; Sheng, Z.; Cai, L. Indocyanine green-loaded polydopamine-iron ions coordination nanoparticles for photoacoustic/magnetic resonance dual-modal imaging-guided cancer photothermal therapy. Nanoscale 2016, 8, 17150–17158. [Google Scholar] [CrossRef] [PubMed]
- Sadaba, N.; Salsamendi, M.; Casado, N.; Zuza, E.; Sardon, H. Catechol End-Functionalized Polylactide by Organocatalyzed Ring-Opening Polymerization. Polymers 2018, 10, 155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mrowczynski, R.; Turcu, R.; Leostean, C.; Scheidt, H.A.; Liebscher, J. New versatile polydopamine coated functionalized magnetic nanoparticles. Mater. Chem. Phys. 2013, 138, 295–302. [Google Scholar] [CrossRef]
- Maruthapandi, M.; Natan, M.; Jacobi, G.; Banin, E.; Luong, J.; Gedanken, A. Antibacterial Activity Against Methicillin-Resistant Staphylococcus aureus of Colloidal Polydopamine Prepared by Carbon Dot Stimulated Polymerization of Dopamine. Nanomaterials 2019, 9, 1731. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Gao, Y.; Wang, W.; Qin, A.; Sun, J.Z.; Tang, B.Z. Different amine-functionalized poly(diphenylsubstituted acetylenes) from the same precursor. Polym. Chem. 2016, 7, 5312–5321. [Google Scholar] [CrossRef]
- Liu, F.; He, X.; Zhang, J.; Chen, H.; Zhang, H.; Wang, Z. Controllable synthesis of polydopamine nanoparticles in microemulsions with pH-activatable properties for cancer detection and treatment. J. Mater. Chem. B 2015, 3, 6731–6739. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qu, J.; Guillory, D.; Cheah, P.; Tian, B.; Zheng, J.; Liu, Y.; Cates, C.; Janorkar, A.V.; Zhao, Y. Synthesis of Biomimetic Melanin-Like Multifunctional Nanoparticles for pH Responsive Magnetic Resonance Imaging and Photothermal Therapy. Nanomaterials 2021, 11, 2107. https://doi.org/10.3390/nano11082107
Qu J, Guillory D, Cheah P, Tian B, Zheng J, Liu Y, Cates C, Janorkar AV, Zhao Y. Synthesis of Biomimetic Melanin-Like Multifunctional Nanoparticles for pH Responsive Magnetic Resonance Imaging and Photothermal Therapy. Nanomaterials. 2021; 11(8):2107. https://doi.org/10.3390/nano11082107
Chicago/Turabian StyleQu, Jing, Devin Guillory, Pohlee Cheah, Bin Tian, Jie Zheng, Yongjian Liu, Courtney Cates, Amol V. Janorkar, and Yongfeng Zhao. 2021. "Synthesis of Biomimetic Melanin-Like Multifunctional Nanoparticles for pH Responsive Magnetic Resonance Imaging and Photothermal Therapy" Nanomaterials 11, no. 8: 2107. https://doi.org/10.3390/nano11082107
APA StyleQu, J., Guillory, D., Cheah, P., Tian, B., Zheng, J., Liu, Y., Cates, C., Janorkar, A. V., & Zhao, Y. (2021). Synthesis of Biomimetic Melanin-Like Multifunctional Nanoparticles for pH Responsive Magnetic Resonance Imaging and Photothermal Therapy. Nanomaterials, 11(8), 2107. https://doi.org/10.3390/nano11082107