Introduction of Rare-Earth Oxide Nanoparticles in CNT-Based Nanocomposites for Improved Detection of Underlying CNT Network
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
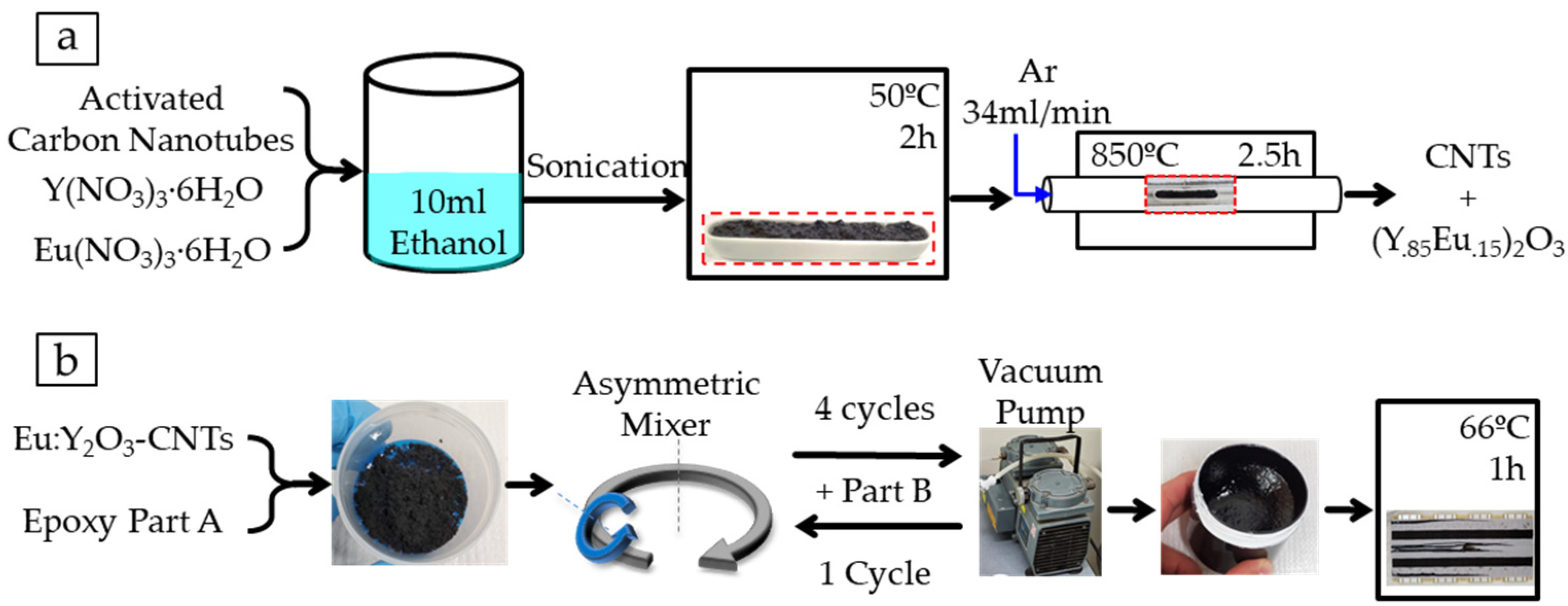
2.2. Carbon Nanotube Preparation and Eu:Y2O3 Nanoparticle Synthesis
2.3. Nanocomposite Fabrication
2.4. Thermo-Gravimetric Analysis
2.5. Material Characterization and Imaging
2.6. Deep Ultraviolet Fluorescence
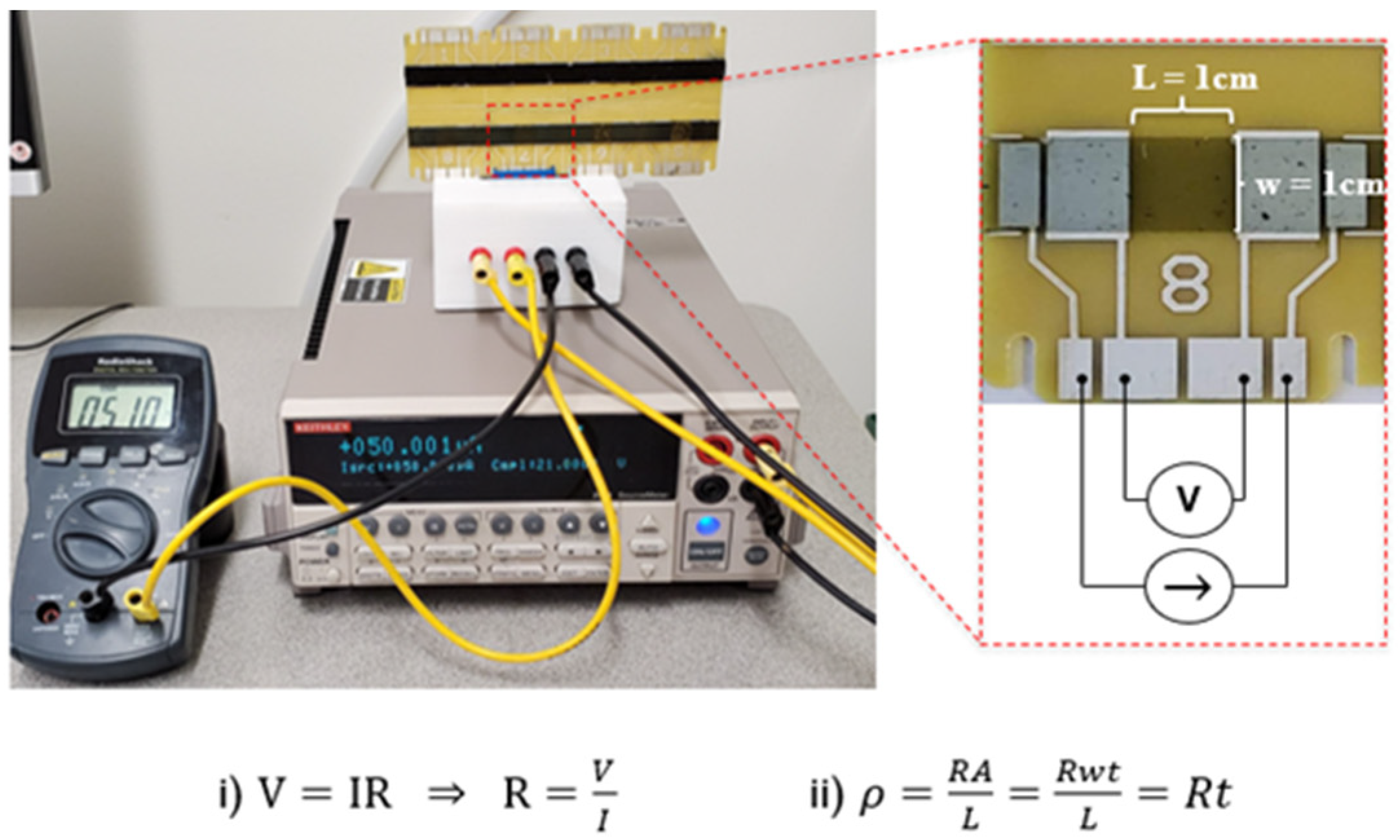
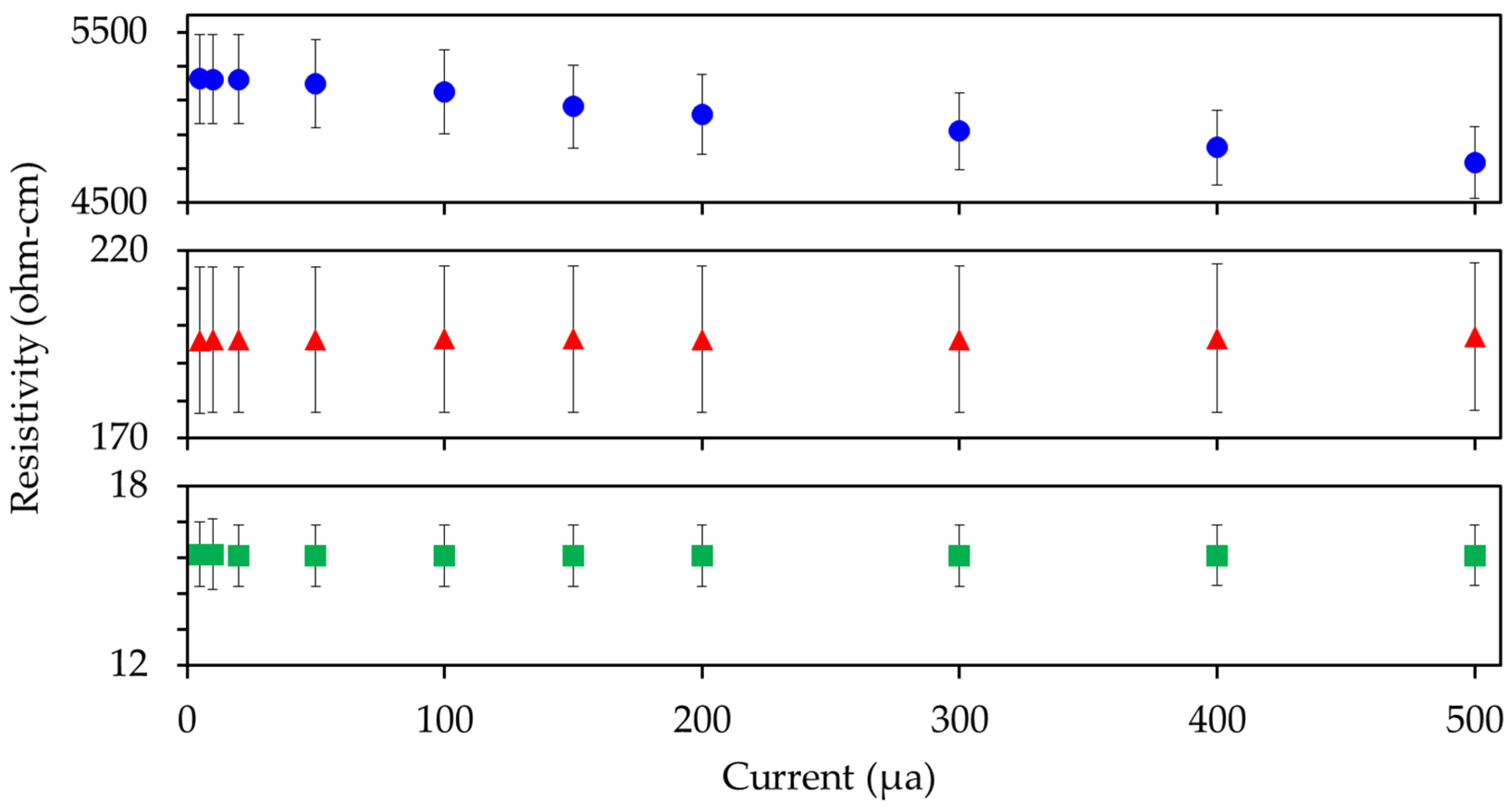
2.7. Conductivity Characterization
3. Results and Discussion
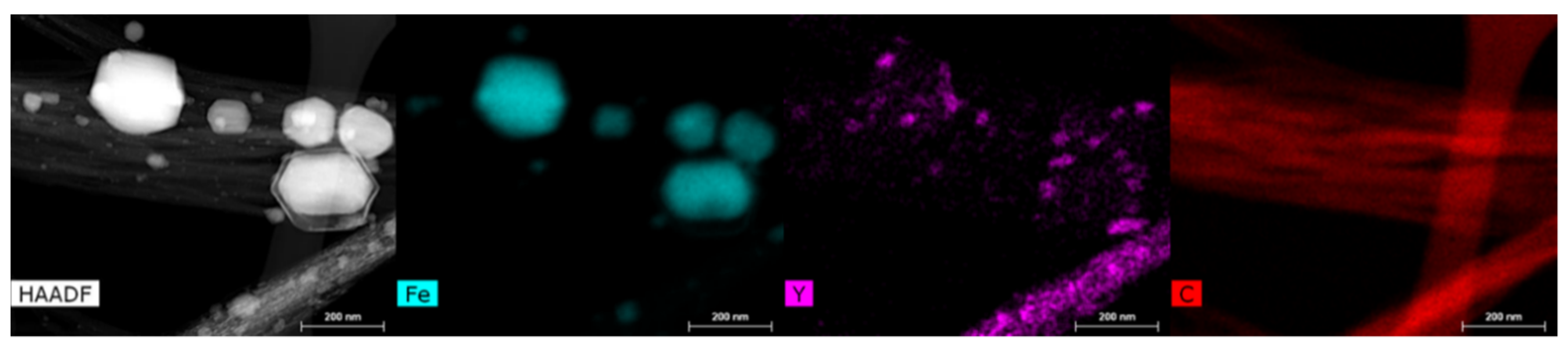
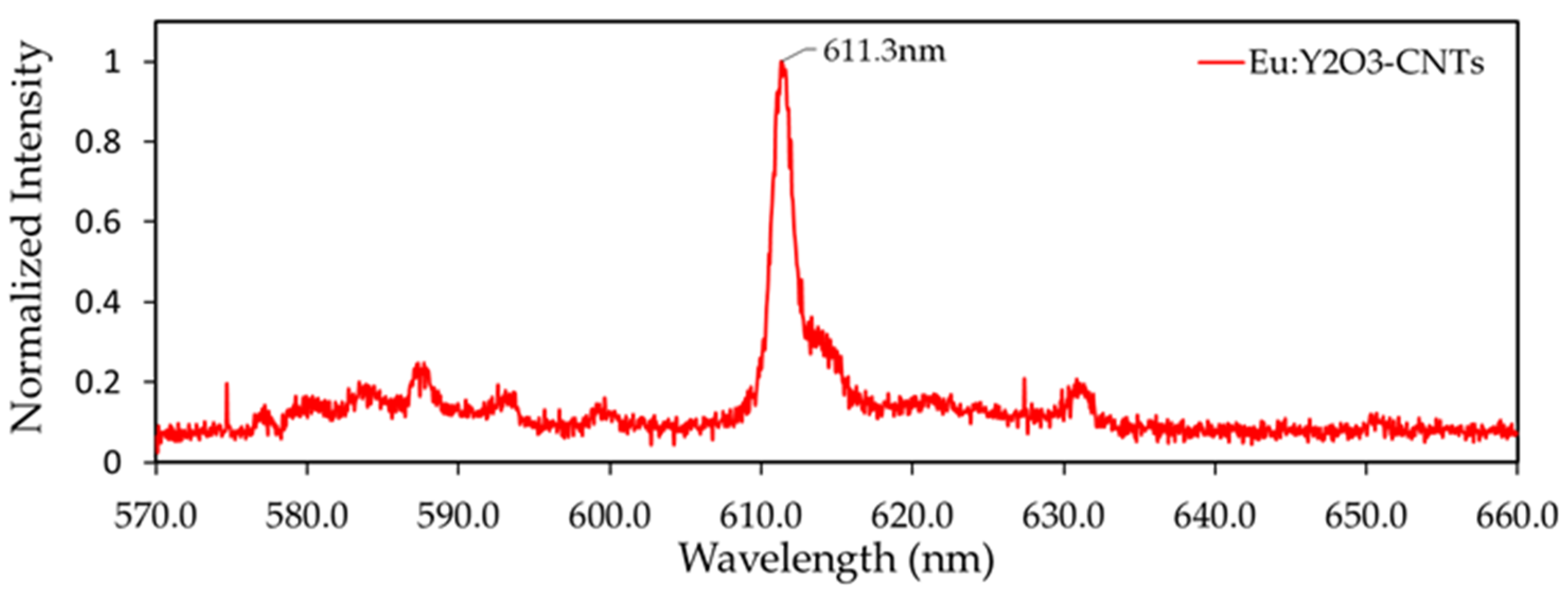
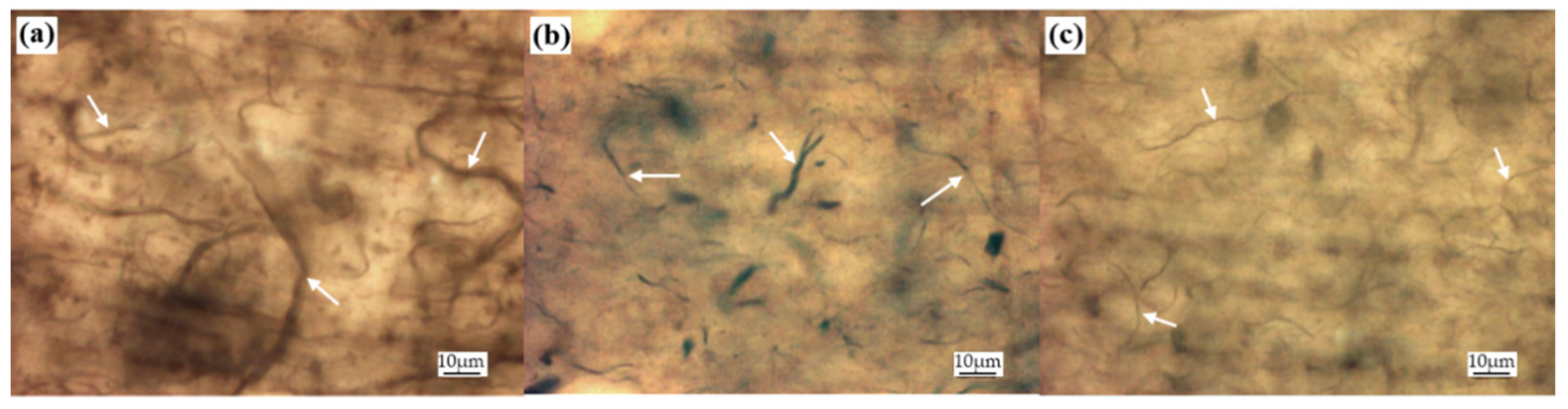
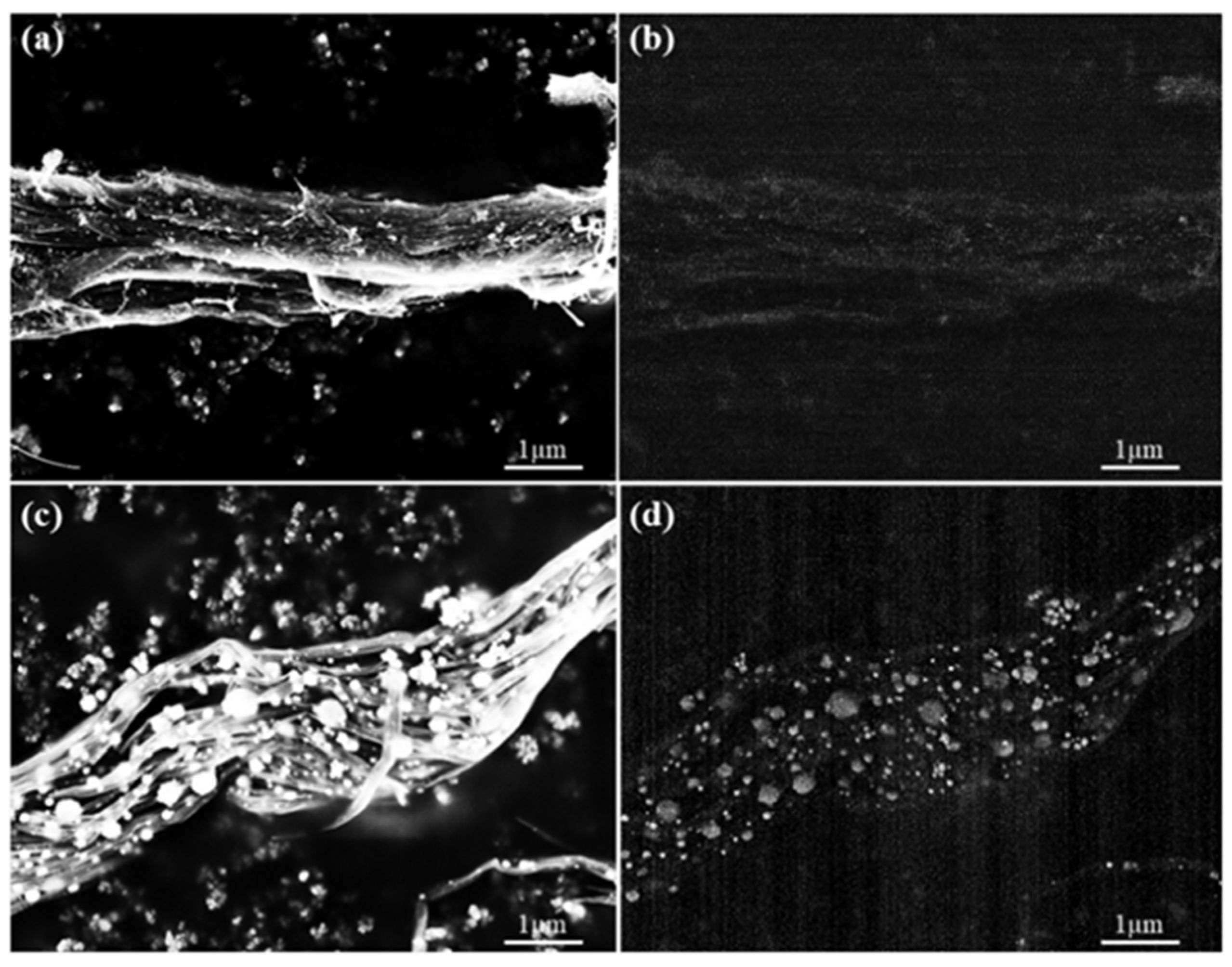
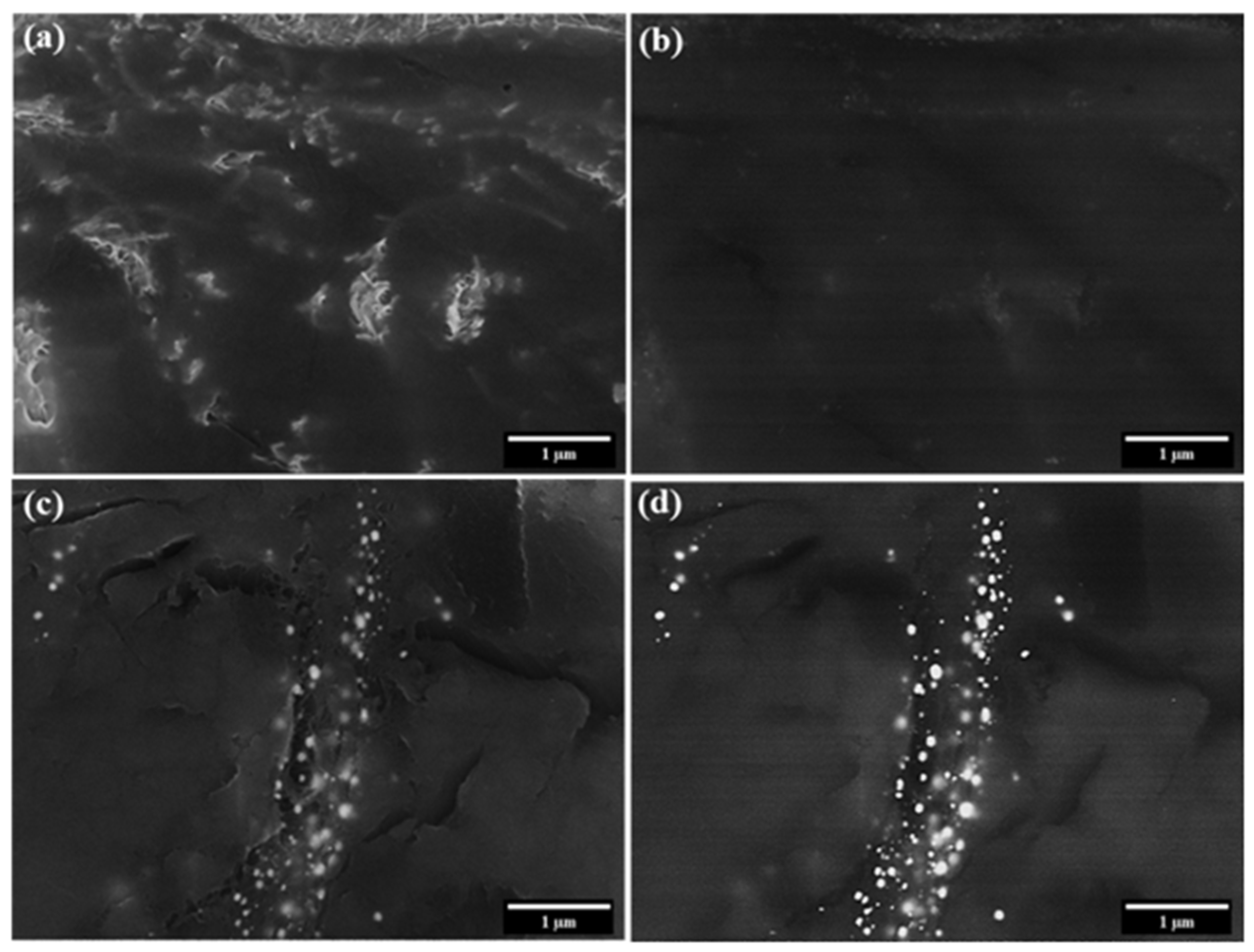
3.1. Characterization of CNT Pulp and Synthesized Eu:Y3O3 Nanoparticles
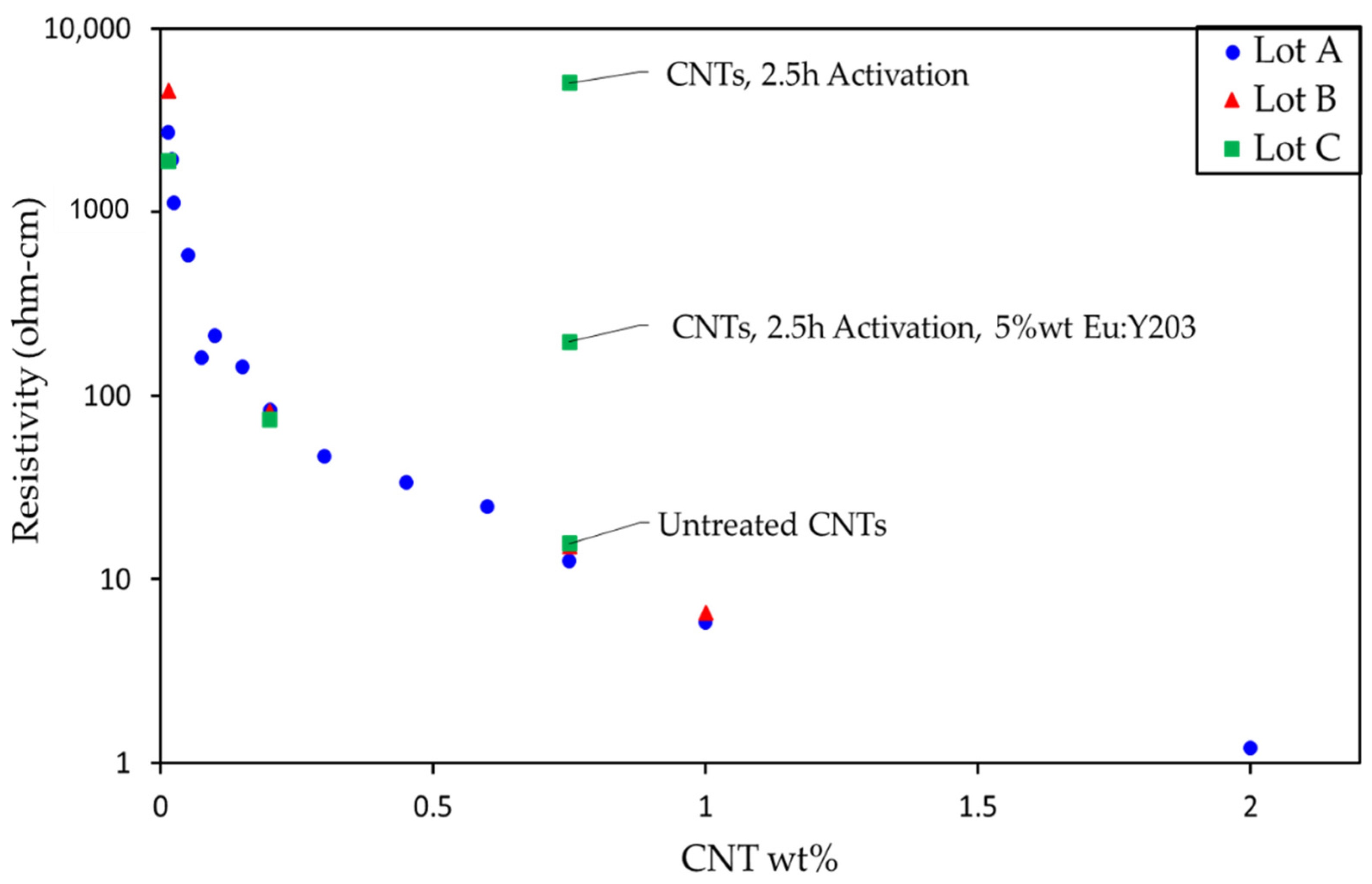
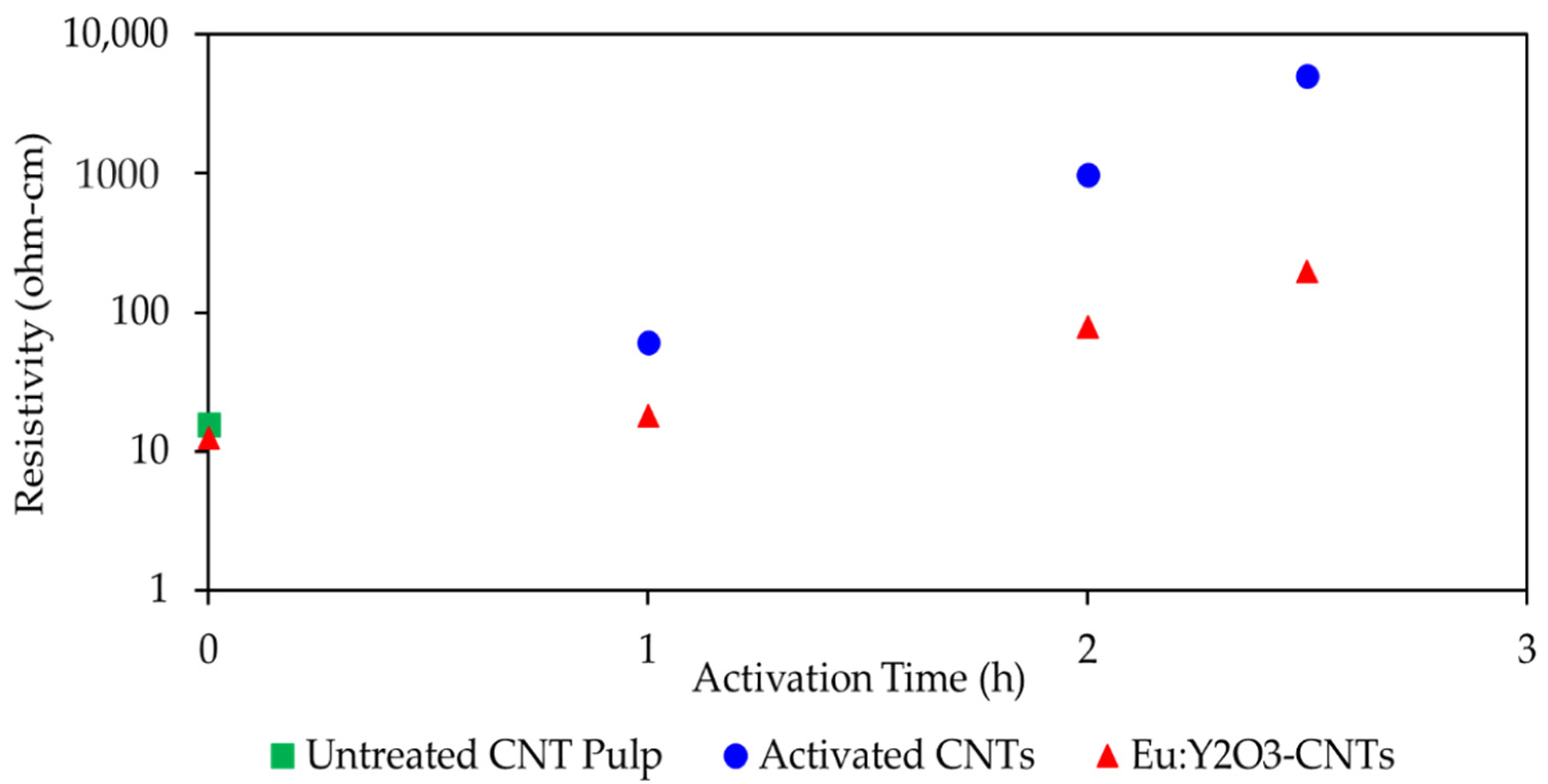
3.2. Characterization of the CNT and Eu:Y2O3-CNT Composites and Their Electrical Performance
3.3. Scanning Electron Microscopy and Micro-Computed Tomography
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Earp, B.; Phillips, J.; Grbovic, D.; Vidmar, S.; Porter, M.; Luhrs, C.C. Impact of Current and Temperature on Extremely Low Loading Epoxy-CNT Conductive Composites. Polymers 2020, 12, 867. [Google Scholar] [CrossRef] [PubMed]
- Bellucci, S.; Balasubramanian, C.; Micciulla, F.; Rinaldi, G. CNT Composites for Aerospace Applications. J. Exp. Nanosci. 2007, 2, 193–206. [Google Scholar] [CrossRef]
- Du, J.-H.; Bai, J.; Cheng, H.-M. The Present Status and Key Problems of Carbon Nanotube Based Polymer Composites. Express Polym. Lett. 2007, 1, 253–273. [Google Scholar] [CrossRef]
- Harris, P.J.F. Carbon Nanotube Composites. Int. Mater. Rev. 2004, 49, 31–43. [Google Scholar] [CrossRef]
- Kausar, A.; Rafique, I.; Muhammad, B. Review of Applications of Polymer/Carbon Nanotubes and Epoxy/CNT Composites. Polym.-Plast. Technol. Eng. 2016, 55, 1167–1191. [Google Scholar] [CrossRef]
- Kumar, P.; Maiti, U.N.; Sikdar, A.; Das, T.K.; Kumar, A.; Sudarsan, V. Recent Advances in Polymer and Polymer Composites for Electromagnetic Interference Shielding: Review and Future Prospects. Polym. Rev. 2019, 59, 687–738. [Google Scholar] [CrossRef]
- Park, S.-H.; Ha, J.-H. Improved Electromagnetic Interference Shielding Properties Through the Use of Segregate Carbon Nanotube Networks. Materials 2019, 12, 1395. [Google Scholar] [CrossRef] [Green Version]
- Spitalsky, Z.; Tasis, D.; Papagelis, K.; Galiotis, C. Carbon Nanotube–Polymer Composites: Chemistry, Processing, Mechanical and Electrical Properties. Prog. Polym. Sci. 2010, 35, 357–401. [Google Scholar] [CrossRef]
- De Volder, M.F.L.; Tawfick, S.H.; Baughman, R.H.; Hart, A.J. Carbon Nanotubes: Present and Future Commercial Applications. Science 2013, 339, 535–539. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Gupta, M.C.; Dudley, K.L. Studies on Electromagnetic Interference Shielding Characteristics of Metal Nanoparticle- and Carbon Nanostructure-Filled Polymer Composites in the Ku-Band Frequency. Micro Nano Lett. 2007, 2, 85–89. [Google Scholar] [CrossRef]
- Khan, W.; Sharma, R.; Saini, P. Carbon Nanotube-Based Polymer Composites: Synthesis, Properties and Applications. In Carbon Nanotubes—Current Progress of Their Polymer Composites; Berber, M., Hafez, I.H., Eds.; Intech Open: London, UK, 2016; pp. 1–45. ISBN 978-953-51-2469-6. [Google Scholar]
- Pantano, A.; Montinaro, N.; Cerniglia, D.; Micciulla, F.; Bistarelli, S.; Cataldo, A.; Bellucci, S. Novel Non-Destructive Evaluation Technique for the Detection of Poor Dispersion of Carbon Nanotubes in Nanocomposites. Compos. Part B Eng. 2019, 163, 52–58. [Google Scholar] [CrossRef]
- Li, W.; Buschhorn, S.T.; Schulte, K.; Bauhofer, W. The Imaging Mechanism, Imaging Depth, and Parameters Influencing the Visibility of Carbon Nanotubes in a Polymer Matrix Using an SEM. Carbon 2011, 49, 1955–1964. [Google Scholar] [CrossRef]
- Finnie, P.; Kaminska, K.; Homma, Y.; Austing, D.G.; Lefebvre, J. Charge Contrast Imaging of Suspended Nanotubes by Scanning Electron Microscopy. Nanotechnology 2008, 19, 335202. [Google Scholar] [CrossRef] [PubMed]
- Loos, J.; Alexeev, A.; Grossiord, N.; Koning, C.E.; Regev, O. Visualization of Single-Wall Carbon Nanotube (SWNT) Networks in Conductive Polystyrene Nanocomposites by Charge Contrast Imaging. Ultramicroscopy 2005, 104, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Lillehei, P.T.; Kim, J.-W.; Gibbons, L.J.; Park, C. A Quantitative Assessment of Carbon Nanotube Dispersion in Polymer Matrices. Nanotechnology 2009, 20, 325708. [Google Scholar] [CrossRef]
- Kovacs, J.Z.; Andresen, K.; Pauls, J.R.; Garcia, C.P.; Schossig, M.; Schulte, K.; Bauhofer, W. Analyzing the Quality of Carbon Nanotube Dispersions in Polymers Using Scanning Electron Microscopy. Carbon 2007, 45, 1279–1288. [Google Scholar] [CrossRef] [Green Version]
- Earp, B.; Simpson, J.; Phillips, J.; Grbovic, D.; Vidmar, S.; McCarthy, J.; Luhrs, C. Electrically Conductive CNT Composites at Loadings below Theoretical Percolation Values. Nanomaterials 2019, 9, 491. [Google Scholar] [CrossRef] [Green Version]
- Chang, N.C. Fluorescence and Stimulated Emission from Trivalent Europium in Yttrium Oxide. J. Appl. Phys. 1963, 34, 3500–3504. [Google Scholar] [CrossRef]
- Chang, N.C.; Gruber, J.B. Spectra and Energy Levels of Eu3+ in Y2O3. J. Chem. Phys. 1964, 41, 3227–3234. [Google Scholar] [CrossRef]
- Blasse, G.; Grabmaier, B.C. Luminescent Materials; Springer: Berlin/Heidelberg, Germany, 1994; ISBN 978-3-642-79017-1. [Google Scholar]
- Mariscal-Becerra, L.; Vázquez-Arreguín, R.; Balderas, U.; Carmona-Téllez, S.; Murrieta Sánchez, H.; Falcony, C. Luminescent Characteristics of Layered Yttrium Oxide Nano-Phosphors Doped with Europium. J. Appl. Phys. 2017, 121, 125111. [Google Scholar] [CrossRef]
- Wakefield, G.; Holland, E.; Dobson, P.J.; Hutchison, J.L. Luminescence Properties of Nanocrystalline Y2O3:Eu. Adv. Mater. 2001, 13, 1557–1560. [Google Scholar] [CrossRef]
- Schmechel, R.; Kennedy, M.; von Seggern, H.; Winkler, H.; Kolbe, M.; Fischer, R.A.; Xaomao, L.; Benker, A.; Winterer, M.; Hahn, H. Luminescence Properties of Nanocrystalline Y2O3:Eu3+ in Different Host Materials. J. Appl. Phys. 2001, 89, 1679–1686. [Google Scholar] [CrossRef]
- Das, G.K. Rare Earth Doped Nanomaterials as Potential Contrast Agents for Optical/Magnetic Resonance Imaging; Nanyang Technological University: Singapore, 2010. [Google Scholar]
- Yang, H.; Zhang, D.; Shi, L.; Fang, J. Synthesis and Strong Red Photoluminescence of Europium Oxide Nanotubes and Nanowires Using Carbon Nanotubes as Templates. Acta Mater. 2008, 56, 955–967. [Google Scholar] [CrossRef]
- Technologies, N. Nanocomp Technologies’ Products|Dispersed Products. Available online: https://www.miralon.com/dispersed-products (accessed on 28 January 2021).
- LOCTITE 9396aero. Available online: https://www.henkel-adhesives.com/vn/en/product/adhesives/loctite_ea_9396_aero.html (accessed on 28 January 2021).
- Tomviz for Tomographic Visualization of Nanoscale Materials. Available online: https://tomviz.org/ (accessed on 29 July 2021).
- Melnikov, P.; Nascimento, V.A.; Consolo, L.Z.Z.; Silva, A.F. Mechanism of Thermal Decomposition of Yttrium Nitrate Hexahydrate, Y(NO3)3·6H2O and Modeling of Intermediate Oxynitrates. J. Therm. Anal. Calorim. 2013, 111, 115–119. [Google Scholar] [CrossRef]
- Melnikov, P.; Arkhangelsky, I.V.; Nascimento, V.A.; de Oliveira, L.C.S.; Silva, A.F.; Zanoni, L.Z. Thermal Properties of Europium Nitrate Hexahydrate Eu(NO3)3·6H2O. J. Therm. Anal. Calorim. 2017, 128, 1353–1358. [Google Scholar] [CrossRef]
- Jack, K.H.; Goodeve, C.F. The Iron-Nitrogen System: The Preparation and the Crystal Structures of Nitrogen-Austenite(γ) and Nitrogen-Martensite(α’). Proc. R. Soc. Lond. Ser. Math. Phys. Sci. 1951, 208, 200–215. [Google Scholar] [CrossRef]
- Tessier, F.; Navrotsky, A.; Niewa, R.; Leineweber, A.; Jacobs, H.; Kikkawa, S.; Takahashi, M.; Kanamaru, F.; DiSalvo, F.J. Energetics of Binary Iron Nitrides. Solid State Sci. 2000, 2, 457–462. [Google Scholar] [CrossRef]
- Hirai, H.; Kondo, K. Modified Phases of Diamond Formed Under Shock Compression and Rapid Quenching. Science 1991, 253, 772. [Google Scholar] [CrossRef]
- Wen, B.; Zhao, J.J.; Li, T.J. Synthesis and Crystal Structure of N-Diamond. Int. Mater. Rev. 2007, 52, 131–151. [Google Scholar] [CrossRef]
- Wen, B.; Zhao, J.; Li, T.; Dong, C.; Jin, J. N-Diamond from Catalysed Carbon Nanotubes: Synthesis and Crystal Structure. J. Phys. Condens. Matter 2005, 17, L513–L519. [Google Scholar] [CrossRef]
- Pang, H.; Xu, L.; Yan, D.-X.; Li, Z.-M. Conductive Polymer Composites with Segregated Structures. Prog. Polym. Sci. 2014, 39, 1908–1933. [Google Scholar] [CrossRef]
- Mochizuki, S. Electrical Conductivity of α-Fe2O3. Phys. Status Solidi A 1977, 41, 591–594. [Google Scholar] [CrossRef]
- Chen, X.; Tang, X.-Z.; Liang, Y.N.; Cheah, J.W.; Hu, P.; Hu, X. Controlled Thermal Functionalization for Dispersion Enhancement of Multi-Wall Carbon Nanotube in Organic Solvents. J. Mater. Sci. 2016, 51, 5625–5634. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hubbard, J.; Isik, T.; Ansell, T.Y.; Ortalan, V.; Luhrs, C. Introduction of Rare-Earth Oxide Nanoparticles in CNT-Based Nanocomposites for Improved Detection of Underlying CNT Network. Nanomaterials 2021, 11, 2168. https://doi.org/10.3390/nano11092168
Hubbard J, Isik T, Ansell TY, Ortalan V, Luhrs C. Introduction of Rare-Earth Oxide Nanoparticles in CNT-Based Nanocomposites for Improved Detection of Underlying CNT Network. Nanomaterials. 2021; 11(9):2168. https://doi.org/10.3390/nano11092168
Chicago/Turabian StyleHubbard, Joel, Tugba Isik, Troy Y. Ansell, Volkan Ortalan, and Claudia Luhrs. 2021. "Introduction of Rare-Earth Oxide Nanoparticles in CNT-Based Nanocomposites for Improved Detection of Underlying CNT Network" Nanomaterials 11, no. 9: 2168. https://doi.org/10.3390/nano11092168





