Efficient Catalytic Degradation of Phenol with Phthalocyanine-Immobilized Reduced Graphene–Bacterial Cellulose Nanocomposite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Preparation of Pc-rGBC Nanocomposite
2.3. Characterization
2.4. Adsorption and Catalytic Degradation of Phenol
3. Results and Discussion
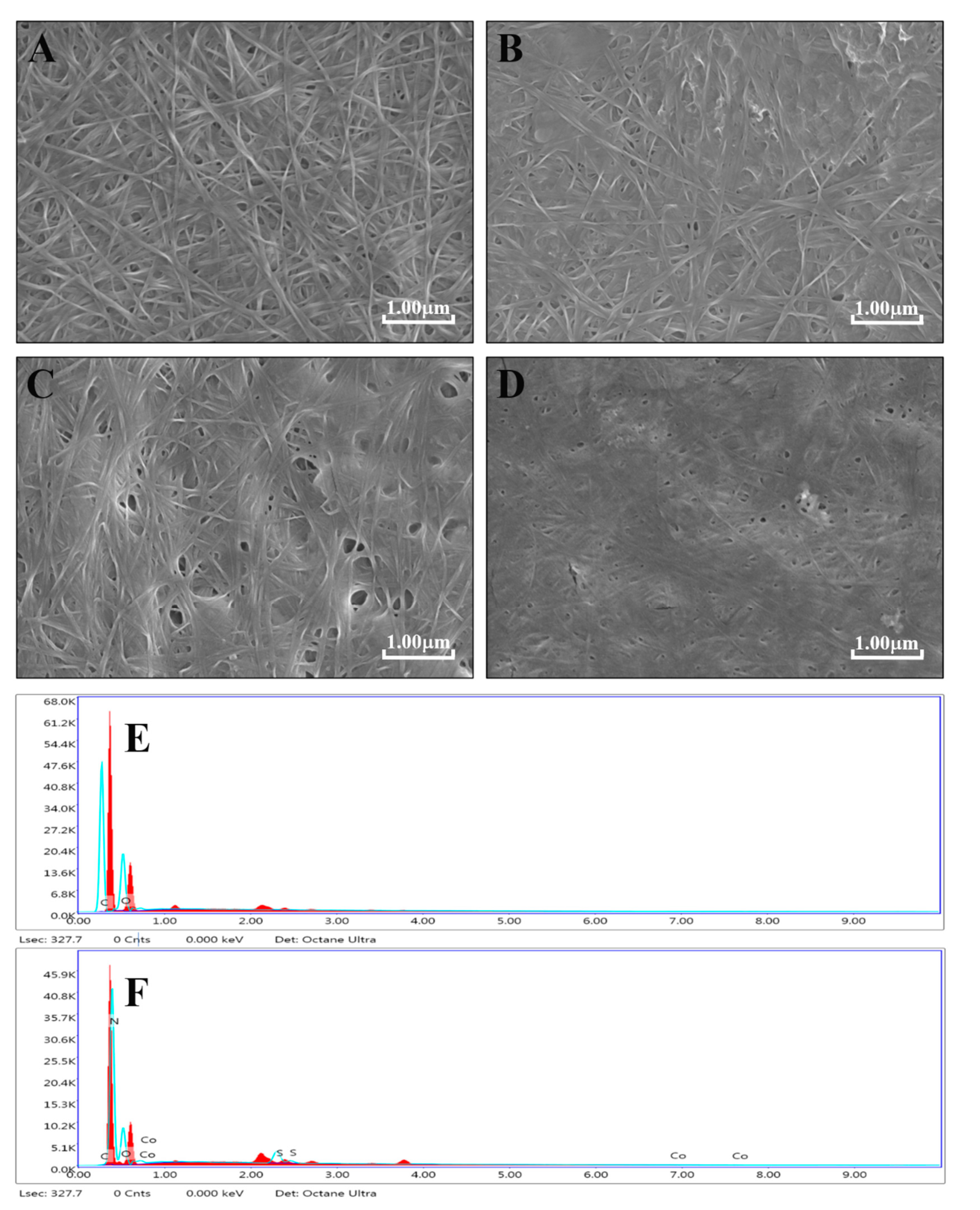
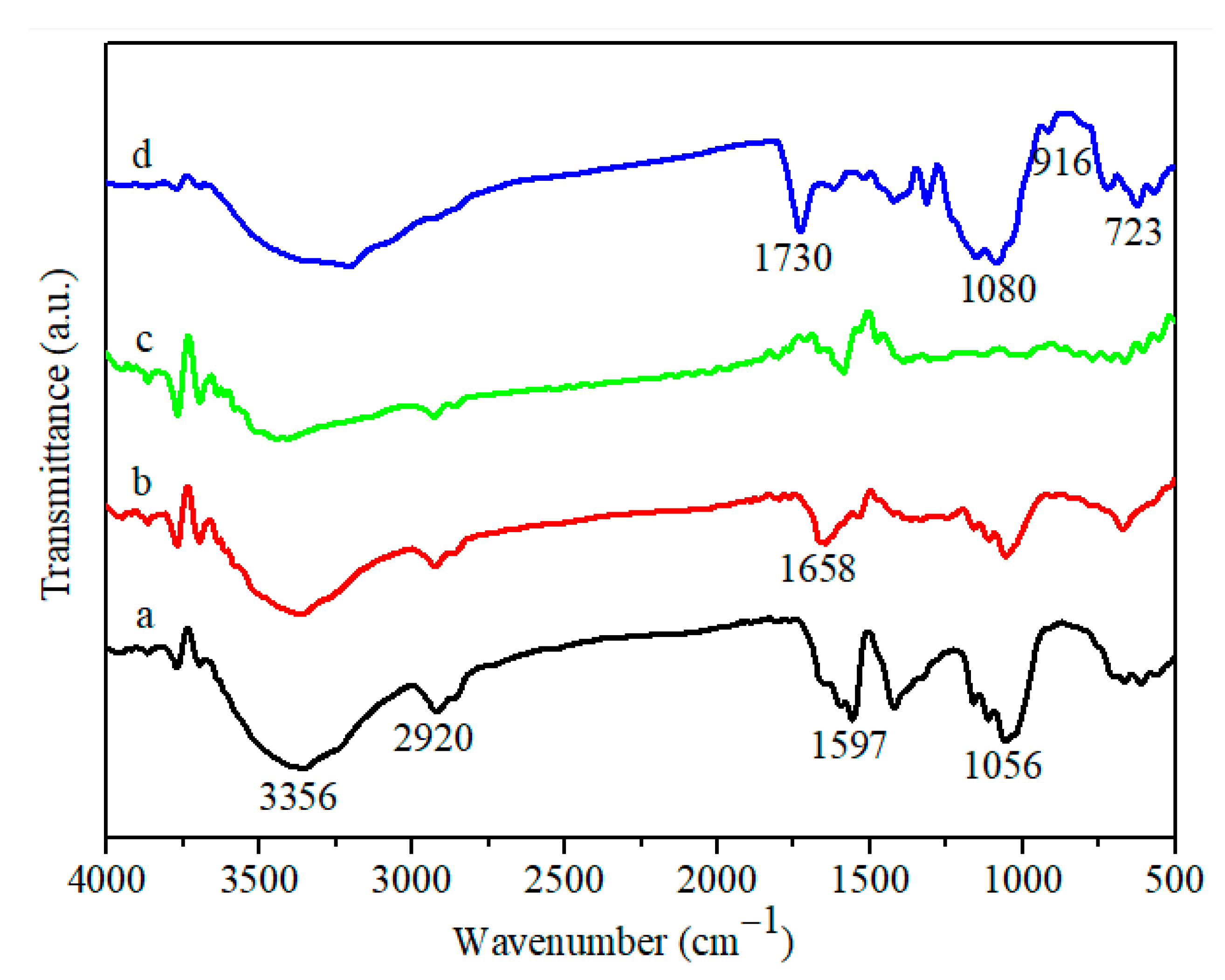
3.1. Materials’ Characterization
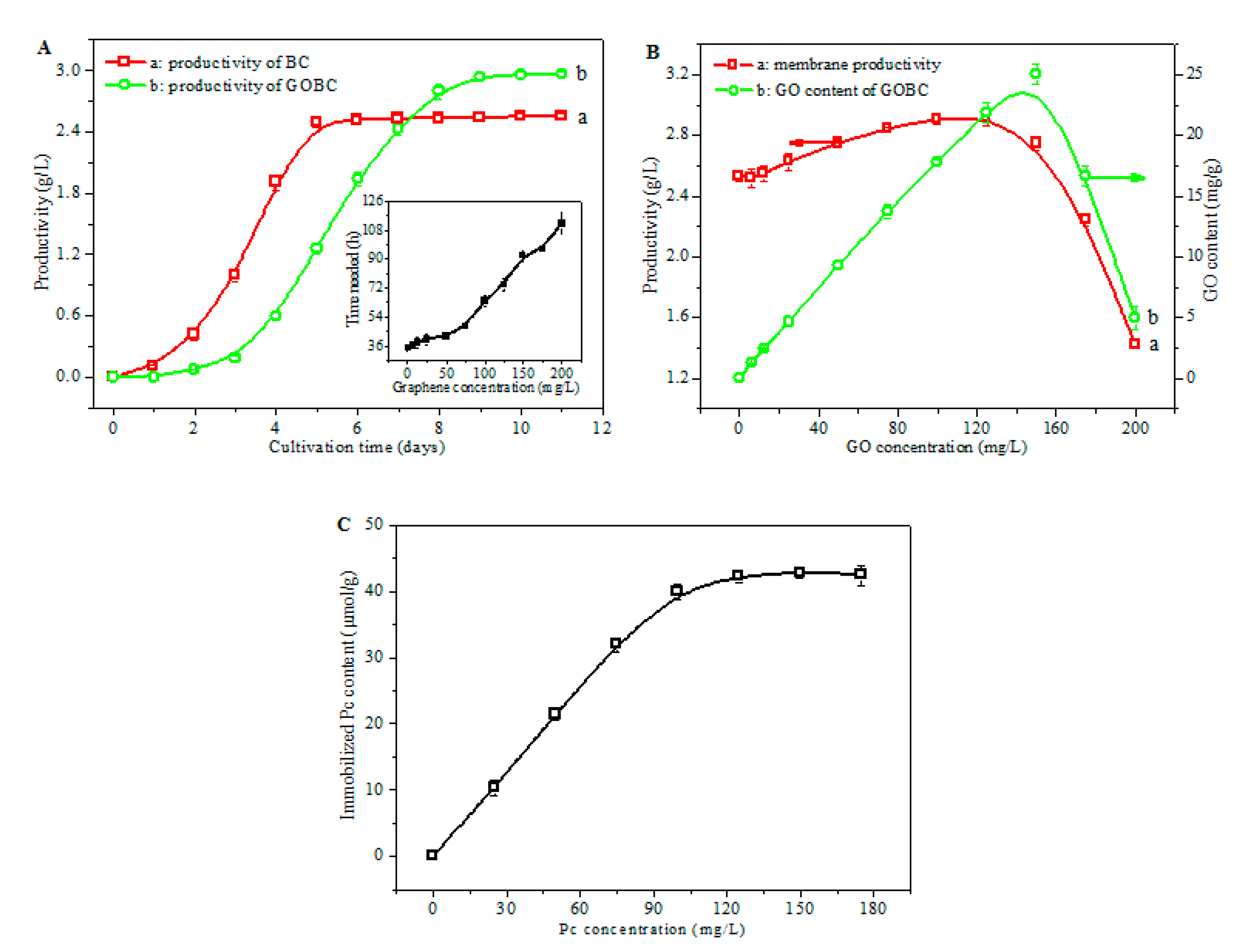
3.2. Study of Cultivation and Pc Immobilization Process
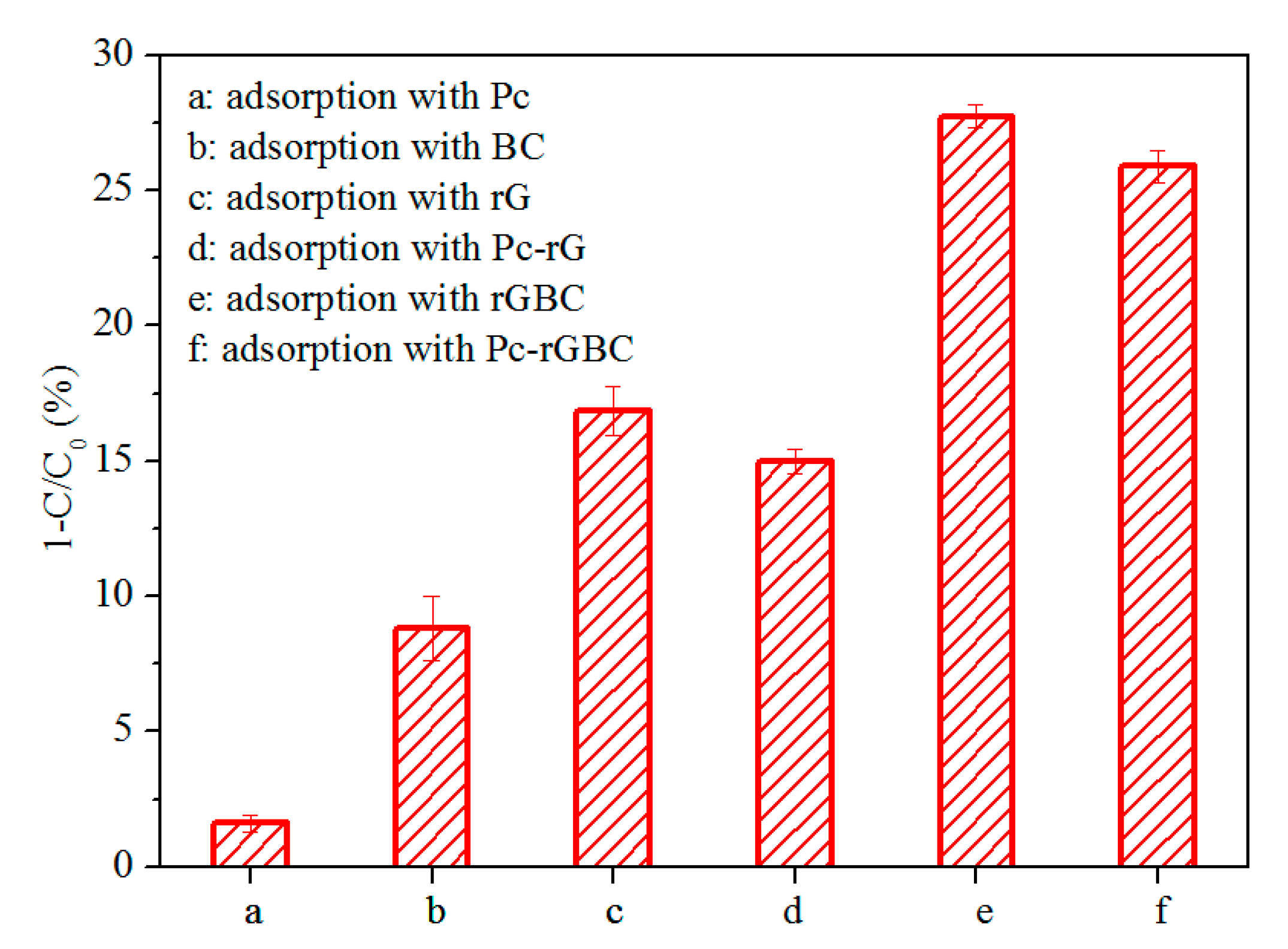
3.3. Adsorption and Catalytic Oxidation of Phenol with Pc-rGBC
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tolosa, L.; Martinez-Sena, T.; Schimming, J.P.; Moro, E.; Escher, S.E.; Braak, B.T.; van Der Water, B.; Miranda, M.A.; van Vugt-Lussenburg, B.M.A.; Castell, J.V. The in vitro assessment of the toxicity of volatile, oxidisable, redox-cycling compounds: Phenols as an example. Arch. Toxicol. 2021, 95, 2109–2121. [Google Scholar] [CrossRef]
- Michalowicz, J.; Duda, W. Phenols—Sources and toxicity. Pol. J. Environ. Studies 2007, 16, 347–362. [Google Scholar]
- Benkhennouche-Bouchene, H.; Mahy, J.G.; Lambert, S.D.; Hayoun, B.; Deflaoui, O.; Bourouina, M.; Bachari, K.; Hamitouche, A.E.; Bacha-Bourouina, S. Statistical modeling and optimization of Escherichia coli growth parameters for the biological treatment of phenol. Biocatal. Agric. Biotechnol. 2021, 34, 102016. [Google Scholar] [CrossRef]
- Pradeep, N.V.; Anupama, S.; Navya, K.; Shalini, H.N.; Idris, M.; Hampannavar, U.S. Biological removal of phenol from wastewaters: A mini review. Appl. Water Sci. 2015, 5, 105–112. [Google Scholar] [CrossRef] [Green Version]
- Zagklis, D.P.; Vavouraki, A.I.; Kornaros, M.E.; Paraskeva, C.A. Purification of olive mill wastewater phenols through membrane filtration and resin adsorption/desorption. J. Hazard. Mater. 2015, 285, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.T.; Xie, J.; Ren, Z.Q.; Zhang, W.D. Solvent extraction of phenol with cumene from wastewater. Desalin. Water Treat. 2013, 51, 3826–3831. [Google Scholar] [CrossRef]
- Zhang, Y.T.; Chang, C.; Tan, B.R.; Xu, D.B.; Wang, Y.; Qi, T. Application of a sustainable bioderived solvent (biodiesel) for phenol extraction. ACS Omega 2019, 4, 10431–10437. [Google Scholar] [CrossRef]
- Yavuz, Y.; Koparal, A.S.; Ogutveren, U.B. Phenol removal through chemical oxidation using Fenton reagent. Chem. Eng. Technol. 2007, 30, 583–586. [Google Scholar] [CrossRef]
- Zabik, N.L.; Virca, C.N.; McCormick, T.M.; Martic-Milne, S. Selective electrochemical versus chemical oxidation of bulky phenol. J. Phys. Chem. B 2016, 120, 8914–8924. [Google Scholar] [CrossRef]
- Zhang, J.C.; Qin, L.; Yang, Y.Z.; Liu, X.G. Porous carbon nanospheres aerogel based molecularly imprinted polymer for efficient phenol adsorption and removal from wastewater. Sep. Purif. Technol. 2021, 274, 119029. [Google Scholar] [CrossRef]
- Liu, X.Y.; Tu, Y.A.; Liu, S.C.; Liu, K.L.; Zhang, L.F.; Li, G.H.; Xu, Z.Q. Adsorption of ammonia nitrogen and phenol onto the lignite surface: An experimental and molecular dynamics simulation study. J. Hazard. Mater. 2021, 416, 125966. [Google Scholar] [CrossRef]
- Chaghaganooj, Z.D.; Asasian-Kolur, N.; Sharifian, S.; Sillanpaa, M. Ce and mn/bio-waste-based activated carbon composite: Characterization, phenol adsorption and regeneration. J. Environ. Chem. Eng. 2021, 9, 105788. [Google Scholar] [CrossRef]
- Ghahghaey, Z.; Hekmati, M.; Ganji, M.D. Theoretical investigation of phenol adsorption on functionalized graphene using DFT calculations for effective removal of organic contaminants from wastewater. J. Mol. Liq. 2021, 324, 114777. [Google Scholar] [CrossRef]
- El Oufir, Z.; Ramezani, H.; Mathieu, N.; Bhatia, S.K.; Delpeux, S. Impact of high adsorbent conductivity on adsorption of polar molecules: Simulation of phenol adsorption on graphene sheets. Adsorption 2020, 26, 537–552. [Google Scholar] [CrossRef]
- Wang, X.M.; Lu, M.X.; Wang, H.; Pei, Y.F.; Rao, H.H.; Du, X.Z. Three-dimensional graphene aerogels-mesoporous silica frameworks for superior adsorption capability of phenols. Sep. Purif. Technol. 2015, 153, 7–13. [Google Scholar] [CrossRef]
- Liu, Q.-S.; Zheng, T.; Wang, P.; Jiang, J.-P.; Li, N. Adsorption isotherm, kinetic and mechanism studies of some substituted phenols on activated carbon fibers. Chem. Eng. J. 2010, 157, 348–356. [Google Scholar] [CrossRef]
- Li, Y.; Du, Q.; Liu, T.; Sun, J.; Jiao, Y.; Xia, Y.; Xia, L.; Wang, Z.; Zhang, W.; Wang, K.; et al. Equilibrium, kinetic and thermodynamic studies on the adsorption of phenol onto graphene. Mater. Res. Bull. 2012, 47, 1898–1904. [Google Scholar] [CrossRef]
- Ma, D.S.; Yi, H.; Lai, C.; Liu, X.G.; Huo, X.Q.; An, Z.W.; Li, L.; Fu, Y.K.; Li, B.S.; Zhang, M.M.; et al. Critical review of advanced oxidation processes in organic wastewater treatment. Chemosphere 2021, 275, 130104. [Google Scholar] [CrossRef] [PubMed]
- Domingues, E.; Fernandes, E.; Gomes, J.; Martins, R.C. Advanced oxidation processes perspective regarding swine wastewater treatment. Sci. Total Environ. 2021, 776, 145958. [Google Scholar] [CrossRef]
- Coha, M.; Farinelli, G.; Tiraferri, A.; Minella, M.; Vione, D. Advanced oxidation processes in the removal of organic substances from produced water: Potential, configurations, and research needs. Chem. Eng. J. 2021, 414, 128668. [Google Scholar] [CrossRef]
- Saka, E.T.; Dugdu, E.; Unver, Y. Degradation of substituted phenols with different oxygen sources catalyzed by Co(II) and Cu(II) phthalocyanine complexes. J. Coord. Chem. 2019, 72, 1119–1130. [Google Scholar] [CrossRef]
- Kluson, P.; Drobek, M.; Krejcikova, S.; Krysa, J.; Kalaji, A.; Cajthaml, T.; Rakusan, J. Molecular structure effects in photodegradation of phenol and its chlorinated derivatives with phthalocyanines. Appl. Catal. B-Environ. 2008, 80, 321–326. [Google Scholar] [CrossRef]
- Cheng, M.; Lai, C.; Liu, Y.; Zeng, G.M.; Huang, D.L.; Zhang, C.; Qin, L.; Hu, L.; Zhou, C.Y.; Xiong, W.P. Metal-organic frameworks for highly efficient heterogeneous Fenton-like catalysis. Coord. Chem. Rev. 2018, 368, 80–92. [Google Scholar] [CrossRef]
- Navalon, S.; Martin, R.; Alvaro, M.; Garcia, H. Gold on diamond nanoparticles as a highly efficient Fenton catalyst. Angew. Chem. Int. Ed. 2010, 49, 8403–8407. [Google Scholar] [CrossRef]
- Liu, W.X.; Zhang, L.; Yang, Q.L.; Zhang, Z.W.; Liu, Y.H.; Guo, R.X.; Torres, O.L.; Yan, Z.Y.; Chen, J.Q. Highly efficient treatment of terephthalic acid wastewater by Fenton-like combined biodegradation. Desalin. Water Treat. 2020, 182, 243–252. [Google Scholar] [CrossRef]
- Shin, S.; Yoon, H.; Jang, J. Polymer-encapsulated iron oxide nanoparticles as highly efficient Fenton catalysts. Catal. Commun. 2008, 10, 178–182. [Google Scholar] [CrossRef]
- Li, B.; Sun, L.; Bian, J.; Sun, N.; Sun, J.; Chen, L.; Li, Z.; Jing, L. Controlled synthesis of novel Z-scheme iron phthalocyanine/porous WO3 nanocomposites as efficient photocatalysts for CO2 reduction. Appl. Catal. B-Environ. 2020, 270, 118849. [Google Scholar] [CrossRef]
- Liu, Y.S.; McCrory, C.C.L. Modulating the mechanism of electrocatalytic CO2 reduction by cobalt phthalocyanine through polymer coordination and encapsulation. Nat. Commun. 2019, 10, 1683. [Google Scholar] [CrossRef]
- Makarov, S.G.; Ketkov, S.Y.; Wohrle, D. A planar binuclear cobalt(ii) phthalocyanine as a highly efficient catalyst for the oxidation of a mercaptan. Chem. Commun. 2020, 56, 5653–5656. [Google Scholar] [CrossRef]
- Zhu, Z.X.; Chen, Y.; Gu, Y.; Wu, F.; Lu, W.Y.; Xu, T.F.; Chen, W.X. Catalytic degradation of recalcitrant pollutants by Fenton-like process using polyacrylonitrile-supported iron (II) phthalocyanine nanofibers: Intermediates and pathway. Water Res. 2016, 93, 296–305. [Google Scholar] [CrossRef]
- da Silva, T.H.; de Souza, T.F.M.; Ribeiro, A.O.; Calefi, P.S.; Ciuffi, K.J.; Nassar, E.J.; Molina, E.F.; Hamer, P.; de Faria, E.H. New strategies for synthesis and immobilization of methalophtalocyanines onto kaolinite: Preparation, characterization and chemical stability evaluation. Dyes Pigment. 2016, 134, 41–50. [Google Scholar] [CrossRef] [Green Version]
- Balkus, K.J.; Eissa, M.; Levado, R. Oxidation of Alkanes Catalyzed by Zeolite-Encapsulated Perfluorinated Ruthenium Phthalocyanines. J. Am. Chem. Soc. 1995, 117, 10753–10754. [Google Scholar] [CrossRef]
- Han, Z.B.; Han, X.; Zhao, X.M.; Yu, J.T.; Xu, H. Iron phthalocyanine supported on amidoximated PAN fiber as effective catalyst for controllable hydrogen peroxide activation in oxidizing organic dyes. J. Hazard. Mater. 2016, 320, 27–35. [Google Scholar] [CrossRef]
- Klaewkla, R.; Arend, M.; Hoelderich, W.F. A review of mass transfer controlling the reaction rate in heterogeneous catalytic systems. In Mass Transfer-Advanced Aspects; IntechOpen: Rijeka, Croatia, 2011. [Google Scholar]
- Hong, Q.L.; Chen, S.L. Facile one-step fabrication of phthalocyanine-graphene-bacterial-cellulose nanocomposite with superior catalytic performance. Nanomaterials 2020, 10, 1673. [Google Scholar] [CrossRef] [PubMed]
- Marinescu, C.; Ben Ali, M.; Hamdi, A.; Cherifi, Y.; Barras, A.; Coffinier, Y.; Somacescu, S.; Raditoiu, V.; Szunerits, S.; Boukherroub, R. Cobalt phthalocyanine-supported reduced graphene oxide: A highly efficient catalyst for heterogeneous activation of peroxymonosulfate for rhodamine B and pentachlorophenol degradation. Chem. Eng. J. 2018, 336, 465–475. [Google Scholar] [CrossRef]
- Wang, Q.L.; Li, H.Y.; Yang, J.H.; Sun, Q.; Li, Q.Y.; Yang, J.J. Iron phthalocyanine-graphene donor-acceptor hybrids for visible-light-assisted degradation of phenol in the presence of H2O2. Appl. Catal. B-Environ. 2016, 192, 182–192. [Google Scholar] [CrossRef]
- Bader, H.; Sturzenegger, V.; Hoigne, J. Photometric method for the determination of low concentrations of hydrogen peroxide by the peroxidase catalyzed oxidation of n n diethyl p phenylenediamine dpd. Water Res. 1988, 22, 1109–1115. [Google Scholar] [CrossRef]
- Park, S.; Lee, K.S.; Bozoklu, G.; Cai, W.; Nguyen, S.T.; Ruoff, R.S. Graphene oxide papers modified by divalent ions - Enhancing mechanical properties via chemical cross-linking. ACS Nano 2008, 2, 572–578. [Google Scholar] [CrossRef]
- Achar, B.; Fohlen, G.; Parker, J.; Keshavayya, J. Synthesis and structural studies of metal (II) 4, 9, 16, 23-phthalocyanine tetraamines. Polyhedron 1987, 6, 1463–1467. [Google Scholar] [CrossRef]
- Reiniati, I.; Hrymak, A.N.; Margaritis, A. Kinetics of cell growth and crystalline nanocellulose production by Komagataeibacter xylinus. Biochem. Eng. J. 2017, 127, 21–31. [Google Scholar] [CrossRef]
- Zou, X.F.; Zhang, L.; Wang, Z.J.; Luo, Y. Mechanisms of the Antimicrobial Activities of Graphene Materials. J. Am. Chem. Soc. 2016, 138, 2064–2077. [Google Scholar] [CrossRef] [PubMed]
- Karahan, H.E.; Wei, L.; Goh, K.; Liu, Z.; Birer, O.; Dehghani, F.; Xu, C.J.; Wei, J.; Chen, Y. Bacterial physiology is a key modulator of the antibacterial activity of graphene oxide. Nanoscale 2016, 8, 17181–17189. [Google Scholar] [CrossRef]
- Chen, S.; Xie, W.; Guo, B.; Pan, T.; Chen, W. Revealing the role of graphene in enhancing the catalytic performance of phthalocyanine immobilized graphene/bacterial cellulose nanocomposite. Cellulose 2019, 26, 7863–7875. [Google Scholar] [CrossRef]
- Ertl, G.; Knözinger, H.; Schüth, F.; Weitkamp, J. Handbook of Heterogeneous Catalysis, Second, Completely Revised and Enlarged Edn.; Wiley: Hoboken, NJ, USA, 2008. [Google Scholar]
- Lousada, C.M.; Jonsson, M. Kinetics, Mechanism, and Activation Energy of H2O2 Decomposition on the Surface of ZrO2. J. Phys. Chem. C 2010, 114, 11202–11208. [Google Scholar] [CrossRef]
- Stoyanova, M.; Christoskova, S.T. Comparative study on the kinetics of catalytic decomposition of hydrogen peroxide on individual and iron-modified Co-oxide systems. Oxid. Commun. 2007, 30, 945–952. [Google Scholar]
- Lin, S.-S.; Gurol, M.D. Catalytic Decomposition of Hydrogen Peroxide on Iron Oxide: Kinetics, Mechanism, and Implications. Environ. Sci. Technol. 1998, 32, 1417–1423. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, B.; Sun, Y.; Fan, Q.; Chen, J.; Fang, W.; Chen, S. Efficient Catalytic Degradation of Phenol with Phthalocyanine-Immobilized Reduced Graphene–Bacterial Cellulose Nanocomposite. Nanomaterials 2021, 11, 2218. https://doi.org/10.3390/nano11092218
Wu B, Sun Y, Fan Q, Chen J, Fang W, Chen S. Efficient Catalytic Degradation of Phenol with Phthalocyanine-Immobilized Reduced Graphene–Bacterial Cellulose Nanocomposite. Nanomaterials. 2021; 11(9):2218. https://doi.org/10.3390/nano11092218
Chicago/Turabian StyleWu, Binbin, Yikai Sun, Qiujin Fan, Jiahui Chen, Weizheng Fang, and Shiliang Chen. 2021. "Efficient Catalytic Degradation of Phenol with Phthalocyanine-Immobilized Reduced Graphene–Bacterial Cellulose Nanocomposite" Nanomaterials 11, no. 9: 2218. https://doi.org/10.3390/nano11092218
APA StyleWu, B., Sun, Y., Fan, Q., Chen, J., Fang, W., & Chen, S. (2021). Efficient Catalytic Degradation of Phenol with Phthalocyanine-Immobilized Reduced Graphene–Bacterial Cellulose Nanocomposite. Nanomaterials, 11(9), 2218. https://doi.org/10.3390/nano11092218




