Preparation of TiSi2 Powders with Enhanced Lithium-Ion Storage via Chemical Oven Self-Propagating High-Temperature Synthesis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of TiSi2
2.2. Synthesis of Graphite Oxide
2.3. Preparation of TiSi2/Graphene (RGO) Hybrid Material
2.4. Preparation of Electrodes
2.5. Material Characterization
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kostopoulou, A.; Vernardou, D.; Makri, D.; Brintakis, K.; Savva, K.; Stratakis, E. Highly stable metal halide perovskite micro-cube anodes for lithium-air batteries. J. Power Sources Adv. 2020, 3, 100015. [Google Scholar] [CrossRef]
- Li, P.; Kim, H.; Myung, S.; Sun, Y. Diverting Exploration of Silicon Anode into Practical Way: A Review Focused on Sili-con-Graphite Composite for Lithium Ion Batteries. Energy Storage Mater. 2021, 35, 550–576. [Google Scholar] [CrossRef]
- Wang, J.; Liao, L.; Lee, H.R.; Shi, F.; Huang, W.; Zhao, J.; Pei, A.; Tang, J.; Zheng, X.; Chen, W.; et al. Surface-engineered meso-porous silicon microparticles as high-Coulombic-efficiency anodes for lithium-ion batteries. Nano Energy 2019, 61, 404–410. [Google Scholar] [CrossRef]
- Hui, X.; Zhao, R.; Zhang, P.; Li, C.; Wang, C.; Yin, L. Low-temperature reduction strategy synthesized Si/Ti3C2 MXene compo-site anodes for high-performance li-ion batteries. Adv. Energy Mater. 2019, 9, 1901065. [Google Scholar] [CrossRef]
- Sung, J.; Ma, J.; Choi, S.; Hong, J.; Kim, N.; Chae, S.; Son, Y.; Kim, S.Y.; Cho, J. Fabrication of lamellar nanosphere structure for effective Stress-Management in Large-Volume-Variation anodes of High-Energy Lithium-Ion batteries. Adv. Mater. 2019, 31, 1900970. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wu, Z.; Su, Z.; Chen, S.; Yan, C.; Al-Mamun, M.; Tang, Y.; Zhang, S. A mechanically robust self-healing binder for silicon anode in lithium ion batteries. Nano Energy 2021, 81, 105654. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, X.; Yan, P.; Cheng, R.; Tang, Y.; Cui, M.; Wang, B.; Zhang, L.; Wang, X.; Jiang, Y.; et al. Dual bond enhanced multidimensional constructed composite silicon anode for High-Performance lithium ion batteries. ACS Nano 2019, 13, 8854–8864. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, N.; Goodman, M.D.; Zhang, H.G.; Epstein, E.S.; Huang, B.; Pan, Z.; Kim, J.; Choi, J.H.; Huang, X.; et al. Mechanically and Chemically Robust Sandwich-Structured C@Si@C Nanotube Array Li-Ion Battery Anodes. ACS Nano 2015, 9, 1985–1994. [Google Scholar] [CrossRef]
- Anani, A.; Huggins, R. Multinary alloy electrodes for solid state batteries I. A phase diagram approach for the selection and storage properties determination of candidate electrode materials. J. Power Sources 1992, 38, 351–362. [Google Scholar] [CrossRef]
- Kasavajjula, U.; Wang, C.; Appleby, A.J. Nano- and bulk-silicon-based insertion anodes for lithium-ion secondary cells. J. Power Sources 2007, 163, 1003–1039. [Google Scholar] [CrossRef]
- Ding, B.; Cai, Z.; Ahsan, Z.; Ma, Y.; Zhang, S.; Song, G.; Yuan, C.; Yang, W.; Wen, C. A Review of Metal Silicides for Lithium-Ion Battery Anode Application. Acta Met. Sin. (English Lett.) 2021, 34, 291–308. [Google Scholar] [CrossRef]
- Liu, Y.; He, Y.; Ma, R.; Gao, M.; Pan, H. Improved lithium storage properties of Mg2Si anode material synthesized by hydro-gen-driven chemical reaction. Electrochem. Commun. 2012, 25, 15–18. [Google Scholar] [CrossRef]
- Yan, J.; Huang, H.; Zhang, J.; Yang, Y. The study of Mg2Si/carbon composites as anode materials for lithium ion batteries. J. Power Sources 2008, 175, 547–552. [Google Scholar] [CrossRef]
- Tamirat, A.G.; Hou, M.; Liu, Y.; Bin, D.; Sun, Y.; Fan, L.; Wang, Y.; Xia, Y. Highly stable carbon coated Mg2Si intermetallic nano-particles for lithium-ion battery anode. J. Power Sources 2018, 384, 10–17. [Google Scholar] [CrossRef]
- Courtel, F.M.; Duguay, D.; Abu-Lebdeh, Y.; Davidson, I.J. Investigation of CrSi2 and MoSi2 as anode materials for lithium-ion batteries. J. Power Sources 2012, 202, 269–275. [Google Scholar] [CrossRef]
- Netz, A.; Huggins, R.A.; Weppner, W. Investigations of a number of alternative negative electrode materials for use in lithium cells. Ionics 2001, 7, 433–439. [Google Scholar] [CrossRef]
- Zhou, S.; Wang, D. Unique Lithiation and Delithiation Processes of Nanostructured Metal Silicides. ACS Nano 2010, 4, 7014–7020. [Google Scholar] [CrossRef]
- Yeh, C.; Chen, W.; Hsu, C. Formation of titanium silicides Ti5Si3 and TiSi2 by self-propagating combustion synthesis. J. Alloy. Compd. 2007, 432, 90–95. [Google Scholar] [CrossRef]
- Yeh, C.; Wang, H.; Chen, W. A comparative study on combustion synthesis of Ti–Si compounds. J. Alloy. Compd. 2008, 450, 200–207. [Google Scholar] [CrossRef]
- Liu, J.; Bai, Y.; Chen, P.; Cui, N.; Yin, H. Reaction synthesis of TiSi2 and Ti5Si3 by ball-milling and shock loading and their photocatalytic activities. J. Alloy. Compd. 2013, 555, 375–380. [Google Scholar] [CrossRef]
- Liang, B.; Liu, Y.; Xu, Y. Silicon-based materials as high capacity anodes for next generation lithium ion batteries. J. Power Sources 2014, 267, 469–490. [Google Scholar] [CrossRef]
- Zhou, S.; Liu, X.; Wang, D. Si/TiSi2 Heteronanostructures as High-Capacity Anode Material for Li Ion Batteries. Nano Lett. 2010, 10, 860–863. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Yang, X.; Lin, Y.; Xie, J.; Wang, D. A Nanonet-Enabled Li Ion Battery Cathode Material with High Power Rate, High Capacity, and Long Cycle Lifetime. ACS Nano 2012, 6, 919–924. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhong, X.; Shaw, J.C.; Liu, L.; Huang, Y.; Duan, X. Very high energy density silicide–air primary batteries. Energy Environ. Sci. 2013, 6, 2621. [Google Scholar] [CrossRef]
- Zhang, X.; Xie, X.; Wang, H.; Zhang, J.; Pan, B.; Xie, Y. Enhanced Photoresponsive Ultrathin Graphitic-Phase C3N4 Nanosheets for Bioimaging. J. Am. Chem. Soc. 2013, 135, 18–21. [Google Scholar] [CrossRef]
- Lindsay, L.; Broido, D.A. Enhanced thermal conductivity and isotope effect in single-layer hexagonal boron nitride. Phys. Rev. B 2011, 84, 155421. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Wu, Q.; Mishra, C.; Kang, J.; Zhang, H.; Cho, K.; Cai, W.; Balandin, A.A.; Ruoff, R.S. Thermal conductivity of isotopically modified graphene. Nat. Mater. 2012, 11, 203–207. [Google Scholar] [CrossRef]
- Yoo, E.; Kim, J.; Hosono, E.; Zhou, H.-S.; Kudo, T.; Honma, I. Large Reversible Li Storage of Graphene Nanosheet Families for Use in Rechargeable Lithium Ion Batteries. Nano Lett. 2008, 8, 2277–2282. [Google Scholar] [CrossRef]
- Li, X.; Geng, D.; Zhang, Y.; Meng, X.; Li, R.; Sun, X. Superior cycle stability of nitrogen-doped graphene nanosheets as anodes for lithium ion batteries. Electrochem. Commun. 2011, 13, 822–825. [Google Scholar] [CrossRef]
- Mukherjee, R.; Thomas, A.V.; Krishnamurthy, A.; Koratkar, N. Photothermally Reduced Graphene as High-Power Anodes for Lithium-Ion Batteries. ACS Nano 2012, 6, 7867–7878. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, Y.; Ren, W.; Tan, Q.; Chen, Y.; Li, H.; Zhong, Z.; Su, F. Scalable synthesis of interconnected porous Sili-con/Carbon composites by the rochow reaction as High-Performance anodes of lithium ion batteries. Angew. Chem. 2014, 126, 5165–5169. [Google Scholar] [CrossRef]
- Yi, R.; Zai, J.; Dai, F.; Gordin, M.L.; Wang, D. Dual conductive network-enabled graphene/Si–C composite anode with high areal capacity for lithium-ion batteries. Nano Energy 2014, 6, 211–218. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, H.; Yan, J.; Zhang, B.; Li, W. Effect of argon atmosphere on the formation of MoSi2 by self-propagating combus-tion method. Int. J. Refract. Metals Hard Mater. 2007, 25, 318–321. [Google Scholar] [CrossRef]
- Chen, F.; Xu, J.; Yan, J.; Tang, S. Effects of Y2O3 on SiC/MoSi2 composite by mechanical-assistant combustion synthesis. Int. J. Refract. Met. Hard Mater. 2013, 36, 143–148. [Google Scholar] [CrossRef]
- Trambukis, J.; Munir, Z.A. Effect of Particle Dispersion on the Mechanism of Combustion Synthesis of Titanium Silicide. J. Am. Ceram. Soc. 1990, 73, 1240–1245. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved syn-thesis of graphene oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef] [PubMed]
- Ahn, W.; Kang, K.; Kim, K. Synthesis of TS-1 by microwave heating of template-impregnated SiO2–TiO2 xerogels. Catal. Lett. 2001, 72, 229–232. [Google Scholar] [CrossRef]
- Han, Y.; Liu, X.; Lu, Z. Systematic Investigation of Prelithiated SiO2 Particles for High-Performance Anodes in Lithium-Ion Battery. Appl. Sci. 2018, 8, 1245. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.; Zheng, G.; Liu, N.; Carney, T.J.; Yang, Y.; Cui, Y. Engineering Empty Space between Si Nanoparticles for Lithium-Ion Battery Anodes. Nano Lett. 2012, 12, 904–909. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, D.; Lu, Z.; Hsu, P.-C.; Lee, H.R.; Liu, N.; Zhao, J.; Wang, H.; Liu, C.; Cui, Y. A high tap density secondary silicon particle anode fabricated by scalable mechanical pressing for lithium-ion batteries. Energy Environ. Sci. 2015, 8, 2371–2376. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, Y.; Qi, J.; Wang, Y.; Luo, J.; Yao, W. Preparation of nanocrystalline MoSi2 with enhanced lithium storage by sol–gel and carbonthermal reduction method. Ceram. Int. 2018, 44, 9494–9498. [Google Scholar] [CrossRef]
- Domi, Y.; Usui, H.; Takaishi, R.; Sakaguchi, H. Lithiation and Delithiation Reactions of Binary Silicide Electrodes in an Ionic Liquid Electrolyte as Novel Anodes for Lithium-Ion Batteries. ChemElectroChem 2018, 6, 581–589. [Google Scholar] [CrossRef] [Green Version]
- Du, Z.; Hatchard, T.D.; Bissonnette, P.; Dunlap, R.A.; Obrovac, M.N. Electrochemical activity of nano-NiSi2 in Li Cells. J. Elec-trochem. Soc. 2016, 163, A2456–A2460. [Google Scholar] [CrossRef]
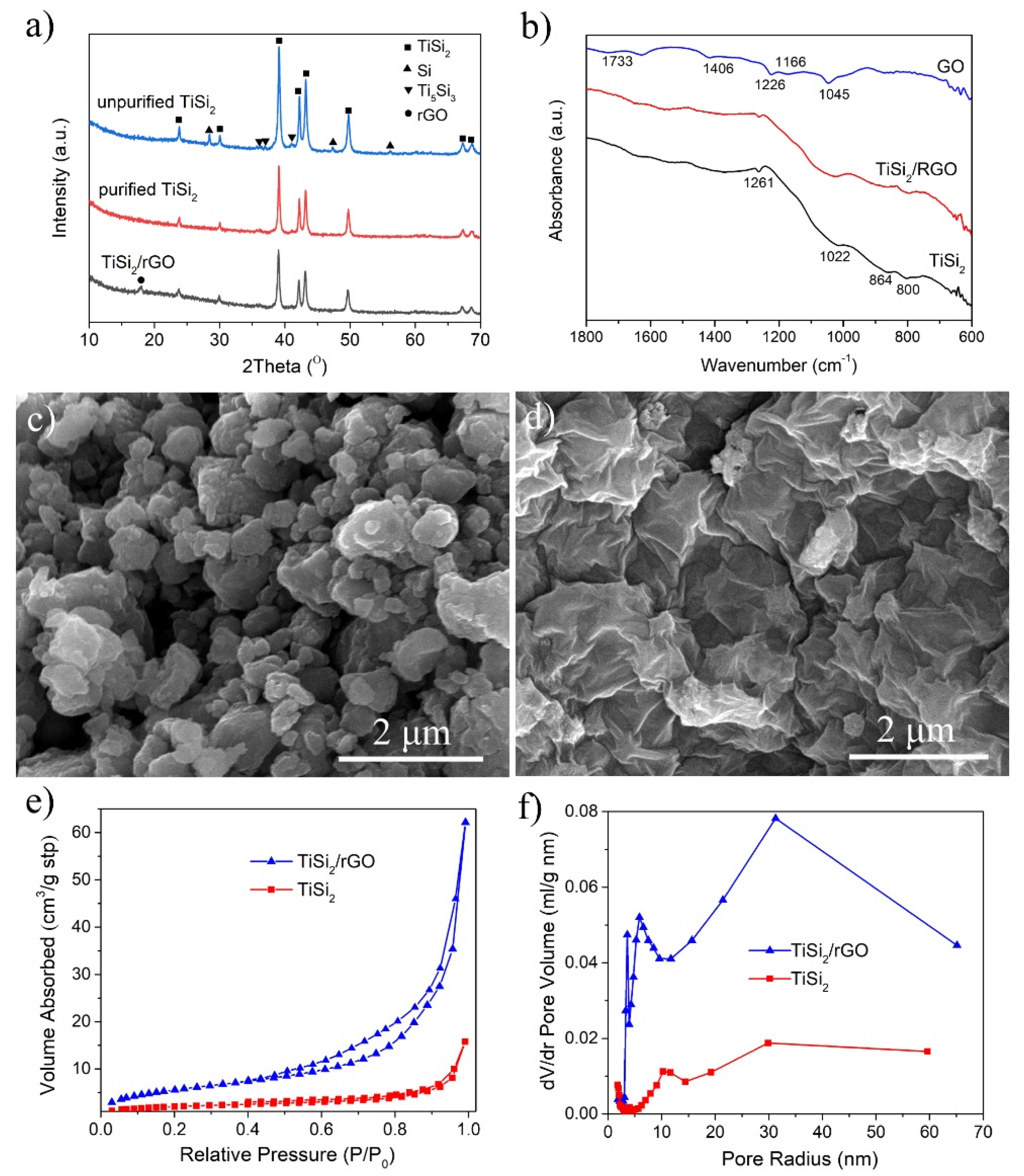
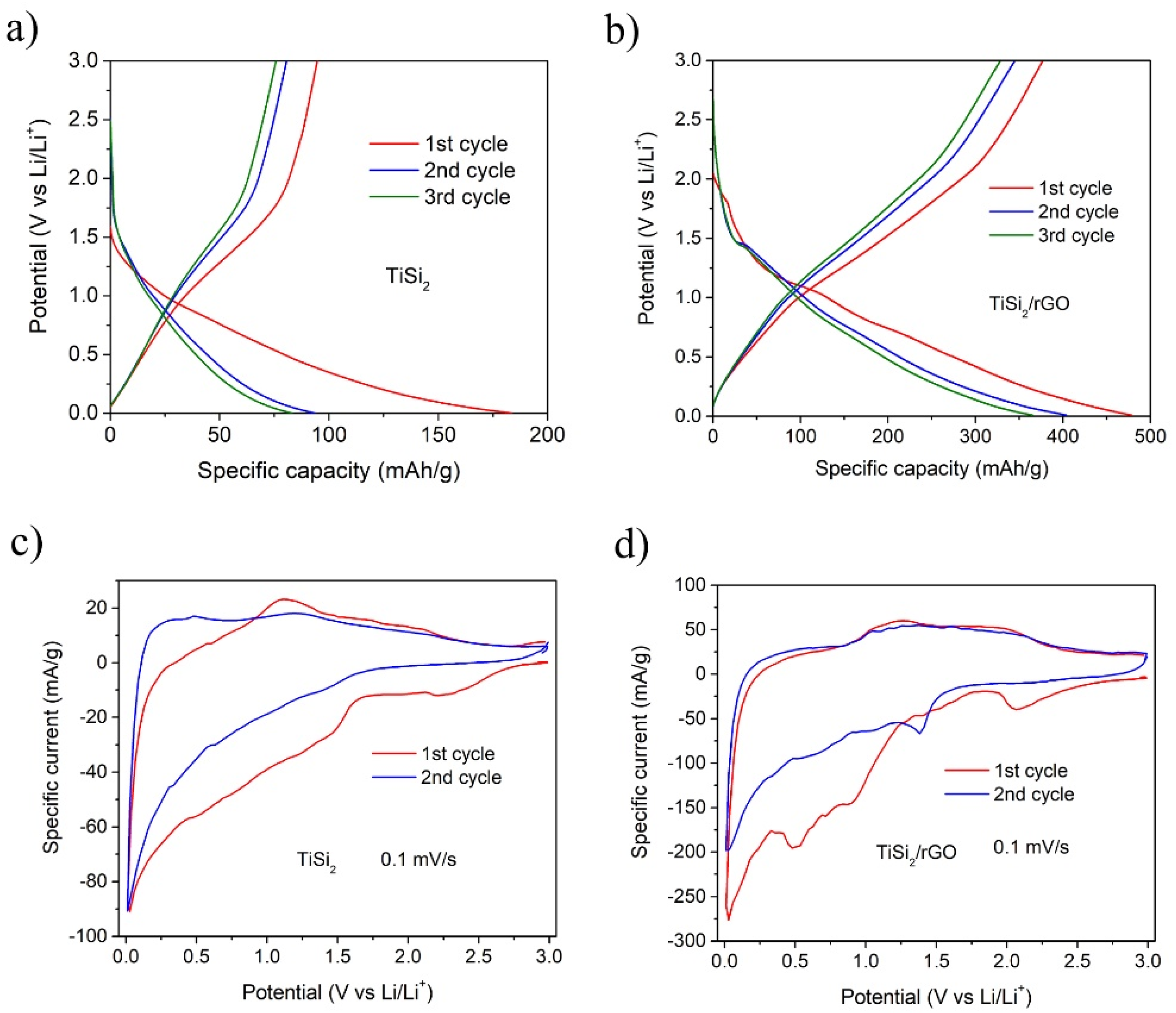

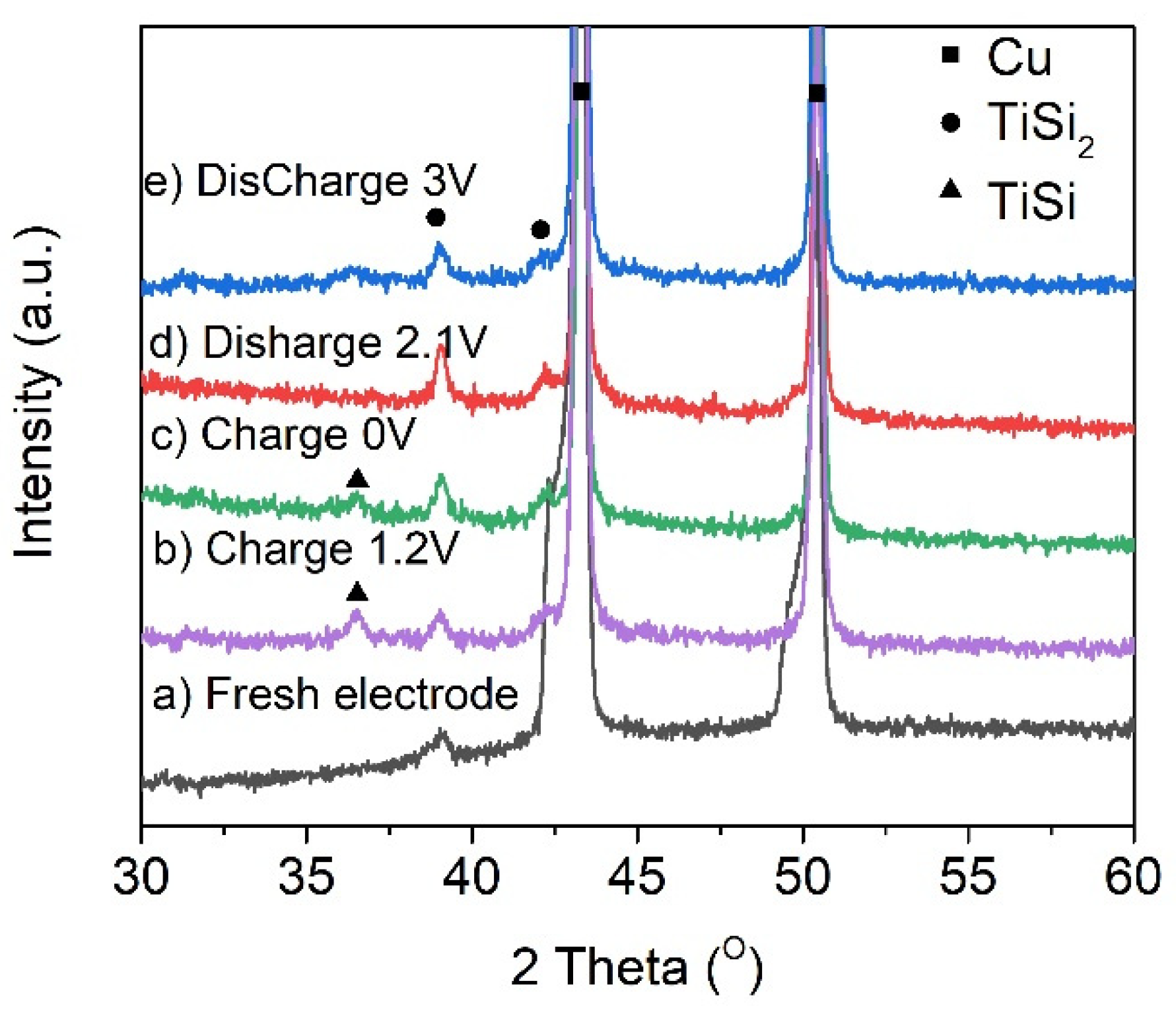
| Materials | Electrolyte | Specific Capacity (mAh/g) | Capacity Retention/Cycles | Ref. |
|---|---|---|---|---|
| CrSi2 | 1 M LiFSA/Py13-FSA | 240 (50 mA/g) | 89%/300 | [42] |
| FeSi2 | 1 M LiFSA/Py13-FSA | 630 (50 mA/g) | 85%/300 | [42] |
| FeSi2 | 1 M LiTFSA/PC | 700 (50 mA/g) | 15%/300 | [42] |
| NiSi2 | 1 M LiFSA/Py13-FSA | 820 (50 mA/g) | 58%/250 | [42] |
| Nano-NiSi2 | 1 M LiPF6/EC-DC-FEC | 313 (87.5 mA/g) | ~88%/60 | [43] |
| MoSi2 | 1 M LiPF6/EC-DMC-EMC | 153 (134 mA/g) | 127%/100 | [41] |
| MoSi2 | 1 M LiPF6/EC-DMC | ~110 (67 mA/g) | - | [15] |
| TiSi2/rGO | 1 M LiPF6/EC-DMC-EMC | 378 (50 mA/g) | 136%/600 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.; Jin, M.; Shi, X.; Li, Q.; Gan, C.; Yao, W. Preparation of TiSi2 Powders with Enhanced Lithium-Ion Storage via Chemical Oven Self-Propagating High-Temperature Synthesis. Nanomaterials 2021, 11, 2279. https://doi.org/10.3390/nano11092279
Xu J, Jin M, Shi X, Li Q, Gan C, Yao W. Preparation of TiSi2 Powders with Enhanced Lithium-Ion Storage via Chemical Oven Self-Propagating High-Temperature Synthesis. Nanomaterials. 2021; 11(9):2279. https://doi.org/10.3390/nano11092279
Chicago/Turabian StyleXu, Jianguang, Menglan Jin, Xinlu Shi, Qiuyu Li, Chengqiang Gan, and Wei Yao. 2021. "Preparation of TiSi2 Powders with Enhanced Lithium-Ion Storage via Chemical Oven Self-Propagating High-Temperature Synthesis" Nanomaterials 11, no. 9: 2279. https://doi.org/10.3390/nano11092279
APA StyleXu, J., Jin, M., Shi, X., Li, Q., Gan, C., & Yao, W. (2021). Preparation of TiSi2 Powders with Enhanced Lithium-Ion Storage via Chemical Oven Self-Propagating High-Temperature Synthesis. Nanomaterials, 11(9), 2279. https://doi.org/10.3390/nano11092279






