Carbon Dioxide-Derived Biodegradable and Cationic Polycarbonates as a New siRNA Carrier for Gene Therapy in Pancreatic Cancer
Abstract
:1. Introduction
2. Materials and Methods
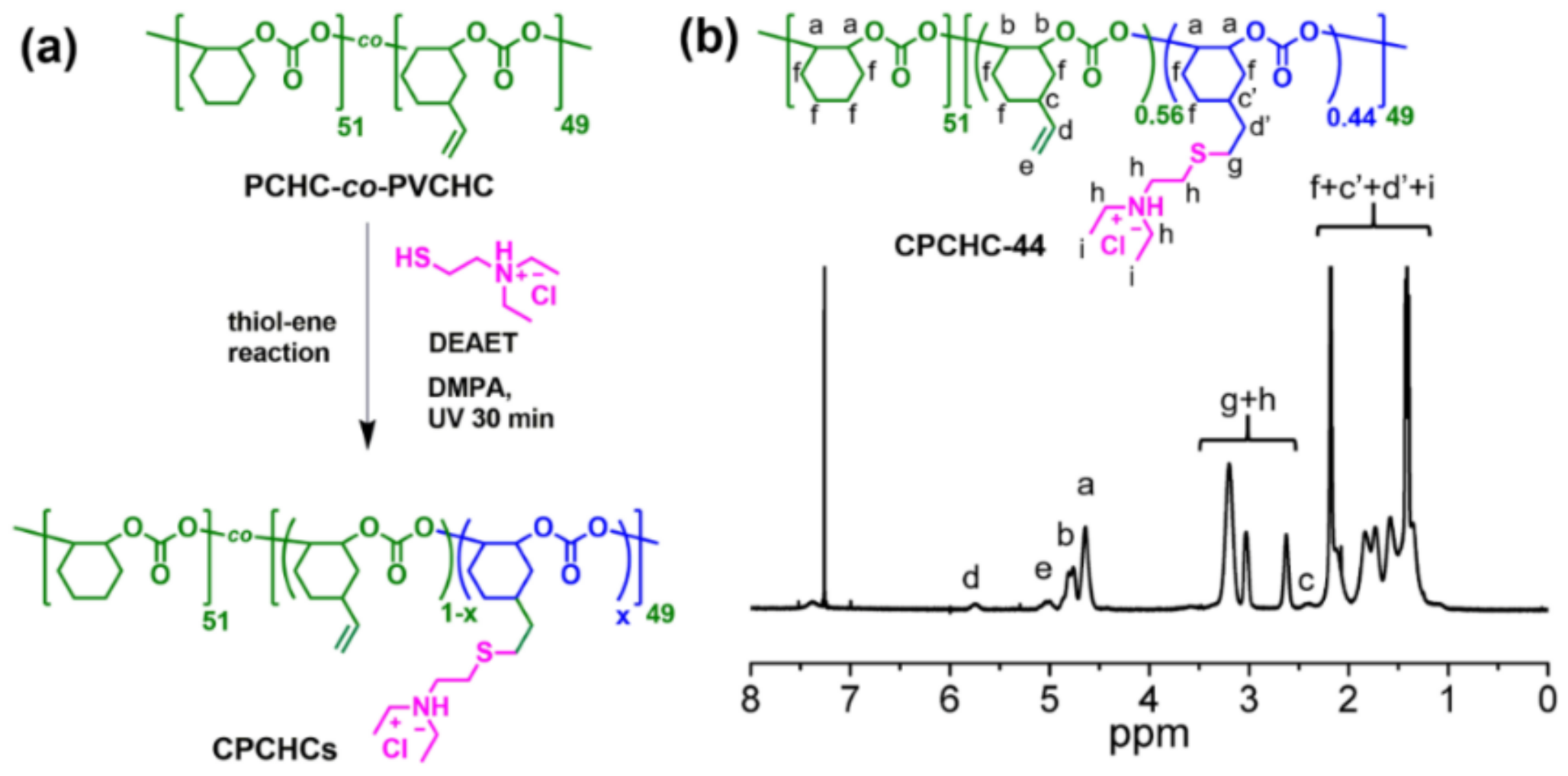
2.1. Synthesis and Characterization of CPCHC-44
2.2. Agarose Gel Retardation Assay
2.3. The Formation and Characterization of CPCHC-44/siRNA NPs
2.4. Serum Enzymatic Protection Assay
2.5. The Cytotoxicity Evaluation of CPCHC-44
2.6. Cell Culture
2.7. siRNA Transfection
2.8. Confocal Imaging
2.9. The Evaluation of Transfection Efficiency
2.10. Gene Expression Assay
2.11. The Assessment of Cell Proliferation and Migration
2.12. Analysis of Apoptosis
2.13. The Formation of 3D Tumor Spheroid Model
2.14. Blood Test of CPCHC-44 NPs
2.15. Xenograft Tumor Model Study
2.16. Statistical Analysis
3. Results and Discussion
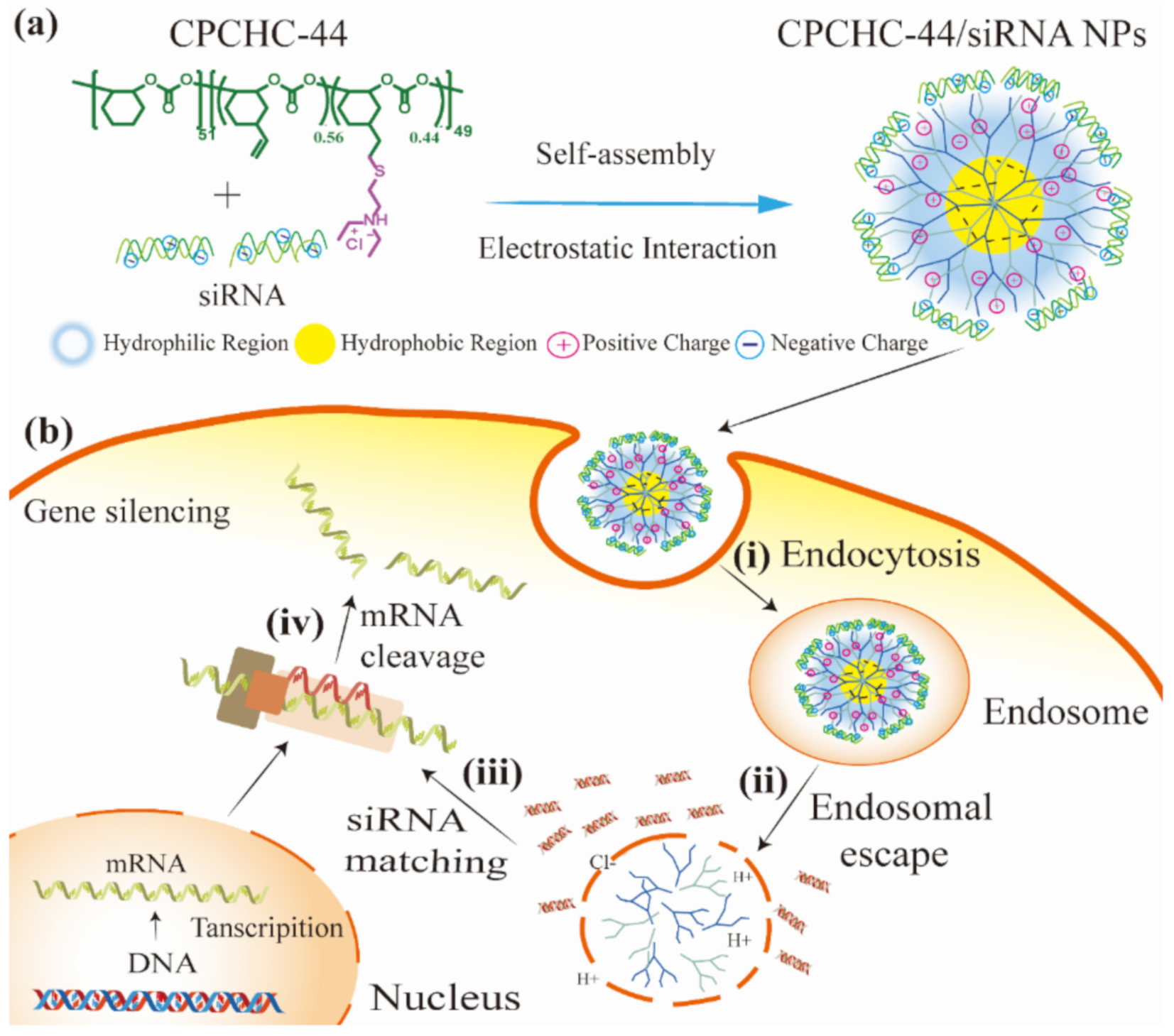
3.1. Synthesis and Characterization of CPCHC-44
3.2. Cytotoxicity of CPCHC-44/siRNA NPs
3.3. Preparation and Characterization of CPCHC-44/siRNA NPs
3.4. Intracellular Uptake and Transfection Efficiency of siRNA
3.5. Endosomal Escape of siRNA Assisted by CPCHC-44
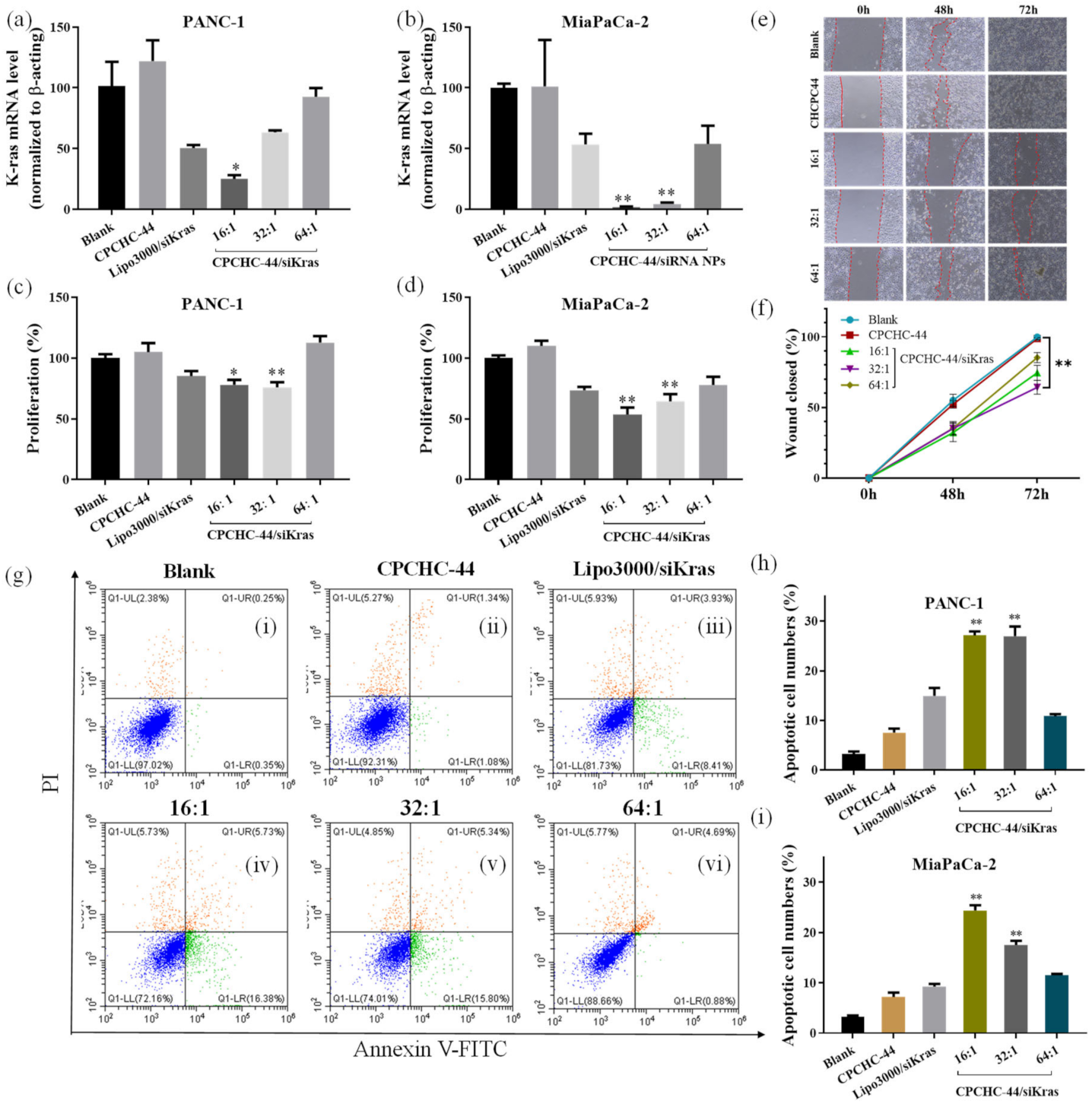
3.6. The Inhibition Effect of Kras Gene Transcription
3.7. Therapeutic Effects of CPCHC-44/siRNA NPs
3.8. The Penetration Ability of CPCHC-44 NPs on 3D Tumor Spheroid Model
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Christenson, E.S.; Jaffee, E.; Azad, N.S. Current and emerging therapies for patients with advanced pancreatic ductal adeno-carcinoma: A bright future. Lancet Oncol. 2020, 21, e135–e145. [Google Scholar] [CrossRef]
- Yoshida, T.; Ohnami, S.; Aoki, K. Development of gene therapy to target pancreatic cancer. Cancer Sci. 2004, 95, 283–289. [Google Scholar] [CrossRef] [Green Version]
- Pei, Y.; Chen, L.; Huang, Y.; Wang, J.; Feng, J.; Xu, M.; Chen, Y.; Song, Q.; Jiang, G.; Gu, X.; et al. Sequential targeting TGF-beta signaling and KRAS mutation increases therapeutic efficacy in pancreatic cancer. Small 2019, 15, e1900631. [Google Scholar] [CrossRef] [PubMed]
- Moore, A.R.; Rosenberg, S.C.; McCormick, F.; Malek, S. RAS-targeted therapies: Is the undruggable drugged? Nat. Rev. Drug Discov. 2020, 19, 533–552. [Google Scholar] [CrossRef] [PubMed]
- Buscail, L.; Bournet, B.; Cordelier, P. Role of oncogenic KRAS in the diagnosis, prognosis and treatment of pancreatic cancer. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 153–168. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Wang, Y.; Li, X. Targeting the untargetable KRAS in cancer therapy. Acta Pharm. Sin. B 2019, 9, 871–879. [Google Scholar] [CrossRef]
- Aslan, M.; Shahbazi, R.; Ulubayram, K.; Ozpolat, B. Targeted therapies for pancreatic cancer and hurdles ahead. Anticancer Res. 2018, 38, 6591–6606. [Google Scholar] [CrossRef] [Green Version]
- Ryan, M.B.; Corcoran, R.B. Therapeutic strategies to target RAS-mutant cancers. Nat. Rev. Clin. Oncol. 2018, 15, 709–720. [Google Scholar] [CrossRef]
- Kessler, D.; Gmachl, M.; Mantoulidis, A.; Martin, L.J.; Zoephel, A.; Mayer, M.; Gollner, A.; Covini, D.; Fischer, S.; Gerstberger, T.; et al. Drugging an undruggable pocket on KRAS. Proc. Natl. Acad. Sci. USA 2019, 116, 15823–15829. [Google Scholar] [CrossRef] [Green Version]
- Hamarsheh, S.a.; Groß, O.; Brummer, T.; Zeiser, R. Immune modulatory effects of oncogenic KRAS in cancer. Nat. Commun. 2020, 11, 5439. [Google Scholar] [CrossRef]
- Titze-de-Almeida, R.; David, C.; Titze-de-Almeida, S.S. The race of 10 synthetic rnai-based drugs to the pharmaceutical market. Pharm. Res. 2017, 34, 1339–1363. [Google Scholar] [CrossRef]
- Kim, B.; Park, J.-H.; Sailor, M.J. Rekindling RNAi therapy: Materials design requirements for in vivo sirna delivery. Adv. Mater. 2019, 31, 1903637. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.; Yang, G.; Ouyang, Q.; Kuang, S.; Song, P.; Xu, G.; Poenar, D.; Zhu, G.; Yong, K.; Wang, Z.J.N.E. Nanowire-array-based gene electro-transfection system driven by human-motion operated triboelectric nanogenerator. Nano Energy 2019, 64, 103901. [Google Scholar] [CrossRef]
- Saw, P.E.; Song, E.W. siRNA therapeutics: A clinical reality. Sci. China Life Sci. 2020, 63, 485–500. [Google Scholar] [CrossRef]
- Yang, C.; Chan, K.K.; Lin, W.-J.; Soehartono, A.M.; Lin, G.; Toh, H.; Yoon, H.S.; Chen, C.-K.; Yong, K.-T. Biodegradable nanocarriers for small interfering ribonucleic acid (siRNA) co-delivery strategy increase the chemosensitivity of pancreatic cancer cells to gemcitabine. Nano Res. 2017, 10, 3049–3067. [Google Scholar] [CrossRef]
- Yang, C.; Yin, M.; Xu, G.; Lin, W.J.; Chen, J.; Zhang, Y.; Feng, T.; Huang, P.; Chen, C.K.; Yong, K.T. Biodegradable polymers as a noncoding miRNA nanocarrier for multiple targeting therapy of human hepatocellular carcinoma. Adv. Healthc. Mater. 2019, 8, e1801318. [Google Scholar] [CrossRef]
- Setten, R.L.; Rossi, J.J.; Han, S.P. The current state and future directions of RNAi-based therapeutics. Nat. Rev. Drug Discovery 2019, 18, 421–446. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Kanasty, R.L.; Eltoukhy, A.A.; Vegas, A.J.; Dorkin, J.R.; Anderson, D.G. Non-viral vectors for gene-based therapy. Nat. Rev. Genet. 2014, 15, 541–555. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.E.; Ehrhardt, A.; Kay, M.A. Progress and problems with the use of viral vectors for gene therapy. Nat. Rev. Genet. 2003, 4, 346–358. [Google Scholar] [CrossRef]
- Chen, C.K.; Huang, P.K.; Law, W.C.; Chu, C.H.; Chen, N.T.; Lo, L.W. Biodegradable polymers for gene-delivery applications. Int. J. Nanomed. 2020, 15, 2131–2150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, T.J.; Tajmir-Riahi, H.A.; Pillai, C.K.S. Biodegradable polymers for gene delivery. Molecules 2019, 24, 3744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hosseinkhani, H.; Domb, A.J. Biodegradable polymers in gene-silencing technology. Polym. Adv. Technol. 2019, 30, 2647–2655. [Google Scholar] [CrossRef]
- Yang, C.; Panwar, N.; Wang, Y.; Zhang, B.; Liu, M.; Toh, H.; Yoon, H.S.; Tjin, S.C.; Chong, P.H.; Law, W.C.; et al. Biodegradable charged polyester-based vectors (BCPVs) as an efficient non-viral transfection nanoagent for gene knockdown of the BCR-ABL hybrid oncogene in a human chronic myeloid leukemia cell line. Nanoscale 2016, 8, 9405–9416. [Google Scholar] [CrossRef]
- Varkouhi, A.K.; Lammers, T.; Schiffelers, R.M.; van Steenbergen, M.J.; Hennink, W.E.; Storm, G. Gene silencing activity of siRNA polyplexes based on biodegradable polymers. Eur. J. Pharm. Biopharm. 2011, 77, 450–457. [Google Scholar] [CrossRef]
- Park, T.G.; Jeong, J.H.; Kim, S.W. Current status of polymeric gene delivery systems. Adv. Drug Deliv. Rev. 2006, 58, 467–486. [Google Scholar] [CrossRef]
- Jones, C.H.; Chen, C.K.; Chen, M.F.; Ravikrishnan, A.; Zhang, H.G.; Gollakota, A.; Chung, T.C.; Cheng, C.; Pfeifer, B.A. PEGylated cationic polylactides for hybrid biosynthetic gene delivery. Mol. Pharmaceutics 2015, 12, 846–856. [Google Scholar] [CrossRef]
- Sabir, M.I.; Xu, X.X.; Li, L. A review on biodegradable polymeric materials for bone tissue engineering applications. J. Mater. Sci. 2009, 44, 5713–5724. [Google Scholar] [CrossRef]
- Zhang, Z.; Kuijer, R.; Bulstra, S.K.; Grijpma, D.W.; Feijen, J. The in vivo and in vitro degradation behavior of poly(trimethylene carbonate). Biomaterials 2006, 27, 1741–1748. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Meng, F.H.; Cheng, R.; Deng, C.; Feijen, J.; Zhong, Z.Y. Advanced drug and gene delivery systems based on functional biodegradable polycarbonates and copolymers. J. Control. Release 2014, 190, 398–414. [Google Scholar] [CrossRef] [PubMed]
- Scharfenberg, M.; Hilf, J.; Frey, H. Functional polycarbonates from carbon dioxide and tailored epoxide monomers: Degradable materials and their application potential. Adv. Funct. Mater. 2018, 28, 1704302. [Google Scholar] [CrossRef]
- Li, Y.J.; Shimizu, H. Compatibilization by homopolymer: Significant improvements in the modulus and tensile strength of PPC/PMMA blends by the addition of a small amount of PVAc. ACS Appl. Mater. Interfaces 2009, 1, 1650–1655. [Google Scholar] [CrossRef]
- Liu, G.-L.; Wu, H.-W.; Lin, Z.-I.; Liao, M.-G.; Su, Y.-C.; Chen, C.-K.; Ko, B.-T. Synthesis of functional CO2-based polycarbonates via dinuclear nickel nitrophenolate-based catalysis for degradable surfactant and drug-loaded nanoparticle applications. Polym. Chem. 2021, 12, 1244–1259. [Google Scholar] [CrossRef]
- Jiang, H.; Liu, X.; Knolhoff, B.L.; Hegde, S.; Lee, K.B.; Jiang, H.; Fields, R.C.; Pachter, J.A.; Lim, K.H.; DeNardo, D.G. Development of resistance to FAK inhibition in pancreatic cancer is linked to stromal depletion. Gut 2020, 69, 122–132. [Google Scholar] [CrossRef]
- Yang, C.; Chan, K.K.; Xu, G.; Yin, M.; Lin, G.; Wang, X.; Lin, W.J.; Birowosuto, M.D.; Zeng, S.; Ogi, T.; et al. Biodegradable polymer-coated multifunctional graphene quantum dots for light-triggered synergetic therapy of pancreatic cancer. ACS Appl. Mater. Interfaces 2019, 11, 2768–2781. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.; Chen, C.K.; Yin, F.; Yang, C.; Tian, J.; Chen, T.; Xu, G.; He, C.; Lin, M.C.; Wang, J.; et al. Biodegradable nanoparticles as siRNA carriers for in vivo gene silencing and pancreatic cancer therapy. J. Mater. Chem. B 2017, 5, 3327–3337. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Hu, R.; Anderson, T.; Wang, Y.; Lin, G.; Law, W.C.; Lin, W.J.; Nguyen, Q.T.; Toh, H.T.; Yoon, H.S.; et al. Biodegradable nanoparticle-mediated K-ras down regulation for pancreatic cancer gene therapy. J. Mater. Chem. B 2015, 3, 2163–2172. [Google Scholar] [CrossRef]
- Lin, G.; Hu, R.; Law, W.C.; Chen, C.K.; Wang, Y.; Li Chin, H.; Nguyen, Q.T.; Lai, C.K.; Yoon, H.S.; Wang, X.; et al. Biodegradable nanocapsules as siRNA carriers for mutant K-Ras gene silencing of human pancreatic carcinoma cells. Small 2013, 9, 2757–2763. [Google Scholar] [CrossRef] [PubMed]
- Fleming, J.B.; Shen, G.L.; Holloway, S.E.; Davis, M.; Brekken, R.A. Molecular consequences of silencing mutant K-ras in pancreatic cancer cells: Justification for K-ras-directed therapy. Mol. Cancer Res. 2005, 3, 413–423. [Google Scholar] [CrossRef] [Green Version]
- Takei, K.; Haucke, V. Clathrin-mediated endocytosis: Membrane factors pull the trigger. Trends Cell Biol. 2001, 11, 385–391. [Google Scholar] [CrossRef]
- Yang, C.; Mo, X.; Lv, J.; Liu, X.; Yuan, M.; Dong, M.; Li, L.; Luo, X.; Fan, X.; Jin, Z.; et al. Lipopolysaccharide enhances FcεRI-mediated mast cell degranulation by increasing Ca2+ entry through store-operated Ca2+ channels: Implications for lipopolysaccharide exacerbating allergic asthma. Exp. Physiol. 2012, 97, 1315–1327. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-H.; Tsai, C.-Y.; Lin, W.-J.; Su, Y.-C.; Chuang, H.-J.; Liu, W.-L.; Chen, C.-T.; Chen, C.-K.; Ko, B.-T. Alternating copolymerization of epoxides with carbon dioxide or cyclic anhydrides using bimetallic nickel and cobalt catalysts: Preparation of hydrophilic nanofibers from functionalized polyesters. Polymer 2018, 141, 1–11. [Google Scholar] [CrossRef]
- Chen, C.K.; Lin, W.J.; Hsia, Y.; Lo, L.W. Synthesis of polylactide-based core-shell interface cross-linked micelles for anticancer drug delivery. Macromol. Biosci. 2017, 17, 1600191. [Google Scholar] [CrossRef]
- Hickey, J.W.; Santos, J.L.; Williford, J.M.; Mao, H.Q. Control of polymeric nanoparticle size to improve therapeutic delivery. J. Control. Release 2015, 219, 536–547. [Google Scholar] [CrossRef] [Green Version]
- Cabral, H.; Matsumoto, Y.; Mizuno, K.; Chen, Q.; Murakami, M.; Kimura, M.; Terada, Y.; Kano, M.R.; Miyazono, K.; Uesaka, M.; et al. Accumulation of sub-100 nm polymeric micelles in poorly permeable tumours depends on size. Nat. Nanotechnol. 2011, 6, 815–823. [Google Scholar] [CrossRef]
- Gao, Y.; Jia, L.; Wang, Q.; Hu, H.; Zhao, X.; Chen, D.; Qiao, M. pH/Redox Dual-responsive polyplex with effective endosomal escape for codelivery of siRNA and doxorubicin against drug-resistant cancer cells. ACS Appl. Mater. Interfaces 2019, 11, 16296–16310. [Google Scholar] [CrossRef] [PubMed]
- Hosseinahli, N.; Aghapour, M.; Duijf, P.H.G.; Baradaran, B. Treating cancer with microRNA replacement therapy: A literature review. J. Cell. Physiol. 2018, 233, 5574–5588. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Jiang, Y.; Peng, H.; Chen, Y.; Zhu, P.; Huang, Y. Recent progress in microRNA delivery for cancer therapy by non-viral synthetic vectors. Adv. Drug Deliv. Rev. 2015, 81, 142–160. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Lin, Z.-I.; Yang, J.; Liu, G.-L.; Hu, Z.; Huang, H.; Li, X.; Liu, Q.; Ma, M.; Xu, Z.; et al. Carbon Dioxide-Derived Biodegradable and Cationic Polycarbonates as a New siRNA Carrier for Gene Therapy in Pancreatic Cancer. Nanomaterials 2021, 11, 2312. https://doi.org/10.3390/nano11092312
Zhang X, Lin Z-I, Yang J, Liu G-L, Hu Z, Huang H, Li X, Liu Q, Ma M, Xu Z, et al. Carbon Dioxide-Derived Biodegradable and Cationic Polycarbonates as a New siRNA Carrier for Gene Therapy in Pancreatic Cancer. Nanomaterials. 2021; 11(9):2312. https://doi.org/10.3390/nano11092312
Chicago/Turabian StyleZhang, Xinmeng, Zheng-Ian Lin, Jingyu Yang, Guan-Lin Liu, Zulu Hu, Haoqiang Huang, Xiang Li, Qiqi Liu, Mingze Ma, Zhourui Xu, and et al. 2021. "Carbon Dioxide-Derived Biodegradable and Cationic Polycarbonates as a New siRNA Carrier for Gene Therapy in Pancreatic Cancer" Nanomaterials 11, no. 9: 2312. https://doi.org/10.3390/nano11092312
APA StyleZhang, X., Lin, Z.-I., Yang, J., Liu, G.-L., Hu, Z., Huang, H., Li, X., Liu, Q., Ma, M., Xu, Z., Xu, G., Yong, K.-T., Tsai, W.-C., Tsai, T.-H., Ko, B.-T., Chen, C.-K., & Yang, C. (2021). Carbon Dioxide-Derived Biodegradable and Cationic Polycarbonates as a New siRNA Carrier for Gene Therapy in Pancreatic Cancer. Nanomaterials, 11(9), 2312. https://doi.org/10.3390/nano11092312






