Facile Sulfurization under Ambient Condition with Na2S to Fabricate Nanostructured Copper Sulfide
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Sulfurization of Cu Foils
2.3. Materials Characterization
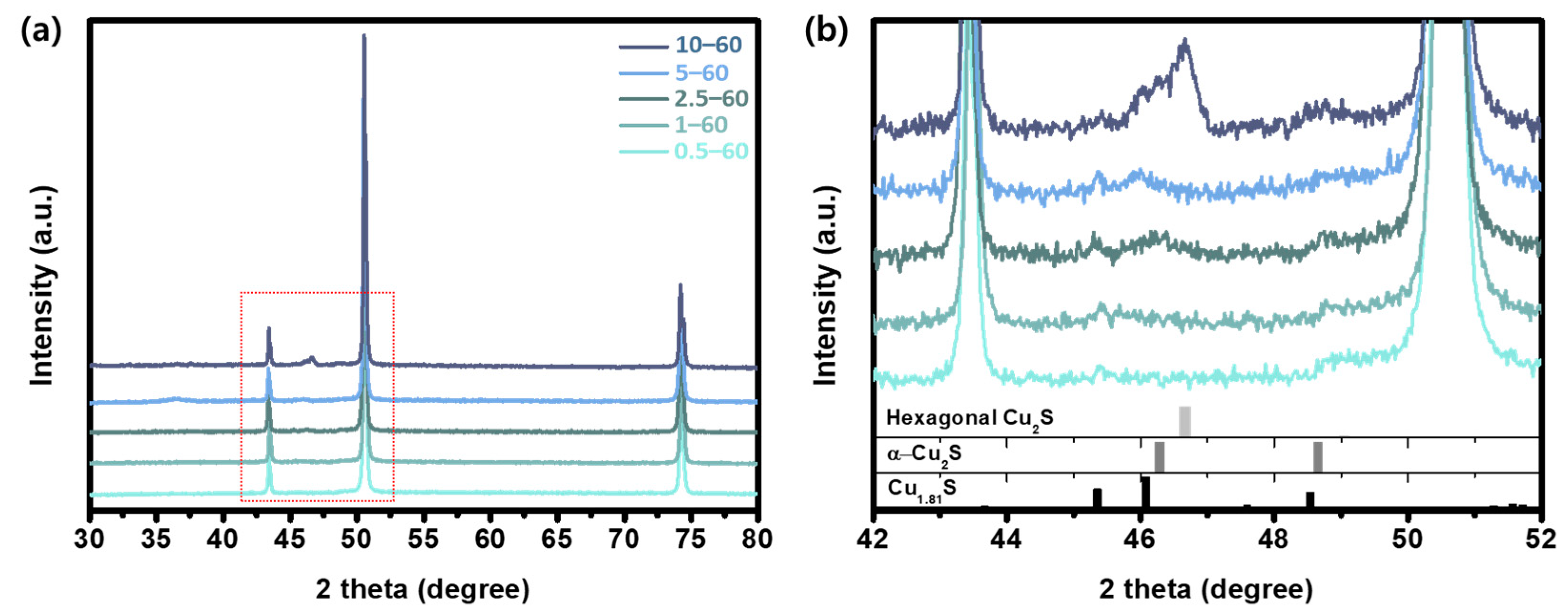
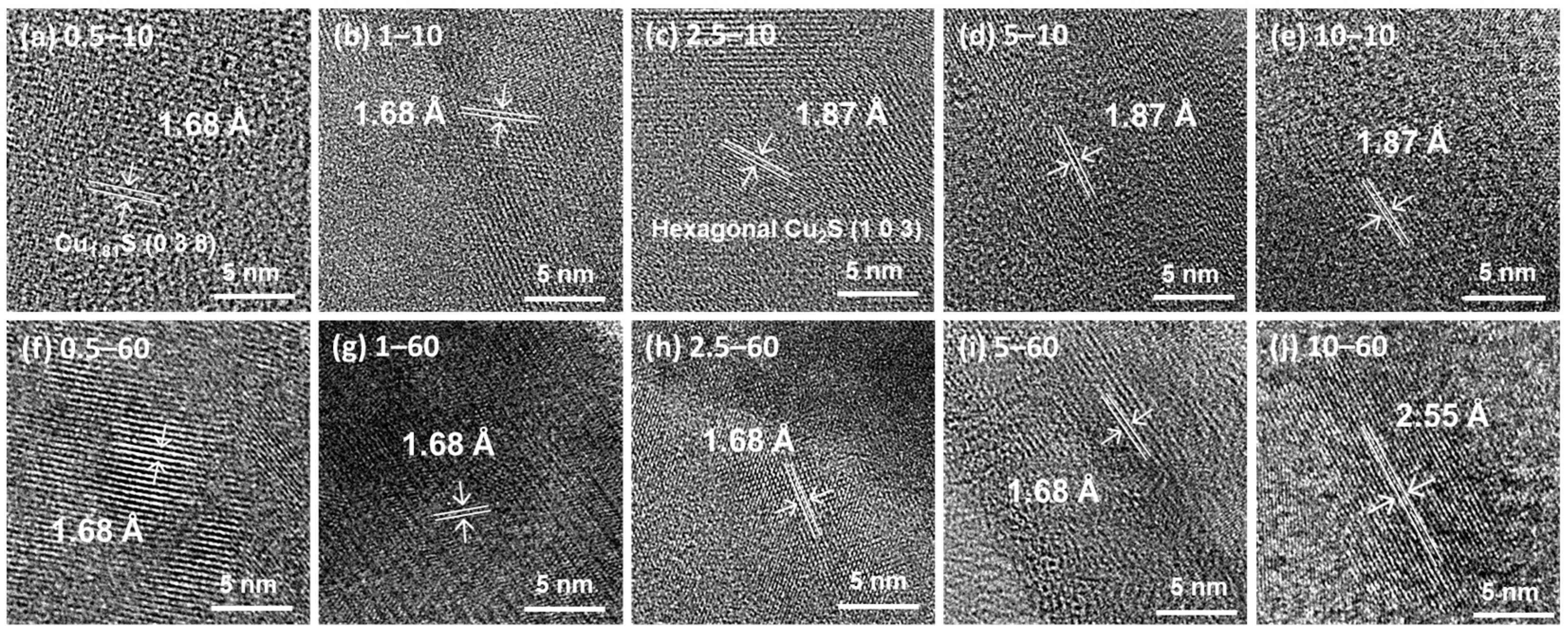
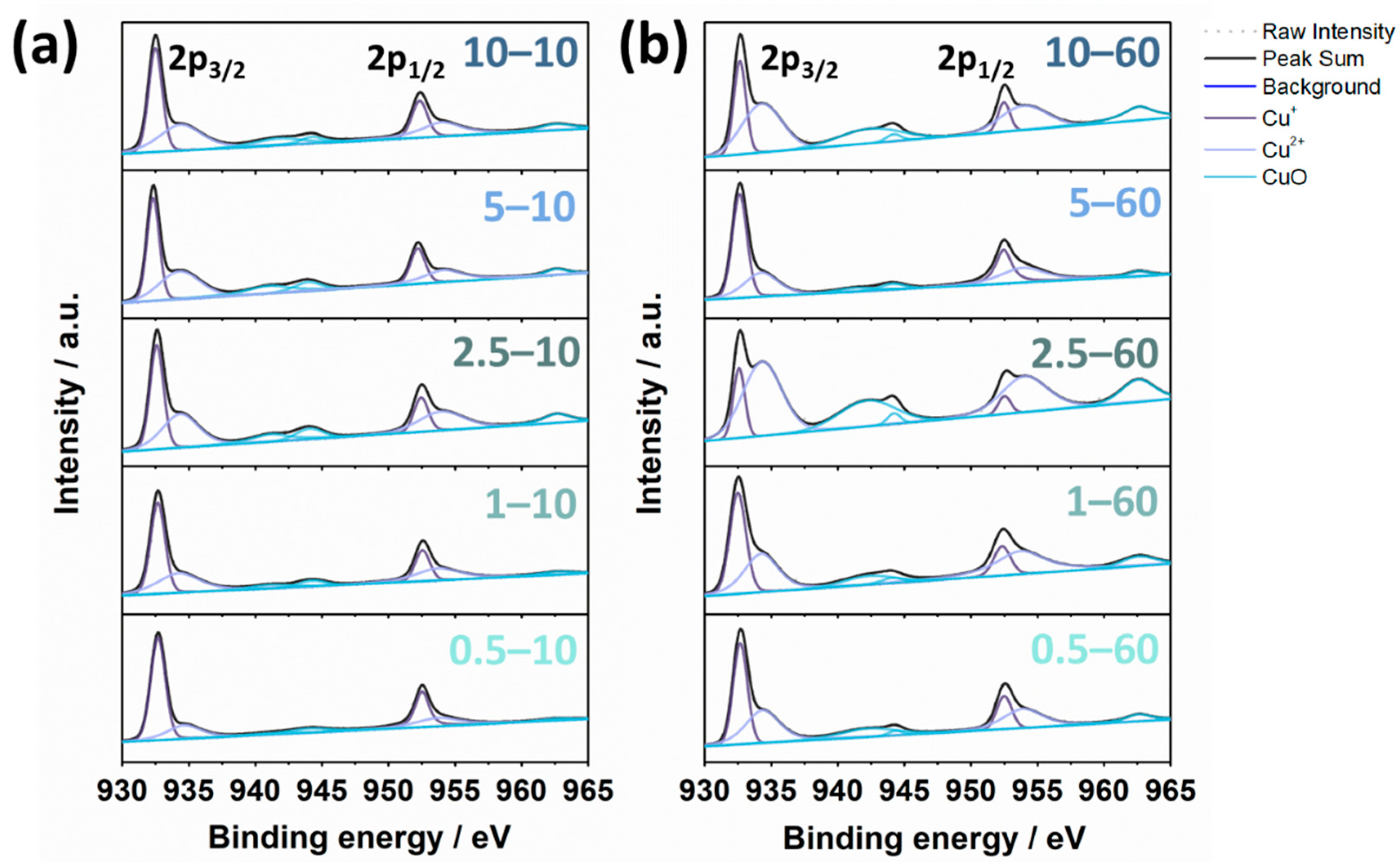
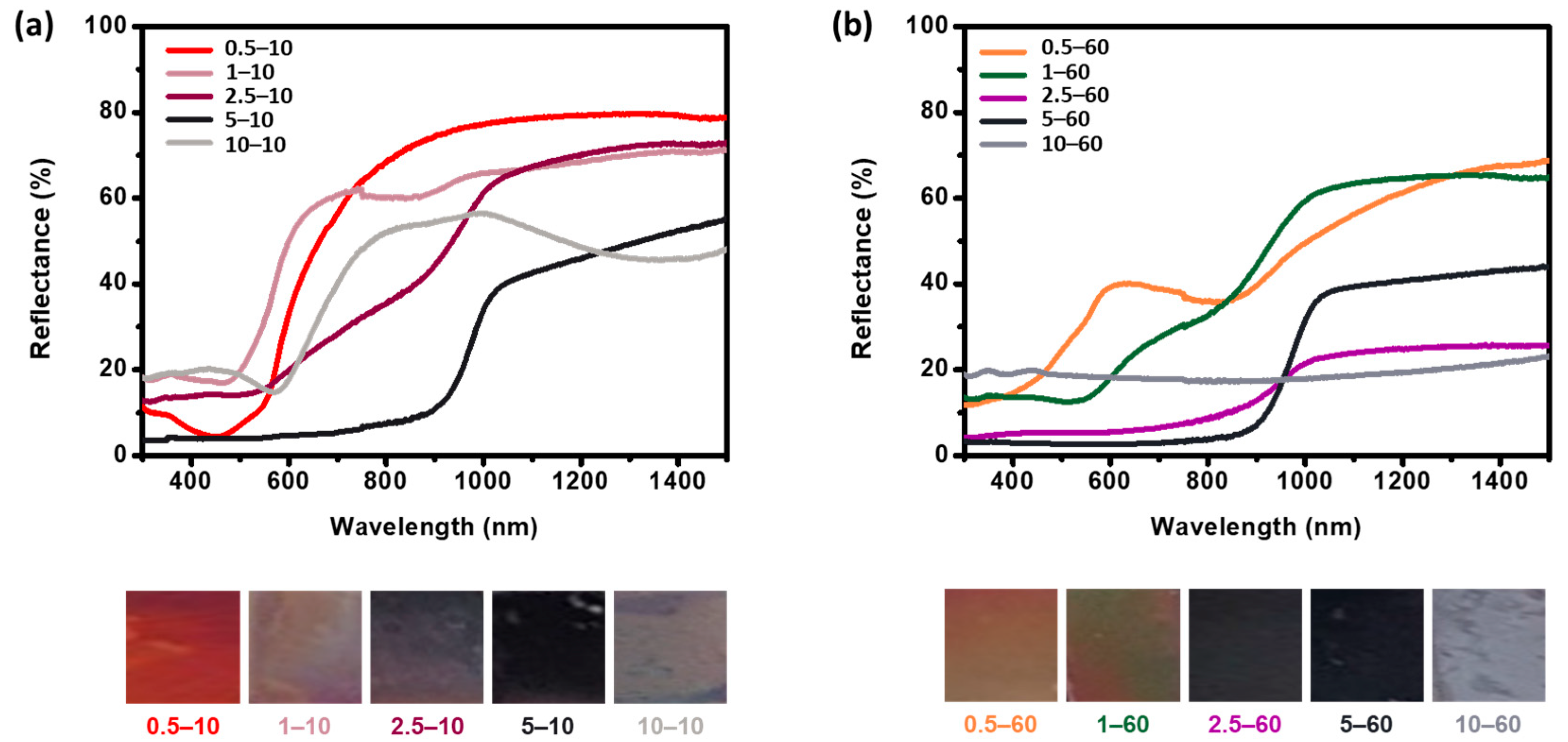
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mohamed, S.H. Photocatalytic, optical and electrical properties of copper-doped zinc sulfide thin films. J. Phys. D Appl. Phys. 2010, 43, 035406. [Google Scholar] [CrossRef]
- Leventis, H.C.; King, S.P.; Sudlow, A.; Hill, M.S.; Molloy, K.C.; Haque, S.A. Nanostructured hybrid polymer−inorganic solar cell active layers formed by controllable in situ growth of semiconducting sulfide networks. Nano Lett. 2010, 10, 1253–1258. [Google Scholar] [CrossRef]
- Salavati-Niasari, M.; Alizadeh, S.; Mousavi-Kamazani, M.; Mir, N.; Rezaei, O.; Ahmadi, E. Surfactant-free fabrication of copper sulfides (CuS, Cu2S) via hydrothermal method. J. Cluster Sci. 2013, 24, 1181–1191. [Google Scholar] [CrossRef]
- Sun, S.; Li, P.; Liang, S.; Yang, Z. Diversified copper sulfide (Cu2−xS) micro-/nanostructures: A comprehensive review on synthesis, modifications and applications. Nanoscale 2017, 9, 11357–11404. [Google Scholar] [CrossRef]
- Sun, Q.; Wang, Y.A.; Li, L.S.; Wang, D.; Zhu, T.; Xu, J.; Yang, C.; Li, Y. Bright, multicoloured light-emitting diodes based on quantum dots. Nat. Photonics 2007, 1, 717–722. [Google Scholar] [CrossRef]
- Minnam Reddy, V.R.; Gedi, S.; Park, C.; R.W., M.; K.T., R.R. Development of sulphurized SnS thin film solar cells. Curr. Appl. Phys. 2015, 15, 588–598. [Google Scholar] [CrossRef]
- Hajimazdarani, M.; Ghasali, E.; Naderi, N.; Orooji, Y. Enhanced optical properties and photodetection behavior of ZnS thin film deposited by electron beam evaporation upon doping with europium oxide. Ceram. Int. 2020, 46, 28382–28389. [Google Scholar] [CrossRef]
- Madarász, J.; Bombicz, P.; Okuya, M.; Kaneko, S. Thermal decomposition of thiourea complexes of Cu(I), Zn(II), and Sn(II) chlorides as precursors for the spray pyrolysis deposition of sulfide thin films. Solid State Ionics 2001, 141–142, 439–446. [Google Scholar] [CrossRef]
- Loranca-Ramos, F.E.; Diliegros-Godines, C.J.; Silva González, R.; Pal, M. Structural, optical and electrical properties of copper antimony sulfide thin films grown by a citrate-assisted single chemical bath deposition. Appl. Surf. Sci. 2018, 427, 1099–1106. [Google Scholar] [CrossRef]
- Aldalbahi, A.; Mkawi, E.M.; Ibrahim, K.; Farrukh, M.A. Effect of sulfurization time on the properties of copper zinc tin sulfide thin films grown by electrochemical deposition. Sci. Rep. 2016, 6, 32431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sobhani, A.; Salavati-Niasari, M. Synthesis, characterization, optical and magnetic properties of a nickel sulfide series by three different methods. Superlattices Microstruct. 2013, 59, 1–12. [Google Scholar] [CrossRef]
- Biswas, S.; Kar, S.; Chaudhuri, S. Optical and magnetic properties of manganese-incorporated zinc sulfide nanorods synthesized by a solvothermal process. J. Phys. Chem. B 2005, 109, 17526–17530. [Google Scholar] [CrossRef]
- Dhas, N.A.; Zaban, A.; Gedanken, A. Surface synthesis of zinc sulfide nanoparticles on silica microspheres: Sonochemical preparation, characterization, and optical properties. Chem. Mater. 1999, 11, 806–813. [Google Scholar] [CrossRef]
- Lebedev, M.V.; Lvova, T.V.; Sedova, I.V. Coordination of the chemical and electronic processes in GaSb(100) surface modification with aqueous sodium sulfide solution. J. Mater. Chem. C 2018, 6, 5760–5768. [Google Scholar] [CrossRef]
- Thalmann, B.; Voegelin, A.; Sinnet, B.; Morgenroth, E.; Kaegi, R. Sulfidation kinetics of silver nanoparticles reacted with metal sulfides. Environ. Sci. Technol. 2014, 48, 4885–4892. [Google Scholar] [CrossRef] [PubMed]
- Fan, D.; Lan, Y.; Tratnyek, P.G.; Johnson, R.L.; Filip, J.; O’Carroll, D.M.; Nunez Garcia, A.; Agrawal, A. Sulfidation of Iron-Based Materials: A Review of Processes and Implications for Water Treatment and Remediation. Environ. Sci. Technol. 2017, 51, 13070–13085. [Google Scholar] [CrossRef]
- Ceylan, A. Synthesis of SnS thin films via high vacuum sulfidation of sputtered Sn thin films. Mater. Lett. 2017, 201, 194–197. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, B.; Wei, L. Sulfidation growth and characterization of nanocrystalline ZnS thin films. Vacuum 2008, 82, 1208–1211. [Google Scholar] [CrossRef]
- Mrowec, S.; Stokłosa, A.; Danielewski, M. A new thermobalance for studying the kinetics of high-temperature sulfidation of metals. Oxid. Met. 1977, 11, 355–363. [Google Scholar] [CrossRef]
- Larson, R.S. A Physical and mathematical model for the atmospheric sulfidation of copper by hydrogen sulfide. J. Electrochem. Soc. 2002, 149, B40. [Google Scholar] [CrossRef]
- Kang, M.-G.; Park, H.-H. Effect of prepared GaAs surface on the sulfidation with (NH4)2Sx solution. J. Vac. Sci. Technol. A 1999, 17, 88–92. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Z. Hydrothermal synthesis and optical properties of CuS nanoplates. Mater. Lett. 2008, 62, 2279–2281. [Google Scholar] [CrossRef]
- Malghan, S.G. Role of sodium sulfide in the flotation of oxidized copper, lead, and zinc ores. Min. Metall. Explor. 1986, 3, 158–163. [Google Scholar] [CrossRef]
- Zhang, Y.C.; Qiao, T.; Ya Hu, X. A simple hydrothermal route to nanocrystalline CuS. J. Cryst. Growth 2004, 268, 64–70. [Google Scholar] [CrossRef]
- Chen, L.; Sakamoto, M.; Haruta, M.; Nemoto, T.; Sato, R.; Kurata, H.; Teranishi, T. Tin Ion Directed Morphology Evolution of Copper Sulfide Nanoparticles and Tuning of Their Plasmonic Properties via Phase Conversion. Langmuir 2016, 32, 7582–7587. [Google Scholar] [CrossRef] [PubMed]
- Ren, K.; Yin, P.; Zhou, Y.; Cao, X.; Dong, C.; Cui, L.; Liu, H.; Du, X. Localized defects on copper sulfide surface for enhanced plasmon resonance and water splitting. Small 2017, 13, 1700867. [Google Scholar] [CrossRef] [PubMed]
- Goel, S.; Chen, F.; Cai, W. Synthesis and biomedical applications of copper sulfide nanoparticles: From sensors to theranostics. Small 2014, 10, 631–645. [Google Scholar] [CrossRef]
- Toe, C.Y.; Zheng, Z.; Wu, H.; Scott, J.; Amal, R.; Ng, Y.H. Transformation of cuprous oxide into hollow copper sulfide cubes for photocatalytic hydrogen generation. J. Phys. Chem. C 2018, 122, 14072–14081. [Google Scholar] [CrossRef]
- Marimuthu, T.; Anandhan, N.; Panneerselvam, R.; Ganesan, K.P.; Roselin, A.A. Synthesis and characterization of copper sulfide thin films for quantum dot sensitized solar cell and supercapacitor applications. Nano-Struct. Nano-Objects 2019, 17, 138–147. [Google Scholar] [CrossRef]
- Grozdanov, I.; Najdoski, M. Optical and electrical properties of copper sulfide films of variable composition. J. Solid State Chem. 1995, 114, 469–475. [Google Scholar] [CrossRef]
- Das, S.R.; Vankar, V.D.; Nath, P.; Chopra, K.L. The preparation of Cu2S films for solar cells. Thin Solid Films 1978, 51, 257–264. [Google Scholar] [CrossRef]
- Cuevas, A.; Romero, R.; Leinen, D.; Dalchiele, E.A.; Ramos-Barrado, J.R.; Martin, F. Effect of the stoichiometry of CuxS thin films on the optical and electrical properties and the solar thermal performance. Sol. Energy Mater. Sol. Cells 2015, 134, 199–208. [Google Scholar] [CrossRef]
- Fu, W.; Liu, M.; Xue, F.; Wang, X.; Diao, Z.; Guo, L. Facile polyol synthesis of CuS nanocrystals with a hierarchical nanoplate structure and their application for electrocatalysis and photocatalysis. RSC Adv. 2016, 6, 80361–80367. [Google Scholar] [CrossRef]
- Buerger, M.J.; Buerger, N.W. Low-chalcocite and high-chalcocite. Am. Mineral. 1944, 29, 55–65. [Google Scholar]
- Zhong, H.; Zhou, Y.; Ye, M.; He, Y.; Ye, J.; He, C.; Yang, C.; Li, Y. Controlled Synthesis and Optical Properties of Colloidal Ternary Chalcogenide CuInS2 Nanocrystals. Chem. Mater. 2008, 20, 6434–6443. [Google Scholar] [CrossRef]
- Llanos, J.; Buljan, A.; Mujica, C.; Ramírez, R. Electron transfer and electronic structure of KCuFeS2. J. Alloys Compd. 1996, 234, 40–42. [Google Scholar] [CrossRef]
- Gan, Z.H.; Yu, G.Q.; Tay, B.K.; Tan, C.M.; Zhao, Z.W.; Fu, Y.Q. Preparation and characterization of copper oxide thin films deposited by filtered cathodic vacuum arc. J. Phys. D Appl. Phys. 2003, 37, 81–85. [Google Scholar] [CrossRef]
- Karikalan, N.; Karthik, R.; Chen, S.-M.; Karuppiah, C.; Elangovan, A. Sonochemical synthesis of sulfur doped reduced graphene oxide supported CuS nanoparticles for the non-enzymatic glucose sensor applications. Sci. Rep. 2017, 7, 2494. [Google Scholar] [CrossRef]
- Liu, P.; Hensen, E.J.M. Highly efficient and robust Au/MgCuCr2O4 catalyst for gas-phase oxidation of ethanol to acetaldehyde. J. Am. Chem. Soc. 2013, 135, 14032–14035. [Google Scholar] [CrossRef]
- Kutty, T.R.N. A controlled copper-coating method for the preparation of ZnS: Mn DC electroluminescent powder phosphors. Mater. Res. Bull. 1991, 26, 399–406. [Google Scholar] [CrossRef]
- Cabrera-German, D.; García-Valenzuela, J.A.; Martínez-Gil, M.; Suárez-Campos, G.; Montiel-González, Z.; Sotelo-Lerma, M.; Cota-Leal, M. Assessing the chemical state of chemically deposited copper sulfide: A quantitative analysis of the X-ray photoelectron spectra of the amorphous-to-covellite transition phases. Appl. Surf. Sci. 2019, 481, 281–295. [Google Scholar] [CrossRef]
- Goh, S.W.; Buckley, A.N.; Skinner, W.M.; Fan, L.-J. An X-ray photoelectron and absorption spectroscopic investigation of the electronic structure of cubanite, CuFe2S3. Phys. Chem. Miner. 2010, 37, 389–405. [Google Scholar] [CrossRef]
- Akgul, F.A.; Akgul, G.; Yildirim, N.; Unalan, H.E.; Turan, R. Influence of thermal annealing on microstructural, morphological, optical properties and surface electronic structure of copper oxide thin films. Mater. Chem. Phys. 2014, 147, 987–995. [Google Scholar] [CrossRef]
- Dubale, A.A.; Pan, C.-J.; Tamirat, A.G.; Chen, H.-M.; Su, W.-N.; Chen, C.-H.; Rick, J.; Ayele, D.W.; Aragaw, B.A.; Lee, J.-F.; et al. Heterostructured Cu2O/CuO decorated with nickel as a highly efficient photocathode for photoelectrochemical water reduction. J. Mater. Chem. A 2015, 3, 12482–12499. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Kim, N.R.; Choi, J.; Yoon, H.J.; Lee, M.E.; Son, S.U.; Jin, H.-J.; Yun, Y.S. Conversion reaction of copper sulfide based nanohybrids for sodium-ion batteries. ACS Sustain. Chem. Eng. 2017, 5, 9802–9808. [Google Scholar] [CrossRef]
- Liu, S.-Q.; Wen, H.-R.; Ying, G.; Zhu, Y.-W.; Fu, X.-Z.; Sun, R.; Wong, C.-P. Amorphous Ni(OH)2 encounter with crystalline CuS in hollow spheres: A mesoporous nano-shelled heterostructure for hydrogen evolution electrocatalysis. Nano Energy 2018, 44, 7–14. [Google Scholar] [CrossRef]
- Fan, L.; Chen, S.; Ma, R.; Wang, J.; Wang, L.; Zhang, Q.; Zhang, E.; Liu, Z.; Lu, B. Ultrastable potassium storage performance realized by highly effective solid electrolyte interphase layer. Small 2018, 14, 1801806. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Jiang, Q.; Feng, C.; Chen, X.; Guo, Z. Synthesis and performance of tungsten disulfide/carbon (WS2/C) Composite as anode material. J. Electron. Mater. 2018, 47, 251–260. [Google Scholar] [CrossRef]
- Fan, L.; Ma, R.; Yang, Y.; Chen, S.; Lu, B. Covalent sulfur for advanced room temperature sodium-sulfur batteries. Nano Energy 2016, 28, 304–310. [Google Scholar] [CrossRef]
- Paraknowitsch, J.P.; Thomas, A.; Schmidt, J. Microporous sulfur-doped carbon from thienyl-based polymer network precursors. Chem. Commun. 2011, 47, 8283–8285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luther, J.M.; Jain, P.K.; Ewers, T.; Alivisatos, A.P. Localized surface plasmon resonances arising from free carriers in doped quantum dots. Nat. Mater. 2011, 10, 361–366. [Google Scholar] [CrossRef]
- Zhao, Y.; Pan, H.; Lou, Y.; Qiu, X.; Zhu, J.; Burda, C. Plasmonic Cu2−xS nanocrystals: Optical and structural properties of copper-deficient copper(I) sulfides. J. Am. Chem. Soc. 2009, 131, 4253–4261. [Google Scholar] [CrossRef] [PubMed]
- Couve, S.; Gouskov, L.; Szepessy, L.; Vedel, J.; Castel, E. Resistivity and optical transmission of CuxS layers as a function of composition. Thin Solid Films 1973, 15, 223–231. [Google Scholar] [CrossRef]
- Zhang, D.-F.; Zhang, H.; Shang, Y.; Guo, L. Stoichiometry-controlled fabrication of CuxS hollow structures with Cu2O as sacrificial templates. Cryst. Growth Des. 2011, 11, 3748–3753. [Google Scholar] [CrossRef]
- Kim, C.S.; Choi, S.H.; Bang, J.H. New insight into copper sulfide electrocatalysts for quantum dot-sensitized solar cells: Composition-dependent electrocatalytic activity and stability. ACS Appl. Mater. Interfaces 2014, 6, 22078–22087. [Google Scholar] [CrossRef]
- Li, F.; Liu, Y.; Cao, Y.; Zhang, Y.; Zhe, T.; Guo, Z.; Sun, X.; Wang, Q.; Wang, L. Copper sulfide nanoparticle-carrageenan films for packaging application. Food Hydrocoll. 2020, 109, 106094. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.-W.; Jaiswal, L. Bioactive agar-based functional composite film incorporated with copper sulfide nanoparticles. Food Hydrocoll. 2019, 93, 156–166. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, E.; Park, Y.; Kim, J.; Paik, T.; Ha, D.-H. Facile Sulfurization under Ambient Condition with Na2S to Fabricate Nanostructured Copper Sulfide. Nanomaterials 2021, 11, 2317. https://doi.org/10.3390/nano11092317
Hwang E, Park Y, Kim J, Paik T, Ha D-H. Facile Sulfurization under Ambient Condition with Na2S to Fabricate Nanostructured Copper Sulfide. Nanomaterials. 2021; 11(9):2317. https://doi.org/10.3390/nano11092317
Chicago/Turabian StyleHwang, Eunseo, Yoonsu Park, Jongbae Kim, Taejong Paik, and Don-Hyung Ha. 2021. "Facile Sulfurization under Ambient Condition with Na2S to Fabricate Nanostructured Copper Sulfide" Nanomaterials 11, no. 9: 2317. https://doi.org/10.3390/nano11092317






