A Study on the Effects of Selenization Temperature on the Properties of Na-Doped Cu2ZnSn(S,Se)4 Thin Film and Its Correlation with the Performance of Solar Cells
Abstract
:1. Introduction
2. Experimental Details
2.1. Preparation of (Na0.1Cu0.9)2ZnSn(S,Se)4 Film
2.2. Device Fabrication
2.3. Characterization
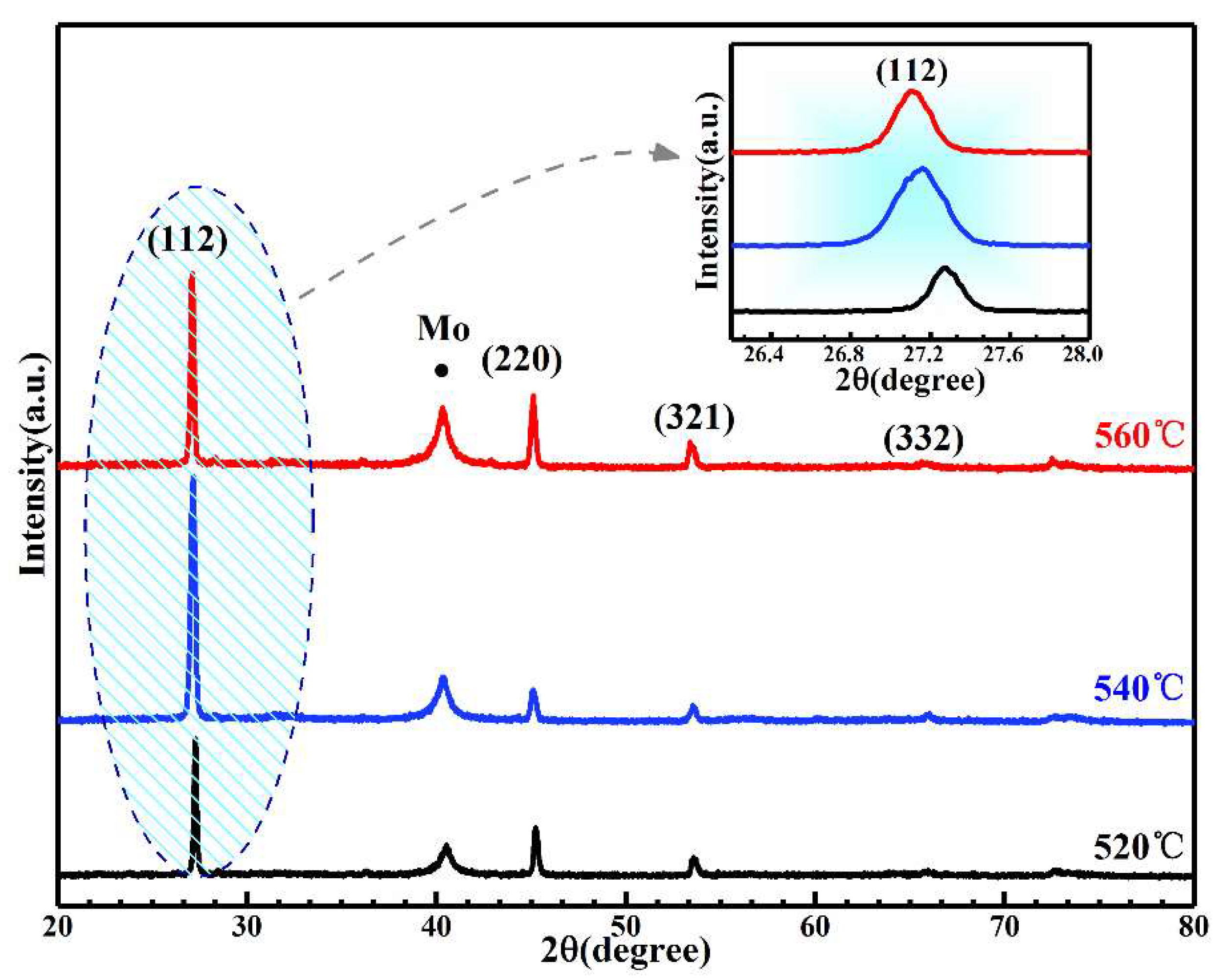
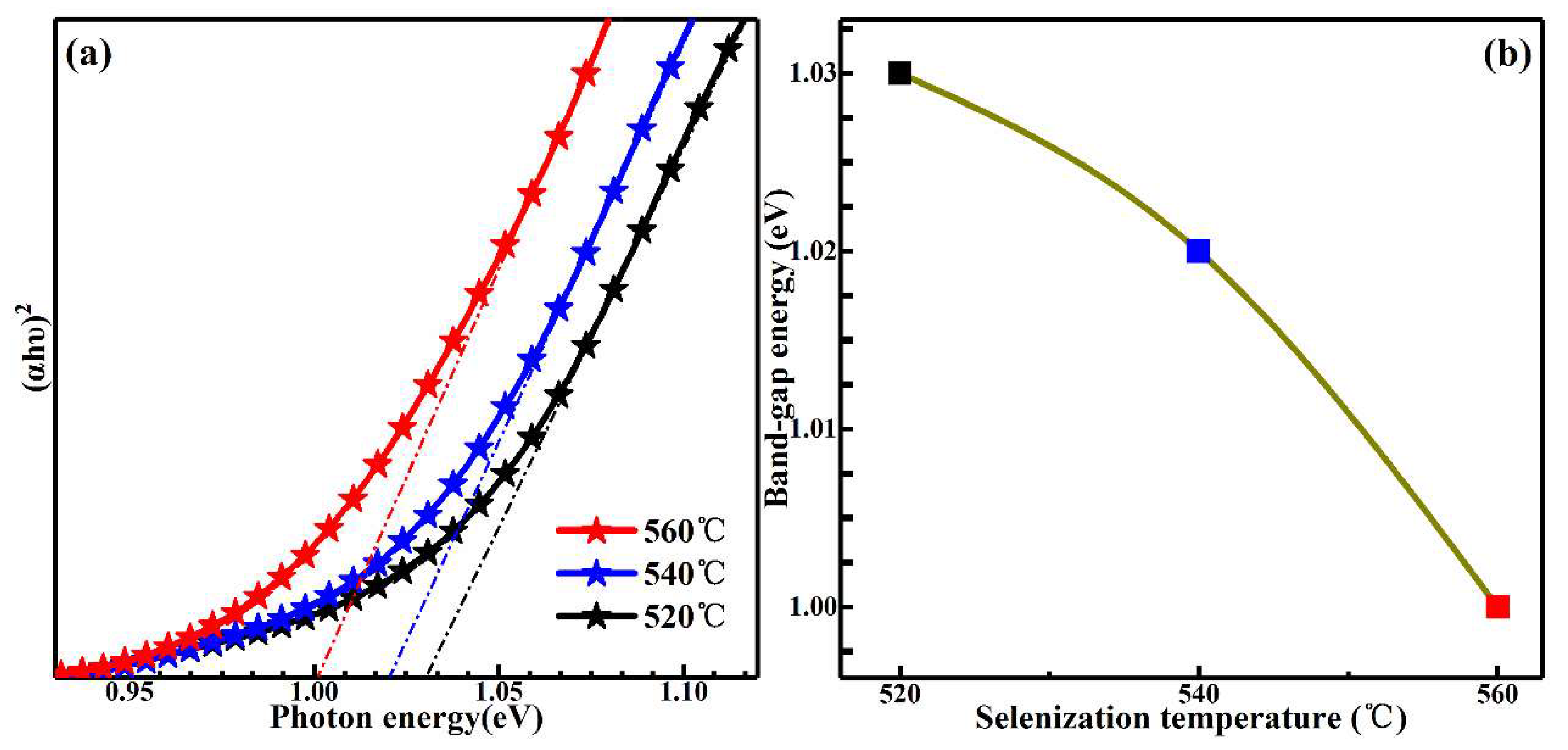
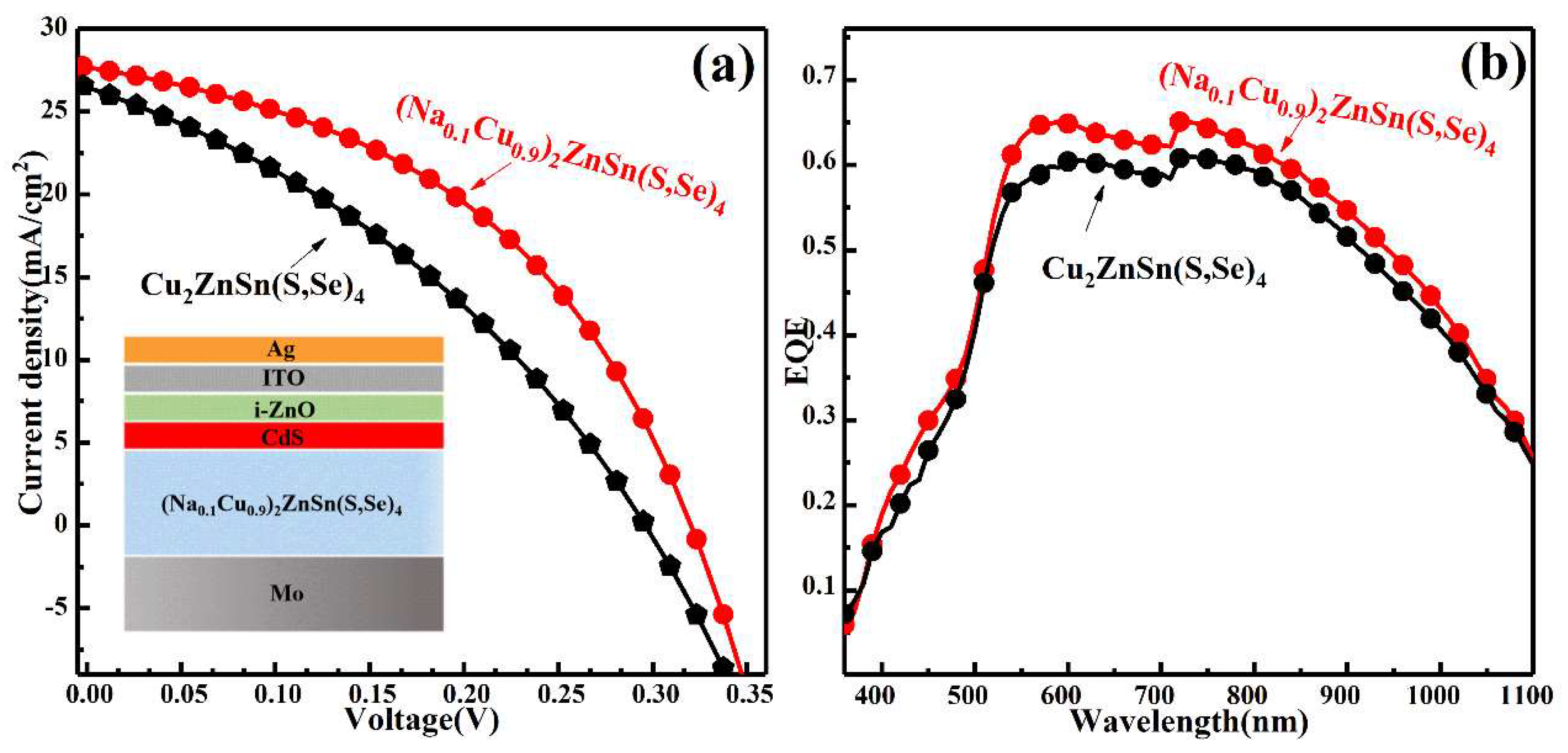
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Todorov, T.K.; Reuter, K.B.; Mitzi, D.B. High-efficiency solar cell with earth-abun-dant liquid-processed absorber. Adv. Mater. 2010, 22, E156–E159. [Google Scholar] [CrossRef]
- Kaur, K.; Kumar, N.; Kumar, M. Strategic review of interface carrier recombination in earth abundant Cu–Zn–Sn–S–Se solar cells: Current challenges and future prospects. J. Mater. Chem. A 2017, 5, 3069–3090. [Google Scholar] [CrossRef]
- Panthani, M.G.; Akhavan, V.; Goodfellow, B.; Schmidtke, J.P.; Dunn, L.; Dodabalapur, A.; Barbara, P.F.; Korgel, B.A. Synthesis of CuInS, CuInSe, and Cu(InxGa1-x)Se(CIGS) Nanocrystal “Inks” for Printable Photovoltaics. J. Am. Chem. Soc. 2008, 130, 16770–16777. [Google Scholar] [CrossRef] [PubMed]
- Lokhande, A.C.; Chalapathy, R.B.V.; He, M.; Jo, E.; Gang, M.; Pawar, S.A.; Lokhande, C.D.; Kim, J.H. Development of Cu2SnS3 (CTS) thin film solar cells by physical techniques: A status review. Sol. Energy Mater. Sol. Cells 2016, 153, 84–107. [Google Scholar] [CrossRef]
- Jackson, P.; Hariskos, D.; Wuerz, R.; Kiowski, O.; Bauer, A.; Friedlmeier, T.M.; Powalla, M. Properties of Cu(In,Ga)Se2 solar cells with new record efficiencies up to 21.7%. Phys. Status Solidi 2015, 9, 28–31. [Google Scholar] [CrossRef]
- Park, J.Y.; Chalapathy, R.B.V.; Lokhande, A.C.; Hong, C.W.; Kim, J.H. Fabrication of earth abundant Cu2ZnSnSSe4 (CZTSSe) thin film solar cells with cadmium free zinc sulfide (ZnS) buffer layers. J. Alloys Compd. 2017, 695, 2652–2660. [Google Scholar] [CrossRef]
- Son, D.H.; Kim, S.H.; Kim, S.Y.; Kim, Y.I.; Sim, J.H.; Park, S.N.; Jeon, D.H.; Hwang, D.K.; Sung, S.J.; Kang, J.K.; et al. Effect of solid-H2S gas reactions on CZTSSe thin film growth and photovoltaic properties of a 12.62% efficiency device. J. Mater. Chem. A 2019, 7, 25279–25289. [Google Scholar] [CrossRef] [Green Version]
- Shockley, W.; Queisser, H.J. Detailed balance limit of efficiency of p–n junction solar cells. J. Appl. Phys. 1961, 32, 510–519. [Google Scholar] [CrossRef]
- Kato, T.; Wu, J.L.; Hirai, Y.; Sugimoto, H.; Bermudez, V. Record Efficiency for Thin Film Polycrystalline Solar Cells Up to 22.9% Achieved by Cs-Treated Cu(In,Ga)(Se,S)2. IEEE J. Photovolt. 2018, 9, 325–330. [Google Scholar] [CrossRef]
- Wang, W.; Winkler, M.T.; Gunawan, O.; Gokmen, T.; Todorov, T.K.; Zhu, Y.; Mitzi, D.B. Device Characteristics of CZTSSe Thin-Film Solar Cells with 12.6% Efficiency. Adv. Energy Mater. 2014, 4, 1301465. [Google Scholar] [CrossRef]
- Tanaka, K.; Oonuki, M.; Moritake, N.; Uchiki, H. Cu2ZnSnS4 thin film solar cells prepared by non-vacuum processing. Sol. Energy Mater. Sol. Cells 2009, 93, 583–587. [Google Scholar] [CrossRef]
- Rey, G.; Redinger, A.; Sendler, J.; Weiss, T.P.; Thevenin, M.; Guennou, M.; El Adib, B.; Siebentritt, S. The bandgap of Cu2ZnSnSe4: Effect of order-disorder. Appl. Phys. Lett. 2014, 105, 112106. [Google Scholar] [CrossRef]
- Sui, Y.R.; Wu, Y.J.; Zhang, Y.; Wang, F.Y.; Gao, Y.B.; Lv, S.Q.; Wang, Z.W.; Sun, Y.F.; Wei, M.B.; Yao, B.; et al. Synthesis of simple, low cost and benign sol–gel Cu2InxZn1-xSnS4 alloy thin films: Influence of different rapid thermal annealing conditions and their photovoltaic solar cells. RSC Adv. 2018, 8, 9038–9048. [Google Scholar] [CrossRef] [Green Version]
- Fu, J.; Tian, Q.; Zhou, Z.; Kou, D.; Meng, Y.; Zhou, W.; Wu, S. Improving the performance of solution-processed Cu2ZnSn(S,Se)4 photovoltaic materials by Cd2+ Substitution. Chem. Mater. 2016, 28, 5821–5828. [Google Scholar] [CrossRef]
- Kim, I.; Kim, K.; Oh, Y.; Woo, K.; Cao, G.Z.; Jeong, S.; Moon, J. Bandgap-graded Cu2Zn(Sn1-xGex)S4 thin-film solar cells derived from metal chalcogenide complex ligand capped nanocrystals. Chem. Mater. 2014, 26, 3957–3965. [Google Scholar] [CrossRef]
- Li, W.; Liu, X.L.; Cui, H.T.; Huang, S.J.; Hao, X.J. The role of Ag in (Ag,Cu)2ZnSnS4 thin film for solar cell application. J. Alloys Compd. 2015, 625, 277–283. [Google Scholar] [CrossRef]
- Yang, Y.C.; Kang, X.J.; Huang, L.J.; Wei, S.; Pan, D.C. A general water-based precursor solution approach to deposit earth abundant Cu2ZnSn(S,Se)4 thin film solar cells. J. Power Sources 2016, 313, 15–20. [Google Scholar] [CrossRef]
- Nagaoka, A.; Scarpulla, M.A.; Yoshino, K. Na-doped Cu2ZnSnS4 single crystal grown by traveling-heater method. J. Cryst. Growth 2016, 453, 119–123. [Google Scholar] [CrossRef] [Green Version]
- Xiao, Z.Y.; Yao, B.; Li, Y.F.; Ding, Z.H.; Gao, Z.M.; Zhao, H.F.; Zhang, L.G.; Zhang, Z.Z.; Sui, Y.R.; Wang, G. Influencing Mechanism of the Selenization Temperature and Time on the Power Conversion Efficiency of Cu2ZnSn(S,Se)4-based Solar Cells. ACS Appl. Mater. Interfaces 2016, 8, 17334–17342. [Google Scholar] [CrossRef]
- Scragg, J.J.; Ericson, T.; Kubart, T.; Edoff, M.; Platzer-Björkman, C. Chemical Insights into the Instability of Cu2ZnSnS4 Films during Annealing. Chem. Mater. 2011, 23, 4625–4633. [Google Scholar] [CrossRef]
- Hsu, C.J.; Duan, H.S.; Yang, W.; Zhou, H.; Yang, Y. Benign Solutions and Innovative Sequential Annealing Processes for High Performance Cu2ZnSn(S,Se)4 Photovoltaics. Adv. Energy Mater. 2014, 4, 1301287. [Google Scholar] [CrossRef]
- Nguyen, D.C.; Ito, S.; Dung, D.V.A. Effects of Annealing Conditions on Crystallization of the CZTS Absorber and Photovoltaic Properties of Cu(Zn,Sn)(S,Se)2 Solar Cells. J. Alloys Compd. 2015, 632, 676–680. [Google Scholar] [CrossRef]
- Ranjbar, S.; Rajesh Menon, M.R.; Fernandes, P.A. Effect of Selenization Conditions on the Growth and Properties of Cu2ZnSn(S,Se)4 Thin Films. Thin Solid Films. 2015, 582, 188–192. [Google Scholar] [CrossRef] [Green Version]
- Kishor Kumar, Y.B.; Suresh Babu, G.; Uday Bhaskar, P.; Sundar Raja, V. Preparation and characterization of spray-deposited Cu2ZnSnS4 thin films. Sol. Energy Mater. Sol. Cells 2009, 93, 1230–1237. [Google Scholar] [CrossRef]
- Wu, Y.J.; Zhang, Y.; Sui, Y.R.; Wang, Z.W.; Lv, S.Q.; Wei, M.B.; Sun, Y.F.; Yao, B.; Liu, X.Y.; Yang, L.L. Bandgap engineering of Cu2InxZn1−xSn(S,Se)4 alloy films for photovoltaic applications. Ceram. Int. 2018, 44, 1942–1950. [Google Scholar] [CrossRef]
- Walsh, A.; Chen, S.; Wei, S.H.; Gong, X.G. Kesterite Thin-Film Solar Cells: Advances in Materials Modelling of Cu2ZnSnS4. Adv. Energy Mater. 2012, 2, 400–409. [Google Scholar] [CrossRef]
- Liu, K.; Yao, B.; Li, Y.; Ding, Z.; Sun, H.; Jiang, Y.; Wang, G.; Pan, D. A versatile strategy for fabricating various Cu2ZnSnS4 precursor solutions. J. Mater. Chem. C 2017, 5, 3035. [Google Scholar] [CrossRef]
- Embden, J.V.; Chesman, A.S.R.; Della Gaspera, E.; Duffy, N.W.; Watkins, S.E.; Jasieniak, J.J. Cu2ZnSnS4xSe4(1-x) solar cells from polar nanocrystal inks. J. Am. Chem. Soc. 2014, 136, 5237–5240. [Google Scholar] [CrossRef] [PubMed]
- Khadka, D.B.; Kim, J.H. Bandgap Engineering of Alloyed Cu2ZnGexSn1-xQ4 (Q=S,Se) Films for Solar Cell. J. Phys. Chem. C 2015, 119, 1706–1713. [Google Scholar] [CrossRef]
- Rondiya, S.; Wadnerkar, N.; Jadhav, Y.; Jadkar, S.; Haram, S.; Kabir, M. Structural, Electronic, and Optical Properties of Cu2NiSnS4: A Combined Experimental and Theoretical Study toward Photovoltaic Applications. Chem. Mater. 2017, 29, 3133–3142. [Google Scholar] [CrossRef]
- Calderón, C.; Gordillo, G.; Becerra, R.; Bartolo-Pérez, P. XPS analysis and characterization of thin films Cu2ZnSnS4 grown using a novel solution based route. Mater. Sci. Semicond. Process. 2015, 39, 492–498. [Google Scholar] [CrossRef]
- Tsega, M.; Dejene, F.B.; Kuo, D.H. Morphological evolution and structural properties of Cu2ZnSn(S,Se)4 thin films deposited from single ceramic target by a one-step sputtering process and selenization without H2Se. J. Alloys Compd. 2015, 642, 140–147. [Google Scholar] [CrossRef]
- Liu, W.C.; Guo, B.L.; Wu, X.S.; Zhang, F.M.; Mak, C.L.; Wong, K.H. Facile hydrothermal synthesis of hydrotropic Cu2ZnSnS4 nanocrystal quantum dots: Band-gap engineering and phonon confinement effect. J. Mater. Chem. A 2013, 1, 3182–3186. [Google Scholar] [CrossRef]
- Wu, Q.H.; ThiBen, A.; Jaegermann, W. XPS and UPS study of Na deposition on thin film V2O5. Appl. Surf. Sci. 2005, 252, 1801–1805. [Google Scholar] [CrossRef]
- Sui, Y.R.; Wu, Y.J.; Zhang, Y.; Wang, Z.W.; Wei, M.B.; Yao, B. Indium effect on structural, optical and electrical properties of Cu2InxZn1-xSnS4 alloy thin films for solar cell. Superlattices Microstruct. 2017, 111, 579–590. [Google Scholar] [CrossRef]
- Li, Y.F.; Yao, B.; Lu, Y.M.; Wei, Z.P.; Gai, Y.Q.; Zheng, C.J.; Zhang, Z.Z.; Li, B.H.; Shen, D.Z.; Fan, X.W.; et al. Realization of p-type conduction in undoped MgxZn1-xO thin films by controlling Mg content. Appl. Phys. Lett. 2007, 91, 232115. [Google Scholar] [CrossRef]




| Sample | Cu (at%) | Zn (at%) | Sn (at%) | Na (at%) | S (at%) | Se (at%) | Se/(S+Se) |
|---|---|---|---|---|---|---|---|
| T520 | 19.57 | 16.12 | 9.18 | 3.19 | 8.47 | 43.47 | 83.69 |
| T540 | 20.79 | 16.63 | 9.71 | 3.31 | 4.22 | 45.34 | 91.49 |
| T560 | 20.56 | 16.63 | 10.21 | 3.88 | 2.08 | 46.64 | 97.74 |
| Sample | ρ (Ω·cm) | n (cm−3) | μ (cm−2V−1s−1) | Conduction Type |
|---|---|---|---|---|
| T520 | 5.59 × 101 | 9.22 × 1016 | 4.63 × 100 | p |
| T540 | 4.77 × 101 | 2.93 × 1017 | 4.63 × 10−1 | p |
| T560 | 5.61 × 101 | 5.54 × 1016 | 2.57 × 100 | p |
| Sample | Voc (mV) | Jsc (mA/cm2) | Rs (Ωcm2) | Rsh (Ωcm2) | FF (%) | PCE (%) |
|---|---|---|---|---|---|---|
| CZTSSe | 296 | 26.42 | 30.37 | 130.80 | 35.16 | 2.75 |
| (Na0.1Cu0.9)2ZnSn(S,Se)4 | 338 | 27.16 | 17.58 | 279.05 | 52.59 | 4.82 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Jiang, D.; Zeng, F.; Sui, Y. A Study on the Effects of Selenization Temperature on the Properties of Na-Doped Cu2ZnSn(S,Se)4 Thin Film and Its Correlation with the Performance of Solar Cells. Nanomaterials 2021, 11, 2434. https://doi.org/10.3390/nano11092434
Wang Z, Jiang D, Zeng F, Sui Y. A Study on the Effects of Selenization Temperature on the Properties of Na-Doped Cu2ZnSn(S,Se)4 Thin Film and Its Correlation with the Performance of Solar Cells. Nanomaterials. 2021; 11(9):2434. https://doi.org/10.3390/nano11092434
Chicago/Turabian StyleWang, Zhanwu, Dongyue Jiang, Fancong Zeng, and Yingrui Sui. 2021. "A Study on the Effects of Selenization Temperature on the Properties of Na-Doped Cu2ZnSn(S,Se)4 Thin Film and Its Correlation with the Performance of Solar Cells" Nanomaterials 11, no. 9: 2434. https://doi.org/10.3390/nano11092434
APA StyleWang, Z., Jiang, D., Zeng, F., & Sui, Y. (2021). A Study on the Effects of Selenization Temperature on the Properties of Na-Doped Cu2ZnSn(S,Se)4 Thin Film and Its Correlation with the Performance of Solar Cells. Nanomaterials, 11(9), 2434. https://doi.org/10.3390/nano11092434





