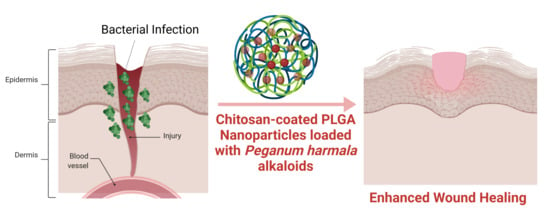
Chitosan-Coated PLGA Nanoparticles Loaded with Peganum harmala Alkaloids with Promising Antibacterial and Wound Healing Activities
Abstract
:1. Introduction
2. Results and Discussion
2.1. Formulation Optimization by Three-Factor, Three-Level Box–Behnken Response Surface Design (33 BBD)
2.1.1. Influence of the Independent Factors on Particle Size (PS)
2.1.2. Influence of the Independent Factors on PDI
2.1.3. Influence of the Independent Factors on ZP
2.1.4. Influence of the Independent Factors on Entrapment Efficiency (EE %)
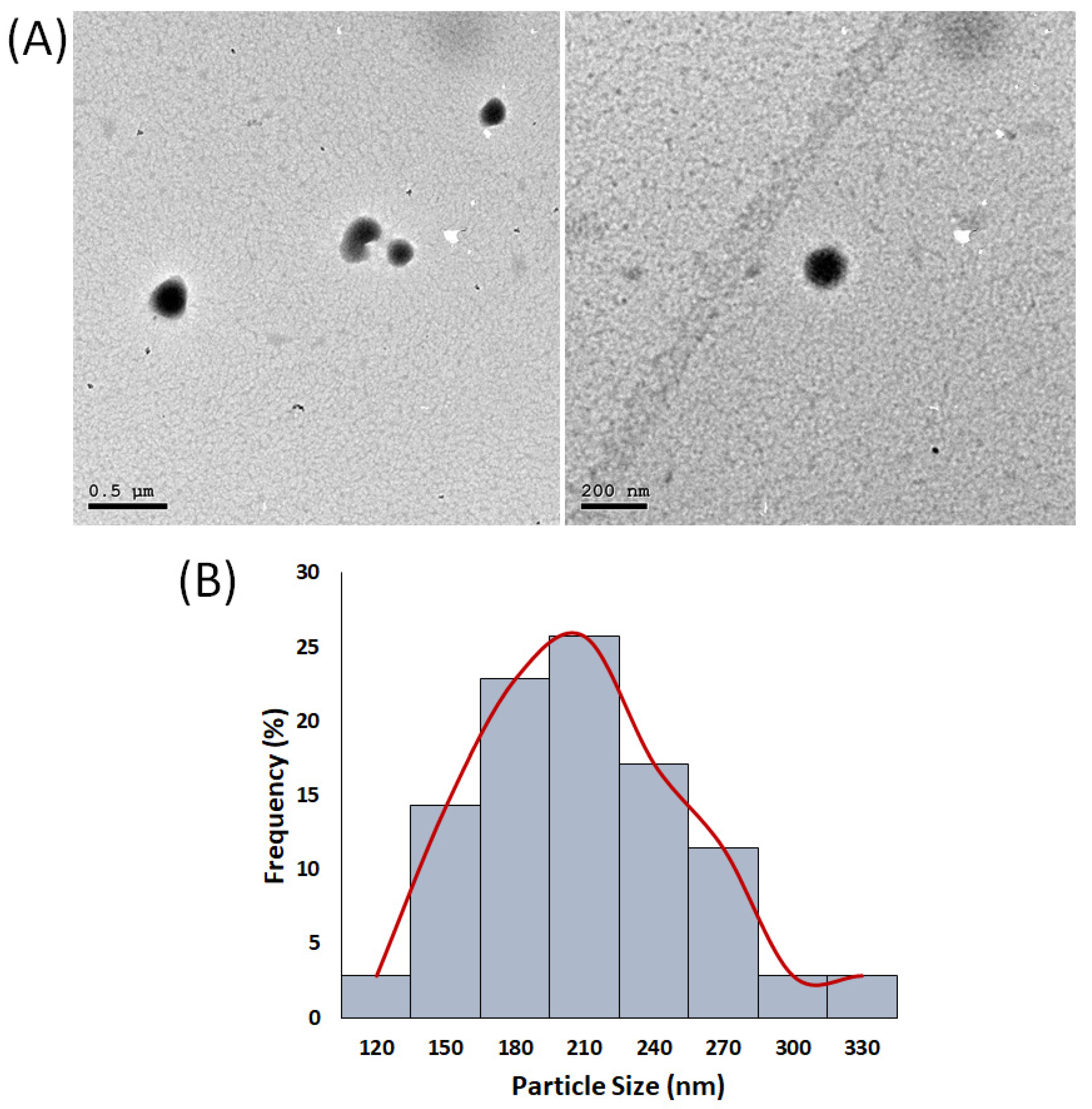
2.2. Characterization of the Optimized H/CS/PLGA NPs
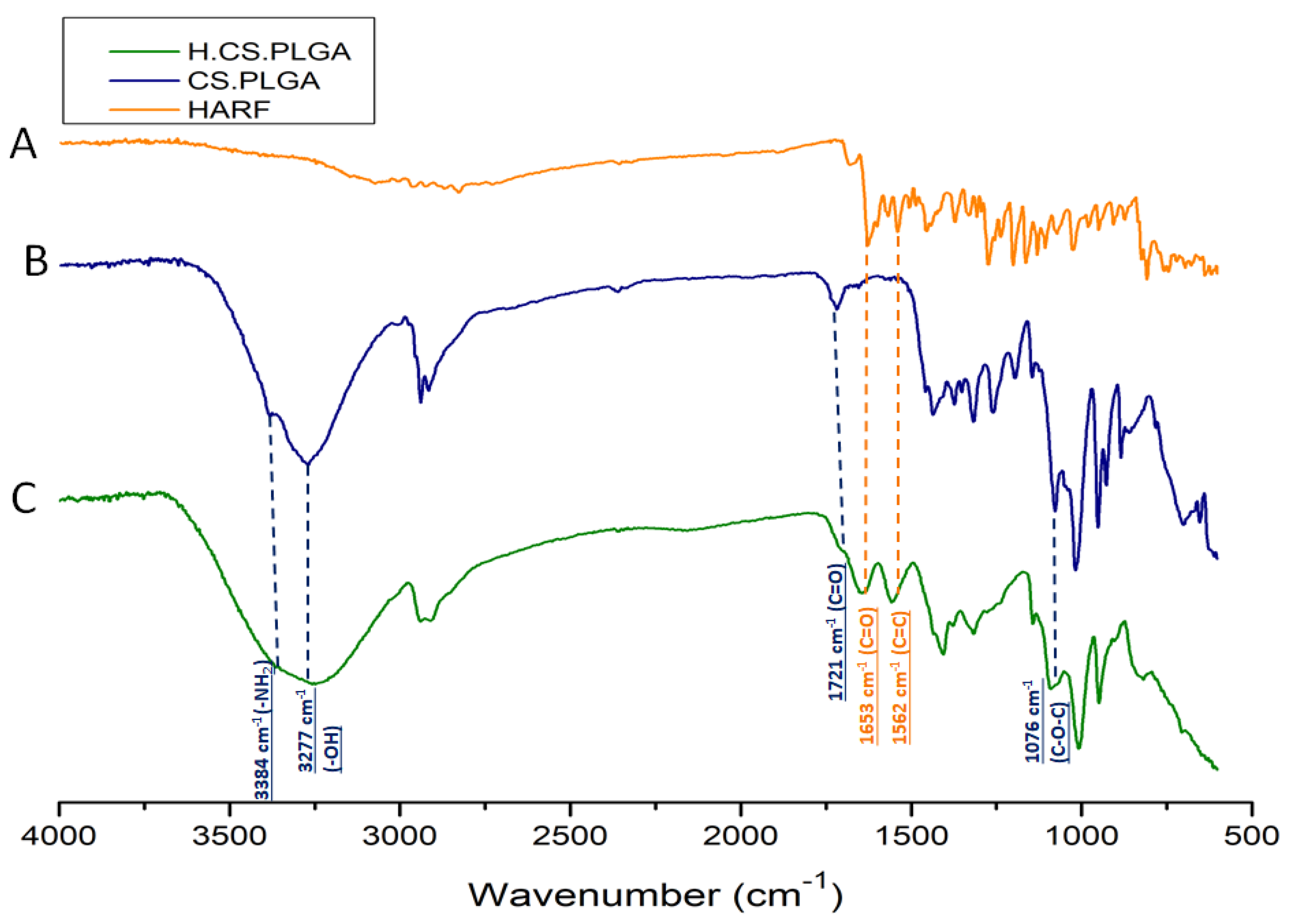
2.3. ATR-FTIR Analysis of the Optimal H/CS/PLGA NPs Formulation
2.4. In Vitro Release Study for the Optimal H/CS/PLGA NPs Formulation
2.5. Antimicrobial Assay for the Optimal H/CS/PLGA NPs Formulation
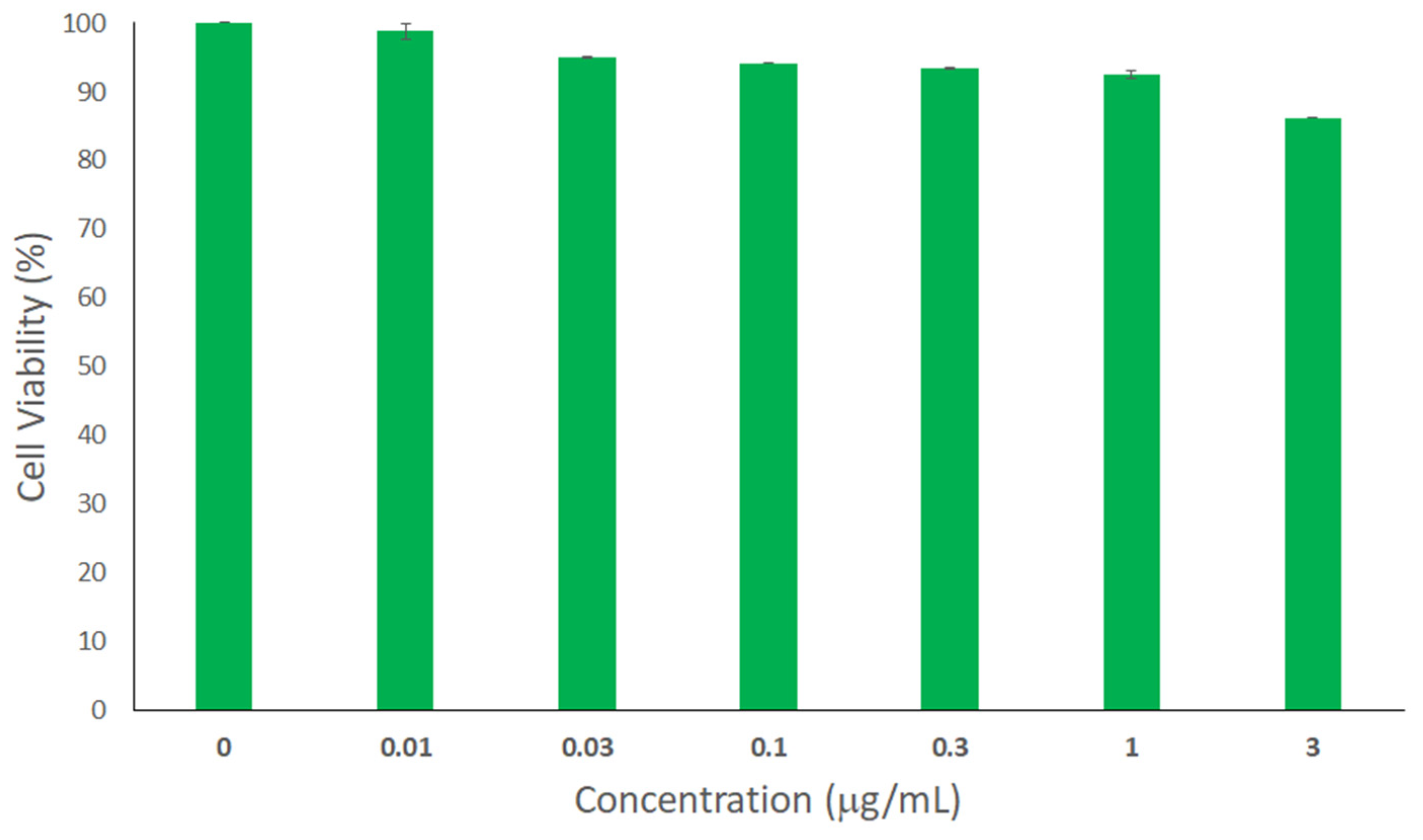
2.6. Cytotoxicity Assay
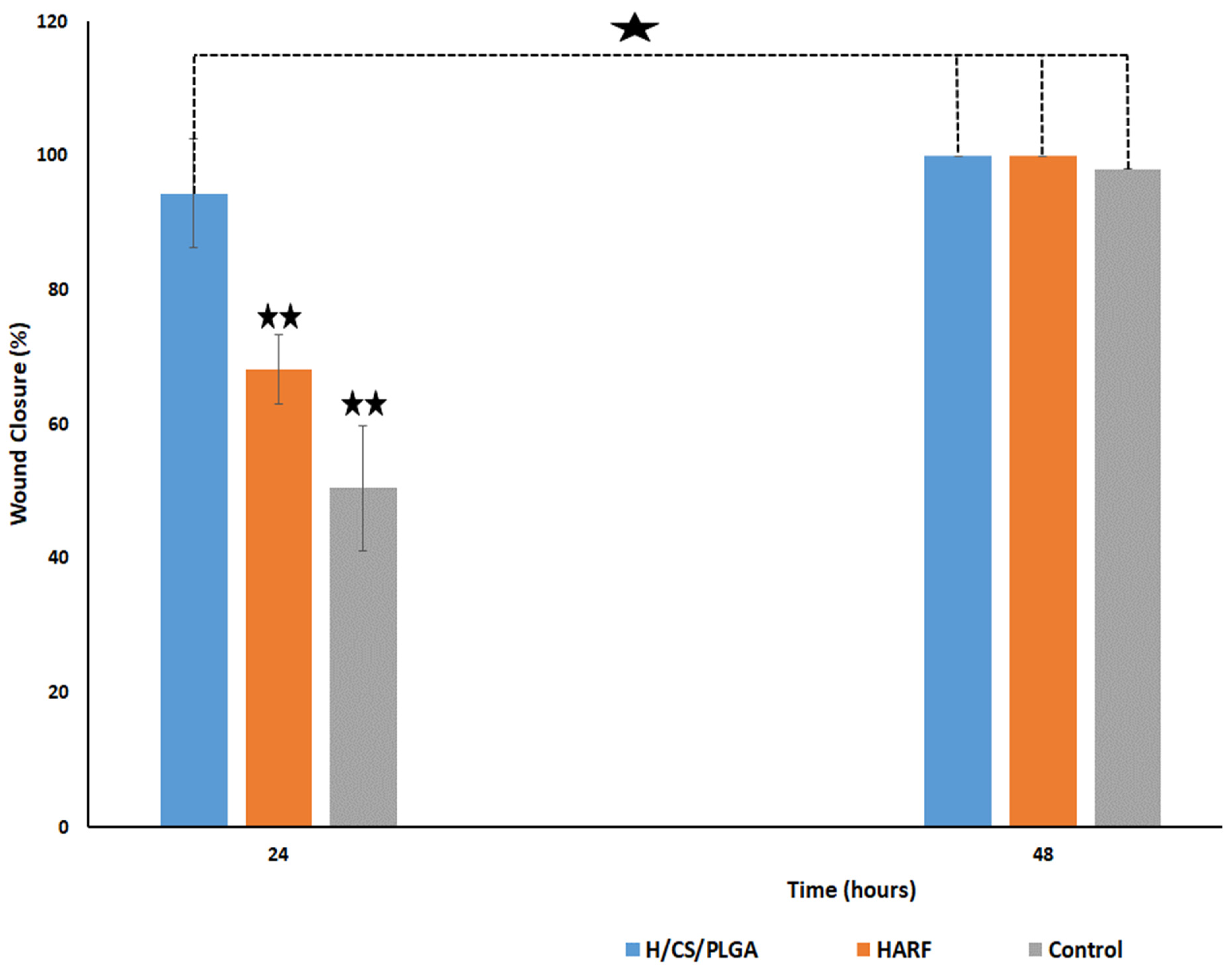
2.7. In Vitro Scratch Wound Healing Assay
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Plant Material
4.3. Methods
4.3.1. Extraction and Isolation of Major P. harmala Alkaloids
4.3.2. Experimental Design
4.3.3. Preparation of H/CS/PLGA NPs
4.3.4. Average Particle Size, Polydispersity Index (PDI), and Zeta Potential Measurements
4.3.5. Determination of Entrapment Efficiency (EE%)
4.3.6. Formulation Optimization
4.3.7. Characterization of the Optimal H/CS/PLGA NPs Formulation
4.3.8. In Vitro Release Study of HARF from the Optimal H/CS/PLGA NPs Formulation
4.3.9. Antimicrobial Assay for the Optimal H/CS/PLGA NPs Formulation
Inoculum Preparation (Colony Suspension Method)
Broth Macrodilution Method
4.3.10. Cytotoxicity Assay
Cell Culture
Sulforhodamine B Colorimetric Assay
4.3.11. In Vitro Scratch Wound Healing Assay
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Ter Horst, B.; Chouhan, G.; Moiemen, N.; Grover, L.M. Advances in keratinocyte delivery in burn wound care. Adv. Drug Deliv. Rev. 2017, 123, 18–32. [Google Scholar] [CrossRef]
- Morey, M.; Pandit, A. Responsive triggering systems for delivery in chronic wound healing. Adv. Drug Deliv. Rev. 2018, 129, 169–193. [Google Scholar] [CrossRef] [PubMed]
- Negut, I.; Grumezescu, V.; Grumezescu, A.M. Treatment Strategies for Infected Wounds. Molecules 2018, 23, 2392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mihai, M.; Holban, A.M.; Giurcăneanu, C.; Popa, L.G.; Buzea, M.; Filipov, M.; Lazăr, V.; Chifiriuc, M.C.; Popa, M.I. Identification and phenotypic characterization of the most frequent bacterial etiologies in chronic skin ulcers. Rom. J. Morphol. Embryol. 2014, 55, 1401–1408. [Google Scholar]
- Fahmy, S.A.; Mamdouh, W. Garlic oil-loaded PLGA nanoparticles with controllable size and shape and enhanced antibacterial activities. J. Appl. Polym. Sci. 2018, 135, 46133. [Google Scholar] [CrossRef]
- Fahmy, S.; Preis, E.; Bakowsky, U.; Azzazy, H. Platinum Nanoparticles: Green Synthesis and Biomedical Applications. Molecules 2020, 25, 4981. [Google Scholar] [CrossRef] [PubMed]
- Fahmy, S.A.; Preis, E.; Bakowsky, U.; Azzazy, H.M.E.-S. Palladium Nanoparticles Fabricated by Green Chemistry: Promising Chemotherapeutic, Antioxidant and Antimicrobial Agents. Materials 2020, 13, 3661. [Google Scholar] [CrossRef]
- Fahmy, S.; Fawzy, I.; Saleh, B.; Issa, M.; Bakowsky, U.; Azzazy, H. Green Synthesis of Platinum and Palladium Nanoparticles Using Peganum harmala L. Seed Alkaloids: Biological and Computational Studies. Nanomaterials 2021, 11, 965. [Google Scholar] [CrossRef]
- Fahmy, S.A.; Issa, M.Y.; Saleh, B.M.; Meselhy, M.R.; Azzazy, H.M.E.-S. Peganum harmala Alkaloids Self-Assembled Supramolecular Nanocapsules with Enhanced Antioxidant and Cytotoxic Activities. ACS Omega 2021, 6, 11954–11963. [Google Scholar] [CrossRef]
- Derakhshanfar, A.; Oloumi, M.M.; Mirzaie, M. Study on the effect of Peganum harmala extract on experimental skin wound healing in rat: Pathological and biomechanical findings. Comp. Haematol. Int. 2009, 19, 169–172. [Google Scholar] [CrossRef]
- Pormohammad, A.; Monych, N.; Ghosh, S.; Turner, D.; Turner, R. Nanomaterials in Wound Healing and Infection Control. Antibiotics 2021, 10, 473. [Google Scholar] [CrossRef] [PubMed]
- El-Shafie, S.; Fahmy, S.A.; Ziko, L.; Elzahed, N.; Shoeib, T.; Kakarougkas, A. Encapsulation of Nedaplatin in Novel PEGylated Liposomes Increases Its Cytotoxicity and Genotoxicity against A549 and U2OS Human Cancer Cells. Pharmaceutics 2020, 12, 863. [Google Scholar] [CrossRef] [PubMed]
- Fahmy, S.A.; Alawak, M.; Brüßler, J.; Bakowsky, U.; El Sayed, M.M.H. Nanoenabled Bioseparations: Current Developments and Future Prospects. BioMed Res. Int. 2019, 2019, 4983291. [Google Scholar] [CrossRef] [PubMed]
- Fahmy, S.A.; Ponte, F.; El-Rahman, M.K.A.; Russo, N.; Sicilia, E.; Shoeib, T. Investigation of the host-guest complexation between 4-sulfocalix[4]arene and nedaplatin for potential use in drug delivery. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 193, 528–536. [Google Scholar] [CrossRef]
- Fahmy, S.A.; Ponte, F.; Sicilia, E.; Azzazy, H.M.E.-S. Experimental and Computational Investigations of Carboplatin Supramolecular Complexes. ACS Omega 2020, 5, 31456–31466. [Google Scholar] [CrossRef]
- Fahmy, S.A.; Ponte, F.; Fawzy, I.M.; Sicilia, E.; Bakowsky, U.; Azzazy, H.M.E.-S. Host-Guest Complexation of Oxaliplatin and Para-Sulfonatocalix[n]Arenes for Potential Use in Cancer Therapy. Molecules 2020, 25, 5926. [Google Scholar] [CrossRef]
- Fahmy, S.A.; Brüßler, J.; Alawak, M.; El-Sayed, M.M.H.; Bakowsky, U.; Shoeib, T. Chemotherapy Based on Supramolecular Chemistry: A Promising Strategy in Cancer Therapy. Pharmaceutics 2019, 11, 292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fahmy, S.A.; Brüßler, J.; Ponte, F.; El-Rahman, M.K.A.; Russo, N.; Sicilia, E.; Bakowsky, U.; Shoeib, T. A study on the physicochemical properties and cytotoxic activity of p-sulfocalix[4]arene-nedaplatin complex. J. Phys. Conf. Ser. 2019, 1310, 012011. [Google Scholar] [CrossRef]
- Shende, P.; Gupta, H. Formulation and comparative characterization of nanoparticles of curcumin using natural, synthetic and semi-synthetic polymers for wound healing. Life Sci. 2020, 253, 117588. [Google Scholar] [CrossRef]
- Karri, V.V.S.R.; Kuppusamy, G.; Talluri, S.V.; Mannemala, S.S.; Kollipara, R.; Wadhwani, A.D.; Mulukutla, S.; Raju, K.R.S.; Malayandi, R. Curcumin loaded chitosan nanoparticles impregnated into collagen-alginate scaffolds for diabetic wound healing. Int. J. Biol. Macromol. 2016, 93, 1519–1529. [Google Scholar] [CrossRef]
- Suh, J.-K.F.; Matthew, H. Application of chitosan-based polysaccharide biomaterials in cartilage tissue engineering: A review. Biomaterials 2000, 21, 2589–2598. [Google Scholar] [CrossRef] [PubMed]
- Soorbaghi, F.P.; Isanejad, M.; Salatin, S.; Ghorbani, M.; Jafari, S.; Derakhshankhah, H. Bioaerogels: Synthesis approaches, cellular uptake, and the biomedical applications. Biomed. Pharmacother. 2019, 111, 964–975. [Google Scholar] [CrossRef]
- Shariatinia, Z.; Jalali, A.M. Chitosan-based hydrogels: Preparation, properties and applications. Int. J. Biol. Macromol. 2018, 115, 194–220. [Google Scholar] [CrossRef]
- Ong, S.-Y.; Wu, J.; Moochhala, S.M.; Tan, M.-H.; Lu, J. Development of a chitosan-based wound dressing with improved hemostatic and antimicrobial properties. Biomaterials 2008, 29, 4323–4332. [Google Scholar] [CrossRef] [PubMed]
- Miguel, S.A.P.; Moreira, A.F.; Correia, I.J. Chitosan based-asymmetric membranes for wound healing: A review. Int. J. Biol. Macromol. 2019, 127, 460–475. [Google Scholar] [CrossRef]
- Anand, P.; Nair, H.B.B.; Sung, B.; Kunnumakkara, A.B.; Yadav, V.R.; Tekmal, R.R.; Aggarwal, B.B. RETRACTED: Design of curcumin-loaded PLGA nanoparticles formulation with enhanced cellular uptake, and increased bioactivity in vitro and superior bioavailability in vivo. Biochem. Pharmacol. 2010, 79, 330–338. [Google Scholar] [CrossRef] [Green Version]
- Nagoba, B.S.; Suryawanshi, N.M.; Wadher, B.; Selkar, S. Acidic Environment and Wound Healing: A Review. Wounds 2015, 27, 5–11. [Google Scholar]
- Fredenberg, S.; Wahlgren, M.; Reslow, M.; Axelsson, A. The mechanisms of drug release in poly(lactic-co-glycolic acid)-based drug delivery systems—A review. Int. J. Pharm. 2011, 415, 34–52. [Google Scholar] [CrossRef]
- Ghitman, J.; Biru, E.I.; Stan, R.; Iovu, H. Review of hybrid PLGA nanoparticles: Future of smart drug delivery and theranostics medicine. Mater. Des. 2020, 193, 108805. [Google Scholar] [CrossRef]
- Takeuchi, I.; Suzuki, T.; Makino, K. Iontophoretic transdermal delivery using chitosan-coated PLGA nanoparticles for transcutaneous immunization. Colloids Surf. A Physicochem. Eng. Asp. 2020, 608, 125607. [Google Scholar] [CrossRef]
- Schiller, S.; Hanefeld, A.; Schneider, M.; Lehr, C.-M. Towards a Continuous Manufacturing Process of Protein-Loaded Polymeric Nanoparticle Powders. AAPS PharmSciTech 2020, 21, 269. [Google Scholar] [CrossRef]
- Rampino, A.; Borgogna, M.; Blasi, P.; Bellich, B.; Cesàro, A. Chitosan nanoparticles: Preparation, size evolution and stability. Int. J. Pharm. 2013, 455, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Taymouri, S.; Ahmadi, Z.; Mirian, M.; Tavakoli, N. Simvastatin Nanosuspensions Prepared Using a Combination of PH-Sensitive and Timed-Release Approaches for Potential Treatment of Colorectal Cancer. Pharm. Dev. Technol. 2021, 26, 335–348. [Google Scholar] [CrossRef] [PubMed]
- Topal, G.R.; Mészáros, M.; Porkoláb, G.; Szecskó, A.; Polgár, T.F.; Siklós, L.; Deli, M.A.; Veszelka, S.; Bozkir, A. ApoE-Targeting Increases the Transfer of Solid Lipid Nanoparticles with Donepezil Cargo across a Culture Model of the Blood–Brain Barrier. Pharmaceutics 2020, 13, 38. [Google Scholar] [CrossRef] [PubMed]
- Gan, Q.; Wang, T. Chitosan nanoparticle as protein delivery carrier—Systematic examination of fabrication conditions for efficient loading and release. Colloids Surf. B Biointerfaces 2007, 59, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Chronopoulou, L.; Massimi, M.; Giardi, M.F.; Cametti, C.; Devirgiliis, L.C.; Dentini, M.; Palocci, C. Chitosan-coated PLGA nanoparticles: A sustained drug release strategy for cell cultures. Colloids Surf. B Biointerfaces 2013, 103, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Sendi, N.; Mkadmini-Hammi, K.; Ben Mansour, R.; Selmi, S.; Trabelsi, N.; Isoda, H.; Ksouri, R.; Megdiche-Ksouri, W. Simultaneous optimization of ultrasound-assisted extraction of flavonoid compounds and antiradical activity from Artemisia herba-Alba using response surface methodology. Prep. Biochem. Biotechnol. 2020, 50, 943–953. [Google Scholar] [CrossRef]
- Delan, W.K.; Zakaria, M.; Elsaadany, B.; ElMeshad, A.N.; Mamdouh, W.; Fares, A.R. Formulation of simvastatin chitosan nanoparticles for controlled delivery in bone regeneration: Optimization using Box-Behnken design, stability and in vivo study. Int. J. Pharm. 2020, 577, 119038. [Google Scholar] [CrossRef]
- Azizi, M.; Sedaghat, S.; Tahvildari, K.; Derakhshi, P.; Ghaemi, A. Synthesis of silver nanoparticles using Peganum harmala extract as a green route. Green Chem. Lett. Rev. 2017, 10, 420–427. [Google Scholar] [CrossRef] [Green Version]
- Lu, B.; Lv, X.; Le, Y. Chitosan-Modified PLGA Nanoparticles for Control-Released Drug Delivery. Polymers 2019, 11, 304. [Google Scholar] [CrossRef] [Green Version]
- Boonsongrit, Y.; Mueller, B.W.; Mitrevej, A. Characterization of drug–chitosan interaction by 1H NMR, FTIR and isothermal titration calorimetry. Eur. J. Pharm. Biopharm. 2008, 69, 388–395. [Google Scholar] [CrossRef]
- Ravi Kumar, M.N.; Bakowsky, U.; Lehr, C.M. Preparation and characterization of cationic PLGA nanospheres as DNA carriers. Biomaterials 2004, 25, 1771–1777. [Google Scholar] [CrossRef]
- Zhang, P.; Liu, C. Enhancement of Skin Wound Healing by rhEGF-Loaded Carboxymethyl Chitosan Nanoparticles. Polymers 2020, 12, 1612. [Google Scholar] [CrossRef]
- Ospina-Villa, J.D.; Gómez-Hoyos, C.; Zuluaga-Gallego, R.; Triana-Chávez, O. Encapsulation of proteins from Leishmania panamensis into PLGA particles by a single emulsion-solvent evaporation method. J. Microbiol. Methods 2019, 162, 1–7. [Google Scholar] [CrossRef]
- Pinnapireddy, S.R.; Duse, L.; Strehlow, B.; Schäfer, J.; Bakowsky, U. Composite liposome-PEI/nucleic acid lipopolyplexes for safe and efficient gene delivery and gene knockdown. Colloids Surf. B Biointerfaces 2017, 158, 93–101. [Google Scholar] [CrossRef]
- Nair, K.L.; Thulasidasan, A.K.T.; Deepa, G.; Anto, R.J.; Kumar, G.V. Purely aqueous PLGA nanoparticulate formulations of curcumin exhibit enhanced anticancer activity with dependence on the combination of the carrier. Int. J. Pharm. 2012, 425, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Motawi, T.K.; El-Maraghy, S.A.; ElMeshad, A.N.; Nady, O.M.; Hammam, O. Cromolyn chitosan nanoparticles as a novel protective approach for colorectal cancer. Chem. Interact. 2017, 275, 1–12. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically: Approved Standard. CLSI (NCCLS) 2018, 26, M7-A7. [Google Scholar]
- Main, K.A.; Mikelis, C.M.; Doçi, C.L. In Vitro Wound Healing Assays to Investigate Epidermal Migration. In Epidermal Cells; Humana: New York, NY, USA, 2019; pp. 147–154. [Google Scholar]
- Dudley, B.A. Role of Ceramide-1 Phosphate in Regulation of Sphingolipid and Eicosanoid Metabolism in Lung Epithelial Cells. Master’s Thesis, University of South Florida, Tampa Bay, FL, USA, 2020. Available online: https://scholarcommons.usf.edu/etd/8534 (accessed on 12 August 2021).
- Rueden, C.; Schindelin, J.E.; Hiner, M.C.; Dezonia, B.E.; Walter, A.E.; Arena, E.T.; Eliceiri, K.W. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinform. 2017, 18, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Chem. Biol. 2012, 9, 676–682. [Google Scholar] [CrossRef] [Green Version]
- Jonkman, J.; Cathcart, J.A.; Xu, F.; Bartolini, M.E.; Amon, J.E.; Stevens, K.M.; Colarusso, P. An introduction to the wound healing assay using live-cell microscopy. Cell Adhes. Migr. 2014, 8, 440–451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez, L.G.; Wu, X.; Guan, J.-L. Wound-Healing Assay. In Cell Migration; Springer: Berlin/Heidelberg, Germany, 2005; pp. 23–29. [Google Scholar]


| Response | Model | R2 | Adjusted R2 | Predicted R2 | Constraints | Predicted | Observed | 95% Prediction Interval | |
|---|---|---|---|---|---|---|---|---|---|
| Y1 | Particle Size (nm) | Quadratic model | 0.96 | 0.90 | 0.61 | In range | 204.84 | 202.27 ± 2.44 | 198.56–211.13 |
| Y2 | PDI | Quadratic model | 0.97 | 0.92 | 0.53 | Minimize | 0.10 | 0.23 ± 0.01 | 0.07–0.13 |
| Y3 | Zeta Potential (mV) | Linear model | 0.91 | 0.89 | 0.87 | Maximize | 8.79 | 9.22 ± 0.94 | 7.12–10.45 |
| Y4 | Entrapment Efficiency (%) | 2FI model | 0.95 | 0.92 | 0.84 | Maximize (lower limit: 80%) | 89.50 | 86.77 ± 4.18 | 79.99–99.01 |
| Factors | Levels of Factors | Levels for the Optimized Formulation | |||
|---|---|---|---|---|---|
| Low (−1) | Medium (0) | High (+1) | |||
| A | HARF:PLGA weight ratio | 0.1:1 | 0.3:1 | 0.5:1 | 0.10 |
| B | CS:PLGA weight ratio | 0 | 0.4:1 | 0.8:1 | 0.60 |
| C | Sonication time (min) | 4 | 8 | 12 | 12 |
| Bacterial Strain | Minimum Inhibitory Concentration (MIC in mg/mL) | ||
|---|---|---|---|
| Harmala Alkaloid Rich Fraction (HARF) | CS/PLGA NPs | H/CS/PLGA NPs | |
| Staphylococcus aureus | 0.5 | 0.18 | 0.13 |
| Escherichia coli | 0.5 | 0.18 | 0.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azzazy, H.M.E.-S.; Fahmy, S.A.; Mahdy, N.K.; Meselhy, M.R.; Bakowsky, U. Chitosan-Coated PLGA Nanoparticles Loaded with Peganum harmala Alkaloids with Promising Antibacterial and Wound Healing Activities. Nanomaterials 2021, 11, 2438. https://doi.org/10.3390/nano11092438
Azzazy HME-S, Fahmy SA, Mahdy NK, Meselhy MR, Bakowsky U. Chitosan-Coated PLGA Nanoparticles Loaded with Peganum harmala Alkaloids with Promising Antibacterial and Wound Healing Activities. Nanomaterials. 2021; 11(9):2438. https://doi.org/10.3390/nano11092438
Chicago/Turabian StyleAzzazy, Hassan Mohamed El-Said, Sherif Ashraf Fahmy, Noha Khalil Mahdy, Meselhy Ragab Meselhy, and Udo Bakowsky. 2021. "Chitosan-Coated PLGA Nanoparticles Loaded with Peganum harmala Alkaloids with Promising Antibacterial and Wound Healing Activities" Nanomaterials 11, no. 9: 2438. https://doi.org/10.3390/nano11092438
APA StyleAzzazy, H. M. E.-S., Fahmy, S. A., Mahdy, N. K., Meselhy, M. R., & Bakowsky, U. (2021). Chitosan-Coated PLGA Nanoparticles Loaded with Peganum harmala Alkaloids with Promising Antibacterial and Wound Healing Activities. Nanomaterials, 11(9), 2438. https://doi.org/10.3390/nano11092438