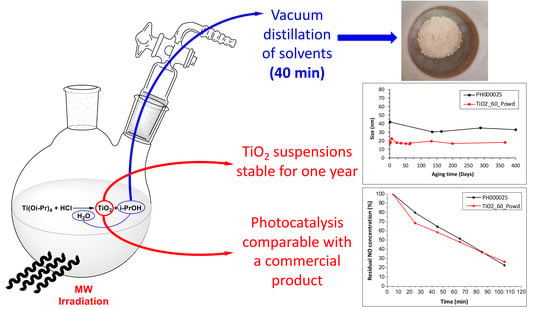
Microwave-Assisted Vacuum Synthesis of TiO2 Nanocrystalline Powders in One-Pot, One-Step Procedure
Abstract
:1. Introduction
2. Materials and Methods
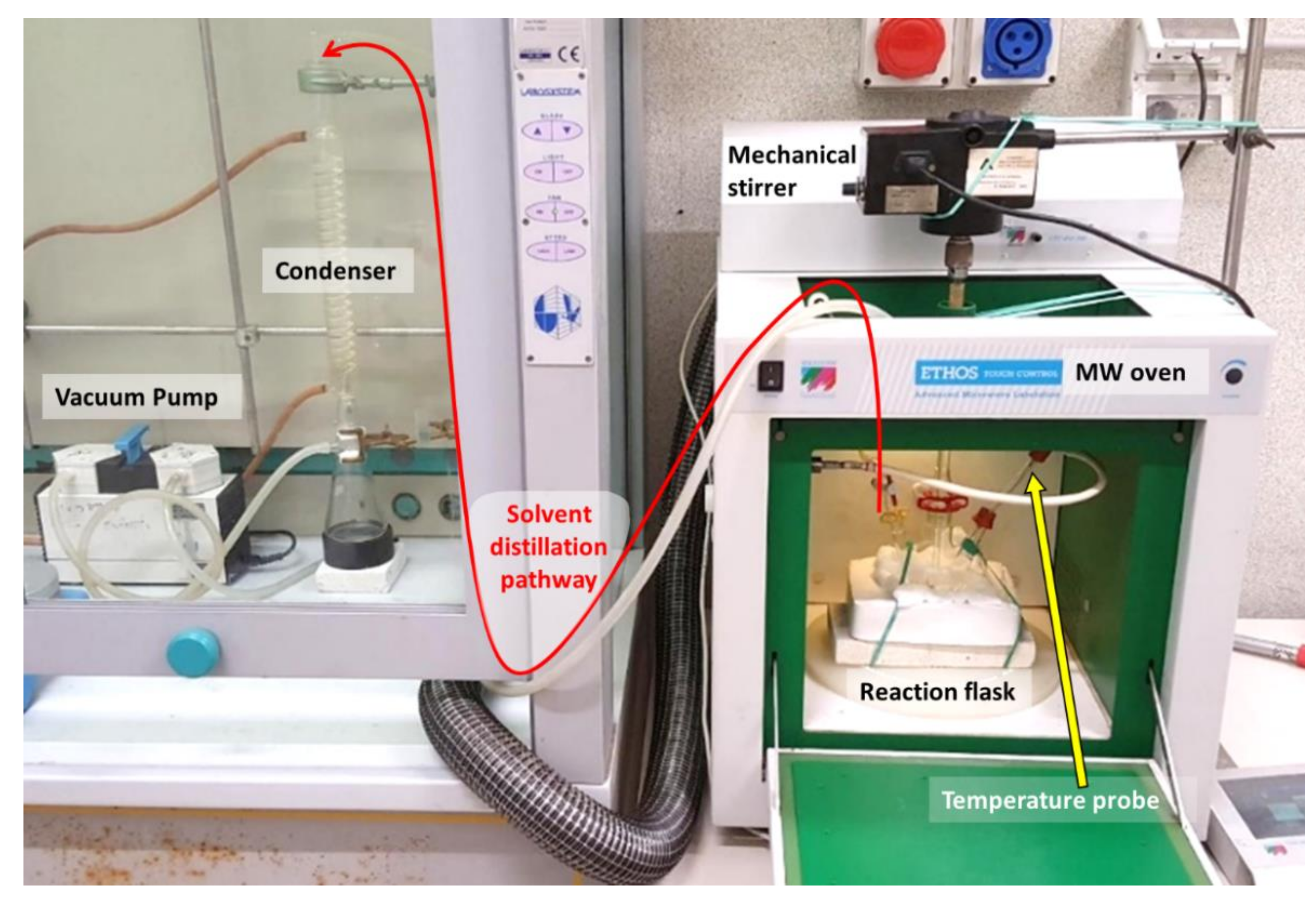
2.1. Experimental Setup
2.2. Materials
2.3. Procedure for TiO2 Nanoparticle Synthesis under Vacuum
2.4. Analytical Techniques Used
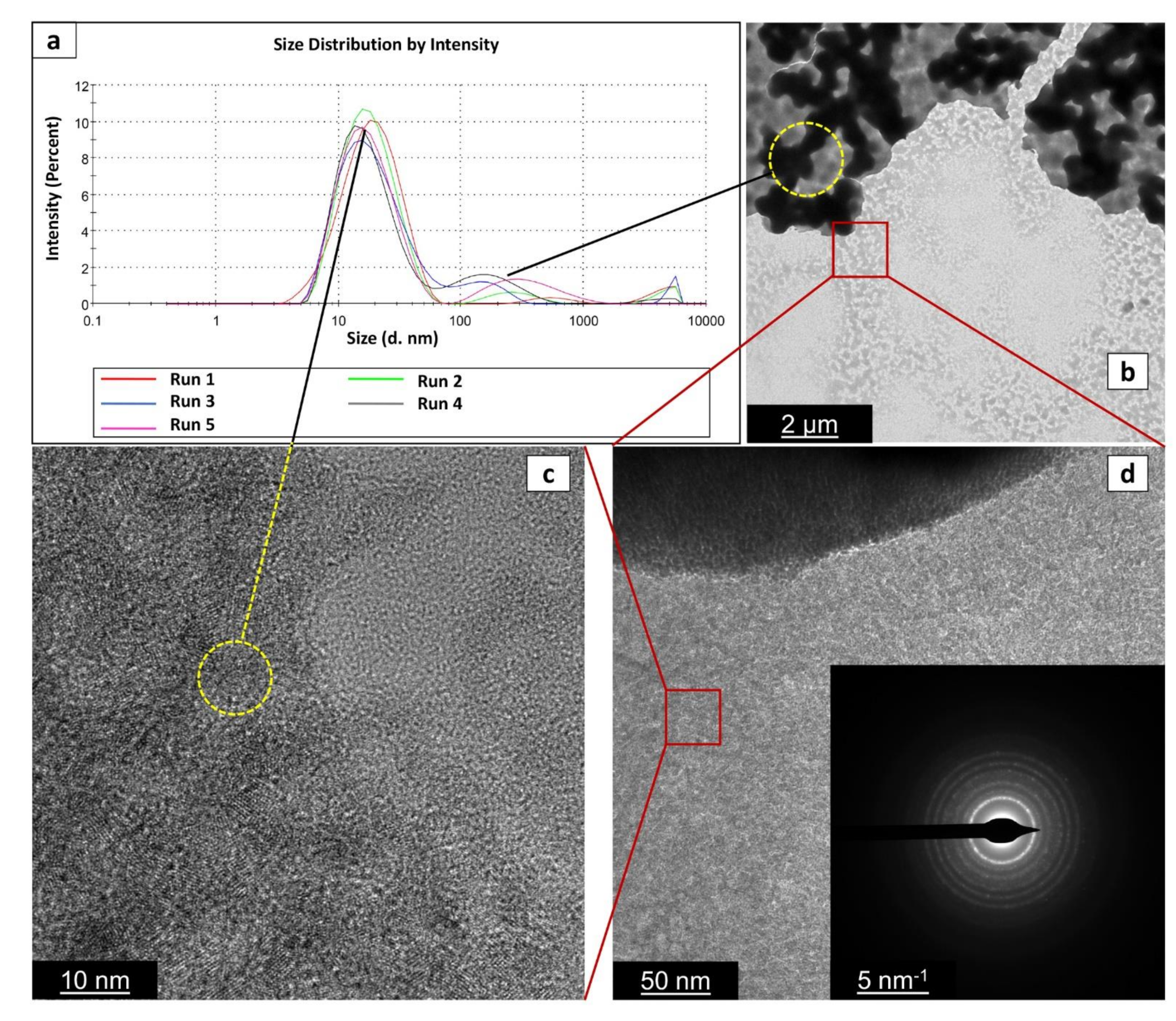
2.4.1. Size Distribution
2.4.2. XRD
2.4.3. TEM Observations
2.4.4. BET Surface Area
2.5. Photocatalytic Tests
3. Results and Discussion
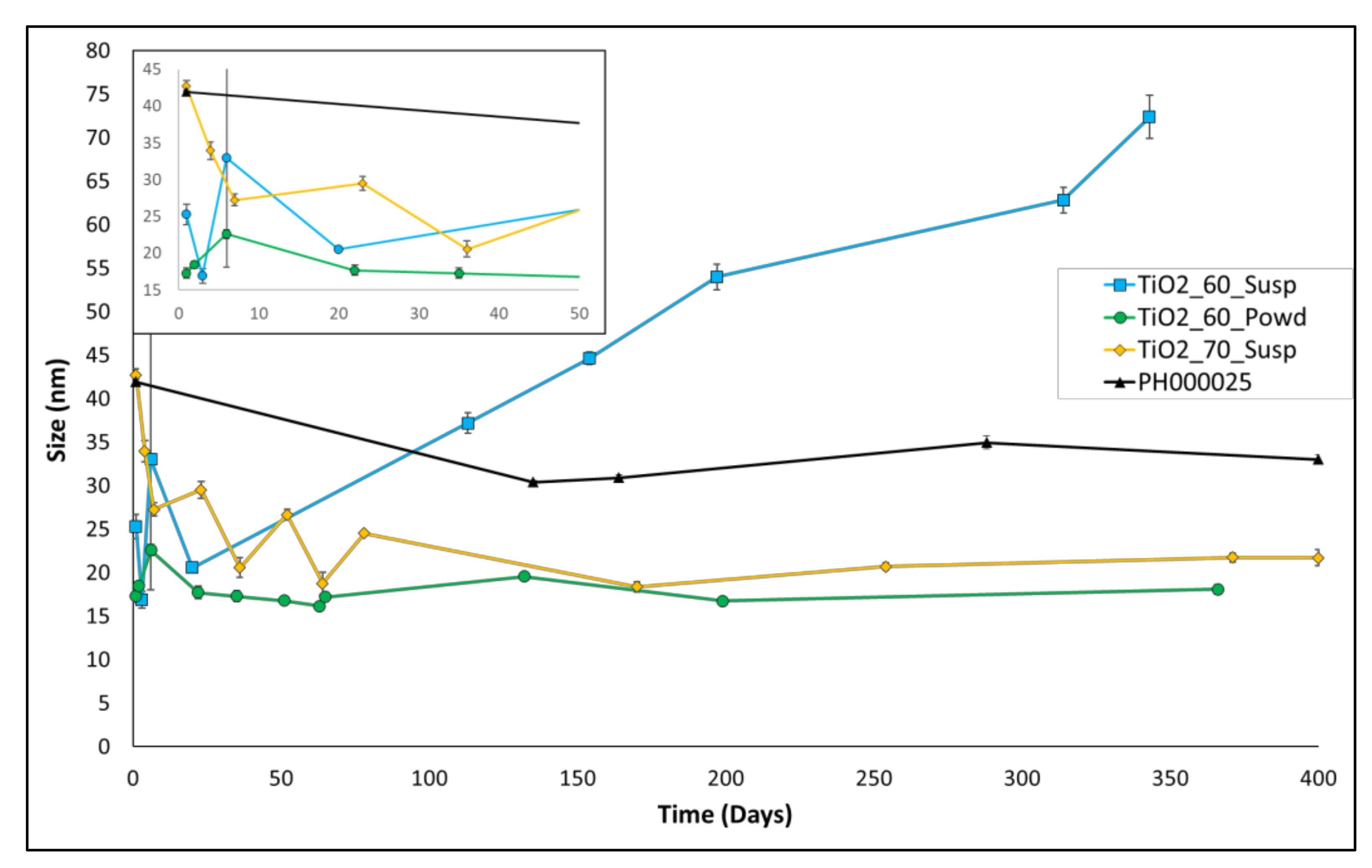
3.1. DLS Results for Long-Term Aging
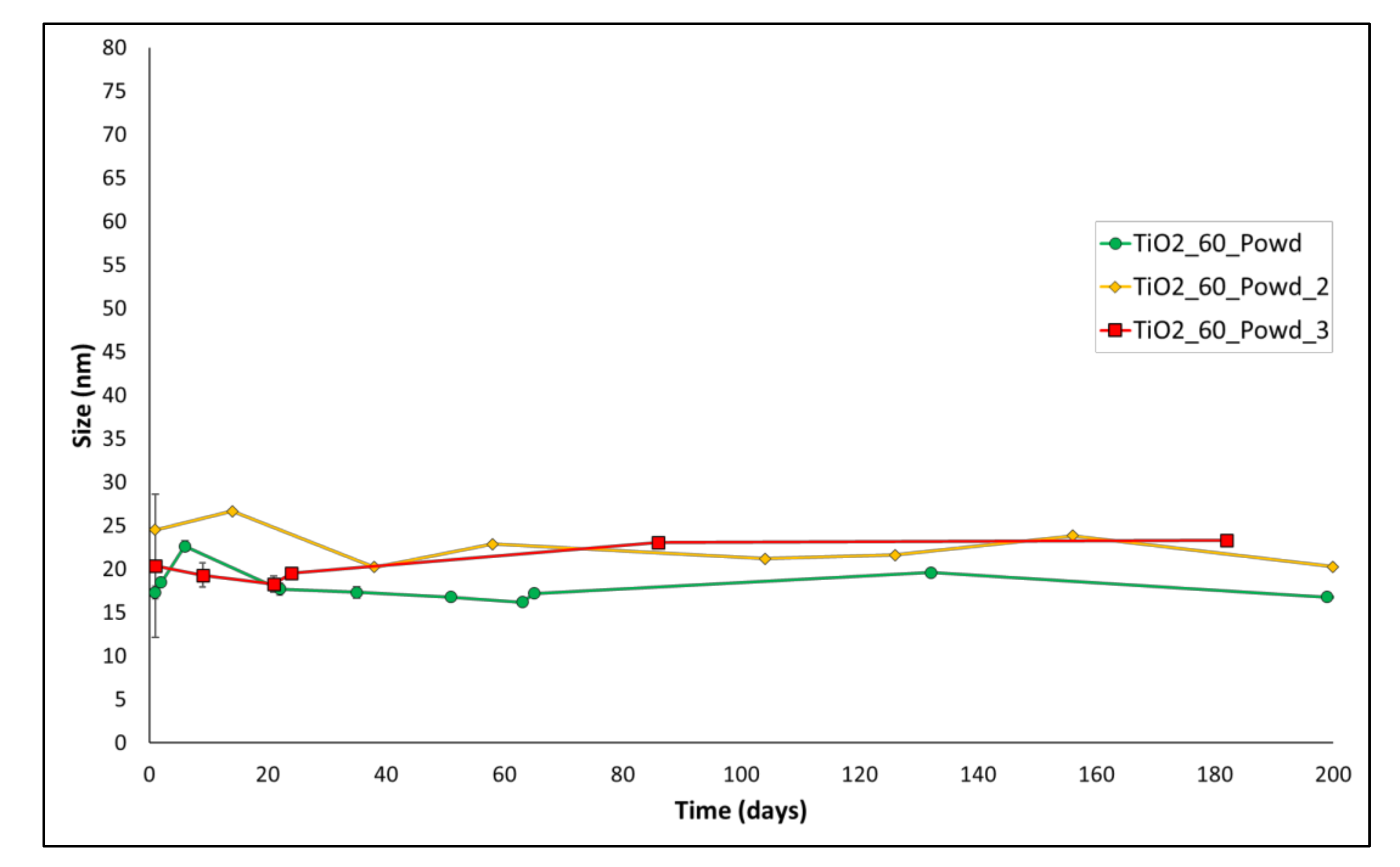
3.2. DLS Results for Reaction Reproducibility
3.3. Products Features
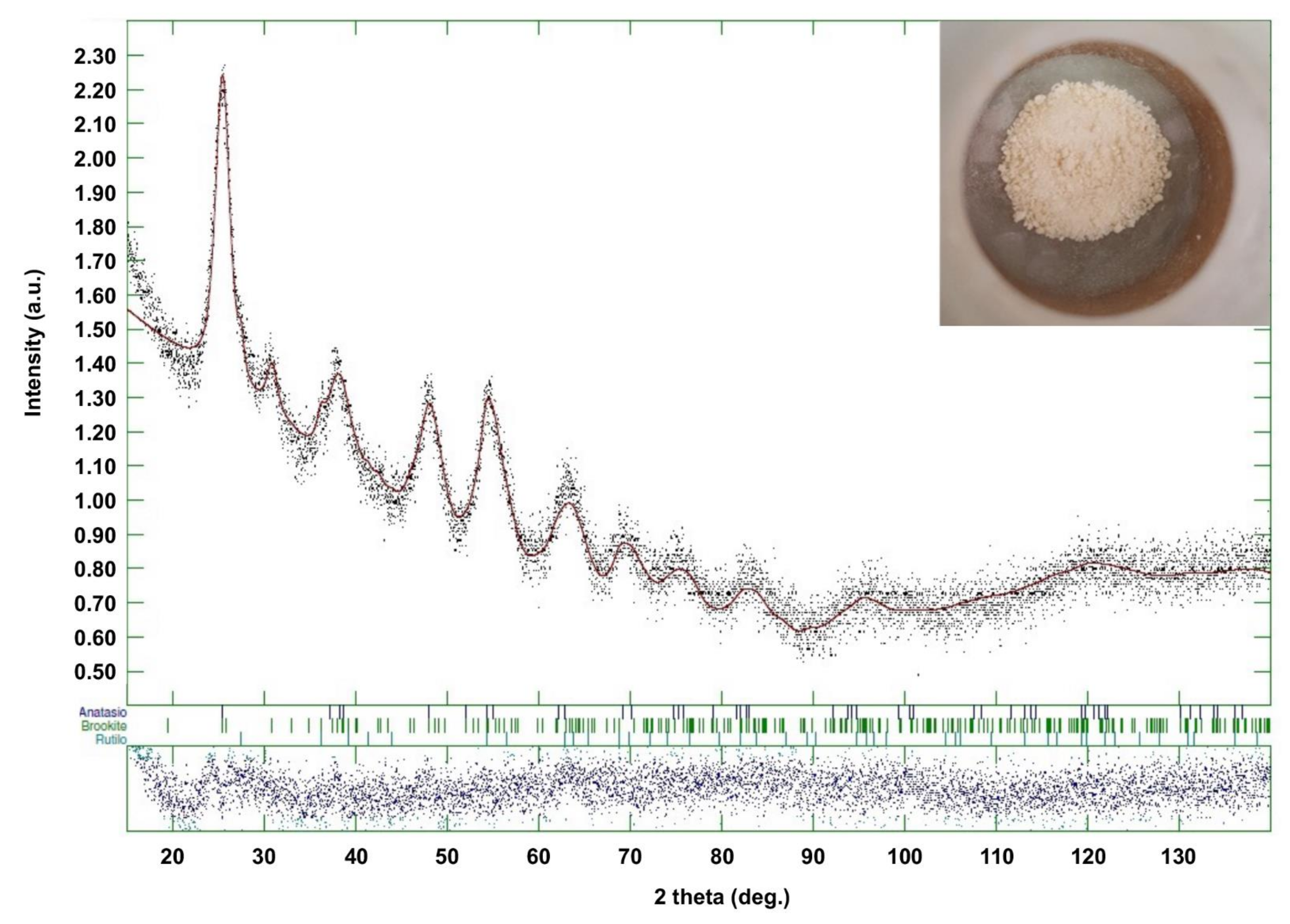
3.3.1. XRD Analysis
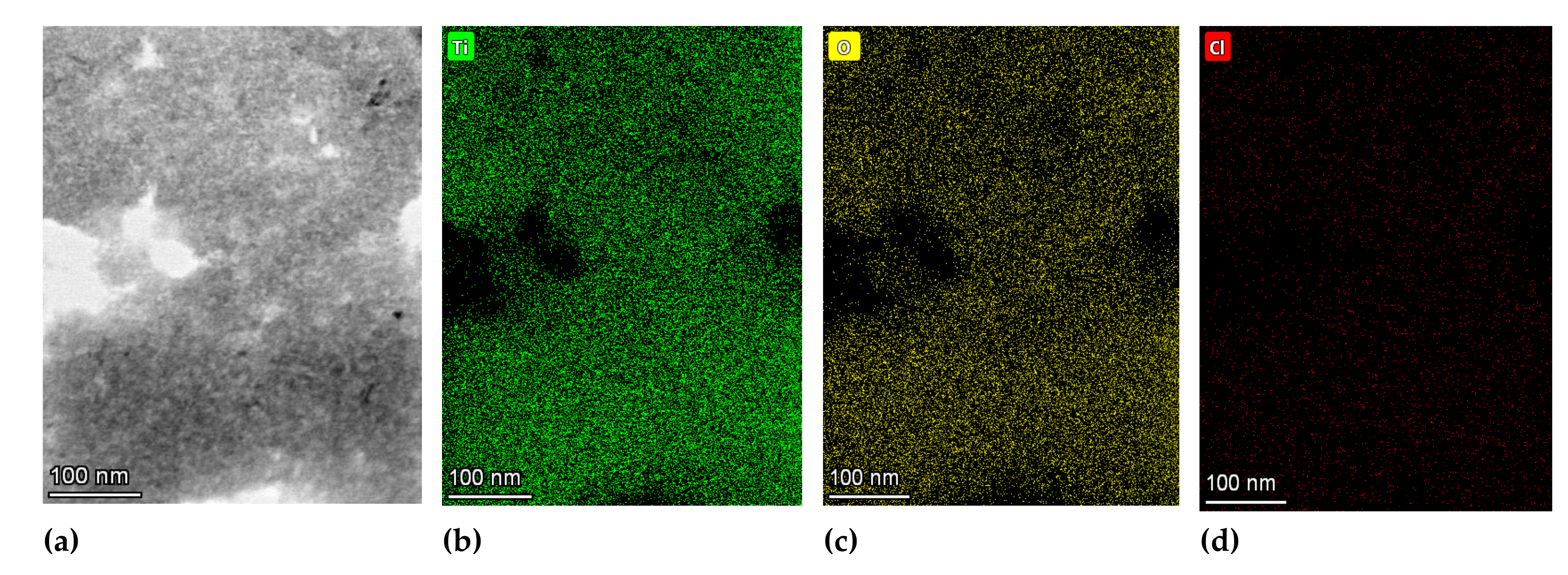
3.3.2. TEM Analysis
3.4. Results of Photocatalytic Tests (NO Removal) and BET Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Robichaud, C.O.; Uyar, A.E.; Darby, M.R.; Zucker, L.G.; Wiesner, A.R. Estimates of Upper Bounds and Trends in Nano-TiO2 Production as a Basis for Exposure Assessment. Environ. Sci. Technol. 2009, 43, 4227–4233. [Google Scholar] [CrossRef] [Green Version]
- Piccinno, F.; Gottschalk, F.; Seeger, S.; Nowack, B. Industrial Production Quantities and Uses of Ten Engineered Nanomaterials in Europe and the World. J. Nanopart. Res. 2012, 14, 1109–1120. [Google Scholar] [CrossRef] [Green Version]
- Cognitive Market Research. Preview of: Global Nano Titanium Dioxide Market Report 2021. 7th Edition. Available online: https://www.cognitivemarketresearch.com/chemical-%26-materials/nano-titanium-dioxide-market-report (accessed on 24 December 2021).
- Varner, K.E.; Rindfusz, K.; Gaglione, A.; Viveiros, E. Nano Titanium Dioxide Environmental Matters: State of the Science Literature Review; U.S. Environmental Protection Agency: Washington, DC, USA, 2010. [Google Scholar]
- Liu, K.; Cao, M.; Fujishima, A.; Jiang, L. Bio-Inspired Titanium Dioxide Materials with Special Wettability and Their Applications. Chem. Rev. 2014, 114, 10044–10094. [Google Scholar] [CrossRef]
- Haider, A.J.; Jameel, Z.N.; Al-Hussaini, I.H.M. Review on: Titanium Dioxide Applications. Energy Procedia 2019, 157, 17–29. [Google Scholar] [CrossRef]
- In 2014 a Whole Issue on the Latest Advances in Research on TiO2 Nanomaterials Was Published in the Journal Chemical Reviews: Chen, X., Selloni, A., Eds.; 2014 Titanium Dioxide Nanomaterials Reviews. Chem. Rev. 2014, 114, 9281–10216. Available online: https://pubs.acs.org/toc/chreay/114/19 (accessed on 24 December 2021).
- Ohno, T.; Sarukawa, K.; Tokieda, K.; Matsumura, M. Morphology of a TiO2 Photocatalyst (Degussa, P-25) Consisting of Anatase and Rutile Crystalline Phases. J. Catal. 2001, 203, 82–86. [Google Scholar] [CrossRef]
- Ohtani, B.; Prieto-Mahaney, O.O.; Li, D.; Abe, R. What Is Degussa (Evonik) P25? Crystalline Composition Analysis, Reconstruction from Isolated Pure Particles and Photocatalytic Activity Test. J. Photochem. Photobiol. A 2010, 216, 179–182. [Google Scholar] [CrossRef] [Green Version]
- Hurum, D.C.; Agrios, A.G.; Gray, K.A.; Rajh, T.; Thurnauer, M.C. Explaining the Enhanced Photocatalytic Activity of Degussa P25 Mixed-Phase TiO2 Using EPR. J. Phys. Chem. B 2003, 107, 4545–4549. [Google Scholar] [CrossRef]
- Stankiewicz, A.I.; Moulijn, J.A. Process Intensification: Transforming Chemical Engineering. Chem. Eng. Prog. 2000, 96, 22–33. [Google Scholar]
- Kumar, V.; Prasad Nigam, K.D. Process Intensification in Green Synthesis. Green Process Synth. 2012, 1, 79–107. [Google Scholar] [CrossRef] [Green Version]
- Keil, F.J. Process Intensification. Rev. Chem. Eng. 2018, 34, 135–200. [Google Scholar] [CrossRef] [Green Version]
- Leonelli, C.; Mason, T.J. Microwave and Ultrasonic Processing: Now a Realistic Option for Industry. Chem. Eng. Process. Process Intensif. 2010, 49, 885–900. [Google Scholar] [CrossRef]
- Martín, Á.; Navarrete, A. Microwave-Assisted Process Intensification Techniques. Curr. Opin. Green Sustain. Chem. 2018, 11, 70–75. [Google Scholar] [CrossRef]
- Li, H.; Zhao, Z.; Xiouras, C.; Stefanidis, G.D.; Li, X.; Gao, X. Fundamentals and Applications of Microwave Heating to Chemicals Separation Processes. Renew. Sust. Energ. Rev. 2019, 114, 109316–109332. [Google Scholar] [CrossRef]
- Paradisi, E.; Rosa, R.; Baldi, G.; Dami, V.; Cioni, A.; Lorenzi, G.; Leonelli, C. Effect of Isopropanol Co-Product on the Long-Term Stability of TiO2 Nanoparticle Suspensions Produced by Microwave-Assisted Synthesis. Chem. Eng. Process. Process Intensif. 2021, 159, 108242–108251. [Google Scholar] [CrossRef]
- Wu, Y.-C.; Tai, Y.-C. Effects of Alcohol Solvents on Anatase TiO2 Nanocrystals Prepared by Microwave-Assisted Solvothermal Method. J. Nanopart. Res. 2013, 15, 1686–1697. [Google Scholar] [CrossRef]
- Zhang, H.; Li, X.; Chen, G. Ionic Liquid-Facilitated Synthesis and Catalytic Activity of Highly Dispersed Ag Nanoclusters Supported on TiO2. J. Mater. Chem. 2009, 19, 8223–8231. [Google Scholar] [CrossRef]
- Feng, Q.; Cai, H.; Lin, H.; Qin, S.; Liu, Z.; Ma, D.; Ye, Y. Synthesis and Structural Characteristics of High Surface Area TiO2 Aerogels by Ultrasonic-Assisted Sol–Gel Method. Nanotechnology 2018, 29, 075702. [Google Scholar]
- Imparato, C.; Iervolino, G.; Fantauzzi, M.; Koral, C.; Macyk, W.; Kobielusz, M.; D’Errico, G.; Rea, I.; Di Girolamo, R.; De Stefano, L.; et al. Photocatalytic Hydrogen Evolution by Co-Catalyst Free TiO2/C Bulk Heterostructures Synthesized Under Mild Conditions. RSC Adv. 2020, 10, 12519–12534. [Google Scholar] [CrossRef] [Green Version]
- Kralova, D.; Slouf, M.; Klementova, M.; Kuzel, R.; Kelnar, I. Preparation of Gram Quantities of High-Quality Titanate Nanotubes and their Composites with Polyamide 6. Mater. Chem. Phys. 2010, 124, 652–657. [Google Scholar]
- Zhao, Y.; Wang, Y.; Zang, J.; Lu, J.; Xu, X. A Novel Support of Nano Titania Modified Graphitized Nanodiamond for Pt Electrocatalyst in Direct Methanol Fuel Cell. Int. J. Hydrogen Energy 2015, 40, 4540–4547. [Google Scholar] [CrossRef]
- Makal, P.; Das, D. Self-Doped TiO2 Nanowires in TiO2-B Single Phase, TiO2-B/Anatase and TiO2-Anatase/Rutile Heterojunctions Demonstrating Individual Superiority in Photocatalytic Activity Under Visible and UV Light. Appl. Surf. Sci. 2018, 455, 1106–1115. [Google Scholar]
- Tan, B.; Zhang, X.; Li, Y.; Chen, H.; Ye, X.; Wang, Y.; Ye, J. Anatase TiO2 Mesocrystals: Green Synthesis, in Situ Conversion to Porous Single Crystals, and Self-Doping Ti3+ for Enhanced Visible Light Driven Photocatalytic Removal of NO. Chem. Eur. J. 2017, 23, 5478–5487. [Google Scholar] [CrossRef] [PubMed]
- Milovac, D.; Weigand, I.; Kovačić, M.; Ivanković, M.; Ivanković, H. Highly Porous Hydroxyapatite Derived from Cuttlefish Bone as TiO2 Catalyst Support. Process. Appl. Ceram. 2018, 12, 136–142. [Google Scholar] [CrossRef]
- Liu, K.-I.; Hsueh, Y.-C.; Chen, H.-S.; Perng, T.-P. Mesoporous TiO2/WO3 Hollow Fibers with Interior Interconnected Nanotubes for Photocatalytic Application. J. Mater. Chem. A 2014, 2, 5387–5393. [Google Scholar]
- Nukunudompanich, M.; Chuangchote, S.; Okamoto, Y.; Shinoda, Y.; Suzuki, Y. TiO2 Nanorods and Semi-Nanotubes Prepared from Anodic Aluminum Oxide Template and their Applications as Photoelectrodes in Dye-Sensitized Solar Cells. J. Ceram. Soc. Jpn 2015, 123, 428–432. [Google Scholar] [CrossRef] [Green Version]
- Favaro, M.; Agnoli, S.; Di Valentin, C.; Mattevi, C.; Cattelan, M.; Artiglia, L.; Magnano, E.; Bondino, F.; Nappini, S.; Granozzi, G. TiO2/Graphene Nanocomposites from the Direct Reduction of Graphene Oxide by Metal Evaporation. Carbon 2014, 68, 319–329. [Google Scholar]
- Jung, C.-K.; Bae, I.-S.; Song, Y.-H.; Boo, J.-H. Plasma Surface Modification of TiO2 Photocatalysts for Improvement of Catalytic Efficiency. Surf. Coat. Technol. 2005, 200, 1320–1324. [Google Scholar] [CrossRef]
- Fan, Z.; Fan, L.; Shuang, S.; Dong, C. Highly Sensitive Photoelectrochemical Sensing of Bisphenol A Based on Zinc Phthalocyanine/TiO2 Nanorod Arrays. Talanta 2018, 189, 16–23. [Google Scholar] [CrossRef]
- Han, T.; Li, J.; Zhao, N.; Shi, C.; Liu, E.; He, F.; Ma, L.; Li, Q.; He, C. In-Situ Fabrication of Nano-Sized TiO2 Reinforced Cu Matrix Composites with Well-Balanced Mechanical Properties and Electrical Conductivity. Powder Technol. 2017, 321, 66–73. [Google Scholar] [CrossRef]
- Zurita-Mendez, N.N.; Carbajal-De la Torre, G.; Estevez, M.; Ballesteros-Almanza, L.; Cadenas, E.; Espinosa-Medina, M.A. Evaluation of the Electrochemical Behavior of TiO2/Al2O3/PCL Composite Coatings in Hank’s Solution. Mater. Chem. Phys. 2019, 235, 121773–121783. [Google Scholar] [CrossRef]
- Hales, M.C.; Steinberg, T.A.; Martens, W.N. Synthesis and Characterization of Titanium Sol–Gels in Varied Gravity. J. Non-Cryst. Solids 2014, 396–397, 13–19. [Google Scholar] [CrossRef] [Green Version]
- Delaney, P.; Hanrahan, J.P.; Copley, M.P.; O’Byrne, J.; Holmes, J.D.; Morris, M.A. Synthesis of Porous Silica Foams via a Novel Vacuum-Induced Sol–Gel Method. J. Am. Ceram. Soc. 2009, 92, 2798–2800. [Google Scholar] [CrossRef]
- Baldi, G.; Bitossi, M.; Barzanti, A. Method for the Preparation of Aqueous Dispersions of TiO2 in the Form of Nanoparticles, and Dispersions Obtainable with this Method. International Patent WO 2007/088151, 9 August 2007. [Google Scholar]
- Baldi, G.; Cioni, A.; Dami, V. Apparatus for Determining the Residual Quantity of Polluting Agents in a Gas Mixture and Process for Determining Such Quantity. International Patent WO 2011/016061, 10 February 2011. [Google Scholar]
- Yin, S.; Aita, Y.; Komatsu, M.; Wang, J.; Tang, Q.; Sato, T. Synthesis of Excellent Visible-Light Responsive TiO2-xNy Photocatalyst by a Homogeneous Precipitation-Solvothermal Process. J. Mater. Chem. 2005, 15, 674–682. [Google Scholar] [CrossRef]
- Esparza, Y.; Ngo, T.-D.; Fraschini, C.; Boluk, Y. Aggregate Morphology and Aqueous Dispersibility of Spray-Dried Powders of Cellulose Nanocrystals. Ind. Eng. Chem. Res. 2019, 58, 19926–19936. [Google Scholar]
- Cano-Casanova, L.; Amorós-Pérez, A.; Lillo-Ródenas, M.Á.; Román-Martínez, M.C. Effect of the Preparation Method (Sol-Gel or Hydrothermal) and Conditions on the TiO2 Properties and Activity for Propene Oxidation. Materials 2018, 11, 2227–2245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Sun, P.; Jiang, P.; Guo, Z.; Liu, W.; Lu, Q.; Cao, W. Formation Mechanism of TiO2 Polymorphs under Hydrothermal Conditions Based on Structure Evolution of [Ti(OH)h(H2O)6-h]4-h Monomers. J. Mater. Chem. C 2019, 7, 5764–5771. [Google Scholar] [CrossRef]
- Andrade-Guel, M.; Díaz-Jiménez, L.; Cortés-Hernández, D.; Cabello-Alvarado, C.; Ávila-Orta, C.; Bartolo-Pérez, P.; Gamero-Melo, P. Microwave Assisted Sol–Gel Synthesis of Titanium Dioxide Using Hydrochloric and Acetic Acid as Catalysts. Bol. Soc. Esp. Ceram. V. 2019, 58, 171–177. [Google Scholar] [CrossRef]
- Yusmaman, W.M.; Gunlazuardi, J. The Role of Citric Acid Modifiers Addition in the Preparation of TiO2 Nanoparticles with the Solvothermal Method. AIP Conf. Proc. 2020, 2243, 020033. [Google Scholar]
- Qamar, M.; Merzougui, B.; Anjum, D.; Hakeem, A.S.; Yamani, Z.H.; Bahnemann, D. Synthesis and Photocatalytic Activity of Mesoporous Nanocrystalline Fe-Doped Titanium Dioxide. Catal. Today 2014, 230, 158–165. [Google Scholar] [CrossRef]
- Wang, Y.; Zou, Y.; Shang, Q.; Tan, X.; Yu, T.; Huang, X.; Shi, W.; Xie, Y.; Yan, G.; Wang, X. Quasi-Spherical Brookite TiO2 Nanostructures Synthesized Using Solvothermal Method in the Presence of Oxalic Acid. Trans. Tianjin Univ. 2018, 24, 326–339. [Google Scholar] [CrossRef] [Green Version]
- Su, C.; Lin, K.-F.; Lin, Y.-H.; You, B.-H. Preparation and Characterization of High-Surface-Area Titanium Dioxide by Sol-Gel Process. J. Porous Mater. 2006, 13, 251–258. [Google Scholar]
- Reza, K.M.; Kurny, A.S.W.; Gulshan, F. Parameters Affecting the Photocatalytic Degradation of Dyes Using TiO2: A Review. Appl. Water Sci. 2017, 7, 1569–1578. [Google Scholar] [CrossRef] [Green Version]
- Azeez, F.; Al-Hetlani, E.; Arafa, M.; Abdelmonem, Y.; Nazeer, A.A.; Amin, M.O.; Madkour, M. The Effect of Surface Charge on Photocatalytic Degradation of Methylene Blue Dye Using Chargeable Titania Nanoparticles. Sci. Rep. 2018, 8, 7104–7113. [Google Scholar] [CrossRef]
- Marugán, J.; Christensen, P.; Egerton, T.; Purnama, H. Influence of the Synthesis pH on the Properties and Activity of Sol-Gel TiO2 Photocatalysts. Int. J. Photoenergy 2008, 2008, 759561. [Google Scholar] [CrossRef]
- Cano-Casanova, L.; Amorós-Pérez, A.; Ouzzine, M.; Lillo-Ródenas, M.A.; Román-Martínez, M.C. One Step Hydrothermal Synthesis of TiO2 with Variable HCl Concentration: Detailed Characterization and Photocatalytic Activity in Propene Oxidation. Appl. Catal. B 2018, 220, 645–653. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.-S.; Zhou, G. Effect of Surface Protonation of TiO2 on Charge Recombination and Conduction Band Edge Movement in Dye-Sensitized Solar Cells. J. Phys. Chem. C 2009, 113, 15417–15421. [Google Scholar] [CrossRef]
- Park, S.-K.; Shin, H. Effect of HCl and H2SO4 Treatment of TiO2 Powder on the Photosensitized Degradation of Aqueous Rhodamine B Under Visible Light. J. Nanosci. Nanotechnol. 2014, 14, 8122–8128. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.-e.; Niu, X.; Li, W.; An, J.; Liu, Y.; Chen, Y.; Wang, P.; Yang, X.; Feng, Q. Microwave-Assisted Synthesis of High-Energy Faceted TiO2 Nanocrystals Derived from Exfoliated Porous Metatitanic Acid Nanosheets with Improved Photocatalytic and Photovoltaic Performance. Materials 2019, 12, 3614–3636. [Google Scholar] [CrossRef] [Green Version]
- Sugimoto, T.; Zhou, X.; Muramatsu, A. Synthesis of Uniform Anatase TiO2 Nanoparticles by Gel–Sol Method. J. Colloid Interface Sci. 2002, 252, 339–346. [Google Scholar] [CrossRef]
- Mahshid, S.; Askari, M.; Sasani Ghamsari, M. Synthesis of TiO2 Nanoparticles by Hydrolysis and Peptization of Titanium Isopropoxide Solution. J. Mater. Process. Technol. 2007, 189, 296–300. [Google Scholar] [CrossRef]
- Tsega, M.; Dejene, F.B. Influence of Acidic pH on the Formulation of TiO2 Nanocrystalline Powders with Enhanced Photoluminescence Property. Heliyon 2017, 3, e00246. [Google Scholar] [CrossRef] [Green Version]
- Vorontsov, A.V.; Valdés, H.; Smirniotis, P.G.; Paz, Y. Recent Advancements in the Understanding of the Surface Chemistry in TiO2 Photocatalysis. Surfaces 2020, 3, 72–92. [Google Scholar] [CrossRef] [Green Version]
- Gomes, J.; Lincho, J.; Domingues, E.; Quinta-Ferreira, R.M.; Martins, R.C. N–TiO2 Photocatalysts: A Review of Their Characteristics and Capacity for Emerging Contaminants Removal. Water 2019, 11, 373–408. [Google Scholar] [CrossRef] [Green Version]
- Vorontsov, A.V.; Kabachkov, E.N.; Balikhin, I.L.; Kurkin, E.N.; Troitskii, V.N.; Smirniotis, P.G. Correlation of Surface Area with Photocatalytic Activity of TiO2. J. Adv. Oxid. Technol. 2018, 21, 127–137. [Google Scholar] [CrossRef]
- Simonsen, M.E.; Jensen, H.; Li, Z.; Søgaard, E.G. Surface Properties and Photocatalytic Activity of Nanocrystalline Titania Films. J. Photochem. Photobiol. A 2008, 200, 192–200. [Google Scholar] [CrossRef] [Green Version]
- Qiu, W.; Ren, C.-J.; Gong, M.-C.; Hou, Y.-Z.; Chen, Y.-Q. Structure, Surface Properties and Photocatalytic Activity of TiO2 and TiO2/SiO2 Catalysts Prepared at Different pH Values. Acta Phys. Chim. Sin. 2011, 27, 1487–1492. [Google Scholar] [CrossRef]
- Yeung, K.L.; Yau, S.T.; Maira, A.J.; Coronado, J.M.; Soria, J.; Yue, P.L. The Influence of Surface Properties on the Photocatalytic Activity of Nanostructured TiO2. J. Catal. 2003, 219, 107–116. [Google Scholar] [CrossRef]
- Di Paola, A.; Bellardita, M.; Palmisano, L.; Barbierikova, Z.; Brezova, V. Influence of Crystallinity and OH Surface Density on the Photocatalytic Activity of TiO2 Powders. J. Photochem. Photobiol. A 2014, 273, 59–67. [Google Scholar] [CrossRef]

| Name | Temperature | Time | Final Appearance | Suspension Appearance |
|---|---|---|---|---|
| TiO2_50_Susp | 50 °C | 30 min | White suspension | White suspension |
| TiO2_60_Susp | 60 °C | 25 min | Thick white gel | Cloudy suspension |
| TiO2_60_Powd | 60 °C | 40 min | Beige powder | Cloudy yellow suspension |
| TiO2_70_Susp | 70 °C | 25 min | Thick white gel | Cloudy suspension |
| TiO2_70_Powd | 70 °C | 40 min | Beige powder | White suspension |
| Entry | Name | Z-Average (24 h of Aging) |
|---|---|---|
| 1 | PH000025 (Industrial product) | 41.9 ± 0.3 nm |
| 2 | TiO2_50_Susp | 273 ± 68 nm |
| 3 | TiO2_60_Susp | 25 ± 1 nm |
| 4 | TiO2_60_Powd | 17.3 ± 0.4 nm |
| 5 | TiO2_70_Susp | 42 ± 1 nm |
| 6 | TiO2_70_Powd | 215 ± 13 nm |
| Entry | Name | Z-Average (24 h of Aging) |
|---|---|---|
| 1 | TiO2_60_Powd | 17.3 ± 0.4 nm |
| 2 | TiO2_60_Powd_2 | 24 ± 2 nm |
| 3 | TiO2_60_Powd_3 | 20 ± 1 nm |
| Batch | Anatase (%) (Crystal Size (nm)) | Brookite (%) (Crystal Size (nm)) | Rutile (%) (Crystal Size (nm)) |
|---|---|---|---|
| PH000025 (Industrial Product) | 63 (5) | 32 (4) | 5 (9) |
| TiO2_60_Powd | 71 (5) | 24 (31) | 4 (6) |
| TiO2_60_Powd_2 | 64 (2) | 33 (4) | 3 (22) |
| TiO2_60_Powd_3 | 62 (4) | 30 (3) | 8 (7) |
| Batch | NO Depletion after 105 min | BET Surface Area |
|---|---|---|
| PH000025 (Industrial product) | 78% | 192.7869 m2/g |
| TiO2_60_Powd | 75% | 123.2593 m2/g |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paradisi, E.; Rosa, R.; Baldi, G.; Dami, V.; Cioni, A.; Lorenzi, G.; Leonelli, C. Microwave-Assisted Vacuum Synthesis of TiO2 Nanocrystalline Powders in One-Pot, One-Step Procedure. Nanomaterials 2022, 12, 149. https://doi.org/10.3390/nano12010149
Paradisi E, Rosa R, Baldi G, Dami V, Cioni A, Lorenzi G, Leonelli C. Microwave-Assisted Vacuum Synthesis of TiO2 Nanocrystalline Powders in One-Pot, One-Step Procedure. Nanomaterials. 2022; 12(1):149. https://doi.org/10.3390/nano12010149
Chicago/Turabian StyleParadisi, Enrico, Roberto Rosa, Giovanni Baldi, Valentina Dami, Andrea Cioni, Giada Lorenzi, and Cristina Leonelli. 2022. "Microwave-Assisted Vacuum Synthesis of TiO2 Nanocrystalline Powders in One-Pot, One-Step Procedure" Nanomaterials 12, no. 1: 149. https://doi.org/10.3390/nano12010149