Improved One- and Multiple-Photon Excited Photoluminescence from Cd2+-Doped CsPbBr3 Perovskite NCs
Abstract
:1. Introduction
2. Materials and Methods
3. Results
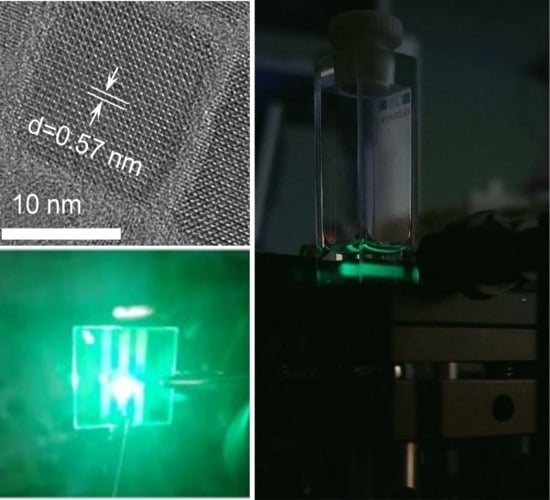
3.1. Synthesis and Characterization of NCs
3.2. One-Photon Excited PL
3.3. LEDs
3.4. Multiphoton-Induced PL
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Calculation of Perovskite NC Molar Concentration
Appendix B. Multiphoton Absorption Cross-Section Calculation
References
- Kumawat, N.K.; Yuan, Z.; Bai, S.; Gao, F. Metal Doping/Alloying of Cesium Lead Halide Perovskite Nanocrystals and their Applications in Light-Emitting Diodes with Enhanced Efficiency and Stability. Isr. J. Chem. 2019, 59, 695–707. [Google Scholar] [CrossRef]
- Xu, L.; Yuan, S.; Zeng, H.; Song, J. A comprehensive review of doping in perovskite nanocrystals/quantum dots: Evolution of structure, electronics, optics, and light-emitting diodes. Mater. Today Nano 2019, 6, 100036. [Google Scholar] [CrossRef]
- Lu, C.-H.; Biesold-McGee, G.V.; Liu, Y.; Kang, Z.; Lin, Z. Doping and ion substitution in colloidal metal halide perovskite nanocrystals. Chem. Soc. Rev. 2020, 49, 4953–5007. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Y.; Hong, M. Cation-doping matters in caesium lead halide perovskite nanocrystals: From physicochemical fundamentals to optoelectronic applications. Nanoscale 2020, 12, 12228–12248. [Google Scholar] [CrossRef]
- Shen, X.; Zhang, Y.; Kershaw, S.V.; Li, T.; Wang, C.; Zhang, X.; Wang, W.; Li, D.; Wang, Y.; Lu, M.; et al. Zn-Alloyed CsPbI3 Nanocrystals for Highly Efficient Perovskite Light-Emitting Devices. Nano Lett. 2019, 19, 1552–1559. [Google Scholar] [CrossRef]
- Zhang, R.; Yuan, Y.; Li, J.; Qin, Z.; Zhang, Q.; Xiong, B.; Wang, Z.; Chen, F.; Du, X.; Yang, W. Ni and K ion doped CsPbX3 NCs for the improvement of luminescence properties by a facile synthesis method in ambient air. J. Lumin. 2020, 221, 117044. [Google Scholar] [CrossRef]
- Chen, J.-K.; Ma, J.-P.; Guo, S.-Q.; Chen, Y.-M.; Zhao, Q.; Zhang, B.-B.; Li, Z.-Y.; Zhou, Y.; Hou, J.; Kuroiwa, Y.; et al. High-Efficiency Violet-Emitting All-Inorganic Perovskite Nanocrystals Enabled by Alkaline-Earth Metal Passivation. Chem. Mater. 2019, 31, 3974–3983. [Google Scholar] [CrossRef]
- Yong, Z.-J.; Guo, S.-Q.; Ma, J.-P.; Zhang, J.-Y.; Li, Z.-Y.; Chen, Y.-M.; Zhang, B.-B.; Zhou, Y.; Shu, J.; Gu, J.-L.; et al. Doping-Enhanced Short-Range Order of Perovskite Nanocrystals for Near-Unity Violet Luminescence Quantum Yield. J. Am. Chem. Soc. 2018, 140, 9942–9951. [Google Scholar] [CrossRef] [Green Version]
- Pan, G.C.; Bai, X.; Xu, W.; Chen, X.; Zhai, Y.; Zhu, J.; Shao, H.; Ding, N.; Xu, L.; Dong, B.; et al. Bright Blue Light Emission of Ni2+ Ion-Doped CsPbClxBr3−x Perovskite Quantum Dots Enabling Efficient Light-Emitting Devices. ACS Appl. Mater. Interfaces 2020, 12, 14195–14202. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Chou, H.-L.; Lin, J.-C.; Lee, Y.-C.; Pao, C.-W.; Chen, J.-L.; Chang, C.-C.; Chi, R.-Y.; Kuo, T.-R.; Lu, C.-W.; et al. Enhanced Luminescence and Stability of Cesium Lead Halide Perovskite CsPbX3 Nanocrystals by Cu2+-Assisted Anion Exchange Reactions. J. Phys. Chem. C 2019, 123, 2353–2360. [Google Scholar] [CrossRef]
- Van der Stam, W.; Geuchies, J.J.; Altantzis, T.; van den Bos, K.H.W.; Meeldijk, J.D.; Van Aert, S.; Bals, S.; Vanmaekelbergh, D.; De Mello Donega, C.D.M. Highly Emissive Divalent-Ion-Doped Colloidal CsPb1−xMxBr3 Perovskite Nanocrystals through Cation Exchange. J. Am. Chem. Soc. 2017, 139, 4087–4097. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; Huang, H.; Kershaw, S.V.; Rogach, A.L. Advances in metal halide perovskite nanocrystals: Synthetic strategies, growth mechanisms, and optoelectronic applications. Mater. Today 2020, 32, 204–221. [Google Scholar] [CrossRef]
- Shamsi, J.; Urban, A.S.; Imran, M.; De Trizio, L.; Manna, L. Metal Halide Perovskite Nanocrystals: Synthesis, Post-Synthesis Modifications, and Their Optical Properties. Chem. Rev. 2019, 119, 3296–3348. [Google Scholar] [CrossRef]
- Liu, M.; Grandhi, G.K.; Matta, S.; Mokurala, K.; Litvin, A.; Russo, S.; Vivo, P. Halide Perovskite Nanocrystal Emitters. Adv. Photonics Res. 2021, 2, 2000118. [Google Scholar] [CrossRef]
- Yan, F.; Tan, S.T.; Li, X.; Demir, H.V. Light Generation in Lead Halide Perovskite Nanocrystals: LEDs, Color Converters, Lasers, and Other Applications. Small 2019, 15, e1902079. [Google Scholar] [CrossRef]
- Gualdrón-Reyes, A.F.; Masi, S.; Mora-Seró, I. Progress in halide-perovskite nanocrystals with near-unity photoluminescence quantum yield. Trends Chem. 2021, 3, 499–511. [Google Scholar] [CrossRef]
- Dey, A.; Ye, J.; De, A.; Debroye, E.; Ha, S.K.; Bladt, E.; Kshirsagar, A.S.; Wang, Z.; Yin, J.; Wang, Y.; et al. State of the Art and Prospects for Halide Perovskite Nanocrystals. ACS Nano 2021, 15, 10775–10981. [Google Scholar] [CrossRef]
- Zou, S.; Liu, Y.; Li, J.; Liu, C.; Feng, R.; Jiang, F.; Li, Y.; Song, J.; Zeng, H.; Hong, M.; et al. Stabilizing Cesium Lead Halide Perovskite Lattice through Mn(II) Substitution for Air-Stable Light-Emitting Diodes. J. Am. Chem. Soc. 2017, 139, 11443–11450. [Google Scholar] [CrossRef]
- Wang, H.-C.; Wang, W.; Tang, A.-C.; Tsai, H.-Y.; Bao, Z.; Ihara, T.; Yarita, N.; Tahara, H.; Kanemitsu, Y.; Chen, S.; et al. High-Performance CsPb1−xSnxBr3 Perovskite Quantum Dots for Light-Emitting Diodes. Angew. Chem. 2017, 129, 13838–13842. [Google Scholar] [CrossRef]
- Yao, J.-S.; Ge, J.; Han, B.-N.; Wang, K.-H.; Yao, H.-B.; Yu, H.-L.; Li, J.-H.; Zhu, B.-S.; Song, J.-Z.; Chen, C.; et al. Ce3+-Doping to Modulate Photoluminescence Kinetics for Efficient CsPbBr3 Nanocrystals Based Light-Emitting Diodes. J. Am. Chem. Soc. 2018, 140, 3626–3634. [Google Scholar] [CrossRef]
- Todorović, P.; Ma, D.; Chen, B.; Quintero-Bermudez, R.; Saidaminov, M.I.; Dong, Y.; Lu, Z.; Sargent, E.H. Spectrally Tunable and Stable Electroluminescence Enabled by Rubidium Doping of CsPbBr3 Nanocrystals. Adv. Opt. Mater. 2019, 7, 1470–1474. [Google Scholar] [CrossRef]
- Yang, H.; Yin, W.; Dong, W.; Gao, L.; Tan, C.-H.; Li, W.; Zhang, X.; Zhang, J. Enhancing the light-emitting performance and stability in CsPbBr3 perovskite quantum dots via simultaneous doping and surface passivation. J. Mater. Chem. C 2020, 8, 14439–14445. [Google Scholar] [CrossRef]
- Hoang, M.T.; Pannu, A.S.; Tang, C.; Yang, Y.; Pham, N.D.; Gui, K.; Wang, X.; Yambem, S.; Sonar, P.; Du, A.; et al. Potassium Doping to Enhance Green Photoemission of Light-Emitting Diodes Based on CsPbBr3 Perovskite Nanocrystals. Adv. Opt. Mater. 2020, 8, 2000742. [Google Scholar] [CrossRef]
- Xu, J.; Li, X.; Xiong, J.; Yuan, C.; Semin, S.; Rasing, T.; Bu, X. Halide Perovskites for Nonlinear Optics. Adv. Mater. 2020, 32, e1806736. [Google Scholar] [CrossRef] [PubMed]
- Denk, W.; Strickler, J.H.; Webb, W.W. Two-photon laser scanning fluorescence microscopy. Science 1990, 248, 73–76. [Google Scholar] [CrossRef] [Green Version]
- Hell, S.W.; Bahlmann, K.; Schrader, M.; Soini, A.; Malak, H.M.; Gryczynski, I.; Lakowicz, J.R. Three-photon excitation in fluorescence microscopy. J. Biomed. Opt. 1996, 1, 71–74. [Google Scholar] [CrossRef]
- Yu, J.H.; Kwon, S.-H.; Petrasek, Z.; Park, O.K.; Jun, S.W.; Shin, K.; Choi, M.; Park, Y.I.; Park, K.; Bin Na, H.; et al. High-resolution three-photon biomedical imaging using doped ZnS nanocrystals. Nat. Mater. 2013, 12, 359–366. [Google Scholar] [CrossRef]
- Tong, L.; Cobley, C.M.; Chen, J.; Xia, Y.; Cheng, J.-X. Bright Three-Photon Luminescence from Gold/Silver Alloyed Nanostructures for Bioimaging with Negligible Photothermal Toxicity. Angew. Chem. Int. Ed. 2010, 49, 3485–3488. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Zhang, F.; Wang, C.; Jia, M.; Zhao, X.; Liu, Z.; Ge, Y.; Zhang, Y.; Zhang, H. Nonlinear Photonics Using Low-Dimensional Metal-Halide Perovskites: Recent Advances and Future Challenges. Adv. Mater. 2021, 33, 2004446. [Google Scholar] [CrossRef]
- Shen, W.; Chen, J.; Wu, J.; Li, X.; Zeng, H. Nonlinear Optics in Lead Halide Perovskites: Mechanisms and Applications. ACS Photonics 2021, 8, 113–124. [Google Scholar] [CrossRef]
- Ketavath, R.; Katturi, N.K.; Ghugal, S.G.; Kolli, H.K.; Swetha, T.; Soma, V.R.; Murali, B. Deciphering the Ultrafast Nonlinear Optical Properties and Dynamics of Pristine and Ni-Doped CsPbBr3 Colloidal Two-Dimensional Nanocrystals. J. Phys. Chem. Lett. 2019, 10, 5577–5584. [Google Scholar] [CrossRef]
- He, T.; Li, J.; Ren, C.; Xiao, S.; Li, Y.; Chen, R.; Lin, X. Strong two-photon absorption of Mn-doped CsPbCl3 perovskite nanocrystals. Appl. Phys. Lett. 2017, 111, 211105. [Google Scholar] [CrossRef] [Green Version]
- He, T.; Li, J.; Qiu, X.; Xiao, S.; Lin, X. Superior multiphoton absorption properties in colloidal Mn-doped CsPbCl3 two-dimensional nanoplatelets. Photonics Res. 2018, 6, 1021–1027. [Google Scholar] [CrossRef]
- Zhao, Y.; Shen, C.; Ding, L.; Liu, J.; Xiang, W.; Liang, X. Novel B-site Cd2+ doped CsPbBr3 quantum dot glass toward strong fluorescence and high stability for wLED. Opt. Mater. 2020, 107, 110046. [Google Scholar] [CrossRef]
- Mondal, N.; De, A.; Samanta, A. Achieving Near-Unity Photoluminescence Efficiency for Blue-Violet-Emitting Perovskite Nanocrystals. ACS Energy Lett. 2019, 4, 32–39. [Google Scholar] [CrossRef]
- Xie, C.; Zhao, Y.; Shi, W.; Yang, P. Postsynthetic Surface-Treatment of CsPbX3 (X = Cl, Br, or I) Nanocrystals via CdX2 Precursor Solution toward High Photoluminescence Quantum Yield. Langmuir 2021, 37, 1183–1193. [Google Scholar] [CrossRef] [PubMed]
- Parfenov, P.S.; Litvin, A.P.; Baranov, A.V.; Ushakova, E.V.; Fedorov, A.V.; Prudnikov, A.V.; Artemyev, M.V. Measurement of the luminescence decay times of PbS quantum dots in the near-IR spectral range. Opt. Spectrosc. 2012, 112, 868–873. [Google Scholar] [CrossRef]
- Parfenov, P.S.; Litvin, A.P.; Ushakova, E.V.; Fedorov, A.V.; Baranov, A.V.; Berwick, K. Note: Near infrared spectral and transient measurements of PbS quantum dots luminescence. Rev. Sci. Instrum. 2013, 84, 116104. [Google Scholar] [CrossRef]
- Skurlov, I.D.; Onishchuk, D.A.; Parfenov, P.S.; Litvin, A.P. An Experimental Setup for Analysis of Weak Photoluminescence in the Near-Infrared Spectral Region. Opt. Spectrosc. 2018, 125, 756–759. [Google Scholar] [CrossRef]
- Han, Q.; Wu, W.; Liu, W.; Yang, Q.; Yang, Y. Temperature-dependent photoluminescence of CsPbX3 nanocrystal films. J. Lumin. 2018, 198, 350–356. [Google Scholar] [CrossRef]
- Das, A.; Arefina, I.A.; Danilov, D.V.; Koroleva, A.V.; Zhizhin, E.V.; Parfenov, P.S.; Kuznetsova, V.A.; Ismagilov, A.O.; Litvin, A.P.; Fedorov, A.V.; et al. Chiral carbon dots based on l/d-cysteine produced via room temperature surface modification and one-pot carbonization. Nanoscale 2021, 13, 8058–8066. [Google Scholar] [CrossRef]
- Albota, M.A.; Xu, C.; Webb, W.W. Two-photon fluorescence excitation cross sections of biomolecular probes from 690 to 960 nm. Appl. Opt. 1998, 37, 7352–7356. [Google Scholar] [CrossRef] [Green Version]
- Kapoor, R.; Friend, C.S.; Patra, A. Two-photon-excited absolute emission cross-sectional measurements calibrated with a luminance meter. J. Opt. Soc. Am. B 2003, 20, 1550–1554. [Google Scholar] [CrossRef]
- Fischer, A.; Cremer, C.; Stelzer, E.H.K. Fluorescence of coumarins and xanthenes after two-photon absorption with a pulsed titanium–sapphire laser. Appl. Opt. 1995, 34, 1989–2003. [Google Scholar] [CrossRef]
- Lanin, A.A.; Chebotarev, A.S.; Pochechuev, M.S.; Kelmanson, I.V.; Kotova, D.A.; Bilan, D.S.; Ermakova, Y.G.; Fedotov, A.B.; Ivanov, A.A.; Belousov, V.V.; et al. Two- and three-photon absorption cross-section characterization for high-brightness, cell-specific multiphoton fluorescence brain imaging. J. Biophotonics 2020, 13, e201900243. [Google Scholar] [CrossRef]
- Navas, J.; Sánchez-Coronilla, A.; Gallardo, J.J.; Cruz Hernández, N.; Piñero, J.C.; Alcántara, R.; Fernández-Lorenzo, C.; De los Santos, D.M.; Aguilar, T.; Martín-Calleja, J. New insights into organic–inorganic hybrid perovskite CH3NH3PbI3 nanoparticles. An experimental and theoretical study of doping in Pb2+ sites with Sn2+, Sr2+, Cd2+ and Ca2+. Nanoscale 2015, 7, 6216–6229. [Google Scholar] [CrossRef]
- Cai, T.; Yang, H.; Hills-Kimball, K.; Song, J.-P.; Zhu, H.; Hofman, E.; Zheng, W.; Rubenstein, B.M.; Chen, O. Synthesis of All-Inorganic Cd-Doped CsPbCl3 Perovskite Nanocrystals with Dual-Wavelength Emission. J. Phys. Chem. Lett. 2018, 9, 7079–7084. [Google Scholar] [CrossRef]
- Varshni, Y.P. Temperature dependence of the energy gap in semiconductors. Physica 1967, 34, 149–154. [Google Scholar] [CrossRef]
- Lee, W.; Li, H.; Wong, A.; Zhang, D.; Lai, M.; Yu, Y.; Kong, Q.; Lin, E.; Urban, J.J.; Grossman, J.C.; et al. Ultralow thermal conductivity in all-inorganic halide perovskites. Proc. Natl. Acad. Sci. USA 2017, 114, 8693–8697. [Google Scholar] [CrossRef] [Green Version]
- Saran, R.; Heuer-Jungemann, A.; Kanaras, A.G.; Curry, R.J. Giant Bandgap Renormalization and Exciton-Phonon Scattering in Perovskite Nanocrystals. Adv. Opt. Mater. 2017, 5, 1700231. [Google Scholar] [CrossRef]
- Li, J.; Yuan, X.; Jing, P.; Li, J.; Wei, M.; Hua, J.; Zhao, J.; Tian, L. Temperature-dependent photoluminescence of inorganic perovskite nanocrystal films. RSC Adv. 2016, 6, 78311–78316. [Google Scholar] [CrossRef]
- Litvin, A.P.; Parfenov, P.S.; Ushakova, E.V.; Simões Gamboa, A.L.; Fedorov, A.V.; Baranov, A.V. Size and Temperature Dependencies of the Low-Energy Electronic Structure of PbS Quantum Dots. J. Phys. Chem. C 2014, 118, 20721–20726. [Google Scholar] [CrossRef]
- Diroll, B.T.; Zhou, H.; Schaller, R.D. Low-Temperature Absorption, Photoluminescence, and Lifetime of CsPbX3 (X = Cl, Br, I) Nanocrystals. Adv. Funct. Mater. 2018, 28, 1800945. [Google Scholar] [CrossRef]
- Baranowski, M.; Plochocka, P. Excitons in Metal-Halide Perovskites. Adv. Energy Mater. 2020, 10, 1903659. [Google Scholar] [CrossRef]
- Protesescu, L.; Yakunin, S.; Bodnarchuk, M.I.; Krieg, F.; Caputo, R.; Hendon, C.H.; Yang, R.X.; Walsh, A.; Kovalenko, M.V. Nanocrystals of Cesium Lead Halide Perovskites (CsPbX3, X = Cl, Br, and I): Novel Optoelectronic Materials Showing Bright Emission with Wide Color Gamut. Nano Lett. 2015, 15, 3692–3696. [Google Scholar] [CrossRef] [Green Version]
- Lu, M.; Zhang, Y.; Wang, S.; Guo, J.; Yu, W.W.; Rogach, A.L. Metal Halide Perovskite Light-Emitting Devices: Promising Technology for Next-Generation Displays. Adv. Funct. Mater. 2019, 29, 1902008. [Google Scholar] [CrossRef]
- Van Le, Q.; Jang, H.W.; Kim, S.Y. Recent Advances toward High-Efficiency Halide Perovskite Light-Emitting Diodes: Review and Perspective. Small Methods 2018, 2, 1700419. [Google Scholar] [CrossRef]
- Zhang, X.; Lin, H.; Huang, H.; Reckmeier, C.; Zhang, Y.; Choy, W.C.H.; Rogach, A.L. Enhancing the Brightness of Cesium Lead Halide Perovskite Nanocrystal Based Green Light-Emitting Devices through the Interface Engineering with Perfluorinated Ionomer. Nano Lett. 2016, 16, 1415–1420. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, C.; Zhang, Y.; Wu, H.; Ji, C.; Chuai, Y.; Wang, P.; Wen, S.; Zhang, C.; Yu, W.W. Bright Perovskite Nanocrystal Films for Efficient Light-Emitting Devices. J. Phys. Chem. Lett. 2016, 7, 4602–4610. [Google Scholar] [CrossRef]
- He, G.S.; Tan, L.-S.; Zheng, A.Q.; Prasad, P.N. Multiphoton Absorbing Materials: Molecular Designs, Characterizations, and Applications. Chem. Rev. 2008, 108, 1245–1330. [Google Scholar] [CrossRef]
- Ganeev, R.A.; Rao, K.S.; Yu, Z.; Yu, W.; Yao, C.; Fu, Y.; Zhang, K.; Guo, C. Strong nonlinear absorption in perovskite films. Opt. Mater. Express 2018, 8, 1472–1483. [Google Scholar] [CrossRef]
- Liu, W.; Xing, J.; Zhao, J.; Wen, X.; Wang, K.; Lu, P.; Xiong, Q. Giant Two-Photon Absorption and Its Saturation in 2D Organic-Inorganic Perovskite. Adv. Opt. Mater. 2017, 5, 1601045. [Google Scholar] [CrossRef]
- Pramanik, A.; Gates, K.; Gao, Y.; Begum, S.; Ray, P.C. Several Orders-of-Magnitude Enhancement of Multiphoton Absorption Property for CsPbX3 Perovskite Quantum Dots by Manipulating Halide Stoichiometry. J. Phys. Chem. C 2019, 123, 5150–5156. [Google Scholar] [CrossRef]
- Chen, J.; Žídek, K.; Chábera, P.; Liu, D.; Cheng, P.; Nuuttila, L.; Al-Marri, M.J.; Lehtivuori, H.; Messing, M.E.; Han, K.; et al. Size- and Wavelength-Dependent Two-Photon Absorption Cross-Section of CsPbBr3 Perovskite Quantum Dots. J. Phys. Chem. Lett. 2017, 8, 2316–2321. [Google Scholar] [CrossRef]
- Krishnakanth, K.N.; Seth, S.; Samanta, A.; Rao, S.V. Broadband femtosecond nonlinear optical properties of CsPbBr_3 perovskite nanocrystals. Opt. Lett. 2018, 43, 603–606. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, X.; Zhao, X.; Xiaoming, L.; Zeng, H.; Sun, H. Nonlinear Absorption and Low-Threshold Multiphoton Pumped Stimulated Emission from All-Inorganic Perovskite Nanocrystals. Nano Lett. 2015, 16, 448–453. [Google Scholar] [CrossRef]
- Han, Q.; Wu, W.; Liu, W.; Yang, Q.; Yang, Y. Two-photon absorption and upconversion luminescence of colloidal CsPbX3 quantum dots. Opt. Mater. 2018, 75, 880–886. [Google Scholar] [CrossRef]
- He, T.; Li, J.; Qiu, X.; Xiao, S.; Yin, C.; Lin, X. Highly Enhanced Normalized-Volume Multiphoton Absorption in CsPbBr3 2D Nanoplates. Adv. Opt. Mater. 2018, 6, 1800843. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, Q.; Zhang, C.; Wang, R.; Wu, H.; Zhang, X.; Xing, G.; Yu, W.W.; Wang, X.; Zhang, Y.; et al. Two-Photon-Pumped Perovskite Semiconductor Nanocrystal Lasers. J. Am. Chem. Soc. 2016, 138, 3761–3768. [Google Scholar] [CrossRef]
- Chen, W.; Bhaumik, S.; Veldhuis, S.A.; Xing, G.; Xu, Q.; Grätzel, M.; Mhaisalkar, S.; Mathews, N.; Sum, T.C. Giant five-photon absorption from multidimensional core-shell halide perovskite colloidal nanocrystals. Nat. Commun. 2017, 8, 15198. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Bhaumik, S.; Goh, T.W.; Kumar, M.S.; Yantara, N.; Grätzel, M.; Mhaisalkar, S.; Mathews, N.; Sum, T.C. Slow cooling and highly efficient extraction of hot carriers in colloidal perovskite nanocrystals. Nat. Commun. 2017, 8, 14350. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Jing, Q.; Xiao, S.; Gao, Y.; Wang, Y.; Zhang, W.; Sun, X.W.; Wang, K.; He, T. Spectral Dynamics and Multiphoton Absorption Properties of All-Inorganic Perovskite Nanorods. J. Phys. Chem. Lett. 2020, 11, 4817–4825. [Google Scholar] [CrossRef] [PubMed]
- Pramanik, A.; Patibandla, S.; Gao, Y.; Gates, K.; Ray, P.C. Water Triggered Synthesis of Highly Stable and Biocompatible 1D Nanowire, 2D Nanoplatelet, and 3D Nanocube CsPbBr3 Perovskites for Multicolor Two-Photon Cell Imaging. JACS Au 2021, 1, 53–65. [Google Scholar] [CrossRef]
- Catalano, I.M.; Cingolani, A. Three-photon absorption coefficient determination by means of nonlinear luminescence experiments. J. Appl. Phys. 1979, 50, 5638–5641. [Google Scholar] [CrossRef]
- Maes, J.; Balcaen, L.; Drijvers, E.; Zhao, Q.; De Roo, J.; Vantomme, A.; Vanhaecke, F.; Geiregat, P.; Hens, Z. Light Absorption Coefficient of CsPbBr3 Perovskite Nanocrystals. J. Phys. Chem. Lett. 2018, 9, 3093–3097. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.; Song, J.; Xiaoming, L.; Zeng, H.; Sun, H. All-Inorganic Colloidal Perovskite Quantum Dots: A New Class of Lasing Materials with Favorable Characteristics. Adv. Mater. 2015, 27, 7101–7108. [Google Scholar] [CrossRef]
- Makarov, N.S.; Guo, S.; Isaienko, O.; Liu, W.; Robel, I.; Klimov, V.I. Spectral and Dynamical Properties of Single Excitons, Biexcitons, and Trions in Cesium–Lead-Halide Perovskite Quantum Dots. Nano Lett. 2016, 16, 2349–2362. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Liu, Y.; Wei, S.; Chen, J.; Zhou, Y.; He, R.; Pullerits, T.; Zheng, K. Microscopic morphology independence in linear absorption cross-section of CsPbBr3 nanocrystals. Sci. China Mater. 2021, 64, 1418–1426. [Google Scholar] [CrossRef]
- Puthenpurayil, J.; Cheng, O.H.-C.; Qiao, T.; Rossi, D.; Son, D.H. On the determination of absorption cross section of colloidal lead halide perovskite quantum dots. J. Chem. Phys. 2019, 151, 154706. [Google Scholar] [CrossRef] [Green Version]
- Beaumont, P.C.; Johnson, D.G.; Parsons, B.J. Photophysical properties of laser dyes: Picosecond laser flash photolysis studies of Rhodamine 6G, Rhodamine B and Rhodamine 101. J. Chem. Soc. Faraday Trans. 1993, 89, 4185–4191. [Google Scholar] [CrossRef]
- Dempster, D.N.; Morrow, T.; Quinn, M.F. The photochemical characteristics of rhodamine 6G-ethanol solutions. J. Photochem. 1973, 2, 343–359. [Google Scholar] [CrossRef]





| Sample | σ2PA (GM) | Volume Normalized σ2PA GM/nm3) | σ3PA (10−80 cm6 s2 Photon−2) | Volume Normalized σ3PA (10−80 cm6 s2 Photon−2 nm−3) | Ref. |
|---|---|---|---|---|---|
| CsPbBr3 NC 12.4 nm | (3.2 ± 1.9) ×105 (800 nm, 30 fs) | 171 ± 112 | (1.7 ± 1.0) ×105 (1300 nm, 30 fs) | 89 ± 18 | This work |
| CsPb0.78Cd0.22Br3 NC 12.7 nm | (2.6 ± 0.8) × 106 (800 nm, 30 fs) | 1265 ± 830 | (2.1 ± 0.7) ×105 (1300 nm, 30 fs) | 102 ± 21 | This work |
| CsPbBr3 NC 7.3 nm | 1.2 × 105 (720 nm 100 fs) | 308 | 2.8 × 104 (1200 nm 100 fs) | 72 | [68] |
| CsPbBr3 NP | 2.28 × 105 (720 nm 100 fs) | 1562 | 1.05 × 105 (1200 nm 100 fs) | 720 | [68] |
| CsPbBr3 NC 9 nm | 2 × 106 (800 nm 100 fs) | 2743 | 1.0 × 106 (1200 nm 100 fs) | 1372 | [71] |
| CsPbBr3 NC 7 nm | 1.8 × 105 (700–1000 nm 100 fs) | 525 | 9.1 × 105 (1000–1500 nm 100 fs) | 2653 | [63] |
| CsPbBr3 NR | 1.5 × 106 (800 nm 100 fs) | 221 | 2.7 × 105 (1300 nm 100 fs) | 40 | [72] |
| CsPbBr3 NC 12 nm | 9.8 × 105 (800 nm 50 fs) | 567 | [65] | ||
| CsPbBr3 NC 9 nm | 1.2 × 105 (800 nm 100 fs) | 164 | [66] | ||
| CsPbBr3 NC 12.4 nm | 2.2 × 105 (800 nm) | 115 | [67] | ||
| CsPbBr3 NC 9 nm | 2.7 × 106 (800 nm 90 fs) | 3704 | [69] | ||
| CsPbBr3 NC 4.6 nm | 1.6 × 104 (800 nm 120 fs) | 164 | [64] | ||
| CsPbBr3 NC 5.2 nm | 2.9 × 104 (800 nm 120 fs) | 206 | [64] | ||
| CsPbBr3 NC 6.9 nm | 6.1 × 104 (800 nm 120 fs) | 186 | [64] | ||
| CsPbBr3 NC 9.4 nm | 1.8 × 105 (800 nm 120 fs) | 217 | [64] | ||
| CsPbBr3 NC 11.4 nm | 4.55 × 105 (800 nm 120 fs) | 307 | [64] | ||
| CsPbBr3 NC 28 nm | 8.1 × 104 (800 nm 100 fs) | 4 | [73] | ||
| CsPbBr3 NP | 4.2 × 105 (800 nm 100 fs) | 300 | [73] | ||
| CsPbBr3 NW | 2.3 × 105 (800 nm 100 fs) | 12 | [73] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skurlov, I.D.; Yin, W.; Ismagilov, A.O.; Tcypkin, A.N.; Hua, H.; Wang, H.; Zhang, X.; Litvin, A.P.; Zheng, W. Improved One- and Multiple-Photon Excited Photoluminescence from Cd2+-Doped CsPbBr3 Perovskite NCs. Nanomaterials 2022, 12, 151. https://doi.org/10.3390/nano12010151
Skurlov ID, Yin W, Ismagilov AO, Tcypkin AN, Hua H, Wang H, Zhang X, Litvin AP, Zheng W. Improved One- and Multiple-Photon Excited Photoluminescence from Cd2+-Doped CsPbBr3 Perovskite NCs. Nanomaterials. 2022; 12(1):151. https://doi.org/10.3390/nano12010151
Chicago/Turabian StyleSkurlov, Ivan D., Wenxu Yin, Azat O. Ismagilov, Anton N. Tcypkin, Haohang Hua, Haibo Wang, Xiaoyu Zhang, Aleksandr P. Litvin, and Weitao Zheng. 2022. "Improved One- and Multiple-Photon Excited Photoluminescence from Cd2+-Doped CsPbBr3 Perovskite NCs" Nanomaterials 12, no. 1: 151. https://doi.org/10.3390/nano12010151
APA StyleSkurlov, I. D., Yin, W., Ismagilov, A. O., Tcypkin, A. N., Hua, H., Wang, H., Zhang, X., Litvin, A. P., & Zheng, W. (2022). Improved One- and Multiple-Photon Excited Photoluminescence from Cd2+-Doped CsPbBr3 Perovskite NCs. Nanomaterials, 12(1), 151. https://doi.org/10.3390/nano12010151