Reuse of Textile Waste to Production of the Fibrous Antibacterial Membrane with Filtration Potential
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials, Chemicals, and Microorganisms
2.2. Preparation of MAG
2.3. Preparation of Fibrous Membranes
2.4. Characterization of the Fibrous Membranes
2.5. Antibacterial Activity
- (1)
- The antibacterial activity of neat rPA or rPA/MAG fibrous membranes was tested by the standard agar diffusion technique [55]. The fibrous layers were cut into circular disks (diameter 9 mm). They were placed on Mueller Hinton Agar (Himedia Laboratories Pvt. Ltd., Mumbai, India) plates inoculated with 1 mL of 0.5 McF turbid bacterial suspension (Escherichia coli, Staphylococcus aureus) in a sterile saline solution. The plates were incubated at 37 °C for 24 h, and the inhibition zones and growth under the samples were evaluated. All experiments were repeated three times.
- (2)
- The growth kinetics of bacterial species were studied using a Tecan microplate reader (M200Pro, Tecan, Männedorf, Switzerland) to examine the fibrous disks (9 mm) containing various concentrations of MAG. The microplate wells were filled with 250 µL Mueller Hinton Broth (Himedia Laboratories Pvt. Ltd., Mumbai, India), 5 µL 0.5 McF turbid bacterial inoculum (rPA fibers with MAG (0, 1, 2, and 3 wt%) or without (control bacterial growth)) and incubated (with shaking) at 37 °C for 24 h. The absorbance values (in nine rounds) were read as optical density (OD600nm) every 30 min. The modified Gompertz equation was used to describe the lag phase of bacterial growth to evaluate the antimicrobial effect of rPA/MAG fibrous membranes [56,57]. A non-linear regression analysis (Marquardt–Levenburgova method) was used for the calculation of the parameters μmax, λ and A for the following conditions: μ > 0, λ > 0 and A > 0. The maximum specific growth rate (μmax) and asymptotic value are given by (Equation (2))where μmax is the maximum specific growth rate (log CFU.l−1.h−1); λ is the lag phase (h); and A is the asymptote defined as the maximum value of relative microorganism counts (log CFU.l−1).
2.6. Biofilm Formation Test
- (1)
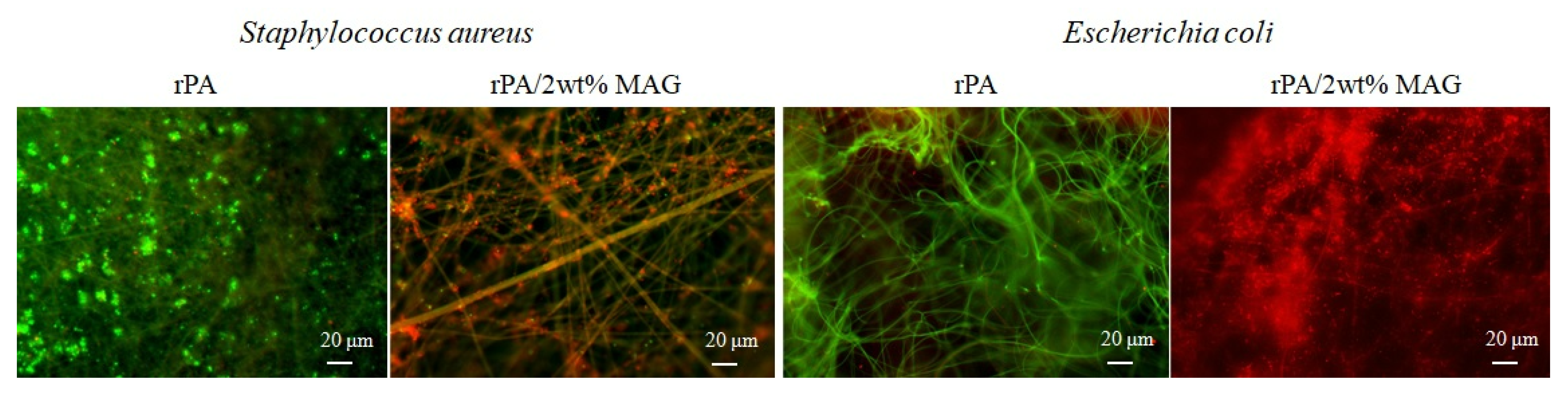
- The samples were washed with sterile saline solution after cultivation and put on sliding glass. They were dyed by fluorescence dye (SYTO®9 and propidium iodide) for 10 s and then covered with a square coverslip. Fluorescence microscopy was performed using a fluorescence microscope Olympus BX53 (Olympus, Tokyo, Japan), equipped with Microscope Digital Camera DP73 (Olympus, Tokyo, Japan) and the cell Sens Standard V1.18 (Olympus, Tokyo, Japan) software. The analysis was carried out on a minimum of 20 positions in three replicates. LIVE/DEAD™ BacLight™ Bacterial Viability Kit (Thermo Fischer, USA), based on the protocol [58], was carried out using slight modifications. SYTO®9 dyed plasma membranes of all bacteria, while propidium iodide can color DNA of only dead cells. The excitation/emission maxima for these dyes are about 480/500 nm for SYTO 9 stain and 490/635 nm for propidium iodide. Thus, bacteria with intact cell membranes stain fluorescent green, whereas bacteria with damaged membranes (dead) stain fluorescent red.
- (2)
- The sample disks were washed after cultivation by sterile saline solution and left dried at 40 °C. SEM microscopy was then performed as it was described earlier (2.4).
2.7. Statistical Analysis
3. Results and Discussion
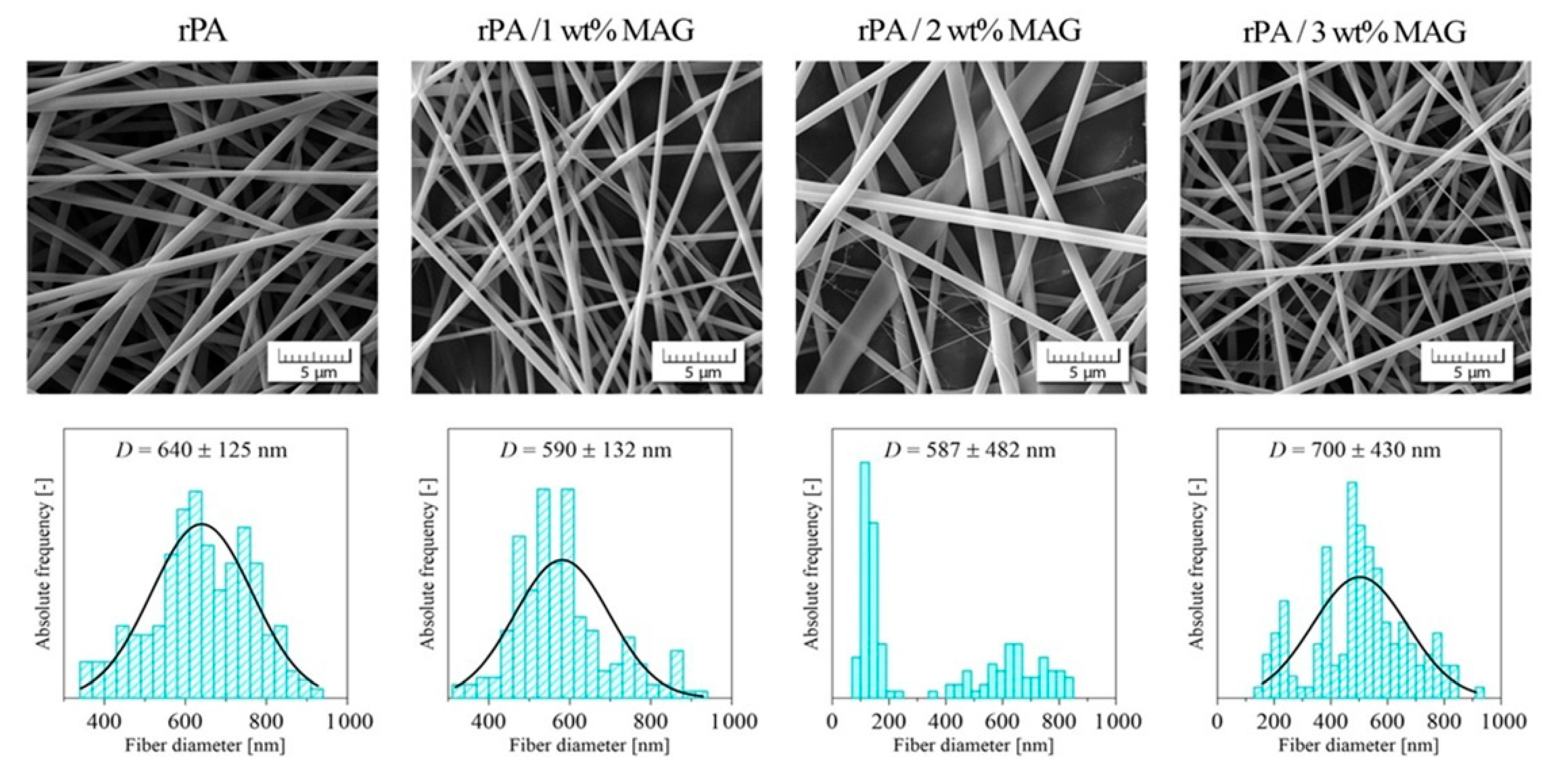
3.1. Morphology of Electrospun Membranes
3.2. ATR-FTIR Analysis
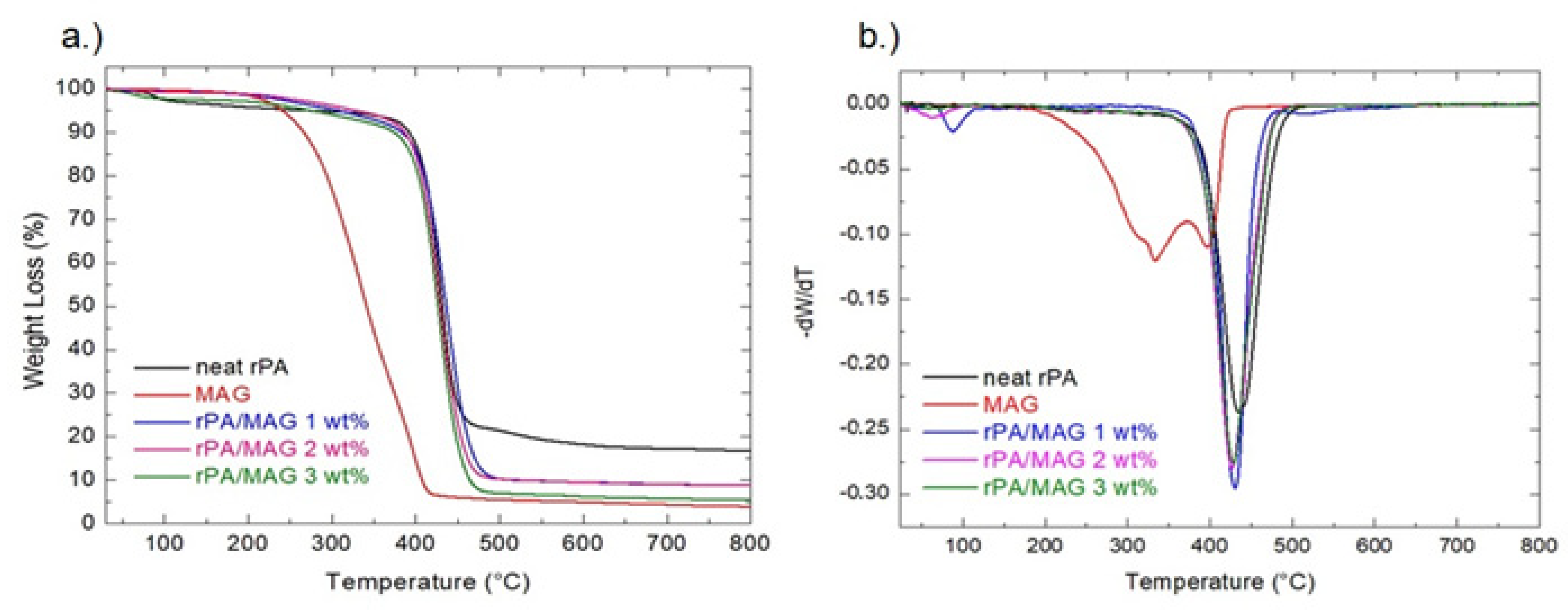
3.3. Thermogravimetric Analysis and First-Order Derivatives (TGA)
3.4. Wettability of Electrospun Membranes
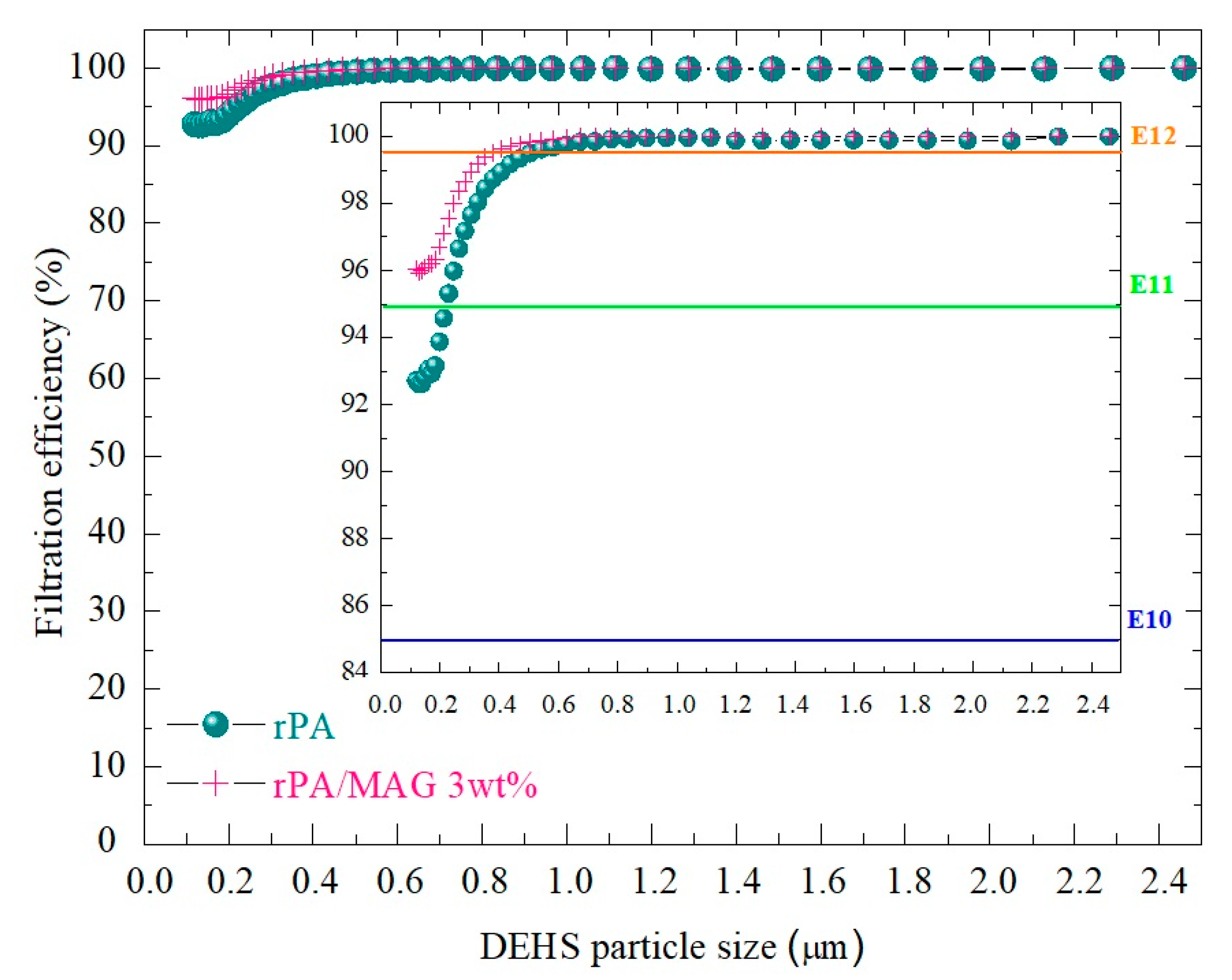
3.5. Assessment of the Filtration Properties of Electrospun rPA and rPA/MAG 3 wt% Membranes
3.6. Antibacterial Activity of Electrospun Membrane
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Žagar, E.; Češarek, U.; Drinčić, A.; Sitar, S.; Shlyapnikov, I.M.; Pahovnik, D. Quantitative determinantion of PA6 and/or PA66 content in polyamide-containing wastes. ACS Sustain. Chem. Eng. 2020, 8, 11818–11826. [Google Scholar] [CrossRef]
- Stanescu, M.D. State of the art of post-consumer textile waste upcycling to reach the zero waste milestone. Environ. Sci. Poll. Res. 2021, 28, 14253–14270. [Google Scholar] [CrossRef] [PubMed]
- Novotna, K.; Cermakova, L.; Pivokonska, L.; Cajthaml, T.; Pivokonsky, M. Microplastics in drinking water treatment—Current knowledge and research needs. Sci. Total Environ. 2019, 667, 730–740. [Google Scholar] [CrossRef] [PubMed]
- Pivokonsky, M.; Cermakova, L.; Novotna, K.; Peer, P.; Cajthaml, T.; Janda, V. Occurrence of microplastics in raw and treated drinking water. Sci. Total Environ. 2018, 643, 1644–1651. [Google Scholar] [CrossRef]
- Statement on the Seventh Meeting of the International Health Regulations (2005) Emergency Committee Regarding the Coronavirus Deseade (COVID-19) Pandemic. Available online: https://www.who.int/news/item/19-04-2021-statement-on-the-seventh-meeting-of-the-international-health-regulations-(2005)-emergency-committee-regarding-the-coronavirus-disease-(covid-19)-pandemic (accessed on 8 December 2021).
- Damayanti, D.; Wulandari, L.A.; Bagaskoro, A.; Rianjanu, A.; Wu, H.S. Possibility rountes for textile recycling technology. Polymers 2021, 13, 3834. [Google Scholar] [CrossRef] [PubMed]
- Harmsen, P.; Scheffer, M.; Bos, H. Textiles for circular fashion: The logic behind recycling options. Sustainability 2021, 13, 9714. [Google Scholar] [CrossRef]
- Patti, A.; Cicala, G.; Acierno, D. Eco-Sustainability of the Textile Production: Waste Recovery and Current Recycling in the Composites World. Polymers 2021, 13, 134. [Google Scholar] [CrossRef]
- Salas, M.A.; Pérez-Acebo, H.; Calderón, V.; Gonzalo-Orden, H. Analysis and economic evaluation of the use of recycled polyamide powder in mansory mortars. Polymers 2020, 12, 2657. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Zou, P.X.W.; Memon, R.A.; Alam, M.D.M.; Sanjayan, J.G.; Kumar, S. Life-cycle cost analysis of building wall and insulation materials. J. Build. Phys. 2019, 43, 428–455. [Google Scholar] [CrossRef]
- Cai, Z.; Faruque, M.A.A.F.; Kiziltas, A.; Mielewski, D.; Naebe, M. Sustainable lightweight insulation materials from textile-based waste for the automobile industry. Materials 2021, 14, 1241. [Google Scholar] [CrossRef]
- Sakthivel, S.; Melese, B.; Edae, A.; Abedom, F.; Mekonnen, S.; Solomon, E. Garment waste recycled cotton/polyester thermal and acoustic properties of air-laid nonwovens. Adv. Mat. Sci. Eng. 2020, 2020, 8304525. [Google Scholar] [CrossRef]
- Singh, R.; Kumar, R.; Ranjan, N.; Penna, R.; Fraternali, F. On the recyclability for sustainable composite structures in civil engineering. Compos. Struct. 2018, 184, 704–713. [Google Scholar] [CrossRef]
- Šišková, A.O.; Peer, P.; Eckstein Andicsová, A.; Jordanov, I.; Rychter, P. Circulatory management of polymer waste: Recycling into fine fibers and their applications. Materials 2021, 14, 4694. [Google Scholar] [CrossRef] [PubMed]
- Moriam, K.; Sawada, D.; Nieminen, K.; Hummel, M.; Ma, Y.; Rissanen, M.; Sixta, H. Towards regenerated cellulose fibers with high toughness. Cellulose 2021, 28, 9547–9566. [Google Scholar] [CrossRef]
- Homem, N.C.; Amorim, M.T.P. Synthesis of cellulose acetate using as raw material textile wastes. Mater. Today Proc. 2020, 31, S315–S317. [Google Scholar] [CrossRef]
- Fockink, D.H.; Andreaus, J.; Ramos, L.P.; Łukasik, R.M. Pretreatment of cotton spinning residues for optimal enzymatic hydrolysis: A case study using green solvents. Renew. Energy 2020, 145, 490–499. [Google Scholar] [CrossRef]
- Hussain, Z.; Sajjad, W.; Khan, T.; Wahid, F. Production of bacterial cellulose from industrial wastes: A review. Cellulose 2019, 26, 2895–2911. [Google Scholar] [CrossRef]
- Yousef, S.; Tatariants, M.; Tichonovas, M.; Kliucininkas, L.; Kukošiūtė, S.I.; Yan, L. Sustainable green technology for recovery of cotton fibers and polyester from textile waste. J. Clean. Prod. 2020, 254, 120078. [Google Scholar] [CrossRef]
- Herzog, B.; Kohan, M.I.; Mestemacher, S.A.; Pagilagan, R.U.; Redmond, K. Polyamides. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim, Germany, 2013. [Google Scholar] [CrossRef]
- Šišková, A.O.; Frajová, J.; Nosko, M. Recycling of poly(ethylene terephthalate) by electrospinning to enhanced the filtration efficiency. Mater. Lett. 2020, 278, 128426. [Google Scholar] [CrossRef]
- Dissanayake, D.G.K.; Weerasinghe, D. Managing post-industrial textile waste: Current status and prospects for Sri Lanka. J. Text. Inst. 2020, 112, 1804–1810. [Google Scholar] [CrossRef]
- Topuz, F.; Abdulhamid, M.A.; Hardian, R.; Holtzl, T.; Szekely, G. Nanofibrous membranes comprising intrinsically microporous polyimides with embedded metal-organic frameworks for capturing volatile organic compounds. J. Hazard. Mater. 2022, 424 Pt A, 127347. [Google Scholar] [CrossRef]
- Isık, T.; Demir, M.M. Tailored electrospun fibers from waste polystyrene for high oil adsorption. Sustain. Mater. Technol. 2018, 18, e00084. [Google Scholar] [CrossRef]
- Zander, N.E.; Sweetser, D.; Cole, D.P.; Gillan, M. Formation of nanofibers from pure and mixed waste streams using electrospinning. Ind Eng. Chem. Res. 2015, 54, 9057–9063. [Google Scholar] [CrossRef]
- Rajak, A.; Hapidin, D.A.; Iskandar, F.; Munir, M.M.; Khairurrijal, K. Controlled morphology of electrospun nanofibers from waste expanded polystyrene for aerosol filtration. Nanotechnology 2019, 30, 425602. [Google Scholar] [CrossRef] [PubMed]
- Baggio, A.; Doan, H.N.; Vo, P.P.; Kinashi, K.; Sakai, W.; Tsutsumi, N.; Fuse, Y.; Sangermano, M. Chitosan-Functionalized Recycled Polyethylene Terephthalate Nanofibrous Membrane for Sustainable On-Demand Oil-Water Separation. Glob. Chall. 2021, 5, 2000107. [Google Scholar] [CrossRef]
- Liu, Y.; Li, K.; Mohideen, M.M.; Ramakrishna, S. Melt Electrospinning: A Green Method to Produce Superfine Fibers; Elsevier Inc.: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Naksuwan, P.; Komárek, M.; Salačová, J.; Militký, J. The study of recycled poly(ethye’ lene terephthalate) nanofibers from PET bottle. Appl. Mech. Mat. 2016, 848, 3–6. [Google Scholar] [CrossRef]
- Heikkilä, P.; Harlin, A. Parameter study of electrospinning of polyamide-6. Eur. Pol. J. 2008, 44, 3067–3079. [Google Scholar] [CrossRef]
- Ge, Q.; Ding, L.; Wu, T.; Xu, G.; Yang, F.; Xiang, M. Effect of surfactant on morphology and pore size of polysulfone membrane. J. Polym. Res. 2018, 25, 21. [Google Scholar] [CrossRef]
- Liu, C.; Hsu, P.C.; Lee, H.W.; Ye, M.; Zheng, G.; Liu, N.; Li, W.; Cui, Y. Transparent air filter for high-efficiency PM2.5 capture. Nat. Commun. 2015, 6, 6205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zulfi, A.; Munir, M.M.; Hapidin, S.A.; Rajak, A.; Edikrednha, D.; Iskandar, F.; Khairurriajal, K. Air filtration media from electrospun waste high-impact polystyrene fiber membrane. Mater. Res. Express 2018, 5, 035049. [Google Scholar] [CrossRef]
- Liu, Y.; Li, S.; Lan, W.; Hossen, M.A.; Qin, W.; Lee, K. Electrospun antibacterial and antiviral poly(ε-caprolactone)/zein/Ag bead-on-string membranes and its application in air filtration. Mater. Today Adv. 2021, 12, 100173. [Google Scholar] [CrossRef]
- Ahne, J.; Li, Q.; Croiset, E.; Tan, Z. Electrospun cellulose acetate nanofibers for airborne nanoparticle filtration. Tex. Res. J. 2019, 89, 3137–3149. [Google Scholar] [CrossRef]
- Šišková, A.O.; Mosnáčková, K.; Hrůza, J.; Frajová, J.; Opálek, A.; Bučková, M.; Kozics, K.; Peer, P.; Eckstein Andicsová, A. Electrospun poly(ethylene terephthalate)/silk fibroin composite for filtration application. Polymers 2021, 13, 2499. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhao, C.; Pan, Z. Porous bead-on-string poly(lactic acid) fibrous membranes for air filtration. J. Colloid. Interface Sci. 2015, 441, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Choi, D.I.; Sung, S.K.; Lee, S.H.; Kim, S.J.; Kim, J.; Han, B.S.; Kim, D.I.; Kim, Y. Eco-friendly poly(vinyl alcohol) nanofiber-based air filter for effectively capturing particulate matter. Appl. Sci. 2021, 11, 3831. [Google Scholar] [CrossRef]
- Orlando, R.; Polat, M.; Afshari, A.; Johnson, M.S.; Fojan, P. Electrospun nanofiber air filters for particles and gaseous pollutants. Sustainability 2021, 13, 6553. [Google Scholar] [CrossRef]
- Sanyal, A.; Sinha-Ray, S. Ultrafine PVDF nanofibers for filtration of air-borne particulate matters: A comprehensive review. Polymers 2021, 13, 1864. [Google Scholar] [CrossRef]
- Liu, H.; Huang, J.; Mao, J.; Chen, Z.; Chen, G.; Lai, Y. Transparent antibacterial nanofiber air filters with highly efficient moisture resistance for sustainable particulate matter capture. iScience 2019, 19, 214–223. [Google Scholar] [CrossRef] [Green Version]
- Bergshoef, M.M.; Vancso, G.J. Transparent nanocomposites with ultrathin, electrospun nylon-6 fiber reinforcement. Adv. Mater. 1999, 11, 1362–1365. [Google Scholar] [CrossRef]
- Pant, H.R.; Pandeya, D.R.; Nam, K.T.; Baek, W.I.; Hong, S.T.; Kim, H.Y. Photocatalytic and antibacterial properties of a TiO2/nylon-6 electrospun nanocomposite mat containing silver nanoparticles. J. Hazard. Mater. 2011, 189, 465–471. [Google Scholar] [CrossRef]
- Abdal-hay, A.; Pant, H.R.; Lim, J.K. Super-hydrophilic electrospun nylon-6/hydroxyapatite membrane for bone tissue engineering. Eur. Polym. J. 2013, 49, 1314–1321. [Google Scholar] [CrossRef]
- Jackman, J.; Yoon, B.K.; Li, D.; Cho, N. Nanotechnology formulations for antibacterial free fatty acids and monoglycerides. Molecules 2016, 21, 305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dolezalkova, I.; Janis, R.; Bunkova, L.; Slobodian, P.; Vicha, R. Preparation, characterization and antibacterial activity of 1-monoacylglycerol of adamantane-1-carboxylic acid. J. Food Biochem. 2013, 34, 544–553. [Google Scholar] [CrossRef]
- Sevcikova, P.; Kasparkova, V.; Hauerlandova, I.; Humpolicek, P.; Kucekova, Z.; Bunkova, L. Formulation, antibacterial activity, and cytotoxicity of 1-monoacylglycerol microemulsions. Eur. J. Lipid Sci. Technol. 2014, 116, 448–457. [Google Scholar] [CrossRef]
- Hauerlandová, I.; Lorencová, E.; Buňka, F.; Navrátil, J.; Janečková, K.; Buňková, L. The influence of fat and monoacylglycerols on growth of spore-forming bacteria in processed cheese. Int. J. Food Microbiol. 2014, 182–183, 37–43. [Google Scholar] [CrossRef]
- Janis, R.; Klasek, A.; Krejci, J.; Bobalova, J. Influence of some chromium complexes on the conversion rate of glycidol—Fatty acid reaction. Tenside Surfact. Det. 2005, 42, 44–48. [Google Scholar] [CrossRef]
- Sutter, M.; Dayoub, W.; Métay, E.; Raoul, Y.; Lemaire, M. 1-O-alkyl (di)glycerol ethers synthesis from methyl esters and triglycerides by two pathways: Catalytic reductive alkylation and transesterification/reduction. Green Chem. 2013, 15, 786–797. [Google Scholar] [CrossRef]
- Zhu, M.; Han, J.; Wang, F.; Shao, W.; Xiong, R.; Zhang, Q.; Pan, H.; Yang, Y.; Samal, S.K.; Zhang, F.; et al. Electrospun nanofibers membranes for effective air filtration. Macromol. Mater. Eng. 2017, 302, 1600353. [Google Scholar] [CrossRef]
- Al-Attabi, E.; Dumée, L.F.; Kong, L.; Schütz, J.A.; Morsi, Y. High efficiency poly(acrylonitrile) electrospun nanofiber membranes for airborne nanomaterials filtration. Adv. Eng. Mater. 2017, 20, 1700572. [Google Scholar] [CrossRef]
- Hes, L. Non-Destructive Determinantion of Comfort Parameters during Marketing of Functional Garments and Clothing. Indian J. Fibre Text Res. 2008, 33, 239–245. Available online: http://nopr.niscair.res.in/bitstream/123456789/2012/1/IJFTR%2033%283%29%20239-245.pdf (accessed on 23 December 2021).
- Razzaque, A.; Tesinova, P.; Hes, L.; Salacova, J.; Abid, H.A. Investigation on hydrostatic resistance and thermal performance of layeres waterproof breathable fabrics. Fiber. Polym. 2017, 18, 1924–1930. [Google Scholar] [CrossRef]
- Irfan, M.; Uddin, Z.; Ahmad, F.; Rasheed, A.; Qadir, M.B.; Ahmad, S.; Aykut, Y.; Nazir, A. Ecofriendly development of electrospun antibacterial membranes loaded with silver nanoparticles. J. Indus. Tex. 2021, 1–14. [Google Scholar] [CrossRef]
- Ferrer, C.; Ramón, D.; Muguerza, B.; Marco, A.; Martínez, A. Effect of olive powder on the growth and inhibition of bacillus cereus. Fodborne Pathog. Dis. 2009, 6, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Zahibi, E.; Babaei, A.; Shahrampour, D.; Arab-Bafrani, Z.; Mirshahidi, K.S.; Majidi, H.J. Facile and rapid in-situ synthesis of chitosan-ZnO nano-hybrids applicable in medical purposes; A novel combination of biomineralization, ultrasound, and bio-safe morphology-conducting agent. Int. J. Biol. Macromol. 2019, 15, 107–116. [Google Scholar] [CrossRef]
- Molecular Probes, Inc. Molecular Probes, Invitrogen Detection Technologies. Available online: https://assets.thermofisher.com/TFS-Assets/LSG/manuals/mp07007.pdf (accessed on 17 May 2021).
- Jabbari, M.; Skrifvars, M.; Åkesson, D.; Taherzadeh, M.J. New solvent for polyamide 66 and use for preparing a single-polymer composite-coated fabric. Int. J. Polym. Sci. 2018, 2018, 6235165. [Google Scholar] [CrossRef]
- Charlet, L.; Mathot, V.; Devaux, J. Crystallization and dissolution behavior of polyamide 6-water systems under pressure. Polym. Int. 2010, 60, 119–125. [Google Scholar] [CrossRef]
- Papadopoulou, E.L.; Pignatelli, F.; Marras, S.; Marini, L.; Davis, A.; Athanassiou, A.; Bayer, I.S. Nylon 6,6/grapheme nanoplatelet composite films obtained from a new solvent. RSC Adv. 2016, 6, 6823–6831. [Google Scholar] [CrossRef]
- Chang, C.W.; Liou, G.S.; Hsiao, S.H. Highly stable anodic green electrochromic aromatic polyamides: Synthesis and electrochromic properties. J. Mater. Chem. 2007, 17, 1007–1015. [Google Scholar] [CrossRef]
- Matulevicius, J.; Kliuciniskas, L.; Martuzevicius, D.; Krugly, E.; Tichonovas, M.; Baltrusaitis, J. Design and characterization of electrospun polyamide nanofiber media for air filtration applications. J. Nanomater. 2014, 2014, 859656. [Google Scholar] [CrossRef] [Green Version]
- Mori, S. Size exclusion chromatography of poly(ethylene terephthalate) using hexafluoro-2-propanol as a mobile phase. Anal. Chem. 1989, 61, 1321–1325. [Google Scholar] [CrossRef]
- Šišková, A.O.; Macová, E.; Berek, D. Liquid chromatography under limiting conditions of desorption 4 separation of blends containing low-solubility polymers. Eur. Polym. J. 2012, 48, 155–162. [Google Scholar] [CrossRef]
- Yu, Y.; Ma, R.; Yan, S.; Fang, J. Preparation of multi-layer nylon-6 nanofibrous membranes by electrospinning and hot pressing methods for dye filtration. RSC Adv. 2018, 8, 12173. [Google Scholar] [CrossRef] [Green Version]
- Parlayici, S.; Avci, A.; Pehlivan, E. Electrospinning of polymeric nanofiber (nylon 6,6/grapheme oxide) for removal of Cr (VI): Synthesis and adsorption studies. J. Anal. Sci. Technol. 2019, 10, 13. [Google Scholar] [CrossRef] [Green Version]
- Razavizadeh, B.M.; Niazmand, R. Characterization of polyamide-6/propolis blended electrospun fibers. Heliyon 2020, 6, e04784. [Google Scholar] [CrossRef]
- Abbasi, A.; Nasef, M.M.; Takeshi, M.; Faridi-Majidi, R. Electrospinning of nylon-6,6 soutions into nanofibers: Rheology and morphology relationships. Chin. J. Polym. Sci. 2014, 32, 793–804. [Google Scholar] [CrossRef]
- Cheremisinoff, N. Industrial Solvents Handbook, 2nd ed.; Marcel Dekker Inc.: New York, NY, USA, 2008; pp. 51–53. [Google Scholar]
- Hou, Q.; Zhen, M.; Qian, H.; Nie, Y.; Bai, X.; Xia, T.; Rehman, M.L.U.; Li, Q.; Ju, M. Upcycling and catalytic degradation of plastic wastes. Cell Rep. Phys. Sci. 2021, 2, 100514. [Google Scholar] [CrossRef]
- Krifa, M.; Yuan, W. Morphology and pore size distribution of electrospun and centrifugal forcespun nylon 6 nanofibe membranes. Tex. Res. J. 2015, 86, 1294–1306. [Google Scholar] [CrossRef]
- Zheng, J.Y.; Zhuang, M.F.; Yu, Z.J.; Zheng, G.F.; Zhao, Y.; Wang, H.; Sun, D.H. The effect of surfactants on the diameter and morphology of electrospun ultrafine nanofiber. J. Nanomater. 2014, 2014, 689298. [Google Scholar] [CrossRef]
- Peer, P.; Sedlarikova, J.; Janalikova, M.; Kucerova, L.; Pleva, P. Novel Polyvinyl Butyral/Monoacylglycerol Nanofibrous Membrane with Antifouling Activity. Materials 2020, 13, 3662. [Google Scholar] [CrossRef] [PubMed]
- Peer, P.; Janalikova, M.; Sedlarikova, J.; Zelenkova, J.; Pleva, P.; Filip, P.; Opalkova Siskova, A. Antibacterial filtration membranes based on PVDF-co-HFP nanofibers with the addition of medium-chain 1-monoacylglycerols. ACS Appl. Mater. Interfaces 2021, 13, 41021–41033. [Google Scholar] [CrossRef]
- European Committee for Standardization. European Standard EN 1822 High Efficiency Air Filters (EPA, HEPA and ULPA); CEN: Brussels, Belgium, 2009; Available online: http://www.gttlab.com/uploads/soft/161025/EN1822-1-2009Highefficiencyairfilters(EPA,HEPAandULPA)Part1Classification,performance.pdf (accessed on 28 October 2021).
- Sambaer, W.; Zatloukal, M.; Kimmer, D. 3D air filtration modeling for nanofiber based filters in the ultrafine particle size range. Chem. Eng. Sci. 2012, 82, 299–311. [Google Scholar] [CrossRef] [Green Version]
- An, A.K.; Lee, E.J.; Guo, J.; Jeong, S.; Lee, J.G.; Ghaffour, N. Enhanced vapor transport in membrane distillation via functionalized carbon nanotubes anchored into electrospun nanofibers. Sci. Rep. 2017, 7, 41562. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Wei, H.; Cui, Y.; Zhao, G.; Feng, F. Antibacterial Interactions of Monolaurin with Commonly Used Antimicrobials and Food Components. J Food Sci. 2009, 74, M418–M421. [Google Scholar] [CrossRef] [PubMed]
- Pletnev, P.; Osterman, I.; Sergiev, P.; Bogdanov, A.; Dontsova, O. Survival Guide: Escherichia coli in the Stationary Phase. Acta Nat. 2015, 7, 22–33. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4717247/pdf/AN20758251-27-022.pdf (accessed on 28 October 2021). [CrossRef]
- Krishnamurthi, V.R.; Niyonshuti, I.I.; Chen, J.; Wang, Y. A new analysis method for evaluating bacterial growth with microplate readers. PLoS ONE 2021, 16, e0245205. [Google Scholar] [CrossRef]
- Harkes, G.; Feijen, J.; Dankert, J. Adhesion of Escherichia coli on to a series of poly(methacrylates) differing in change and hydrophobicity. Biomaterials 1991, 12, 853–860. [Google Scholar] [CrossRef] [Green Version]
- Tang, Y.-W. Molecular Medical Microbiology, 2nd ed.; Academic Press: Cambridge, MA, USA, 2015; Volume 1, Chapter 5. [Google Scholar]






| Textile Recycling | Recycling Methods | Product of Recycling | Drawbacks | Ref. |
|---|---|---|---|---|
| Fiber recycling | Mechanical methods | Fibers |
| [7,8,9,10,11,12] |
| Polymer recycling | Mechanical methods | Fibers | As fiber recycling | [2,8] |
| Physical methods | Polymer suitable for reprocessing (in the form of melt or solution) |
| [6,7,8,13,14,15] | |
| Monomer recycling | Mechanical methods | Fibers | As fiber recycling | [2,8] |
| Chemical/Biological methods | Monomers |
| [7,8,12,16,17,18] |
| Type of Spinning | Advantages | Disadvantages | Ref. |
|---|---|---|---|
| Melt spinning |
|
| [14] |
Solution spinning
|
|
| [15] |
Electrospinning
|
Solution:
|
| [21,24,25,26,27] |
Melt:
| Difficulties in melting the polymers | [28,29] |
| The Concentration of MAG [wt%] | MIN Pore Size [nm] | Average Pore Size [nm] | MAX Pore Size [nm] |
|---|---|---|---|
| 0 | 192 | 840 ± 350 | 2185 |
| 1 | 365 | 1130 ± 600 | 3225 |
| 2 | 345 | 1190 ± 820 | 5350 |
| 3 | 530 | 1740 ± 1070 | 5540 |
| Sample | Basis Weight (g.m−2) | E100nm (%) | E300nm (%) | E600nm (%) | Qf (Pa−1) | ΔP (Pa) | B (L.mm.s−1) | RWVP (%) | Filter Class * |
|---|---|---|---|---|---|---|---|---|---|
| rPA | 8.7 ± 0.02 | 92.7 ± 1.7 | 97.67 ± 2.0 | 98.49 ± 1.9 | 0.035 ± 0.001 | 129 ± 9 | 78.6 ± 3.5 | 95.5 ± 1.1 | E10 |
| rPA/MAG 3 wt% | 9.5 ± 0.02 | 96.1 ± 3.4 | 98.18 ± 1.8 | 99.87 ± 1.8 | 0.024 ± 0.001 | 189 ± 12 | 78.4 ± 3.8 | 98.2 ± 1.3 | E11 |
| Sample | Fiber Diameter (nm) | Basis Weight (g.m−2) | PM Size (nm) | E (%) | Qf (Pa−1) | ΔP (Pa) | Ref. |
|---|---|---|---|---|---|---|---|
| Poly(lactic acid) (PLA) | 150–300 | 5.18 | 260 | 99.97 | 0.065 | 165 | [37] |
| Polyacrilonitrile (PAN) | 200 | NA | ≤100 | 96.12 | 0.024 | 133 | [32] |
| Polyurethane (PU) | 120 | 0.4–0.9 | 20–400 | 99.66 | 0.059–0.029 | 96–190 | [77] |
| Polyamide 6.6 (PA) | 60 | 0.46 | 300 | 90.9 | 0.034 | 69 | [63] |
| Poly(ε-caprolactone) (PCL) | 922 | NA | 300–1000 | 90–97 | 0.010–0.020 | 72–510 | [34] |
| Polyvinylalcohol (PVA) | 150 | NA | ≤1000 | 89.07 | 0.001 | 220 | [38] |
| PVA | 213–430 | 16.6–67.6 | 12–480 | ≥97 | NA | 195–2693 | [39] |
| Poly(methyl methacrylate) (PMMA)/polydimethylsiloxane (PDMS) | 300–1000 | NA | 2500 | 98 | NA | 21 | [41] |
| Polystyrene (PS) | 272–937 | 12.22 | 300 | 99.99 | 0.065 | 50–350 | [33] |
| Poly(ethylene terephthalate) (PET) | 95 | 14 | 120 | 98.28 | NA | NA | [21] |
| PET | 230 | 12 | 120 | 99.97 | 0.019 | 414 | [36] |
| PET/silk | 127 | 2.08 | 120 | 93.38 | 0.030 | 92 | [36] |
| Cellulose acetate (CA) | 175–890 | NA | 4–240 | 14.80–99.80 | max. 0.14 | NA | [35] |
| Bacteria | Fibers | λ(h) | μmax | A | Adj. R2 |
|---|---|---|---|---|---|
| E. coli | - | 3.2705 ± 0.0166 a | 1.0022 ± 0.0013 a | 0.6912 ± 0.0043 a | 0.99859 |
| E. coli | rPA/0 wt%MAG | 3.2541 ± 0.0249 a | 0.9904 ± 0.0018 b | 0.6574 ± 0.0055 a | 0.99707 |
| E. coli | rPA/1 wt%MAG | 3.3277 ± 0.0433 b | 0.9923 ± 0.0039 b | 0.7005 ± 0.0059 b | 0.99724 |
| E. coli | rPA/2 wt%MAG | 3.3145 ± 0.0310 a,c | 0.8573 ± 0.0022 b | 0.6520 ± 0.0044 b | 0.99645 |
| E. coli | rPA/3 wt%MAG | 3.4674 ± 0.0551 c | 0.9264 ± 0.0051 b | 0.6219 ± 0.0039 b | 0.98548 |
| S. aureus | - | 4.2365 ± 0.0334 a | 0.4068 ± 0.0015 a | 0.4024 ± 0.0029 a | 0.99899 |
| S. aureus | rPA/0 wt%MAG | 4.1653 ± 0.0268a, b | 0.3272 ± 0.0009 b | 0.3168 ± 0.0019 b | 0.99615 |
| S. aureus | rPA/1 wt%MAG | 4.5644 ± 0.0333 a, b | 0.3168 ± 0.0017 b | 0.3167 ± 0.0012 b | 0.99424 |
| S. aureus | rPA/2 wt%MAG | 5.0106 ± 0.0532 c | 0.2722 ± 0.0016 b | 0.2722 ± 0.0036 c | 0.99364 |
| S. aureus | rPA/3 wt%MAG | 5.3916 ± 0.0563 c | 0.2716 ± 0.0018 b | 0.2765 ± 0.0031 c | 0.99176 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Opálková Šišková, A.; Pleva, P.; Hrůza, J.; Frajová, J.; Sedlaříková, J.; Peer, P.; Kleinová, A.; Janalíková, M. Reuse of Textile Waste to Production of the Fibrous Antibacterial Membrane with Filtration Potential. Nanomaterials 2022, 12, 50. https://doi.org/10.3390/nano12010050
Opálková Šišková A, Pleva P, Hrůza J, Frajová J, Sedlaříková J, Peer P, Kleinová A, Janalíková M. Reuse of Textile Waste to Production of the Fibrous Antibacterial Membrane with Filtration Potential. Nanomaterials. 2022; 12(1):50. https://doi.org/10.3390/nano12010050
Chicago/Turabian StyleOpálková Šišková, Alena, Pavel Pleva, Jakub Hrůza, Jaroslava Frajová, Jana Sedlaříková, Petra Peer, Angela Kleinová, and Magda Janalíková. 2022. "Reuse of Textile Waste to Production of the Fibrous Antibacterial Membrane with Filtration Potential" Nanomaterials 12, no. 1: 50. https://doi.org/10.3390/nano12010050






