Effective Inhibition of Invasive Pulmonary Aspergillosis by Silver Nanoparticles Biosynthesized with Artemisia sieberi Leaf Extract
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microorganism and Culture Conditions
2.2. Plant Material
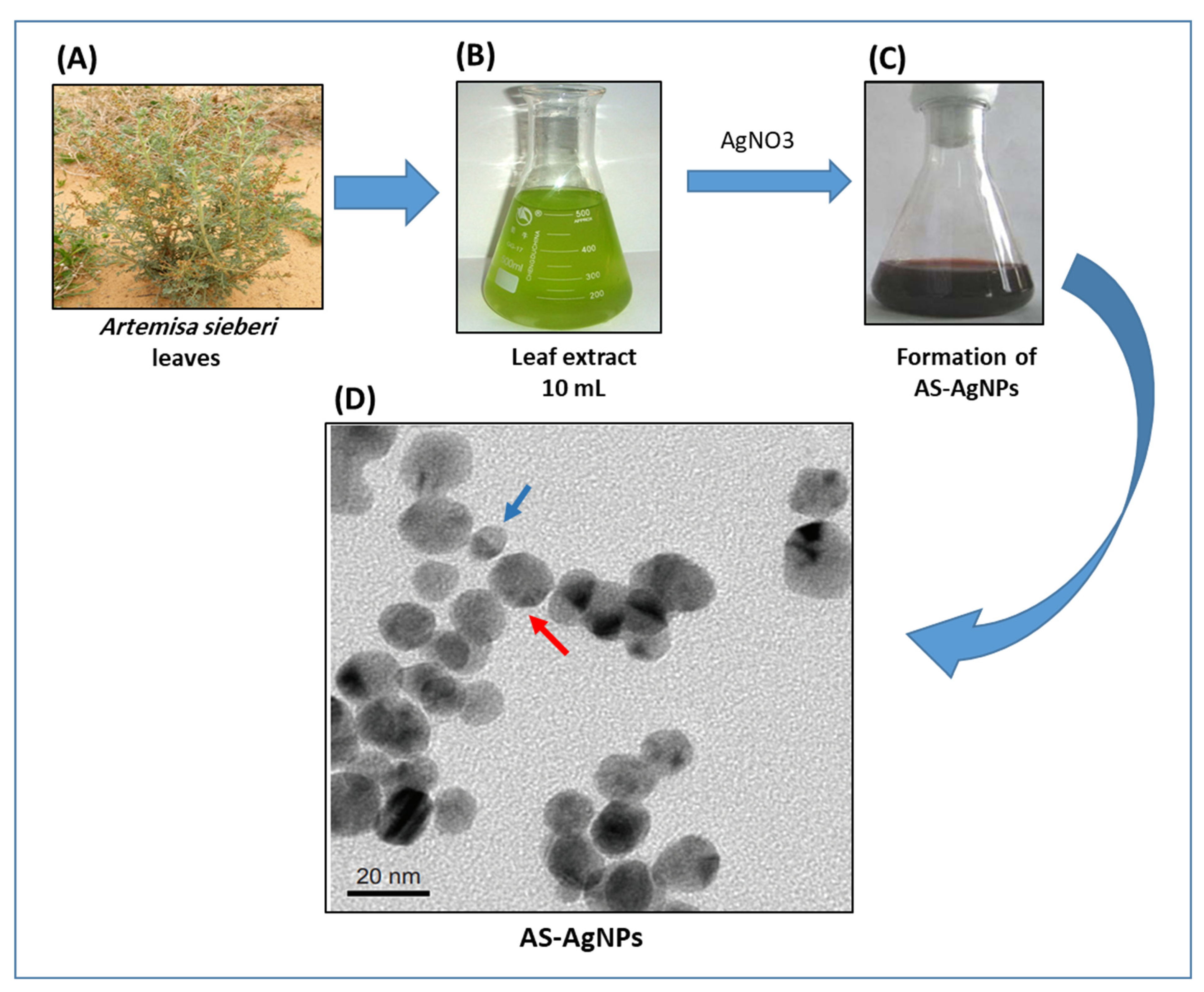
2.3. Biosynthesis and Characterization of AgNPs
2.4. In Vitro Antifungal Susceptibility Testing
2.5. In Vitro Antifungal Activity Assay
2.6. Time Kill Assay
2.7. Scanning Electron Microscopy (SEM)
2.8. Effect of AS-AgNP Treatment on A. fumigatus Biofilm Morphology and Structure
2.9. Cell Culture and Cytotoxicity Assay
2.10. In Vivo Experiment Design
2.11. Histological Study
2.12. Biochemical Assays
2.13. Determination of Fungal Load in Lung Tissue
2.14. Assessment of Gliotoxin in Lung Tissue
2.15. Statistical Analysis
3. Results
3.1. Biosynthesis and Characterization of AS-AgNPs
3.2. In Vitro Antifungal Activity of AS-AgNPs against A. fumigatus
3.3. Ultrastructural Analysis of the Interaction between AS-AgNPs and A. fumigatus Cells Using Scanning Electron Microscopy
3.4. Effect of AS-AgNPs on Morphology and Structure of A. fumigatus Biofilm
3.5. AS-AgNPs Exert No Cytotoxicity on Mouse and Human Cells
3.6. AS-AgNPs Significantly Reduce Inflammation and Repair Lung Tissue Damage in IPA Mice
3.7. AS-AgNPs Decrease Colonization of A. fumigatus and Inhibit Gliotoxin Production in Lung Tissue of IPA Mice
3.8. AS-AgNPs Reduce the Oxidative Stress in IPA Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Seidler, M.J.; Salvenmoser, S.; Müller, F.M. Aspergillus fumigatus forms biofilms with reduced antifungal drug susceptibility on bronchial epithelial cells. Antimicrob. Agents Chemother. 2008, 52, 4130–4136. [Google Scholar] [CrossRef] [Green Version]
- Hage, C.A.; Carmona, E.M.; Epelbaum, O.; Evans, S.E.; Gabe, L.M.; Haydour, Q.; Knox, K.S.; Kolls, J.K.; Murad, M.H.; Wengenack, N.L.; et al. Microbiological Laboratory Testing in the Diagnosis of Fungal Infections in Pulmonary and Critical Care Practice. An Official American Thoracic Society Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2019, 200, 535–550. [Google Scholar] [CrossRef]
- Diekema, D.; Arbefeville, S.; Boyken, L.; Kroeger, J.; Pfaller, M. The changing epidemiology of healthcare-associated candidemia over three decades. Diagn. Microbiol. Infect. Dis. 2012, 73, 45–48. [Google Scholar] [CrossRef]
- Garcia-Vidal, C.; Viasus, D.; Carratalà, J. Pathogenesis of invasive fungal infections. Curr. Opin. Infect. Dis. 2013, 26, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Borghi, E.; Borgo, F.; Morace, G. Fungal Biofilms: Update on Resistance. Adv. Exp. Med. Biol. 2016, 931, 37–47. [Google Scholar] [PubMed]
- Kaur, S.; Singh, S. Biofilm formation by Aspergillus fumigatus. Med. Mycol. 2014, 52, 2–9. [Google Scholar] [PubMed] [Green Version]
- Raffa, N.; Keller, N.P. A call to arms: Mustering secondary metabolites for success and survival of an opportunistic pathogen. PLoS Pathog. 2019, 15, e1007606. [Google Scholar] [CrossRef]
- Dolan, S.K.; O’Keeffe, G.; Jones, G.W.; Doyle, S. Resistance is not futile: Gliotoxin biosynthesis, functionality and utility. Trends Microbiol. 2015, 23, 419–428. [Google Scholar] [CrossRef]
- Lewis, R.E.; Wiederhold, N.P.; Chi, J.; Han, X.Y.; Komanduri, K.V.; Kontoyiannis, D.P.; Prince, R.A. Detection of gliotoxin in experimental and human aspergillosis. Infect. Immun. 2005, 73, 635–637. [Google Scholar] [CrossRef] [Green Version]
- Gardiner, D.M.; Howlett, B.J. Bioinformatic and expression analysis of the putative gliotoxin biosynthetic gene cluster of Aspergillus fumigatus. FEMS Microbiol. Lett. 2005, 248, 241–248. [Google Scholar] [CrossRef] [Green Version]
- Nouri, M.A.; Al-Halbosiy, M.M.F.; Dheeb, B.I.; Hashim, A.J. Cytotoxicity and genotoxicity of gliotoxin on human lymphocytes in vitro. J. King Saud Univ.-Sci. 2015, 27, 193–197. [Google Scholar] [CrossRef] [Green Version]
- Perdoni, F.; Signorelli, P.; Cirasola, D.; Caretti, A.; Galimberti, V.; Biggiogera, M.; Gasco, P.; Musicanti, C.; Morace, G.; Borghi, E. Antifungal activity of Myriocin on clinically relevant Aspergillus fumigatus strains producing biofilm. BMC Microbiol. 2015, 15, 248. [Google Scholar] [CrossRef] [Green Version]
- Patterson, T.F.; Thompson, G.R., 3rd; Denning, D.W.; Fishman, J.A.; Hadley, S.; Herbrecht, R.; Kontoyiannis, D.P.; Marr, K.A.; Morrison, V.A.; Nguyen, M.H.; et al. Executive Summary: Practice Guidelines for the Diagnosis and Management of Aspergillosis: 2016 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2016, 63, 433–442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verweij, P.E.; Chowdhary, A.; Melchers, W.J.; Meis, J.F. Azole Resistance in Aspergillus fumigatus: Can We Retain the Clinical Use of Mold-Active Antifungal Azoles? Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2016, 62, 362–368. [Google Scholar] [CrossRef] [Green Version]
- Reichenberger, F.; Habicht, J.M.; Gratwohl, A.; Tamm, M. Diagnosis and treatment of invasive pulmonary aspergillosis in neutropenic patients. Eur. Respir. J. 2002, 19, 743–755. [Google Scholar] [CrossRef] [Green Version]
- Denning, D.W.; Lee, J.Y.; Hostetler, J.S.; Pappas, P.; Kauffman, C.A.; Dewsnup, D.H.; Galgiani, J.N.; Graybill, J.R.; Sugar, A.M.; Catanzaro, A.; et al. NIAID Mycoses Study Group Multicenter Trial of Oral Itraconazole Therapy for Invasive Aspergillosis. Am. J. Med. 1994, 97, 135–144. [Google Scholar] [CrossRef] [Green Version]
- Talbot, J.J.; Subedi, S.; Halliday, C.L.; Hibbs, D.E.; Lai, F.; Lopez-Ruiz, F.J.; Harper, L.; Park, R.F.; Cuddy, W.S.; Biswas, C.; et al. Surveillance for azole resistance in clinical and environmental isolates of Aspergillus fumigatus in Australia and cyp51A homology modelling of azole-resistant isolates. J. Antimicrob. Chemother. 2018, 73, 2347–2351. [Google Scholar] [CrossRef] [Green Version]
- Correa-Royero, J.; Tangarife Castaño, V.; Durán, D.; Stashenko, E.; Mesa, A. In vitro antifungal activity and cytotoxic effect of essential oils and extracts of medicinal and aromatic plants against Candida krusei and Aspergillus fumigatus. Rev. Bras. Farmacogn. 2010, 20, 734. [Google Scholar] [CrossRef] [Green Version]
- Bansod, S.; Rai, M. Antifungal Activity of Essential Oils from Indian Medicinal Plants Against Human Pathogenic Aspergillus fumigatus and A. niger. World J. Med. Sci. 2008, 3, 88. [Google Scholar]
- Wahab, M.A.; Luming, L.; Matin, M.A.; Karim, M.R.; Aijaz, M.O.; Alharbi, H.F.; Abdala, A.; Haque, R. Silver Micro-Nanoparticle-Based Nanoarchitectures: Synthesis Routes, Biomedical Applications, and Mechanisms of Action. Polymers 2021, 13, 2870. [Google Scholar] [CrossRef]
- You, C.; Han, C.; Wang, X.; Zheng, Y.; Li, Q.; Hu, X.; Sun, H. The progress of silver nanoparticles in the antibacterial mechanism, clinical application and cytotoxicity. Mol. Biol. Rep. 2012, 39, 9193–9201. [Google Scholar] [CrossRef] [PubMed]
- Bashardoust, B.; Mohammadi, S.; Roudbary, M.; Nikoomanesh, F. Susceptibility Evaluation of Aspergillus fumigatus to Silver Nanoparticles Compared with Voriconazole. Infect. Epidemiol. Med. 2016, 2, 20–23. [Google Scholar] [CrossRef]
- León-Buitimea, A.; Garza-Cervantes, J.A.; Gallegos-Alvarado, D.Y.; Osorio-Concepción, M.; Morones-Ramírez, J.R. Nanomaterial-Based Antifungal Therapies to Combat Fungal Diseases Aspergillosis, Coccidioidomycosis, Mucormycosis, and Candidiasis. Pathogens 2021, 10, 1303. [Google Scholar] [CrossRef]
- Ogar, A.; Tylko, G.; Turnau, K. Antifungal properties of silver nanoparticles against indoor mould growth. Sci. Total Environ. 2015, 521–522, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Ali, E.M.; Abdallah, B.M. Effective Inhibition of Candidiasis Using an Eco-Friendly Leaf Extract of Calotropis-gigantean-Mediated Silver Nanoparticles. Nanomaterials 2020, 10, 422. [Google Scholar] [CrossRef] [Green Version]
- Abdallah, B.M.; Ali, E.M. Green Synthesis of Silver Nanoparticles Using the Lotus lalambensis Aqueous Leaf Extract and Their Anti-Candidal Activity against Oral Candidiasis. ACS Omega 2021, 6, 8151–8162. [Google Scholar] [CrossRef]
- Mahboubi, M. Artemisia sieberi Besser essential oil and treatment of fungal infections. Biomed. Pharmacother. 2017, 89, 1422–1430. [Google Scholar] [CrossRef] [PubMed]
- Mahboubi, M.; Kazempour, N. The antifungal activity of Artemisia sieberi essential oil from different localities of Iran against dermatophyte fungi. J. De Mycol. Med. 2015, 25, e65–e71. [Google Scholar] [CrossRef] [PubMed]
- Negahban, M.; Moharramipour, S.; Sefidkon FNegahban, M.; Moharamipour, S.; Sefidkon, F. Fumigant toxicity of essential oil from Artemisia sieberi Besser against three stored-product insects. Journal of Stored Product Research. J. Stored Prod. Res. 2007, 43, 123–128. [Google Scholar] [CrossRef]
- Ardakani, A.S.; Parhizkar, S. Inhibitory effects of Teucrium polium L., Artemisia sieberi Besser. and Achillea wilhelmsii C. Koch on Meloidogyne incognita (Kofoid and White) Chitwood (in vitro and under greenhouse conditions). Int. J. Med. Aromat. Plants 2012, 2, 596–602. [Google Scholar]
- Nahrevanian, H.; Sheykhkanlooye Milan, B.; Kazemi, M.; Hajhosseini, R.; Soleymani Mashhadi, S.; Nahrevanian, S. Antimalarial Effects of Iranian Flora Artemisia sieberi on Plasmodium berghei In Vivo in Mice and Phytochemistry Analysis of Its Herbal Extracts. Malar. Res. Treat. 2012, 2012, 727032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arab, H.-A.; Rahbari, S.; Rassouli, A.; Moslemi, M.; Khosravirad, F.D.A. Determination of artemisinin in Artemisia sieberi and anticoccidial effects of the plant extract in broiler chickens. Trop. Anim. Health Prod. 2006, 38, 497–503. [Google Scholar] [CrossRef]
- Fahmy, S.R.; Soliman, A.M.; Ali, E.M. Antifungal and antihepatotoxic effects of sepia ink extract against oxidative stress as a risk factor of invasive pulmonary aspergillosis in neutropenic mice. Afr. J. Tradit. Complementary Altern. Med. 2014, 11, 148–159. [Google Scholar] [CrossRef] [Green Version]
- Manavathu, E.K.; Cutright, J.L.; Loebenberg, D.; Chandrasekar, P.H. A comparative study of the in vitro susceptibilities of clinical and laboratory-selected resistant isolates of Aspergillus spp. to amphotericin B, itraconazole, voriconazole and posaconazole (SCH 56592). J. Antimicrob. Chemother. 2000, 46, 229–234. [Google Scholar] [CrossRef] [Green Version]
- Espinel-Ingroff, A.; Cuenca-Estrella, M.; Fothergill, A.; Fuller, J.; Ghannoum, M.; Johnson, E.; Pelaez, T.; Pfaller, M.A.; Turnidge, J. Wild-type MIC distributions and epidemiological cutoff values for amphotericin B and Aspergillus spp. for the CLSI broth microdilution method (M38-A2 document). Antimicrob. Agents Chemother. 2011, 55, 5150–5154. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.H.; Campbell, B.C.; Mahoney, N.; Chan, K.L.; Molyneux, R.J.; May, G.S. Enhanced activity of strobilurin and fludioxonil by using berberine and phenolic compounds to target fungal antioxidative stress response. Lett. Appl. Microbiol. 2007, 45, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Bauer, A.W.; Kirby, W.M.; Sherris, J.C.; Turck, M. Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol. 1966, 45, 493–496. [Google Scholar] [CrossRef]
- Nakai, T.; Hatano, K.; Ikeda, F.; Shibuya, K. Electron microscopic findings for micafungin-treated experimental pulmonary aspergillosis in mice. Med. Mycol. 2005, 43, 439–445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mowat, E.; Rajendran, R.; Williams, C.; McCulloch, E.; Jones, B.; Lang, S.; Ramage, G. Pseudomonas aeruginosa and their small diffusible extracellular molecules inhibit Aspergillus fumigatus biofilm formation. FEMS Microbiol. Lett. 2010, 313, 96–102. [Google Scholar] [CrossRef] [Green Version]
- González-Ramírez, A.I.; Ramírez-Granillo, A.; Medina-Canales, M.G.; Rodríguez-Tovar, A.V.; Martínez-Rivera, M.A. Analysis and description of the stages of Aspergillus fumigatus biofilm formation using scanning electron microscopy. BMC Microbiol. 2016, 16, 243. [Google Scholar] [CrossRef] [Green Version]
- Abdallah, B.M.; Alzahrani, A.M.; Abdel-Moneim, A.M.; Ditzel, N.; Kassem, M. A simple and reliable protocol for long-term culture of murine bone marrow stromal (mesenchymal) stem cells that retained their in vitro and in vivo stemness in long-term culture. Biol. Proced. Online 2019, 21, 3. [Google Scholar] [CrossRef]
- Abdallah, B.M.; Ali, E.M. 5′-hydroxy Auraptene stimulates osteoblast differentiation of bone marrow-derived mesenchymal stem cells via a BMP-dependent mechanism. J. Biomed. Sci. 2019, 26, 51. [Google Scholar] [CrossRef]
- Sbaraglia, G.; D’Errico, P.; Serafini, S.; Vecchiarelli, L.; Perito, S. Pathogenicity of various species of Candida in mice immunodepressed with cyclophosphamide. Boll. Della Soc. Ital. Biol. Sper. 1984, 60, 1421–1426. [Google Scholar]
- Fahmy, S.R.; Ali, E.M.; Ahmed, N.S. Therapeutic effect of Sepia ink extract against invasive pulmonary aspergillosis in mice. J. Basic Appl. Zool. 2014, 67, 196–204. [Google Scholar] [CrossRef] [Green Version]
- Botelho, D.; Leo, B.F.; Massa, C.; Sarkar, S.; Tetley, T.; Chung, K.F.; Chen, S.; Ryan, M.P.; Porter, A.; Atochina-Vasserman, E.N.; et al. Exposure to Silver Nanospheres Leads to Altered Respiratory Mechanics and Delayed Immune Response in an in Vivo Murine Model. Front. Pharmacol. 2018, 9, 213. [Google Scholar] [CrossRef] [Green Version]
- Teranishi, Y.; Tanaka, A.; Osumi, M.; Fukui, S. Catalase Activities of Hydrocarbon-utilizing Candida Yeasts. Agric. Biol. Chem. 1974, 38, 1213–1220. [Google Scholar] [CrossRef]
- McCord, J.M.; Fridovich, I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J. Biol. Chem. 1969, 244, 6049–6055. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Sathishkumar, M.; Sneha, K.; Won, S.W.; Cho, C.W.; Kim, S.; Yun, Y.S. Cinnamon zeylanicum bark extract and powder mediated green synthesis of nano-crystalline silver particles and its bactericidal activity. Colloids Surf. B Biointerfaces 2009, 73, 332–338. [Google Scholar] [CrossRef]
- Stepanov, A.L.; Krenn, J.R.; Ditlbacher, H.; Hohenau, A.; Drezet, A.; Steinberger, B.; Leitner, A.; Aussenegg, F.R. Quantitative analysis of surface plasmon interaction with silver nanoparticles. Opt. Lett. 2005, 30, 1524–1526. [Google Scholar] [CrossRef]
- Lin, P.C.; Lin, S.; Wang, P.C.; Sridhar, R. Techniques for physicochemical characterization of nanomaterials. Biotechnol. Adv. 2014, 32, 711–726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Song, W.; Chen, Z.; Li, Q.; Liu, L. Ameliorative effect of synthesized silver nanoparticles by green route method from Zingiber zerumbet on mycoplasmal pneumonia in experimental mice. Artif. Cells Nanomed. Biotechnol. 2019, 47, 2146–2154. [Google Scholar] [CrossRef] [Green Version]
- David, A.; Ponvel, K.M.; Fathima, M.; Anita, S.; Ashli, J.; Athilakshmi, A. Biosynthesis of silver nanoparticles by Momordica charantia leaf extract: Characterization and their antimicrobial activities. J Nat Prod Plant Resour 2015, 4, 1–8. [Google Scholar]
- Tripathy, A.; Raichur, A.M.; Chandrasekaran, N.; Prathna, T.C.; Mukherjee, A. Process variables in biomimetic synthesis of silver nanoparticles by aqueous extract of Azadirachta indica (Neem) leaves. J. Nanoparticle Res. 2010, 12, 237–246. [Google Scholar] [CrossRef]
- Ifuku, S.; Tsuji, M.; Morimoto, M.; Saimoto, H.; Yano, H. Synthesis of silver nanoparticles templated by TEMPO-mediated oxidized bacterial cellulose nanofibers. Biomacromolecules 2009, 10, 2714–2717. [Google Scholar] [CrossRef] [PubMed]
- Jalal, M.; Ansari, M.A.; Alzohairy, M.A.; Ali, S.G.; Khan, H.M.; Almatroudi, A.; Siddiqui, M.I. Anticandidal activity of biosynthesized silver nanoparticles: Effect on growth, cell morphology, and key virulence attributes of Candida species. Int. J. Nanomed. 2019, 14, 4667–4679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muthamil, S.; Devi, V.A.; Balasubramaniam, B.; Balamurugan, K.; Pandian, S.K. Green synthesized silver nanoparticles demonstrating enhanced in vitro and in vivo antibiofilm activity against Candida spp. J. Basic Microbiol. 2018, 58, 343–357. [Google Scholar] [CrossRef] [PubMed]
- Balashanmugam, P.; Balakumaran, M.D.; Murugan, R.; Dhanapal, K.; Kalaichelvan, P.T. Phytogenic synthesis of silver nanoparticles, optimization and evaluation of in vitro antifungal activity against human and plant pathogens. Microbiol. Res. 2016, 192, 52–64. [Google Scholar] [CrossRef]
- Medda, S.; Hajra, A.; Dey, U.; Bose, P.; Mondal, N.K. Biosynthesis of silver nanoparticles from Aloe vera leaf extract and antifungal activity against Rhizopus sp. and Aspergillus sp. Appl. Nanosci. 2015, 5, 875–880. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, D.H.; Vo, T.N.N.; Nguyen, N.T.; Ching, Y.C.; Hoang Thi, T.T. Comparison of biogenic silver nanoparticles formed by Momordica charantia and Psidium guajava leaf extract and antifungal evaluation. PLoS ONE 2020, 15, e0239360. [Google Scholar]
- Carmona, E.M.; Limper, A.H. Overview of Treatment Approaches for Fungal Infections. Clin. Chest Med. 2017, 38, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Stewart, E.R.; Thompson, G.R. Treatment of Primary Pulmonary Aspergillosis: An Assessment of the Evidence. J. Fungi 2016, 2, 25. [Google Scholar] [CrossRef] [Green Version]
- Denning, D.W. Therapeutic outcome in invasive aspergillosis. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 1996, 23, 608–615. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Seth, A.; Wibowo, N.; Zhao, C.X.; Mitter, N.; Yu, C.; Middelberg, A.P. Nanoparticle vaccines. Vaccine 2014, 32, 327–337. [Google Scholar] [CrossRef] [Green Version]
- Bidgoli, R.D.; Pessarakli, M.; Heshmati, G.A.; Barani, H.; Saeedfar, M. Bioactive and Fragrant Constituents of Artemisia sieberi Besser Grown on Two Different Soil Types in Central Iran. Commun. Soil Sci. Plant Anal. 2013, 44, 2713–2719. [Google Scholar] [CrossRef]
- Galal, A.M.; Ross, S.A.; Jacob, M.; ElSohly, M.A. Antifungal activity of artemisinin derivatives. J. Nat. Prod. 2005, 68, 1274–1276. [Google Scholar] [CrossRef]
- Singh, G.; Kumar, P.; Joshi, S.C. Treatment of dermatophytosis by a new antifungal agent ‘apigenin’. Mycoses 2014, 57, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, M.; Kannan, A.; Stojković, D.S.; Glamočlija, J.; Calhelha, R.C.; Ferreira, I.; Sanglard, D.; Soković, M. Flavones, Flavonols, and Glycosylated Derivatives-Impact on Candida albicans Growth and Virulence, Expression of CDR1 and ERG11, Cytotoxicity. Pharmaceuticals 2020, 14, 27. [Google Scholar] [CrossRef] [PubMed]
- Khosravi, A.R.; Shokri, H.; Saffarian, Z. Anti-fungal activity of some native essential oils against emerging multi-drug resistant human nondermatophytic moulds. J. Herb. Med. 2020, 23, 100370. [Google Scholar] [CrossRef]
- Khosravi, A.R.; Shokri, H.; Kermani, S.; Dakhili, M.; Madani, M.; Parsa, S. Antifungal properties of Artemisia sieberi and Origanum vulgare essential oils against Candida glabrata isolates obtained from patients with vulvovaginal candidiasis. J. Mycol. Med. 2011, 21, 93–99. [Google Scholar] [CrossRef]
- Khatoon, N.; Sharma, Y.; Sardar, M.; Manzoor, N. Mode of action and anti-Candida activity of Artemisia annua mediated-synthesized silver nanoparticles. J. Mycol. Med. 2019, 29, 201–209. [Google Scholar] [CrossRef]
- Mikhailova, E.O. Silver Nanoparticles: Mechanism of Action and Probable Bio-Application. J. Funct. Biomater. 2020, 11, 84. [Google Scholar] [CrossRef]
- Lamsal, K.; Kim, S.W.; Jung, J.H.; Kim, Y.S.; Kim, K.S.; Lee, Y.S. Inhibition Effects of Silver Nanoparticles against Powdery Mildews on Cucumber and Pumpkin. Mycobiology 2011, 39, 26–32. [Google Scholar] [CrossRef] [Green Version]
- Shehata, E.; Mostafa, I.; Aziz, W.; Abd Allah, G.E. The Toxic Effect of Magnetic and Non- Magnetic Cinnamic Essential Oil against the Cotton Leafworm, Spodoptera littoralis. Egypt. Acad. J. Biol. Sci. F Toxicol. Pest Control 2019, 11, 107–112. [Google Scholar] [CrossRef]
- Kim, S.W.; Kim, K.S.; Lamsal, K.; Kim, Y.J.; Kim, S.B.; Jung, M.; Sim, S.J.; Kim, H.S.; Chang, S.J.; Kim, J.K.; et al. An in vitro study of the antifungal effect of silver nanoparticles on oak wilt pathogen Raffaelea sp. J. Microbiol. Biotechnol. 2009, 19, 760–764. [Google Scholar]
- Khalil, N. Biogenic silver nanoparticles by Aspergillus terreus as a powerful nanoweapon against Aspergillus fumigatus. Afr. J. Microbiol. Res. 2013, 7, 5645–5651. [Google Scholar]
- Beauvais, A.; Latgé, J.P. Aspergillus Biofilm In Vitro and In Vivo. Microbiol. Spectr. 2015, 3, 149–161. [Google Scholar] [CrossRef] [Green Version]
- Estevez, M.B.; Raffaelli, S.; Mitchell, S.G.; Faccio, R.; Alborés, S. Biofilm Eradication Using Biogenic Silver Nanoparticles. Molecules 2020, 25, 2023. [Google Scholar] [CrossRef] [PubMed]
- Mohanta, Y.; Biswas, K.; Jena, S.; Hashem, A.; Abd Allah, E.F.; Mohanta, T. Corrigendum: Anti-biofilm and Antibacterial Activities of Silver Nanoparticles Synthesized by the Reducing Activity of Phytoconstituents Present in the Indian Medicinal Plants. Front. Microbiol. 2020, 11, 784. [Google Scholar] [CrossRef] [PubMed]
- Lara, H.H.; Romero-Urbina, D.G.; Pierce, C.; Lopez-Ribot, J.L.; Arellano-Jiménez, M.J.; Jose-Yacaman, M. Effect of silver nanoparticles on Candida albicans biofilms: An ultrastructural study. J. Nanobiotechnology 2015, 13, 91. [Google Scholar] [CrossRef] [Green Version]
- Samberg, M.E.; Loboa, E.G.; Oldenburg, S.J.; Monteiro-Riviere, N.A. Silver nanoparticles do not influence stem cell differentiation but cause minimal toxicity. Nanomedicine 2012, 7, 1197–1209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gliga, A.R.; Skoglund, S.; Odnevall Wallinder, I.; Fadeel, B.; Karlsson, H.L. Size-dependent cytotoxicity of silver nanoparticles in human lung cells: The role of cellular uptake, agglomeration and Ag release. Part. Fibre Toxicol. 2014, 11, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez-Cantero, A.; Serrano, D.R.; Navarro-Rodríguez, P.; Schätzlein, A.G.; Uchegbu, I.F.; Torrado, J.J.; Capilla, J. Increased Efficacy of Oral Fixed-Dose Combination of Amphotericin B and AHCC(®) Natural Adjuvant against Aspergillosis. Pharmaceutics 2019, 11, 456. [Google Scholar] [CrossRef] [Green Version]
- Shirkhani, K.; Teo, I.; Armstrong-James, D.; Shaunak, S. Nebulised amphotericin B-polymethacrylic acid nanoparticle prophylaxis prevents invasive aspergillosis. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1217–1226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Italia, J.L.; Sharp, A.; Carter, K.C.; Warn, P.; Kumar, M.N. Peroral amphotericin B polymer nanoparticles lead to comparable or superior in vivo antifungal activity to that of intravenous Ambisome® or Fungizone™. PLoS ONE 2011, 6, e25744. [Google Scholar] [CrossRef] [Green Version]
- Yadav, B.; Specht, C.A.; Lee, C.K.; Pokrovskii, M.; Huh, J.R.; Littman, D.R.; Levitz, S.M. Lung eosinophils elicited during allergic and acute aspergillosis express RORγt and IL-23R but do not require IL-23 for IL-17 production. PLoS Pathog 2021, 17, e1009891. [Google Scholar] [CrossRef] [PubMed]
- Amarsaikhan, N.; Tsoggerel, A.; Hug, C.; Templeton, S.P. The Metabolic Cytokine Adiponectin Inhibits Inflammatory Lung Pathology in Invasive Aspergillosis. J. Immunol. 2019, 203, 956–963. [Google Scholar] [CrossRef] [PubMed]
- Schelenz, S.; Smith, D.A.; Bancroft, G.J. Cytokine and chemokine responses following pulmonary challenge with Aspergillus fumigatus: Obligatory role of TNF-alpha and GM-CSF in neutrophil recruitment. Med. Mycol. 1999, 37, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Heinekamp, T.; Schmidt, H.; Lapp, K.; Pähtz, V.; Shopova, I.; Köster-Eiserfunke, N.; Krüger, T.; Kniemeyer, O.; Brakhage, A.A. Interference of Aspergillus fumigatus with the immune response. Semin. Immunopathol. 2015, 37, 141–152. [Google Scholar] [CrossRef] [Green Version]
- Hof, H.; Kupfahl, C. Gliotoxin in Aspergillus fumigatus: An example that mycotoxins are potential virulence factors. Mycotoxin Res. 2009, 25, 123–131. [Google Scholar] [CrossRef]
- Gayathri, L.; Akbarsha, M.A.; Ruckmani, K. In vitro study on aspects of molecular mechanisms underlying invasive aspergillosis caused by gliotoxin and fumagillin, alone and in combination. Sci. Rep. 2020, 10, 14473. [Google Scholar] [CrossRef]
- Horky, P.; Skalickova, S.; Baholet, D.; Skladanka, J. Nanoparticles as a Solution for Eliminating the Risk of Mycotoxins. Nanomaterials 2018, 8, 727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mousavi, S.A.P.; Pourtalebi, S.M. Inhibitory Effects of Silver Nanoparticles on Growth and Aflatoxin B1 Production by Aspergillus Parasiticus. Iran. J. Med. Sci. 2015, 40, 501–506. [Google Scholar]
- Pietrzak, K.; Twarużek, M.; Czyżowska, A.; Kosicki, R.; Gutarowska, B. Influence of silver nanoparticles on metabolism and toxicity of moulds. Acta Biochim. Pol. 2015, 62, 851–857. [Google Scholar] [CrossRef] [PubMed]
- Khalil, N.M.; Abd El-Ghany, M.N.; Rodríguez-Couto, S. Antifungal and anti-mycotoxin efficacy of biogenic silver nanoparticles produced by Fusarium chlamydosporum and Penicillium chrysogenum at non-cytotoxic doses. Chemosphere 2019, 218, 477–486. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, E.M.; Abdallah, B.M. Effective Inhibition of Invasive Pulmonary Aspergillosis by Silver Nanoparticles Biosynthesized with Artemisia sieberi Leaf Extract. Nanomaterials 2022, 12, 51. https://doi.org/10.3390/nano12010051
Ali EM, Abdallah BM. Effective Inhibition of Invasive Pulmonary Aspergillosis by Silver Nanoparticles Biosynthesized with Artemisia sieberi Leaf Extract. Nanomaterials. 2022; 12(1):51. https://doi.org/10.3390/nano12010051
Chicago/Turabian StyleAli, Enas M., and Basem M. Abdallah. 2022. "Effective Inhibition of Invasive Pulmonary Aspergillosis by Silver Nanoparticles Biosynthesized with Artemisia sieberi Leaf Extract" Nanomaterials 12, no. 1: 51. https://doi.org/10.3390/nano12010051





