Oral Cancer Theranostic Application of FeAu Bimetallic Nanoparticles Conjugated with MMP-1 Antibody
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material and Characterization
2.2. Synthesis of Iron-Gold (FeAu) Bimetallic NPs
2.3. Surface Modification and Synthesis of MMP-1 Antibody Conjugated FeAu NPs
2.4. Magnetic Stimulation-Induced Concentration-Dependent Temperature Elevation
2.5. Cell Culture
2.6. In Vitro Cytotoxicity Analysis
2.7. The Evaluation of Cell-Specific MMP-1 Antibody-Conjugated FeAu NPs Ingestion
2.8. The Effect of Magnetic Field-Induced Hyperthermia on HSC-3
2.9. Analysis of Nanoparticle Ingestion Using Bio-TEM
2.10. In Vivo Mouse Model for Evaluation of Anti-Cancer Treatment
2.11. The Measurement of Tumor Growth in Nude Mice
2.12. The Growth of HSC-3 Cells Was Inhibited by Magnetic Hyperthermia Therapy
2.13. Statistics
3. Results and Discussions
3.1. The Characterization of FeAu Nanoparticles
3.2. The Confirmation of FeAu-Cys NPs Formation
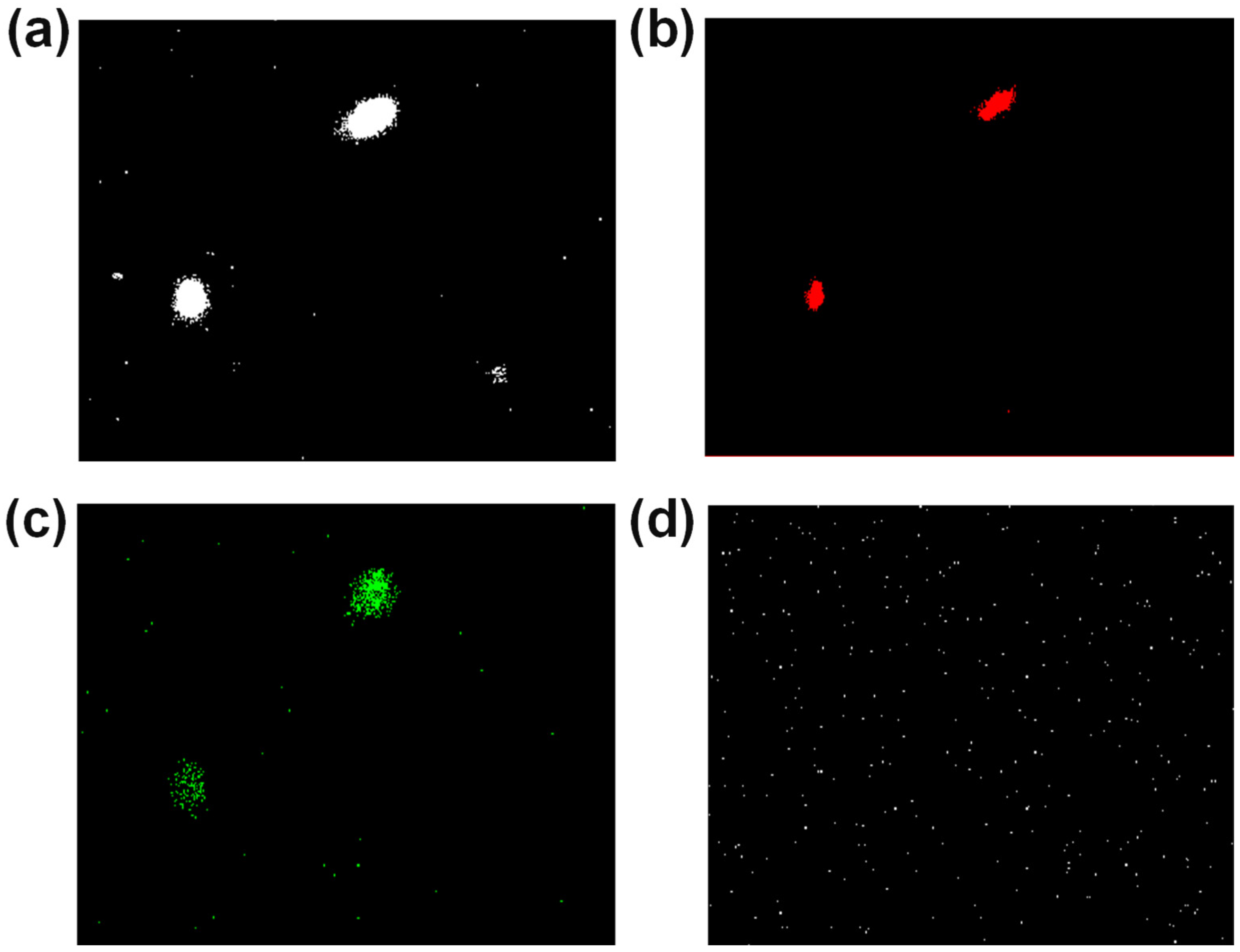
3.3. Confocal Analysis
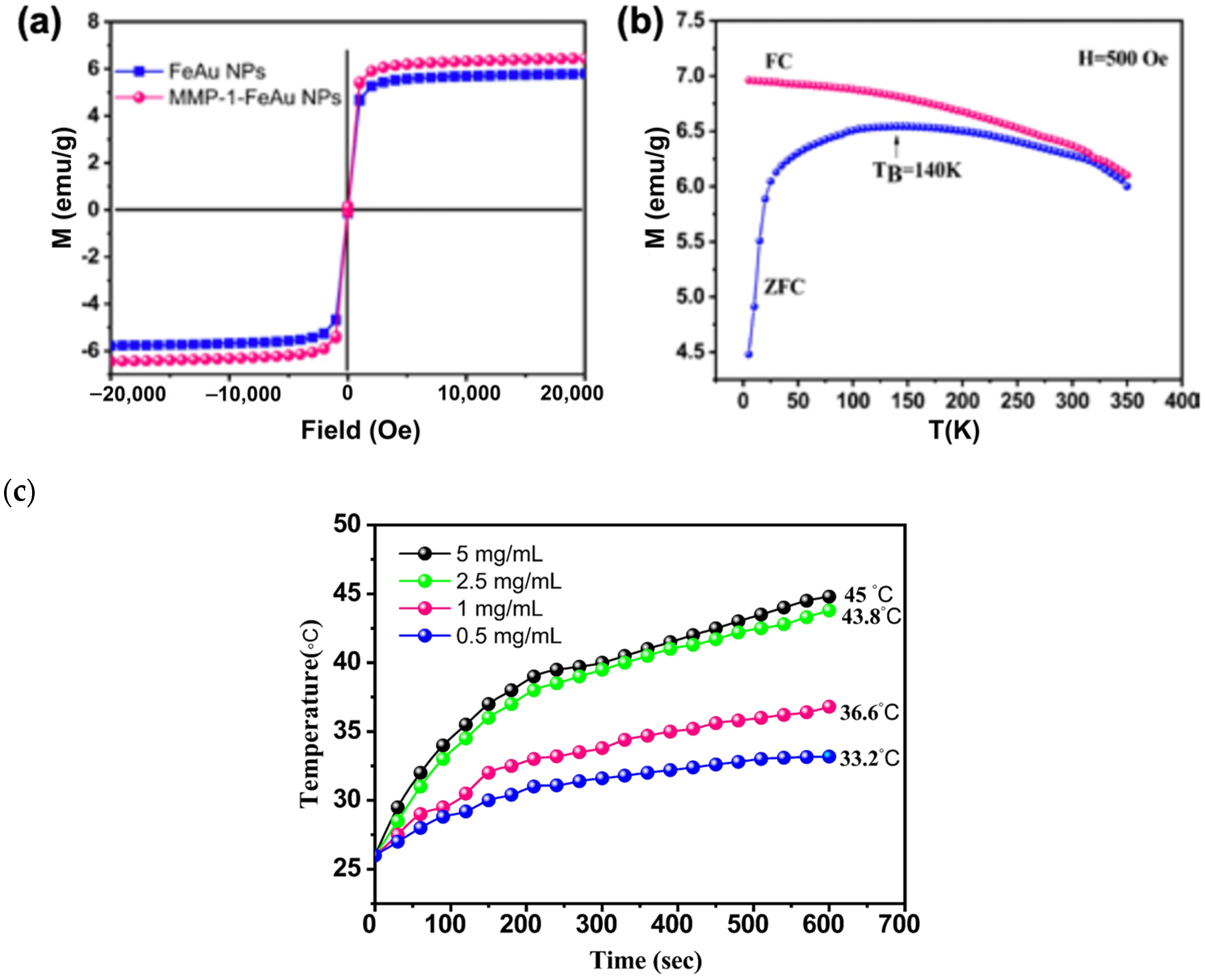
3.4. Analysis of Magnetic Properties of FeAu NPs and MMP-1 Antibody-Conjugated FeAu NPs
3.5. Heat Generation upon Magnetic Field Stimulation
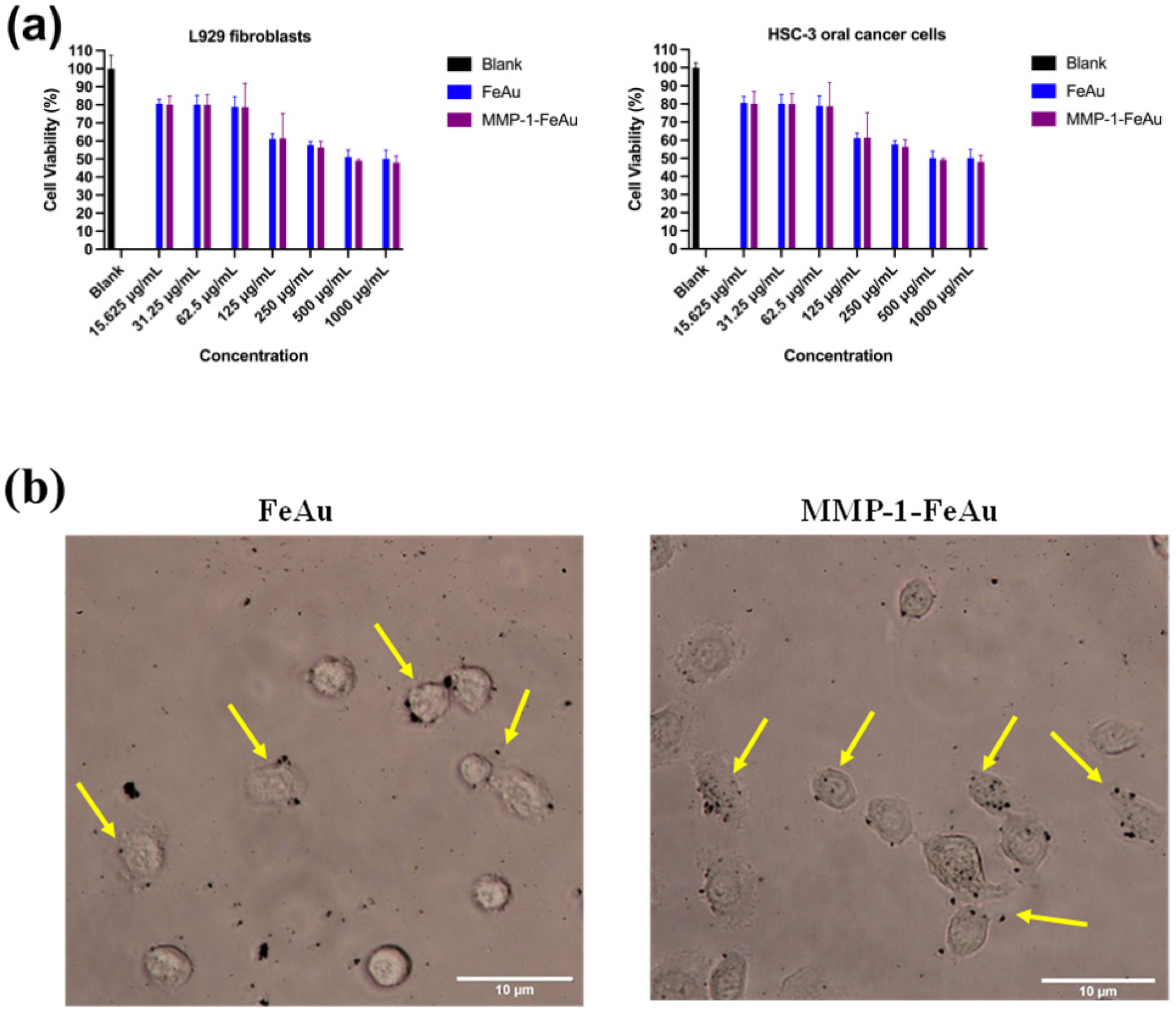
3.6. In Vitro Cytotoxicity Analysis
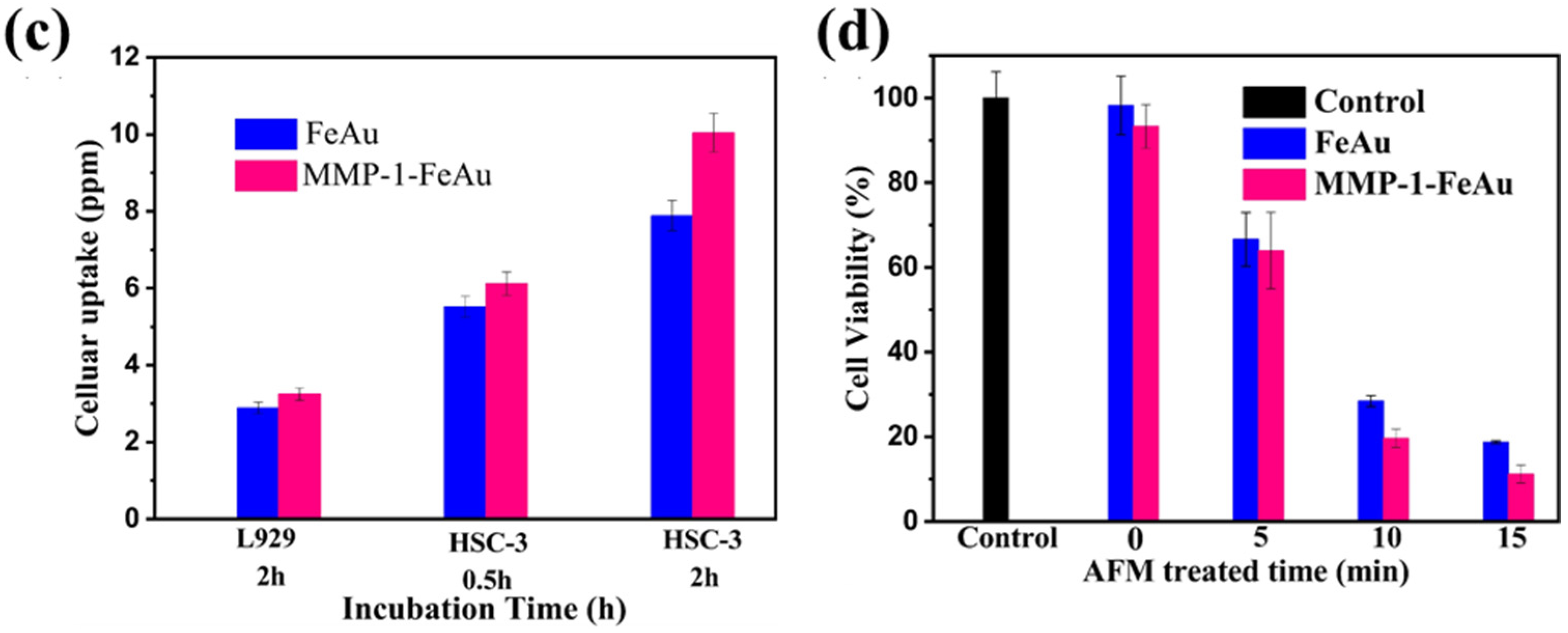
3.7. Analysis of Nanoparticle Ingestion Using Bio-TEM
3.8. The Effects of NP-Mediated Hyperthermia on HSC-3 Cells
3.9. The Effects of NP-Mediated Magnetic Hyperthermia Therapy on Nude Mice
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Chen, Z.G. The cancer stem cell concept in progression of head and neck cancer. J. Oncol. 2009, 2009, 894064. [Google Scholar] [CrossRef] [Green Version]
- Tahir, A.; Nagi, A.H.; Ullah, E.; Janjua, O.S. The role of mast cells and angiogenesis in well-differentiated oral squamous cell carcinoma. J. Cancer Res. Ther. 2013, 9, 387. [Google Scholar]
- Oji, C.; Chukwuneke, F. Poor oral hygiene may be the sole cause of oral cancer. J. Maxillofac. Oral Surg. 2012, 11, 379–383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bagan, J.; Sarrion, G.; Jimenez, Y. Oral cancer: Clinical features. Oral Oncol. 2010, 46, 414–417. [Google Scholar] [CrossRef]
- Ong, T.; Murphy, C.; Smith, A.; Kanatas, A.; Mitchell, D. Survival after surgery for oral cancer: A 30-year experience. Br. J. Oral Maxillofac. Surg. 2017, 55, 911–916. [Google Scholar] [CrossRef]
- Vermorken, J.; Specenier, P. Optimal treatment for recurrent/metastatic head and neck cancer. Ann. Oncol. 2010, 21, vii252–vii261. [Google Scholar] [CrossRef] [PubMed]
- Haen, S.P.; Pereira, P.L.; Salih, H.R.; Rammensee, H.-G.; Gouttefangeas, C. More than just tumor destruction: Immunomodulation by thermal ablation of cancer. Clin. Dev. Immunol. 2011, 2011, 160250. [Google Scholar] [CrossRef]
- Zhao, Q.; Wang, L.; Cheng, R.; Mao, L.; Arnold, R.D.; Howerth, E.W.; Chen, Z.G.; Platt, S. Magnetic nanoparticle-based hyperthermia for head & neck cancer in mouse models. Theranostics 2012, 2, 113. [Google Scholar] [PubMed] [Green Version]
- Al Moustafa, A.-E. Development of Oral Cancer: Risk Factors and Prevention Strategies; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Li, Y.; Dhawan, U.; Wang, H.Y.; Liu, X.; Ku, H.H.; Tsai, M.T.; Yen, H.W.; Chung, R.J. Theranostic Iron@ Gold Core–Shell Nanoparticles for Simultaneous Hyperthermia-Chemotherapy upon Photo-Stimulation. Part. Part. Syst. Charact. 2019, 36, 1800419. [Google Scholar] [CrossRef]
- Christodoulou, E.; Nerantzaki, M.; Nanaki, S.; Barmpalexis, P.; Giannousi, K.; Dendrinou-Samara, C.; Angelakeris, M.; Gounari, E.; Anastasiou, A.D.; Bikiaris, D.N. Paclitaxel Magnetic Core–Shell Nanoparticles Based on Poly (lactic acid) Semitelechelic Novel Block Copolymers for Combined Hyperthermia and Chemotherapy Treatment of Cancer. Pharmaceutics 2019, 11, 213. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.-H.; Xie, J.; Lai, Z.-Y.; Yang, M.-D.; Zhang, G.-H.; Li, Y.; Mu, J.-B.; Xu, J. Radiofrequency deep hyperthermia combined with chemotherapy in the treatment of advanced non-small cell lung cancer. Chin. Med. J. 2019, 132, 922. [Google Scholar] [CrossRef] [PubMed]
- Ha, P.T.; Le, T.T.H.; Bui, T.Q.; Pham, H.N.; Ho, A.S.; Nguyen, L.T. Doxorubicin release by magnetic inductive heating and in vivo hyperthermia-chemotherapy combined cancer treatment of multifunctional magnetic nanoparticles. New J. Chem. 2019, 43, 5404–5413. [Google Scholar] [CrossRef]
- Bharath, G.; Rambabu, K.; Banat, F.; Ponpandian, N.; Alsharaeh, E.; Harrath, A.H.; Alrezaki, A.; Alwasel, S. Shape-controlled rapid synthesis of magnetic nanoparticles and their morphological dependent magnetic and thermal studies for cancer therapy applications. Mater. Res. Express 2019, 6, 66104. [Google Scholar] [CrossRef]
- Daboin, V.; Briceño, S.; Suárez, J.; Carrizales-Silva, L.; Alcalá, O.; Silva, P.; Gonzalez, G. Magnetic SiO2-Mn1-xCoxFe2O4 nanocomposites decorated with Au@ Fe3O4 nanoparticles for hyperthermia. J. Magn. Magn. Mater. 2019, 479, 91–98. [Google Scholar] [CrossRef]
- Farzin, A.; Hassan, S.; Emadi, R.; Etesami, S.A.; Ai, J. Comparative evaluation of magnetic hyperthermia performance and biocompatibility of magnetite and novel Fe-doped hardystonite nanoparticles for potential bone cancer therapy. Mater. Sci. Eng. C 2019, 98, 930–938. [Google Scholar] [CrossRef]
- Goswami, M.M.; Das, A.; De, D. Wetchemical synthesis of FePt nanoparticles: Tuning of magnetic properties and biofunctionalization for hyperthermia therapy. J. Magn. Magn. Mater. 2019, 475, 93–97. [Google Scholar] [CrossRef]
- Gupta, R.; Sharma, D. Evolution of magnetic hyperthermia for glioblastoma multiforme therapy. ACS Chem. Neurosci. 2019, 10, 1157–1172. [Google Scholar] [CrossRef] [PubMed]
- Dabbagh, A.; Hedayatnasab, Z.; Karimian, H.; Sarraf, M.; Yeong, C.H.; Madaah Hosseini, H.R.; Abu Kasim, N.H.; Wong, T.W.; Rahman, N.A. Polyethylene glycol-coated porous magnetic nanoparticles for targeted delivery of chemotherapeutics under magnetic hyperthermia condition. Int. J. Hyperth. 2019, 36, 104–114. [Google Scholar] [CrossRef]
- Lachowicz, D.; Kaczyńska, A.; Wirecka, R.; Kmita, A.; Szczerba, W.; Bodzoń-Kułakowska, A.; Sikora, M.; Karewicz, A.; Zapotoczny, S. A hybrid system for magnetic hyperthermia and drug delivery: SPION functionalized by curcumin conjugate. Materials 2018, 11, 2388. [Google Scholar] [CrossRef] [Green Version]
- Srinoi, P.; Chen, Y.-T.; Vittur, V.; Marquez, M.D.; Lee, T.R. Bimetallic nanoparticles: Enhanced magnetic and optical properties for emerging biological applications. Appl. Sci. 2018, 8, 1106. [Google Scholar] [CrossRef] [Green Version]
- Yuan, M.; Wang, Y.; Qin, Y.X. Promoting neuroregeneration by applying dynamic magnetic fields to a novel nanomedicine: Superparamagnetic iron oxide (SPIO)-gold nanoparticles bounded with nerve growth factor (NGF). Nanomedicine 2018, 14, 1337–1347. [Google Scholar] [CrossRef]
- Torresan, V.; Forrer, D.; Guadagnini, A.; Badocco, D.; Pastore, P.; Casarin, M.; Selloni, A.; Coral, D.; Ceolin, M.; Fernández van Raap, M.B.; et al. 4D Multimodal Nanomedicines Made of Nonequilibrium Au–Fe Alloy Nanoparticles. Acs Nano 2020, 14, 12840–12853. [Google Scholar] [CrossRef]
- Song, J.; Wu, B.; Zhou, Z.; Zhu, G.; Liu, Y.; Yang, Z.; Lin, L.; Yu, G.; Zhang, F.; Zhang, G.; et al. Double-Layered Plasmonic-Magnetic Vesicles by Self-Assembly of Janus Amphiphilic Gold-Iron(II, III) Oxide Nanoparticles. Angew Chem. Int. Ed Engl. 2017, 56, 8110–8114. [Google Scholar] [CrossRef] [PubMed]
- Chung, R.-J.; Wang, H.-Y.; Wu, K.-T. Preparation and characterization of Fe-Au alloy nanoparticles for hyperthermia application. J. Med. Biol. Eng. 2014, 34, 251–255. [Google Scholar] [CrossRef]
- Bañobre-López, M.; Teijeiro, A.; Rivas, J. Magnetic nanoparticle-based hyperthermia for cancer treatment. Rep. Pract. Oncol. Radiother. 2013, 18, 397–400. [Google Scholar] [CrossRef] [Green Version]
- Gurav, D.; Varghese, O.P.; Hamad, O.A.; Nilsson, B.; Hilborn, J.; Oommen, O.P. Chondroitin sulfate coated gold nanoparticles: A new strategy to resolve multidrug resistance and thromboinflammation. Chem. Commun. 2016, 52, 966–969. [Google Scholar] [CrossRef]
- Kalyane, D.; Raval, N.; Maheshwari, R.; Tambe, V.; Kalia, K.; Tekade, R.K. Employment of enhanced permeability and retention effect (EPR): Nanoparticle-based precision tools for targeting of therapeutic and diagnostic agent in cancer. Mater. Sci. Eng. C 2019, 98, 1252–1276. [Google Scholar] [CrossRef] [PubMed]
- George, A.; Ranganathan, K.; Rao, U.K. Expression of MMP-1 in histopathological different grades of oral squamous cell carcinoma and in normal buccal mucosa–an immunohistochemical study. Cancer Biomark. 2010, 7, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Ruan, S.; Cao, X.; Cun, X.; Hu, G.; Zhou, Y.; Zhang, Y.; Lu, L.; He, Q.; Gao, H. Matrix metalloproteinase-sensitive size-shrinkable nanoparticles for deep tumor penetration and pH triggered doxorubicin release. Biomaterials 2015, 60, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.C.; do Carmo, E.D.; da Silva, M.A.D.; Rosa, L.E.B. Matrix metalloproteinase gene polymorphisms and oral cancer. J. Clin. Exp. Dent. 2012, 4, e297. [Google Scholar] [CrossRef]
- Chaudhary, A.K.; Pandya, S.; Mehrotra, R.; Bharti, A.C.; Jain, S.; Singh, M. Functional polymorphism of the MMP-1 promoter (-1607 1G/2G) in potentially malignant and malignant head and neck lesions in an Indian population. Biomarkers 2010, 15, 684–692. [Google Scholar] [CrossRef]
- Tang, M.-L.; Bai, X.-J.; Li, Y.; Dai, X.-J.; Yang, F. MMP-1 Over-expression Promotes Malignancy and Stem-Like Properties of Human Osteosarcoma MG-63 Cells In Vitro. Curr. Med. Sci. 2018, 38, 809–817. [Google Scholar] [CrossRef] [PubMed]
- Rao, V.H.; Singh, R.K.; Finnell, R.H.; Dave, B.J.; Beuhler, B.A.; Sanger, W.G.; Schaefer, G.B. Matrix metalloproteinases and their inhibitors in tumor invasion and metastasis. In Proceedings of the Indian Academy of Sciences-Chemical Sciences; Springer: New Delhi, India, 1999; pp. 239–254. [Google Scholar]
- Hadler-Olsen, E.; Winberg, J.-O.; Uhlin-Hansen, L. Matrix metalloproteinases in cancer: Their value as diagnostic and prognostic markers and therapeutic targets. Tumor Biol. 2013, 34, 2041–2051. [Google Scholar] [CrossRef] [PubMed]
- Pulukuri, S.M.K.; Rao, J.S. Matrix metalloproteinase-1 promotes prostate tumor growth and metastasis. Int. J. Oncol. 2008, 32, 757–765. [Google Scholar]
- Hsu, S.P.; Dhawan, U.; Tseng, Y.-Y.; Lin, C.-P.; Kuo, C.-Y.; Wang, L.-F.; Chung, R.-J. Glioma-sensitive delivery of Angiopep-2 conjugated iron gold alloy nanoparticles ensuring simultaneous tumor imaging and hyperthermia mediated cancer theranostics. Appl. Mater. Today 2020, 18, 100510. [Google Scholar] [CrossRef]


| Element | Weight% | Atomic% |
|---|---|---|
| Fe | 18.40 | 44.29 |
| Au | 81.60 | 55.71 |
| Element | Atomic% | Molar Ratio% |
|---|---|---|
| Fe | 49 | 0.96 |
| Au | 51 | 1.04 |
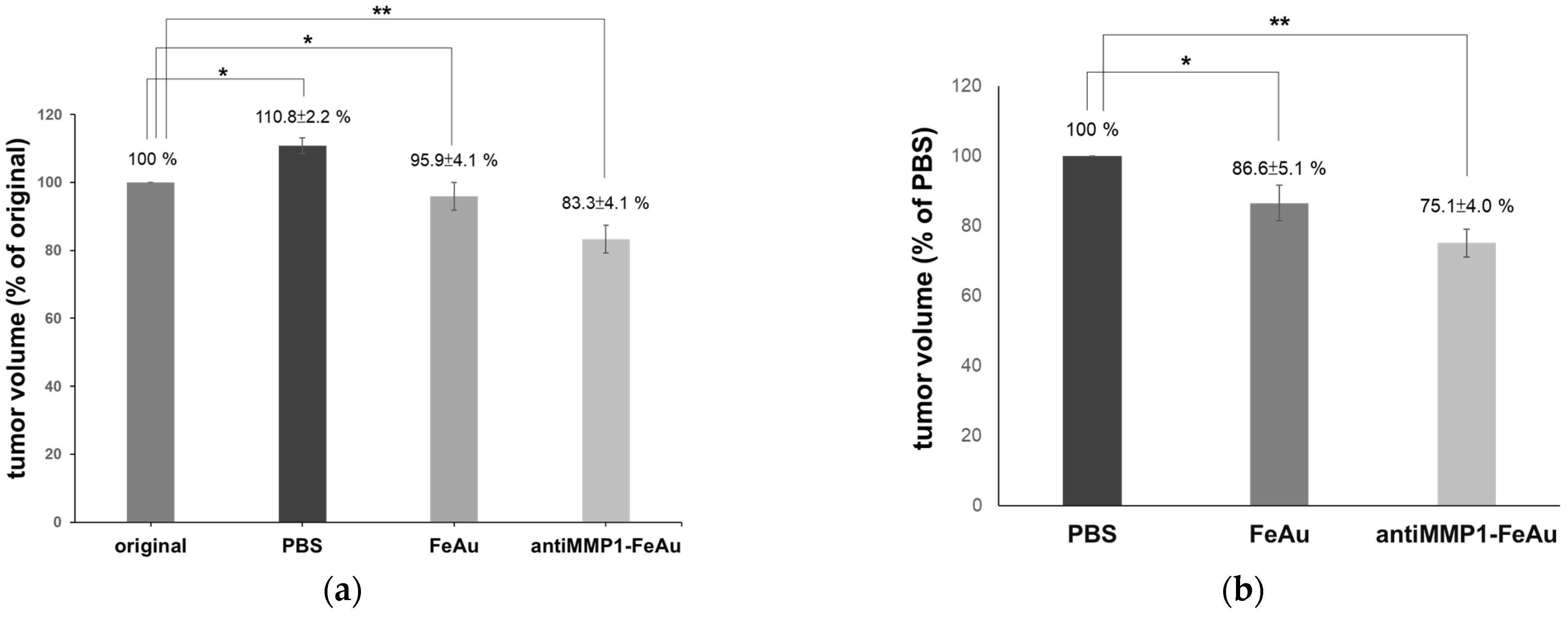
| Volume (mm3) | Variation (%) | |
|---|---|---|
| Original (before magnetic heat treat) | 39.5 ± 0.6 | - |
| Control (magnetic heat treat after 30 day) | 43.79 ± 1.5 | +10.9 |
| FeAu NPs (magnetic heat treat after 30 day) | 37.87 ± 1.0 | −4.1 |
| antiMMP1-FeAu NPs (magnetic heat treat after 30 day) | 32.88 ± 1.3 | −16.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, M.-T.; Sun, Y.-S.; Keerthi, M.; Panda, A.K.; Dhawan, U.; Chang, Y.-H.; Lai, C.-F.; Hsiao, M.; Wang, H.-Y.; Chung, R.-J. Oral Cancer Theranostic Application of FeAu Bimetallic Nanoparticles Conjugated with MMP-1 Antibody. Nanomaterials 2022, 12, 61. https://doi.org/10.3390/nano12010061
Tsai M-T, Sun Y-S, Keerthi M, Panda AK, Dhawan U, Chang Y-H, Lai C-F, Hsiao M, Wang H-Y, Chung R-J. Oral Cancer Theranostic Application of FeAu Bimetallic Nanoparticles Conjugated with MMP-1 Antibody. Nanomaterials. 2022; 12(1):61. https://doi.org/10.3390/nano12010061
Chicago/Turabian StyleTsai, Meng-Tsan, Ying-Sui Sun, Murugan Keerthi, Asit Kumar Panda, Udesh Dhawan, Yung-Hsiang Chang, Chih-Fang Lai, Michael Hsiao, Huey-Yuan Wang, and Ren-Jei Chung. 2022. "Oral Cancer Theranostic Application of FeAu Bimetallic Nanoparticles Conjugated with MMP-1 Antibody" Nanomaterials 12, no. 1: 61. https://doi.org/10.3390/nano12010061
APA StyleTsai, M. -T., Sun, Y. -S., Keerthi, M., Panda, A. K., Dhawan, U., Chang, Y. -H., Lai, C. -F., Hsiao, M., Wang, H. -Y., & Chung, R. -J. (2022). Oral Cancer Theranostic Application of FeAu Bimetallic Nanoparticles Conjugated with MMP-1 Antibody. Nanomaterials, 12(1), 61. https://doi.org/10.3390/nano12010061









