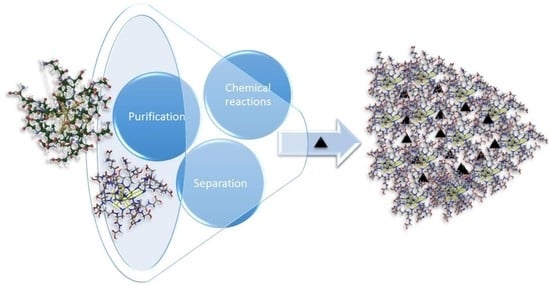
Controlling the Chemistry of Nanoclusters: From Atomic Precision to Controlled Assembly
Abstract
:1. Introduction
2. Ligand-Protected Metal Nanoclusters. Comparing Gold, Silver, Copper and Nickel
3. Purification, Separation, and Isolation of Nanoclusters
4. Chemical Reactions of Nanoclusters
4.1. Intermolecular Chemical Reactions of Nanoclusters. Example of Zinc Ion Induced Aggregation Strategy


4.2. Intramolecular Chemical Reactions of Nanoclusters. Example of Oxygen and Ligand and Metal Exchange Reactions
5. Routes to Self-Assembled Structures of Nanoclusters. from Crystalline Assembly to Directed Assembly of NCs

6. Concluding Remarks
Funding
Conflicts of Interest
References
- Overbeek, J.T.G. Monodisperse colloidal systems, fascinating and useful. Adv. Colloid Interface Sci. 1982, 15, 251–277. [Google Scholar] [CrossRef]
- Jin, R.; Zeng, C.; Zhou, M.; Chen, Y. Atomically Precise Colloidal Metal Nanoclusters and Nanoparticles: Fundamentals and Opportunities. Chem. Rev. 2016, 116, 10346–10413. [Google Scholar] [CrossRef] [PubMed]
- Jin, R. Quantum sized, thiolate-protected gold nanoclusters. Nanoscale 2010, 2, 343–362. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, I.; Pradeep, T. Atomically Precise Clusters of Noble Metals: Emerging Link between Atoms and Nanoparticles. Chem. Rev. 2017, 117, 8208–8271. [Google Scholar] [CrossRef]
- Heuer-Jungemann, A.; Feliu, N.; Bakaimi, I.; Hamaly, M.; Alkilany, A.; Chakraborty, I.; Masood, A.; Casula, M.F.; Kostopoulou, A.; Oh, E.; et al. The Role of Ligands in the Chemical Synthesis and Applications of Inorganic Nanoparticles. Chem. Rev. 2019, 119, 4819–4880. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Zhou, M.; Jin, R. Programmable Metal Nanoclusters with Atomic Precision. Adv. Mater. 2021, 33, 2006591. [Google Scholar] [CrossRef]
- Jin, R. Atomically precise metal nanoclusters: Stable sizes and optical properties. Nanoscale 2015, 7, 1549–1565. [Google Scholar] [CrossRef]
- Negishi, Y.; Hashimoto, S.; Ebina, A.; Hamada, K.; Hossain, S.; Kawawaki, T. Atomic-level separation of thiolate-protected metal clusters. Nanoscale 2020, 12, 8017–8039. [Google Scholar] [CrossRef]
- Comby-Zerbino, C.; Dagany, X.; Chirot, F.; Dugourd, P.; Antoine, R. The emergence of mass spectrometry for characterizing nanomaterials. Atomically precise nanoclusters and beyond. Mater. Adv. 2021, 2, 4896–4913. [Google Scholar] [CrossRef]
- Xiao, Y.; Wu, Z.N.; Yao, Q.F.; Xie, J.P. Luminescent metal nanoclusters: Biosensing strategies and bioimaging applications. Aggregate 2021, 2, 114–132. [Google Scholar] [CrossRef]
- Sun, Y.F.; Zhou, Z.P.; Shu, T.; Qian, L.S.; Su, L.; Zhang, X.J. Multicolor Luminescent Gold Nanoclusters: From Structure to Biosensing and Bioimaging. Prog. Chem. 2021, 33, 179–187. [Google Scholar] [CrossRef]
- Jin, R.; Li, G.; Sharma, S.; Li, Y.; Du, X. Toward Active-Site Tailoring in Heterogeneous Catalysis by Atomically Precise Metal Nanoclusters with Crystallographic Structures. Chem. Rev. 2021, 121, 567–648. [Google Scholar] [CrossRef] [PubMed]
- Higaki, T.; Li, Y.; Zhao, S.; Li, Q.; Li, S.; Du, X.-S.; Yang, S.; Chai, J.; Jin, R. Atomically Tailored Gold Nanoclusters for Catalytic Application. Angew. Chem. Int. Ed. 2019, 58, 8291–8302. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Li, M.; Ren, J.; Qu, X. Metal nanoclusters: Novel probes for diagnostic and therapeutic applications. Chem. Soc. Rev. 2015, 44, 8636–8663. [Google Scholar] [CrossRef] [PubMed]
- Combes, G.F.; Vučković, A.-M.; Perić Bakulić, M.; Antoine, R.; Bonačić-Koutecky, V.; Trajković, K. Nanotechnology in Tumor Biomarker Detection: The Potential of Liganded Nanoclusters as Nonlinear Optical Contrast Agents for Molecular Diagnostics of Cancer. Cancers 2021, 13, 4206. [Google Scholar] [CrossRef] [PubMed]
- Porret, E.; Le Guével, X.; Coll, J.L. Gold nanoclusters for biomedical applications: Toward in vivo studies. J. Mater. Chemistry. B 2020, 8, 2216–2232. [Google Scholar] [CrossRef] [PubMed]
- Rival, J.V.; Mymoona, P.; Lakshmi, K.M.; Nonappa; Pradeep, T.; Shibu, E.S. Self-Assembly of Precision Noble Metal Nanoclusters: Hierarchical Structural Complexity, Colloidal Superstructures, and Applications. Small 2021, 17, 2005718. [Google Scholar] [CrossRef] [PubMed]
- Ebina, A.; Hossain, S.; Horihata, H.; Ozaki, S.; Kato, S.; Kawawaki, T.; Negishi, Y. One-, Two-, and Three-Dimensional Self-Assembly of Atomically Precise Metal Nanoclusters. Nanomaterials 2020, 10, 1105. [Google Scholar] [CrossRef]
- Wang, J.X.; Lin, X.F.; Shu, T.; Su, L.; Liang, F.; Zhang, X.J. Self-Assembly of Metal Nanoclusters for Aggregation-Induced Emission. Int. J. Mol. Sci. 2019, 20, 1891. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Jin, R. Seeing Ligands on Nanoclusters and in Their Assemblies by X-ray Crystallography: Atomically Precise Nanochemistry and Beyond. J. Am. Chem. Soc. 2020, 142, 13627–13644. [Google Scholar] [CrossRef]
- Kang, X.; Zhu, M. Intra-cluster growth meets inter-cluster assembly: The molecular and supramolecular chemistry of atomically precise nanoclusters. Coord. Chem. Rev. 2019, 394, 1–38. [Google Scholar] [CrossRef]
- Wei, X.; Kang, X.; Zuo, Z.; Song, F.; Wang, S.; Zhu, M. Hierarchical structural complexity in atomically precise nanocluster frameworks. Natl. Sci. Rev. 2020, 8, nwaa077. [Google Scholar] [CrossRef]
- Wu, Z.; Yao, Q.; Zang, S.; Xie, J. Directed Self-Assembly of Ultrasmall Metal Nanoclusters. ACS Mater. Lett. 2019, 1, 237–248. [Google Scholar] [CrossRef]
- Antoine, R. Supramolecular Gold Chemistry: From Atomically Precise Thiolate-Protected Gold Nanoclusters to Gold-Thiolate Nanostructures. Nanomaterials 2020, 10, 377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maity, P.; Xie, S.; Yamauchi, M.; Tsukuda, T. Stabilized gold clusters: From isolation toward controlled synthesis. Nanoscale 2012, 4, 4027–4037. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Petty, J.T.; Dickson, R.M. High Quantum Yield Blue Emission from Water-Soluble Au8 Nanodots. J. Am. Chem. Soc. 2003, 125, 7780–7781. [Google Scholar] [CrossRef] [PubMed]
- Zare, I.; Chevrier, D.M.; Cifuentes-Rius, A.; Moradi, N.; Xianyu, Y.; Ghosh, S.; Trapiella-Alfonso, L.; Tian, Y.; Shourangiz-Haghighi, A.; Mukherjee, S.; et al. Protein-protected metal nanoclusters as diagnostic and therapeutic platforms for biomedical applications. Mater. Today 2021, 308, 118323. [Google Scholar] [CrossRef]
- Xie, J.; Zheng, Y.; Ying, J.Y. Protein-Directed Synthesis of Highly Fluorescent Gold Nanoclusters. J. Am. Chem. Soc. 2009, 131, 888–889. [Google Scholar] [CrossRef] [PubMed]
- Chan, P.-H.; Chen, Y.-C. Human Serum Albumin Stabilized Gold Nanoclusters as Selective Luminescent Probes for Staphylococcus aureus and Methicillin-Resistant Staphylococcus aureus. Anal. Chem. 2012, 84, 8952–8956. [Google Scholar] [CrossRef]
- Liu, C.-L.; Wu, H.-T.; Hsiao, Y.-H.; Lai, C.-W.; Shih, C.-W.; Peng, Y.-K.; Tang, K.-C.; Chang, H.-W.; Chien, Y.-C.; Hsiao, J.-K.; et al. Insulin-Directed Synthesis of Fluorescent Gold Nanoclusters: Preservation of Insulin Bioactivity and Versatility in Cell Imaging. Angew. Chem. Int. Ed. 2011, 50, 7056–7060. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.; Liu, L.; Li, F.; Shuang, S.; Li, Y.; Choi, M.M.F.; Dong, C. Lysozyme-stabilized gold nanoclusters as a novel fluorescence probe for cyanide recognition. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 121, 77–80. [Google Scholar] [CrossRef]
- Wen, F.; Dong, Y.; Feng, L.; Wang, S.; Zhang, S.; Zhang, X. Horseradish Peroxidase Functionalized Fluorescent Gold Nanoclusters for Hydrogen Peroxide Sensing. Anal. Chem. 2011, 83, 1193–1196. [Google Scholar] [CrossRef]
- Chakraborty, S.; Babanova, S.; Rocha, R.C.; Desireddy, A.; Artyushkova, K.; Boncella, A.E.; Atanassov, P.; Martinez, J.S. A Hybrid DNA-Templated Gold Nanocluster for Enhanced Enzymatic Reduction of Oxygen. J. Am. Chem. Soc. 2015, 137, 11678–11687. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, A.K.; Sailapu, S.K.; Dutta, D.; Banerjee, S.; Ghosh, S.S.; Chattopadhyay, A. DNA-Templated Single Thermal Cycle Based Synthesis of Highly Luminescent Au Nanoclusters for Probing Gene Expression. ACS Sustain. Chem. Eng. 2018, 6, 2142–2151. [Google Scholar] [CrossRef]
- Bertorelle, F.; Russier-Antoine, I.; Calin, N.; Comby-Zerbino, C.; Bensalah-Ledoux, A.; Guy, S.; Dugourd, P.; Brevet, P.-F.; Sanader, Ž.; Krstić, M.; et al. Au10(SG)10: A Chiral Gold Catenane Nanocluster with Zero Confined Electrons. Optical Properties and First-Principles Theoretical Analysis. J. Phys. Chem. Lett. 2017, 8, 1979–1985. [Google Scholar] [CrossRef] [PubMed]
- Negishi, Y.; Nobusada, K.; Tsukuda, T. Glutathione-Protected Gold Clusters Revisited: Bridging the Gap between Gold(I)−Thiolate Complexes and Thiolate-Protected Gold Nanocrystals. J. Am. Chem. Soc. 2005, 127, 5261–5270. [Google Scholar] [CrossRef]
- Hamouda, R.; Bellina, B.; Bertorelle, F.; Compagnon, I.; Antoine, R.; Broyer, M.; Rayane, D.; Dugourd, P. Electron Emission of Gas-Phase [Au25(SG)18-6H]7− Gold Cluster and Its Action Spectroscopy. J. Phys. Chem. Lett. 2010, 1, 3189–3194. [Google Scholar] [CrossRef]
- Sahoo, A.K.; Banerjee, S.; Ghosh, S.S.; Chattopadhyay, A. Simultaneous RGB Emitting Au Nanoclusters in Chitosan Nanoparticles for Anticancer Gene Theranostics. ACS Appl. Mater. Interfaces 2014, 6, 712–724. [Google Scholar] [CrossRef] [PubMed]
- Tvedte, L.M.; Ackerson, C.J. Size-Focusing Synthesis of Gold Nanoclusters with p-Mercaptobenzoic Acid. J. Phys. Chem. A 2014, 118, 8124–8128. [Google Scholar] [CrossRef]
- Yang, X.; Gan, L.; Han, L.; Li, D.; Wang, J.; Wang, E. Facile preparation of chiral penicillamine protected gold nanoclusters and their applications in cell imaging. Chem. Commun. 2013, 49, 2302–2304. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Shi, M.; Zhou, R.; Chen, X.; Chen, H. Blending of HAuCl4 and histidine in aqueous solution: A simple approach to the Au10 cluster. Nanoscale 2011, 3, 2596–2601. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, W.; Wang, Y.; Yang, X.; Chen, J.; Jiang, Y.; Yu, C.; Lin, Q. Cysteine-directed fluorescent gold nanoclusters for the sensing of pyrophosphate and alkaline phosphatase. J. Mater. Chem. C 2014, 2, 4080–4085. [Google Scholar] [CrossRef]
- Goswami, U.; Basu, S.; Paul, A.; Ghosh, S.S.; Chattopadhyay, A. White light emission from gold nanoclusters embedded bacteria. J. Mater. Chem. C 2017, 5, 12360–12364. [Google Scholar] [CrossRef]
- Udaya Bhaskara Rao, T.; Pradeep, T. Luminescent Ag7 and Ag8 Clusters by Interfacial Synthesis. Angew. Chem. Int. Ed. 2010, 49, 3925–3929. [Google Scholar] [CrossRef]
- Dhanalakshmi, L.; Udayabhaskararao, T.; Pradeep, T. Conversion of double layer charge-stabilized Ag@citrate colloids to thiol passivated luminescent quantum clusters. Chem. Commun. 2012, 48, 859–861. [Google Scholar] [CrossRef] [PubMed]
- Bootharaju, M.S.; Burlakov, V.M.; Besong, T.M.D.; Joshi, C.P.; AbdulHalim, L.G.; Black, D.M.; Whetten, R.L.; Goriely, A.; Bakr, O.M. Reversible Size Control of Silver Nanoclusters via Ligand-Exchange. Chem. Mater. 2015, 27, 4289–4297. [Google Scholar] [CrossRef] [Green Version]
- Wei, W.; Lu, Y.; Chen, W.; Chen, S. One-Pot Synthesis, Photoluminescence, and Electrocatalytic Properties of Subnanometer-Sized Copper Clusters. J. Am. Chem. Soc. 2011, 133, 2060–2063. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, R.; Sahoo, A.K.; Ghosh, S.S.; Paul, A.; Chattopadhyay, A. Blue-Emitting Copper Nanoclusters Synthesized in the Presence of Lysozyme as Candidates for Cell Labeling. ACS Appl. Mater. Interfaces 2014, 6, 3822–3828. [Google Scholar] [CrossRef]
- Singh, R.; Majhi, S.; Sharma, K.; Ali, M.; Sharma, S.; Choudhary, D.; Tripathi, C.S.P.; Guin, D. BSA stabilized copper nanoclusters as a highly sensitive and selective probe for fluorescence sensing of Fe3+ ions. Chem. Phys. Lett. 2021, 76, 139226. [Google Scholar] [CrossRef]
- Rajamanikandan, R.; Ilanchelian, M. Red emitting human serum albumin templated copper nanoclusters as effective candidates for highly specific biosensing of bilirubin. Mater. Sci. Eng. C 2019, 98, 1064–1072. [Google Scholar] [CrossRef]
- Miao, H.; Zhong, D.; Zhou, Z.; Yang, X. Papain-templated Cu nanoclusters: Assaying and exhibiting dramatic antibacterial activity cooperating with H2O2. Nanoscale 2015, 7, 19066–19072. [Google Scholar] [CrossRef]
- Goswami, U.; Dutta, A.; Raza, A.; Kandimalla, R.; Kalita, S.; Ghosh, S.S.; Chattopadhyay, A. Transferrin–Copper Nanocluster–Doxorubicin Nanoparticles as Targeted Theranostic Cancer Nanodrug. ACS Appl. Mater. Interfaces 2018, 10, 3282–3294. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Chen, Y.; Han, Y.; Liu, J.; Ma, S.; Zhang, H.; Chen, X. pH-Regulated Synthesis of Trypsin-Templated Copper Nanoclusters with Blue and Yellow Fluorescent Emission. ACS Omega 2017, 2, 9109–9117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, X.; Li, J.; Han, L.; Ren, J.; Yang, X.; Wang, E. DNA-Hosted Copper Nanoclusters for Fluorescent Identification of Single Nucleotide Polymorphisms. ACS Nano 2012, 6, 3311–3317. [Google Scholar] [CrossRef]
- Das, N.K.; Ghosh, S.; Priya, A.; Datta, S.; Mukherjee, S. Luminescent Copper Nanoclusters as a Specific Cell-Imaging Probe and a Selective Metal Ion Sensor. J. Phys. Chem. C 2015, 119, 24657–24664. [Google Scholar] [CrossRef]
- Lin, Y.-S.; Chiu, T.-C.; Hu, C.-C. Fluorescence-tunable copper nanoclusters and their application in hexavalent chromium sensing. RSC Adv. 2019, 9, 9228–9234. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Feng, Y.; Zhu, S.; Luo, Y.; Zhuo, Y.; Dou, Y. One-step synthesis and applications of fluorescent Cu nanoclusters stabilized by l-cysteine in aqueous solution. Anal. Chim. Acta 2014, 847, 49–54. [Google Scholar] [CrossRef]
- Ghosh, R.; Goswami, U.; Ghosh, S.S.; Paul, A.; Chattopadhyay, A. Synergistic Anticancer Activity of Fluorescent Copper Nanoclusters and Cisplatin Delivered through a Hydrogel Nanocarrier. ACS Appl. Mater. Interfaces 2015, 7, 209–222. [Google Scholar] [CrossRef]
- Lin, Y.-J.; Chen, P.-C.; Yuan, Z.; Ma, J.-Y.; Chang, H.-T. The isomeric effect of mercaptobenzoic acids on the preparation and fluorescence properties of copper nanoclusters. Chem. Commun. 2015, 51, 11983–11986. [Google Scholar] [CrossRef]
- Shahsavari, S.; Hadian-Ghazvini, S.; Hooriabad Saboor, F.; Menbari Oskouie, I.; Hasany, M.; Simchi, A.; Rogach, A.L. Ligand functionalized copper nanoclusters for versatile applications in catalysis, sensing, bioimaging, and optoelectronics. Mater. Chem. Front. 2019, 3, 2326–2356. [Google Scholar] [CrossRef]
- Rieck, D.F.; Gavney, J.A.; Norman, R.L.; Hayashi, R.K.; Dahl, L.F. Synthesis, chromatographic separation, and stereophysical analysis of the homologous [Ni12-x(PMe)x(CO)24-3x]2- series (x = 2, 3, 4) containing noncentered Ni12-xPx icosahedral cages and the [Ni10(.mu.5-PMe)2(.mu.4-PMe)5(CO)10]2- dianion containing a structurally unprecedented heptacapped pentagonal prismatic metal cage: Structural, spectroscopic, and electrochemical consequences due to replacement of Ni(CO)3 fragments with electronically equivalent (isolobal) PMe fragments. J. Am. Chem. Soc. 1992, 114, 10369–10379. [Google Scholar] [CrossRef]
- Ji, J.; Wang, G.; Wang, T.; You, X.; Xu, X. Thiolate-protected Ni39 and Ni41 nanoclusters: Synthesis, self-assembly and magnetic properties. Nanoscale 2014, 6, 9185–9191. [Google Scholar] [CrossRef]
- Joya, K.S.; Sinatra, L.; AbdulHalim, L.G.; Joshi, C.P.; Hedhili, M.N.; Bakr, O.M.; Hussain, I. Atomically monodisperse nickel nanoclusters as highly active electrocatalysts for water oxidation. Nanoscale 2016, 8, 9695–9703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guan, G.; Zhang, S.-Y.; Cai, Y.; Liu, S.; Bharathi, M.S.; Low, M.; Yu, Y.; Xie, J.; Zheng, Y.; Zhang, Y.-W.; et al. Convenient purification of gold clusters by co-precipitation for improved sensing of hydrogen peroxide, mercury ions and pesticides. Chem. Commun. 2014, 50, 5703–5705. [Google Scholar] [CrossRef]
- Galchenko, M.; Schuster, R.; Black, A.; Riedner, M.; Klinke, C. Preparation of high-yield and ultra-pure Au25 nanoclusters: Towards their implementation in real-world applications. Nanoscale 2019, 11, 1988–1994. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; Yue, Y.; Wang, P.; He, H.; Jin, Y. Facile and rapid synthesis of water-soluble fluorescent gold nanoclusters for sensitive and selective detection of Ag+. J. Mater. Chem. C 2013, 1, 908–913. [Google Scholar] [CrossRef]
- Tang, Y.; Xu, J.; Xiong, C.; Xiao, Y.; Zhang, X.; Wang, S. Enhanced electrochemiluminescence of gold nanoclusters via silver doping and their application for ultrasensitive detection of dopamine. Analyst 2019, 144, 2643–2648. [Google Scholar] [CrossRef]
- Pramanik, G.; Humpolickova, J.; Valenta, J.; Kundu, P.; Bals, S.; Bour, P.; Dracinsky, M.; Cigler, P. Gold nanoclusters with bright near-infrared photoluminescence. Nanoscale 2018, 10, 3792–3798. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Feng, L.; Yan, W.; Hussain, I.; Su, L.; Tan, B. PVP-templated highly luminescent copper nanoclusters for sensing trinitrophenol and living cell imaging. Nanoscale 2019, 11, 1286–1294. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Hassinen, J.; Pulkkinen, P.; Tenhu, H.; Ras, R.H.A.; Pradeep, T. Simple and Efficient Separation of Atomically Precise Noble Metal Clusters. Anal. Chem. 2014, 86, 12185–12190. [Google Scholar] [CrossRef] [Green Version]
- Niihori, Y.; Kikuchi, Y.; Shima, D.; Uchida, C.; Sharma, S.; Hossain, S.; Kurashige, W.; Negishi, Y. Separation of Glutathionate-Protected Gold Clusters by Reversed-Phase Ion-Pair High-Performance Liquid Chromatography. Ind. Eng. Chem. Res. 2017, 56, 1029–1035. [Google Scholar] [CrossRef]
- Comby-Zerbino, C.; Perić, M.; Bertorelle, F.; Chirot, F.; Dugourd, P.; Bonačić-Koutecký, V.; Antoine, R. Catenane Structures of Homoleptic Thioglycolic Acid-Protected Gold Nanoclusters Evidenced by Ion Mobility-Mass Spectrometry and DFT Calculations. Nanomaterials 2019, 9, 457. [Google Scholar] [CrossRef] [Green Version]
- Bertorelle, F.; Russier-Antoine, I.; Comby-Zerbino, C.; Chirot, F.; Dugourd, P.; Brevet, P.-F.; Antoine, R. Isomeric Effect of Mercaptobenzoic Acids on the Synthesis, Stability, and Optical Properties of Au25(MBA)18 Nanoclusters. ACS Omega 2018, 3, 15635–15642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soleilhac, A.; Bertorelle, F.; Comby-Zerbino, C.; Chirot, F.; Calin, N.; Dugourd, P.; Antoine, R. Size Characterization of Glutathione-Protected Gold Nanoclusters in the Solid, Liquid and Gas Phases. J. Phys. Chem. C 2017, 121, 27733–27740. [Google Scholar] [CrossRef]
- Ligare, M.R.; Baker, E.S.; Laskin, J.; Johnson, G.E. Ligand induced structural isomerism in phosphine coordinated gold clusters revealed by ion mobility mass spectrometry. Chem. Commun. 2017, 53, 7389–7392. [Google Scholar] [CrossRef] [PubMed]
- Baksi, A.; Ghosh, A.; Mudedla, S.K.; Chakraborty, P.; Bhat, S.; Mondal, B.; Krishnadas, K.R.; Subramanian, V.; Pradeep, T. Isomerism in Monolayer Protected Silver Cluster Ions: An Ion Mobility-Mass Spectrometry Approach. J. Phys. Chem. C 2017, 121, 13421–13427. [Google Scholar] [CrossRef]
- Angel, L.A.; Majors, L.T.; Dharmaratne, A.C.; Dass, A. Ion mobility mass spectrometry of Au25(SCH2CH2Ph)18 nanoclusters. ACS Nano 2010, 4, 4691–4700. [Google Scholar] [CrossRef] [PubMed]
- Kalenius, E.; Malola, S.; Matus, M.F.; Kazan, R.; Bürgi, T.; Häkkinen, H. Experimental Confirmation of a Topological Isomer of the Ubiquitous Au25(SR)18 Cluster in the Gas Phase. J. Am. Chem. Soc. 2021, 143, 1273–1277. [Google Scholar] [CrossRef]
- Gayen, C.; Basu, S.; Pan, U.N.; Paul, A. Few Particle-Level Chromaticity Index-Based Discrimination of Biothiols Using Chemically Interactive Dual-Emitting Nanoprobe. ACS Omega 2018, 3, 17220–17226. [Google Scholar] [CrossRef]
- Gayen, C.; Goswami, U.; Gogoi, K.; Basu, S.; Paul, A. Crystallization-Induced Emission Enhancement of Nanoclusters and One-Step Conversion of “Nanoclusters to Nanoparticles” as the Basis for Intracellular Logic Operations. ChemPhysChem 2019, 20, 953–958. [Google Scholar] [CrossRef]
- Gayen, C.; Basu, S.; Goswami, U.; Paul, A. Visible Light Excitation-Induced Luminescence from Gold Nanoclusters Following Surface Ligand Complexation with Zn2+ for Daylight Sensing and Cellular Imaging. Langmuir 2019, 35, 9037–9043. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Fakhouri, H.; Moulin, C.; Dolai, S.; Russier-Antoine, I.; Brevet, P.-F.; Antoine, R.; Paul, A. Four orders-of-magnitude enhancement in the two-photon excited photoluminescence of homoleptic gold thiolate nanoclusters following zinc ion-induced aggregation. Nanoscale 2021, 13, 4439–4443. [Google Scholar] [CrossRef]
- Basu, S.; Nawaj, M.W.; Gayen, C.; Paul, A. Photo induced chemical modification of surface ligands for aggregation and luminescence modulation of copper nanoclusters in the presence of oxygen. Phys. Chem. Chem. Phys. 2019, 21, 21776–21781. [Google Scholar] [CrossRef]
- Basu, S.; Gayen, C.; Dolai, S.; Paul, A. Tailoring the luminescence of atomic clusters via ligand exchange reaction mediated post synthetic modification. Phys. Chem. Chem. Phys. 2020, 22, 3959–3964. [Google Scholar] [CrossRef]
- Combes, G.F.; Fakhouri, H.; Moulin, C.; Girod, M.; Bertorelle, F.; Basu, S.; Ladouce, R.; Bakulić, M.P.; Maršić, Ž.S.; Russier-Antoine, I.; et al. Functionalized Au15 nanoclusters as luminescent probes for protein carbonylation detection. Commun. Chem. 2021, 4, 69. [Google Scholar] [CrossRef]
- Wang, Y.; Bürgi, T. Ligand exchange reactions on thiolate-protected gold nanoclusters. Nanoscale Adv. 2021, 3, 2710–2727. [Google Scholar] [CrossRef] [PubMed]
- Krishnadas, K.R.; Ghosh, A.; Baksi, A.; Chakraborty, I.; Natarajan, G.; Pradeep, T. Intercluster Reactions between Au25(SR)18 and Ag44(SR)30. J. Am. Chem. Soc. 2016, 138, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Khatun, E.; Chakraborty, P.; Jacob, B.R.; Paramasivam, G.; Bodiuzzaman, M.; Dar, W.A.; Pradeep, T. Intercluster Reactions Resulting in Silver-Rich Trimetallic Nanoclusters. Chem. Mater. 2020, 32, 611–619. [Google Scholar] [CrossRef]
- Basu, S.; Bakulić, M.P.; Fakhouri, H.; Russier-Antoine, I.; Moulin, C.; Brevet, P.-F.; Bonačić-Koutecký, V.; Antoine, R. Rationale Strategy to Tune the Optical Properties of Gold Catenane Nanoclusters by Doping with Silver Atoms. J. Phys. Chem. C 2020, 124, 19368–19374. [Google Scholar] [CrossRef]
- Basu, S.; Paul, A.; Chattopadhyay, A. Zinc mediated crystalline assembly of gold nanoclusters for expedient hydrogen storage and sensing. J. Mater. Chem. A 2016, 4, 1218–1223. [Google Scholar] [CrossRef]
- Paul, M.; Basu, S.; Chattopadhyay, A. Complexation Reaction-Based Two-Dimensional Luminescent Crystalline Assembly of Atomic Clusters for Recyclable Storage of Oxygen. Langmuir 2020, 36, 754–759. [Google Scholar] [CrossRef]
- Basu, S.; Paul, A.; Chattopadhyay, A. Zinc-Coordinated Hierarchical Organization of Ligand-Stabilized Gold Nanoclusters for Chiral Recognition and Separation. Chem.–Eur. J. 2017, 23, 9137–9143. [Google Scholar] [CrossRef]
- Basu, S.; Goswami, U.; Paul, A.; Chattopadhyay, A. Crystalline assembly of gold nanoclusters for mitochondria targeted cancer theranostics. J. Mater. Chem. B 2018, 6, 1650–1657. [Google Scholar] [CrossRef]
- Basu, S.; Bhandari, S.; Pan, U.N.; Paul, A.; Chattopadhyay, A. Crystalline nanoscale assembly of gold clusters for reversible storage and sensing of CO2 via modulation of photoluminescence intermittency. J. Mater. Chem. C 2018, 6, 8205–8211. [Google Scholar] [CrossRef]
- Basu, S.; Chattopadhyay, A. Room-Temperature Delayed Fluorescence of Gold Nanoclusters in Zinc-Mediated Two-Dimensional Crystalline Assembly. Langmuir 2019, 35, 5264–5270. [Google Scholar] [CrossRef] [PubMed]
- Yoon, B.; Luedtke, W.D.; Barnett, R.N.; Gao, J.; Desireddy, A.; Conn, B.E.; Bigioni, T.; Landman, U. Hydrogen-bonded structure and mechanical chiral response of a silver nanoparticle superlattice. Nat. Mater. 2014, 13, 807–811. [Google Scholar] [CrossRef] [PubMed]
- Yao, Q.; Yu, Y.; Yuan, X.; Yu, Y.; Zhao, D.; Xie, J.; Lee, J.Y. Counterion-Assisted Shaping of Nanocluster Supracrystals. Angew. Chem. Int. Ed. 2015, 54, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, A.; Hou, W.; Li, T.; Wang, K.; Zhang, Q.; de la Fuente, J.M.; Jin, W.; Cui, D. Mimicking Pathogenic Invasion with the Complexes of Au22(SG)18-Engineered Assemblies and Folic Acid. ACS Nano 2018, 12, 4408–4418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Z.; Dong, C.; Li, Y.; Hao, H.; Zhang, H.; Lu, Z.; Yang, B. Self-assembly of Au15 into single-cluster-thick sheets at the interface of two miscible high-boiling solvents. Angew. Chem. 2013, 52, 9952–9955. [Google Scholar] [CrossRef]
- Ouyang, X.; Wang, M.; Guo, L.; Cui, C.; Liu, T.; Ren, Y.; Zhao, Y.; Ge, Z.; Guo, X.; Xie, G.; et al. DNA Nanoribbon-Templated Self-Assembly of Ultrasmall Fluorescent Copper Nanoclusters with Enhanced Luminescence. Angew. Chem. Int. Ed. 2020, 59, 11836–11844. [Google Scholar] [CrossRef]
- Jiang, T.; Qu, G.; Wang, J.; Ma, X.; Tian, H. Cucurbiturils brighten Au nanoclusters in water. Chem. Sci. 2020, 11, 3531–3537. [Google Scholar] [CrossRef] [Green Version]
- Hembury, M.; Beztsinna, N.; Asadi, H.; van den Dikkenberg, J.B.; Meeldijk, J.D.; Hennink, W.E.; Vermonden, T. Luminescent Gold Nanocluster-Decorated Polymeric Hybrid Particles with Assembly-Induced Emission. Biomacromolecules 2018, 19, 2841–2848. [Google Scholar] [CrossRef]
- Bertorelle, F.; Basu, S.; Fakhouri, H.; Perić Bakulić, M.; Mignon, P.; Russier-Antoine, I.; Brevet, P.-F.; Thomas, S.; Kalarikkal, N.; Antoine, R. Covalent anchoring of atomically precise glutathione-protected gold nanoclusters on graphene oxide nanosheets. Nano Express 2020, 1, 030005. [Google Scholar] [CrossRef]
- Shen, J.; Wang, Z.; Sun, D.; Xia, C.; Yuan, S.; Sun, P.; Xin, X. pH-Responsive Nanovesicles with Enhanced Emission Co-Assembled by Ag(I) Nanoclusters and Polyethyleneimine as a Superior Sensor for Al3+. ACS Appl. Mater. Interfaces 2018, 10, 3955–3963. [Google Scholar] [CrossRef]
- Benavides, J.; Quijada-Garrido, I.; García, O. The synthesis of switch-off fluorescent water-stable copper nanocluster Hg2+ sensors via a simple one-pot approach by an in situ metal reduction strategy in the presence of a thiolated polymer ligand template. Nanoscale 2020, 12, 944–955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ju, E.; Li, T.; Ramos da Silva, S.; Gao, S.-J. Gold Nanocluster-Mediated Efficient Delivery of Cas9 Protein through pH-Induced Assembly-Disassembly for Inactivation of Virus Oncogenes. ACS Appl. Mater. Interfaces 2019, 11, 34717–34724. [Google Scholar] [CrossRef] [PubMed]
- Qing, Z.; He, X.; He, D.; Wang, K.; Xu, F.; Qing, T.; Yang, X. Poly(thymine)-Templated Selective Formation of Fluorescent Copper Nanoparticles. Angew. Chem. Int. Ed. 2013, 52, 9719–9722. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Chen, Y.; Cai, W.; Feng, H.; Du, T.; Liu, W.; Jiang, H.; Pasquarelli, A.; Weizmann, Y.; Wang, X. In situ self-assembling Au-DNA complexes for targeted cancer bioimaging and inhibition. Proc. Natl. Acad. Sci. USA 2020, 117, 308–316. [Google Scholar] [CrossRef]
- Chakraborty, P.; Nag, A.; Sugi, K.S.; Ahuja, T.; Varghese, B.; Pradeep, T. Crystallization of a Supramolecular Coassembly of an Atomically Precise Nanoparticle with a Crown Ether. ACS Mater. Lett. 2019, 1, 534–540. [Google Scholar] [CrossRef]
- Huang, Y.; Ji, J.; Zhang, J.; Wang, F.; Lei, J. Host–guest recognition-regulated aggregation-induced emission for in situ imaging of MUC1 protein. Chem. Commun. 2020, 56, 313–316. [Google Scholar] [CrossRef]
- Su, X.; Liu, J. pH-Guided Self-Assembly of Copper Nanoclusters with Aggregation-Induced Emission. ACS Appl. Mater. Interfaces 2017, 9, 3902–3910. [Google Scholar] [CrossRef]
- Dutta, A.; Goswami, U.; Chattopadhyay, A. Probing Cancer Cells through Intracellular Aggregation-Induced Emission Kinetic Rate of Copper Nanoclusters. ACS Appl. Mater. Interfaces 2018, 10, 19459–19472. [Google Scholar] [CrossRef]
- Cheng, L.; Ren, C.; Zhang, X.; Yang, J. New insight into the electronic shell of Au38(SR)24: A superatomic molecule. Nanoscale 2013, 5, 1475–1478. [Google Scholar] [CrossRef]
- Häkkinen, H. Electronic shell structures in bare and protected metal nanoclusters. Adv. Phys. X 2016, 1, 467–491. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Li, P.; Yuan, L.-F.; Cheng, L.; Yang, J. From isosuperatoms to isosupermolecules: New concepts in cluster science. Nanoscale 2016, 8, 12787–12792. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Jin, R. On the Ligand’s Role in the Fluorescence of Gold Nanoclusters. Nano Lett. 2010, 10, 2568–2573. [Google Scholar] [CrossRef] [PubMed]
- Gran, E.R.; Bertorelle, F.; Fakhouri, H.; Antoine, R.; Perić Bakulić, M.; Sanader Maršić, Ž.; Bonačić-Koutecký, V.; Blain, M.; Antel, J.; Maysinger, D. Size and ligand effects of gold nanoclusters in alteration of organellar state and translocation of transcription factors in human primary astrocytes. Nanoscale 2021, 13, 3173–3183. [Google Scholar] [CrossRef] [PubMed]
- Perić, M.; Sanader Maršić, Ž.; Russier-Antoine, I.; Fakhouri, H.; Bertorelle, F.; Brevet, P.-F.; le Guével, X.; Antoine, R.; Bonačić-Koutecký, V. Ligand shell size effects on one- and two-photon excitation fluorescence of zwitterion functionalized gold nanoclusters. Phys. Chem. Chem. Phys. 2019, 21, 23916–23921. [Google Scholar] [CrossRef]
- Bonačić-Koutecký, V.; Antoine, R. Enhanced two-photon absorption of ligated silver and gold nanoclusters: Theoretical and experimental assessments. Nanoscale 2019, 11, 12436–12448. [Google Scholar] [CrossRef]
- Russier-Antoine, I.; Bertorelle, F.; Calin, N.; Sanader, Z.; Krstic, M.; Comby-Zerbino, C.; Dugourd, P.; Brevet, P.-F.; Bonacic-Koutecky, V.; Antoine, R. Ligand-core NLO-phores: A combined experimental and theoretical approach to the two-photon absorption and two-photon excited emission properties of small-ligated silver nanoclusters. Nanoscale 2017, 9, 1221–1228. [Google Scholar] [CrossRef] [Green Version]
- Sanader, Z.; Krstic, M.; Russier-Antoine, I.; Bertorelle, F.; Dugourd, P.; Brevet, P.-F.; Antoine, R.; Bonacic-Koutecky, V. Two-photon absorption of ligand-protected Ag15 nanoclusters. Towards a new class of nonlinear optics nanomaterials. Phys. Chem. Chem. Phys. 2016, 18, 12404–12408. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Metal | Stabilizer | No. of Metal Atoms Comprising the Clusters | Emission Color | Reference |
|---|---|---|---|---|
| gold | Polyamido amine (4th generation) | 8 | blue | 26 |
| gold | BSA | 25 | red | 28 |
| gold | HSA | 25 | red | 29 |
| gold | DNA | 7 | red | 33 |
| gold | glutathione | 10 | Non-emissive | 35 |
| gold | MPA + chitosan | 20 | orange-red (pH dependent) | 38 |
| gold | histidine | 10 | blue | 41 |
| gold | MPA + bacteria | Not determined | white | 43 |
| silver | produced by Ag NPs protected by mercapto-succininc acid | 8, 7 | red, blue green | 44 |
| silver | produced by etching of Ag NPs capped by citrate ions | 38 | red | 45 |
| copper | lysozymes | 2–9 | blue | 48 |
| copper | glutathione | 15 | blue | 55 |
| copper | cysteine | 4 | cyan | 57 |
| copper | dihydrolipoic acid | 4 | red | 58 |
| nickel | phenylethanethiol | 4, 6 | Not reported | 63 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basu, S.; Paul, A.; Antoine, R. Controlling the Chemistry of Nanoclusters: From Atomic Precision to Controlled Assembly. Nanomaterials 2022, 12, 62. https://doi.org/10.3390/nano12010062
Basu S, Paul A, Antoine R. Controlling the Chemistry of Nanoclusters: From Atomic Precision to Controlled Assembly. Nanomaterials. 2022; 12(1):62. https://doi.org/10.3390/nano12010062
Chicago/Turabian StyleBasu, Srestha, Anumita Paul, and Rodolphe Antoine. 2022. "Controlling the Chemistry of Nanoclusters: From Atomic Precision to Controlled Assembly" Nanomaterials 12, no. 1: 62. https://doi.org/10.3390/nano12010062
APA StyleBasu, S., Paul, A., & Antoine, R. (2022). Controlling the Chemistry of Nanoclusters: From Atomic Precision to Controlled Assembly. Nanomaterials, 12(1), 62. https://doi.org/10.3390/nano12010062