Ni-Doped Ordered Nanoporous Carbon Prepared from Chestnut Wood Tannins for the Removal and Photocatalytic Degradation of Methylene Blue
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of the Ni-OMC Sample
2.2. Batch Adsorption Studies
2.3. Photocatalytic Studies
2.4. Kinetics Studies
2.5. Recyclability Studies
2.6. X-ray Photoelectron Spectroscopy (XPS)
2.7. X-ray Diffraction (XRD)
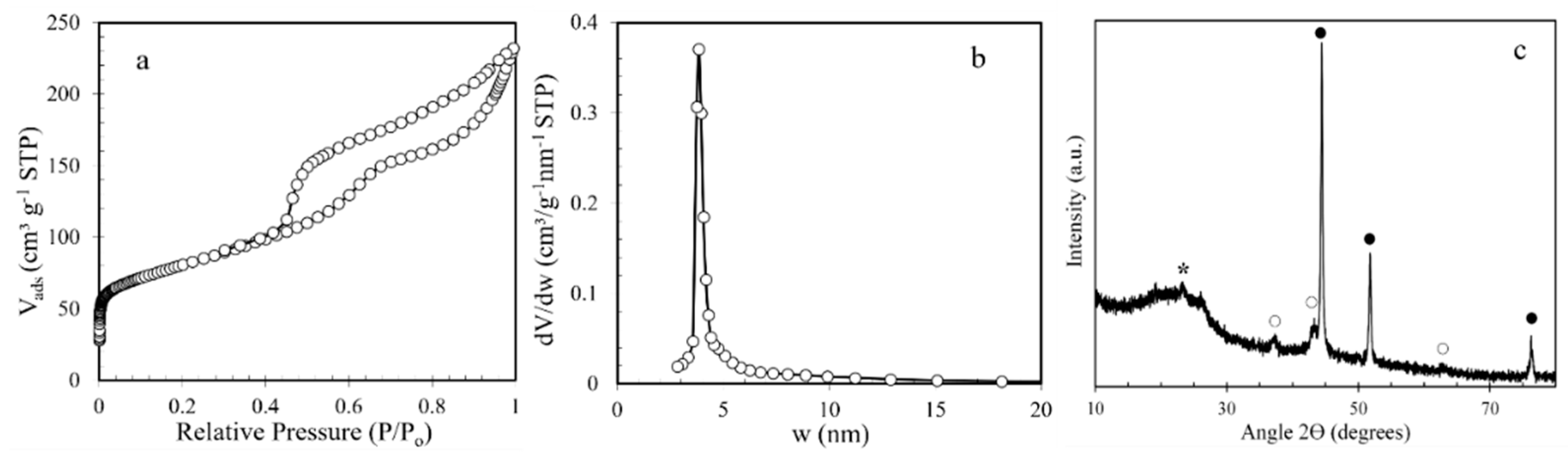
2.8. Nitrogen Sorption
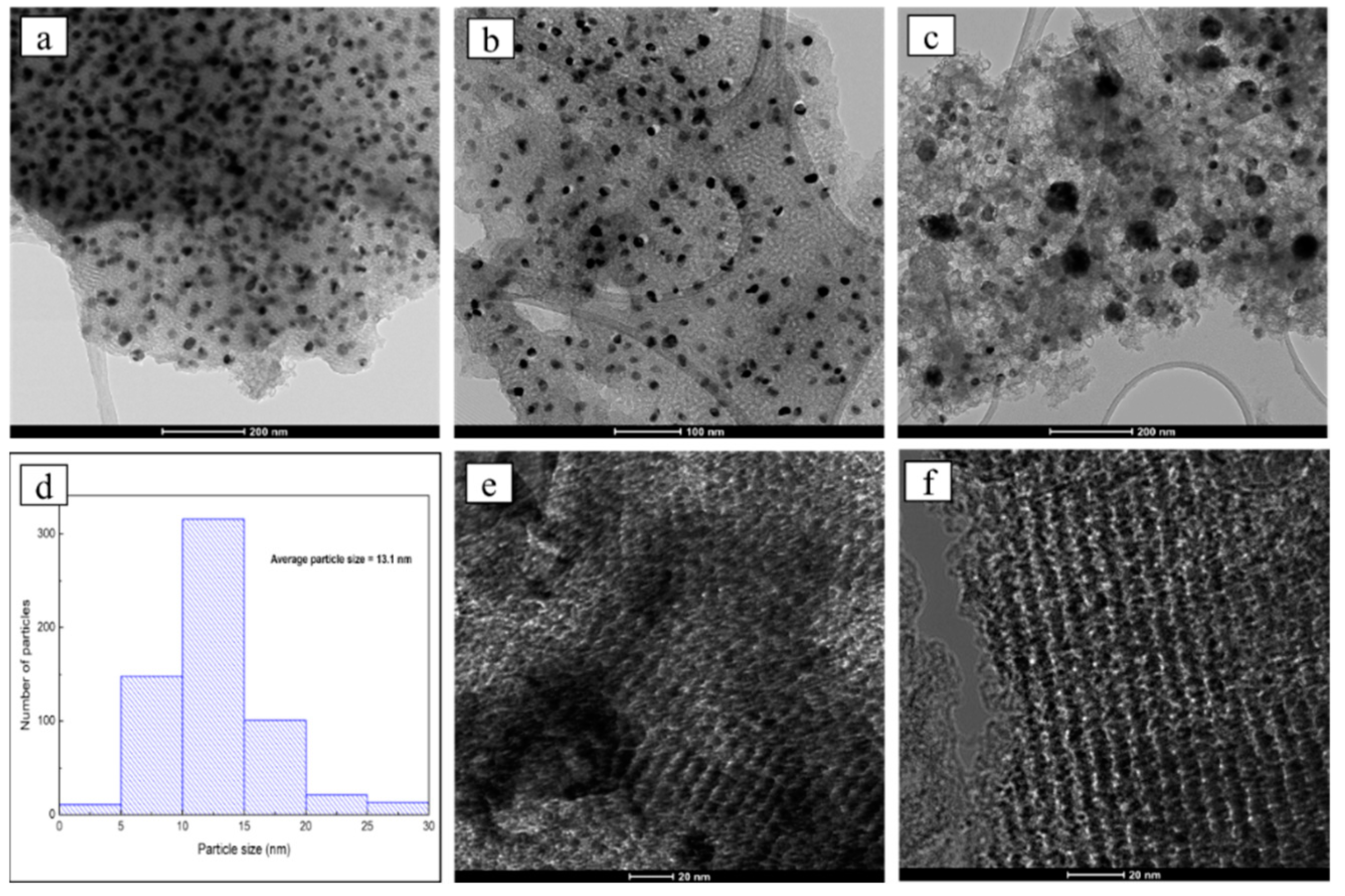
2.9. Transmission Electron Microscopy (TEM)
3. Results
3.1. Textural and Structural Characterization
3.2. Chemical Characterization
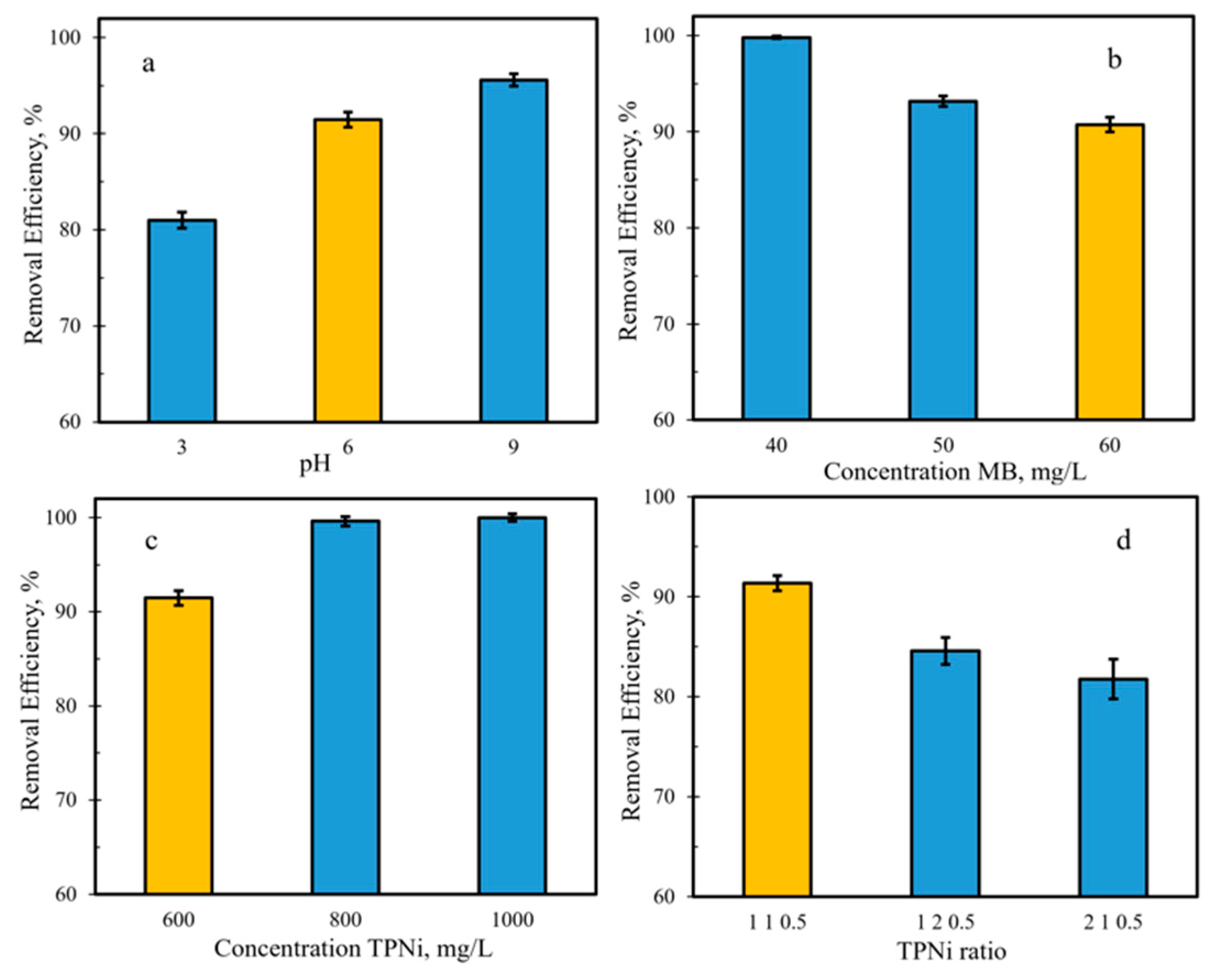
3.3. Adsorption Studies
3.4. Photocatalytic Studies
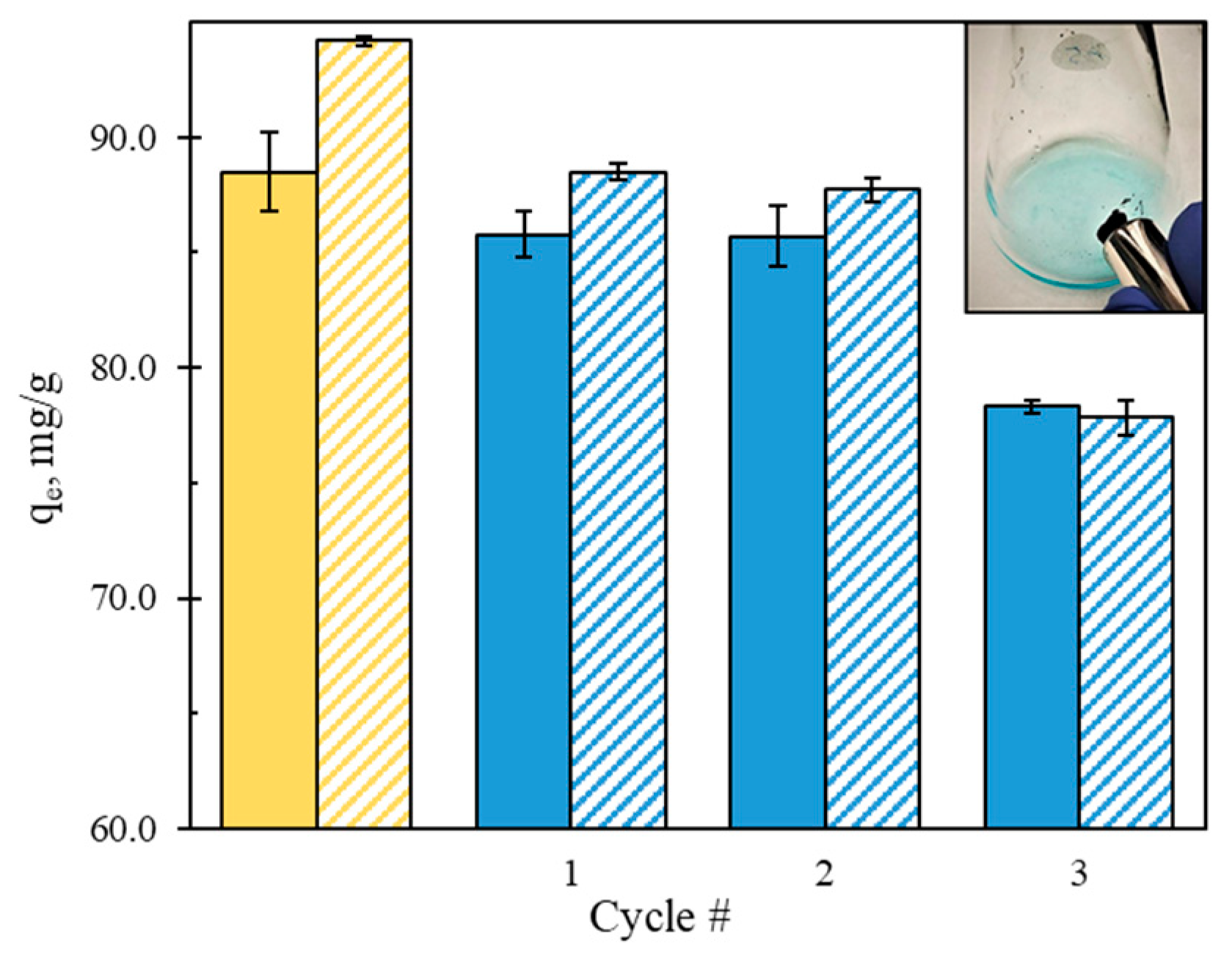
3.5. Recyclability Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, W.; Liu, J.; Zhao, D. Mesoporous materials for energy conversion and storage devices. Nat. Rev. Mater. 2016, 1, 16023–16040. [Google Scholar] [CrossRef]
- Sanchez-Sanchez, A.; Izquierdo, M.T.; Medjahdi, G.; Ghanbaja, J.; Celzard, A.; Fierro, V. Ordered mesoporous carbons obtained by soft-templating of tannin in mild conditions. Microporous Mesoporous Mater. 2018, 270, 127–139. [Google Scholar] [CrossRef]
- Ryoo, R.; Joo, S.H.; Kruk, M.; Jaroniec, M. Ordered Mesoporous Carbons. Adv. Mater. 2001, 13, 677–681. [Google Scholar] [CrossRef]
- Liu, L.; Deng, Q.-F.; Agula, B.; Zhao, X.; Ren, T.-Z.; Yuan, Z.-Y. Ordered mesoporous carbon catalyst for dehydrogenation of propane to propylene. Chem. Commun. 2011, 47, 8334–8336. [Google Scholar] [CrossRef]
- Ambrosio, E.P.; Dumitrescu, M.A.; Francia, C.; Gerbaldi, C.; Spinelli, P. Ordered Mesoporous Carbons as Catalyst Support for PEM Fuel Cells. Fuel Cells 2009, 9, 197–200. [Google Scholar] [CrossRef]
- Teng, W.; Wu, Z.; Fan, J.; Zhang, W.-X.; Zhao, D. Amino-functionalized ordered mesoporous carbon for the separation of toxic microcystin-LR. J. Mater. Chem. A 2015, 3, 19168–19176. [Google Scholar] [CrossRef]
- Chew, T.-L.; Ahmad, A.L.; Bhatia, S. Ordered mesoporous silica (OMS) as an adsorbent and membrane for separation of carbon dioxide (CO2). Adv. Colloid Interface Sci. 2010, 153, 43–57. [Google Scholar] [CrossRef]
- Baek, D.S.; Lee, K.A.; Park, J.; Kim, J.H.; Lee, J.; Lim, J.S.; Lee, S.Y.; Shin, T.J.; Jeong, H.Y.; Son, J.S.; et al. Ordered Mesoporous Carbons with Graphitic Tubular Frameworks by Dual Templating for Efficient Electrocatalysis and Energy Storage. Angew. Chem. Int. Ed. Engl. 2021, 60, 1441–1449. [Google Scholar] [CrossRef]
- Zhou, J.; Bao, L.; Wu, S.; Yang, W.; Wang, H. Nitrogen-doped ordered mesoporous carbon using task-specific ionic liquid as a dopant for high-performance supercapacitors. J. Mater. Res. 2017, 32, 404–413. [Google Scholar] [CrossRef]
- Libbrecht, W.; Verberckmoes, A.; Thybaut, J.W.; Van Der Voort, P.; Clercq, J.D. Soft templated mesoporous carbons: Tuning the porosity for the adsorption of large organic pollutants. Carbon 2017, 116, 528–546. [Google Scholar] [CrossRef]
- Russo, P.A.; Ribeiro Carrott, M.M.L.; Carrotta, P.J.M. Hydrocarbons adsorption on templated mesoporous materials: Effect of the pore size, geometry and surface chemistry. New J. Chem. 2011, 35, 407–416. [Google Scholar] [CrossRef]
- Gang, D.; Ahmad, Z.U.; Lian, Q.; Yao, L.; Zappi, M.E. A review of adsorptive remediation of environmental pollutants from aqueous phase by ordered mesoporous carbon. Chem. Eng. J. 2021, 403, 1385–8947. [Google Scholar] [CrossRef]
- Tanaka, S.; Nakatani, N.; Doi, A.; Miyake, Y. Preparation of ordered mesoporous carbon membranes by a soft-templating method. Carbon 2011, 49, 3184–3189. [Google Scholar] [CrossRef]
- Saha, D.; Warren, K.E.; Naskar, A.K. Soft-templated mesoporous carbons as potential materials for oral drug delivery. Carbon 2014, 71, 47–57. [Google Scholar] [CrossRef]
- Castro-Gutiérrez, J.; Sanchez-Sanchez, A.; Ghanbaja, J.; Díez, N.; Sevilla, M.; Celzard, A.; Fierro, V. Synthesis of perfectly ordered mesoporous carbons by water-assisted mechanochemical self-assembly of tannin. Green Chem. 2018, 20, 5123–5132. [Google Scholar] [CrossRef] [Green Version]
- Fu, J.; Shen, F.; Liu, X.; Qi, X. Synthesis of MgO-doped ordered mesoporous carbons by Mg2+-tannin coordination for efficient isomerization of glucose to fructose. Green Energy Environ. 2021. [Google Scholar] [CrossRef]
- Song, Y.; Liu, J.; Sun, K.; Xu, W. Synthesis of sustainable lignin-derived mesoporous carbon for supercapacitors using a nano-sized MgO template coupled with Pluronic F127. RSC Adv. 2017, 7, 48324–48332. [Google Scholar] [CrossRef] [Green Version]
- Herou, S.; Ribadeneyra, M.C.; Madhu, R.; Araullo-Peters, V.; Jensen, A.; Schlee, P.; Titirici, M. Ordered mesoporous carbons from lignin: A new class of biobased electrodes for supercapacitors. Green Chem. 2019, 21, 550–559. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, L.; Yang, S.; Schott, J.A.; Liu, X.; Mahurin, S.M.; Huang, C.; Zhang, Y.; Fulvio, P.F.; Chisholm, M.F.; et al. Solid-state synthesis of ordered mesoporous carbon catalysts via a mechanochemical assembly through coordination cross-linking. Nat. Commun. 2017, 8, 15020. [Google Scholar] [CrossRef]
- Tang, Y.; Qiu, M.; Yang, J.; Shen, F.; Wang, X.; Qi, X. One-pot self-assembly synthesis of Ni-doped ordered mesoporous carbon for quantitative hydrogenation of furfural to furfuryl alcohol. Green Chem. 2021, 23, 1861–1870. [Google Scholar] [CrossRef]
- Tian, Y.; Wang, X.; Pan, Y. Simple synthesis of Ni-containing ordered mesoporous carbons and their adsorption/desorption of methylene orange. J. Hazard. Mater. 2012, 213–214, 361–368. [Google Scholar] [CrossRef] [PubMed]
- García, A.; Nieto, A.; Vila, M.; Vallet-Regí, M. Easy synthesis of ordered mesoporous carbon containing nickel nanoparticles by a low temperature hydrothermal method. Carbon 2013, 51, 410–418. [Google Scholar] [CrossRef]
- Wang, X.; Dai, S. A simple method to ordered mesoporous carbons containing nickel nanoparticles. Adsorption 2009, 15, 138–144. [Google Scholar] [CrossRef]
- Kou, W.; Zhang, Y.; Dong, J.; Mu, C.; Xu, L. Nickel−Nitrogen-Doped Three-Dimensional Ordered Macro-/Mesoporous Carbon as an Efficient Electrocatalyst for CO2 Reduction to CO. ACS Appl. Energy Mater. 2020, 3, 1875–1882. [Google Scholar] [CrossRef]
- Song, Y.; Yin, F.; Zhang, C.; Guo, W.; Han, L.; Yuan, Y. Three-Dimensional Ordered Mesoporous Carbon Spheres Modified with Ultrafine Zinc Oxide Nanoparticles for Enhanced Microwave Absorption Properties. Nano-Micro Lett. 2021, 13, 76. [Google Scholar] [CrossRef] [PubMed]
- Kansal, S.K.; Singh, M.; Sud, D. Studies on photodegradation of two commercial dyes in aqueous phase using different photocatalysts. J. Hazard. Mater. 2007, 141, 581–590. [Google Scholar] [CrossRef]
- Spagnoli, A.A.; Giannakoudakis, D.A.; Bashkova, S. Adsorption of methylene blue on cashew nut shell based carbons activated with zinc chloride: The role of surface and structural parameters. J. Mol. Liq. 2017, 229, 465–471. [Google Scholar] [CrossRef]
- Giannakoudakis, D.A.; Qayyum, A.; Łomot, D.; Besenhard, M.O.; Lisovytskiy, D.; Bandosz, T.J.; Colmenares, J.C. Boosting the Photoactivity of Grafted Titania: Ultrasound-Driven Synthesis of a Multi-Phase Heterogeneous Nano-Architected Photocatalyst. Adv. Funct. Mater. 2021, 31, 2007115. [Google Scholar] [CrossRef]
- Chen, X.; Rangaiah, G.P.; Zhao, X.S. Photocatalytic Degradation of Methylene Blue by Titanium Dioxide: Experimental and Modeling Study. Ind. Eng. Chem. Res. 2014, 53, 14641–14649. [Google Scholar] [CrossRef]
- Chatterjee, D.; Dasgupta, S. Visible light induced photocatalytic degradation of organic pollutants. J. Photochem. Photobiol. C 2005, 6, 186–205. [Google Scholar] [CrossRef]
- Fujishima, A.; Rao, T.N.; Tryk, D.A. Titanium dioxide photocatalysis. J. Photochem. Photobiol. C 2000, 1, 1–21. [Google Scholar] [CrossRef]
- Humayun, M.; Wang, C.; Luo, W. Recent Progress in the Synthesis and Applications of Composite Photocatalysts: A Critical Review. Small Methods 2022, 6, 2101385. [Google Scholar] [CrossRef] [PubMed]
- Akbari, A.; Sabouri, Z.; Hosseini, H.A.; Hashemzadeh, A.; Khatami, M.; Darroudi, M. Effect of nickel oxide nanoparticles as a photocatalyst in dyes degradation and evaluation of effective parameters in their removal from aqueous environments. Inorg. Chem. Commun. 2020, 115, 107867. [Google Scholar] [CrossRef]
- Vilé, G.; Sharma, P.; Nachtegaal, M.; Tollini, F.; Moscatelli, D.; Sroka-Bartnicka, A.; Tomanec, O.; Petr, M.; Filip, J.; Pieta, I.S.; et al. An Earth-Abundant Ni-Based Single-Atom Catalyst for Selective Photodegradation of Pollutants. Sol. RRL 2021, 5, 2100176. [Google Scholar] [CrossRef]
- Fazlali, F.; Mahjoub, A.; Abazari, R. A new route for synthesis of spherical NiO nanoparticles via emulsion nano-reactors with enhanced photocatalytic activity. Solid State Sci. 2015, 48, 263–269. [Google Scholar] [CrossRef]
- Rafiq, A.; Ikram, M.; Ali, S.; Niaz, F.; Khan, M.; Khan, Q.; Maqbool, M. Photocatalytic degradation of dyes using semiconductor photocatalysts to clean industrial water pollution. J. Ind. Eng. Chem. 2021, 97, 111–128. [Google Scholar] [CrossRef]
- Lagergren, S. Zur Theorie der Sogenannten Adsorption Gelöster Stoffe, Kungliga Svenska Vetenskapsakademiens. Handlingar 1898, 24, 1–3. [Google Scholar]
- Ho, Y.S.; McKay, G. Pseudo-second-order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Weber, W.J., Jr.; Morris, J.C. Kinetics of adsorption on carbon from solution. J. Sanit. Eng. ASCE 1963, 89, 31–59. [Google Scholar] [CrossRef]
- Gregg, S.J.; Sing, K.S.W. Adsorption, Surface Area, and Porosity, 2nd ed.; Academic Press: London, UK, 1982. [Google Scholar]
- Terzyk, A.P.; Furmaniak, S.; Gauden, P.A.; Harris, P.J.F.; Kowalczyk, P. Chapter 3—Virtual Porous Carbons. In Novel Carbon Adsorbents; Tascón, J.M.D., Ed.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 61–104. [Google Scholar] [CrossRef]
- Masuda, H.; Iinoya, K. Theoretical study of the scatter of experimental data due to particle-size-distribution. J. Chem. Eng. Jpn. 1970, 4, 60–66. [Google Scholar] [CrossRef] [Green Version]
- Rice, S.B.; Chan, C.; Brown, S.C.; Eschbach, P.; Han, L.; Ensor, D.S.; Stefaniak, A.B.; Bonevich, J.; Vladár, A.E.; Hight Walker, A.R.; et al. Particle size distributions by transmission electron microscopy: An interlaboratory comparison case study. Metrologia 2013, 50, 663–678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, X.; Yuan, H.; Hu, M. A simple method for synthesis of ordered mesoporous carbon. Diam. Relat. Mater. 2019, 98, 107480. [Google Scholar] [CrossRef]
- Jun, S.; Joo, S.H.; Ryoo, R.; Kruk, M.; Jaroniec, M.; Liu, Z.; Ohsuna, T.; Terasaki, O. Synthesis of new, nanoporous carbon with hexagonally ordered mesostructured. J. Am. Chem. Soc. 2000, 122, 10712–10713. [Google Scholar] [CrossRef]
- Smith, V.A.; Rivera, J.F.A.; Bello, R.; Rodríguez-Aguado, E.; Elshaer, M.R.; Wodzinski, R.L.; Bashkova, S. The Role of Surface Chemistry and Polyethylenimine Grafting in the Removal of Cr (VI) by Activated Carbons from Cashew Nut Shells. C 2021, 7, 27. [Google Scholar] [CrossRef]
- Major, G.H.; Fairley, N.; Sherwood, P.M.A.; Linford, M.A.; Terry, J.; Fernandez, V.; Artyushkova, K. Practical guide for curve fitting in x-ray photoelectron spectroscopy. J. Vac. Sci. Technol. A 2020, 38, 061203. [Google Scholar] [CrossRef]
- Dupin, J.-C.; Gonbeau, D.; Vinatier, P.; Levasseur, A. Systematic XPS studies of metal oxides, hydroxides and peroxides. Phys. Chem. Chem. Phys. 2000, 2, 1319–1324. [Google Scholar] [CrossRef]
- Portillo, J.G.; Hernández-Paz, J.F.; Gomez-Mares, M.; Olivas-Armendariz, I.; Rodriguez González, C.A. Synthesis of nanostructured Nickel compounds on conductive metallic substrates. Mater. Lett. 2019, 257, 126676. [Google Scholar] [CrossRef]
- Grosvenor, A.P.; Biesinger, M.C.; Smart, R.S.C.; McIntyre, N.S. New interpretations of XPS spectra of nickel metal and oxides. Surf. Sci. 2006, 600, 1771–1779. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Lau, L.W.M.; Gerson, A.; Smart, R.S.C. X-ray photoelectron spectroscopic chemical state quantification of mixed nickel metal, oxide and hydroxide systems. Surf. Interface Anal. 2009, 41, 324–332. [Google Scholar] [CrossRef]
- Fan, L.; Liu, P.; Yan, X.; Gu, L.; Yang, Z.Z.; Yang, H.G.; Qiu, S.; Yao, X. Atomically isolated nickel species anchored on graphitized carbon for efficient hydrogen evolution electrocatalysis. Nat. Commun. 2016, 7, 10667. [Google Scholar] [CrossRef]
- Sakamoto, K.; Hayashi, F.; Sato, K.; Hirano, M.; Ohtsu, N. XPS spectral analysis for a multiple oxide comprising NiO, TiO2, and NiTiO3. Appl. Surf. Sci. 2020, 526, 146729. [Google Scholar] [CrossRef]
- Wang, Y.; Pan, J.; Li, Y.; Zhang, P.; Li, M.; Zheng, H.; Zhang, X.; Li, H.; Du, Q. Methylene blue adsorption by activated carbon, nickel alginate/activated carbon aerogel, and nickel alginate/graphene oxide aerogel: A comparison study. J. Mater. Res. Technol. 2020, 9, 12443–12460. [Google Scholar] [CrossRef]
- El Qada, E.N.; Allen, S.J.; Walker, G.M. Adsorption of basic dyes from aqueous solution onto activated carbons. Chem. Eng. J. 2008, 135, 174–184. [Google Scholar] [CrossRef]
- Yagub, M.T.; Sen, T.K.; Afroze, S.; Ang, H.M. Dye and its removal from aqueous solution by adsorption: A review. Adv. Colloid Interface Sci. 2014, 209, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Matos, J.; García-López, E.; Palmisano, L.; García, A.; Marcì, G. Influence of activated carbon in TiO2 and ZnO mediated photo-assisted degradation of 2-propanol in gas–solid regime. Appl. Catal. B 2010, 99, 170–180. [Google Scholar] [CrossRef]
- Liu, F.; Jamal, R.; Wang, Y.; Wang, M.; Yang, L.; Abdiryim, T. Photodegradation of methylene blue by photocatalyst of D-A-D type polymer/functionalized multi-walled carbon nanotubes composite under visible-light irradiation. Chemosphere 2017, 168, 1669–1676. [Google Scholar] [CrossRef]
- Heng, C.W.; Chong, W.C.; Pang, Y.L.; Sim, L.C. Photocatalytic degradation of methylene blue under visible light using carbon dot/titanium dioxide nanohybrid. IOP Conf. Ser. Mater. Sci. Eng. 2020, 991, 012092. [Google Scholar] [CrossRef]
- Vallejo, W.; Cantillo, A.; Díaz-Uribe, C. Methylene blue photodegradation under visible irradiation on Ag-Doped ZnO thin films. Int. J. Photoenergy 2020, 2020, 1627498. [Google Scholar] [CrossRef] [Green Version]
- Shang, H.; Chen, L.; Wang, Y.; Liu, H. Efficient photocatalytic degradation of methylene blue by using GO/hemin/TiO2 nanocomposite under visible irradiation. Micro Nano Lett. 2018, 13, 646–651. [Google Scholar] [CrossRef]
- Alberoni, C.; Barroso-Martín, I.; Infantes-Molina, A.; Castellón, E.R.; Talon, A.; Zhao, H.; You, S.; Vomiero, A.; Moretti, E. Ceria doping boosts methylene blue photodegradation in titania nanostructures. Mater. Chem. Front. 2021, 5, 4138. [Google Scholar] [CrossRef]
- Liang, Y.-C.; Xu, N.-C.; Chiang, K.-J. Surface morphology-dependent functionality of titanium dioxide–nickel oxide nanocomposite semiconductors. Nanomaterials 2019, 9, 1651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bandosz, T.J.; Matos, J.; Seredych, M.; Islam, M.N.; Alfano, R.R. Photoactivity of S-doped nanoporous activated carbons: A new perspective for harvesting solar energy on carbon-based semiconductors. Appl. Catal. A 2012, 445, 159–165. [Google Scholar] [CrossRef]
- Guo, X.; Meng, Q.; Wang, D.; Zha, F.; Tang, X.; Tian, H. S-doped NiFe-based catalyst for fast degradation of methylene blue by heterogeneous photo-Fenton reaction. Environ. Sci. Pollut. Res. 2021, 28, 36112–36121. [Google Scholar] [CrossRef] [PubMed]
- Franca, A.S.; Oliveira, L.S.; Ferreira, M.E. Kinetics and equilibrium studies of methylene blue adsorption by spent coffee grounds. Desalination 2009, 249, 267–272. [Google Scholar] [CrossRef]
- Fierro, V.; Torné-Fernández, V.; Montané, D.; Celzard, A. Adsorption of phenol onto activated carbons having different textural and surface properties. Microporous Mesoporous Mater. 2008, 111, 276–284. [Google Scholar] [CrossRef]
- Sabzehmeidani, M.M.; Mahnaee, S.; Ghaedi, M.; Heidari, H.; Roy, V.A.L. Carbon based materials: A review of adsorbents for inorganic and organic compounds. Mater. Adv. 2021, 2, 598–627. [Google Scholar] [CrossRef]
- Wu, F.-C.; Tseng, R.-L.; Juang, R.-S. Initial behavior of intraparticle diffusion model used in the description of adsorption kinetics. Chem. Eng. J. 2009, 153, 1–8. [Google Scholar] [CrossRef]




| SBET (m2/g) | Vtot (cm3/g) | Vmic (cm3/g) | 1 Vmes (cm3/g) | w (nm) | % Yield |
|---|---|---|---|---|---|
| 284 | 0.304 | 0.010 | 0.294 | 4.7 | 22.0 ± 1.9 |
| BE, eV | C1s | % | BE, eV | O1s | % | BE, eV | Ni2p | % |
|---|---|---|---|---|---|---|---|---|
| C | 92.73 | O | 6.53 | Ni | 0.74 | |||
| 284.5 | C–C | 75 | 530.5 | O2− | 21 | 852.5 | Ni(0) | 4 |
| 285.8 | C–O | 15 | 532.1 | O=C/Ni(OH)2 | 53 | 854.5 | NiO | 29 |
| 287.0 | C=O | 5 | 533.7 | O–C | 26 | 856.6 | Ni(OH)2 | 30 |
| 288.3 | O–C=O | 2 | 860.7 | Ni 2p3/2 satellites | 22 | |||
| 290.0 | π–π* | 3 | 863.5 | 14 |
| Material | Method | Degradation % | Ref. |
|---|---|---|---|
| NiO nanoparticles | Thermal decomposition | 70.2 | [33] |
| ZnO-NiO composite | Solvothermal | 85 | [33] |
| NiO-Ag heterostructure | Hydrothermal | 70 | [33] |
| NiO-ZnO composite | Electrospinning | 65.4 | [33] |
| NiO-CuO nanoparticles | Sol-gel | 40 | [33] |
| Polymer-multiwall carbon nanotube composite | Oxidative polymerization | 22–67 | [58] |
| Carbon dot-TiO2 nanohybrid | Hydrothermal | 16–41 | [59] |
| Ag-doped ZnO thin films | Sol-gel | 45.1 | [60] |
| GO-hemin-TiO2 nanocomposite | Adsorption | 90 | [61] |
| Ceria-doped titania | Hydrothermal | 85 | [62] |
| TiO2-NiO heterostructures | Hydrothermal/chemical bath deposition | 75–95 | [63] |
| S-doped nanoporous carbon | Doping | 55–100 | [64] |
| S-doped NiFe catalyst | Solvothermal | 73.1 | [65] |
| Ni-doped nanoporous carbon | EISA | 84 | this work |
| Pseudo First-Order | Pseudo Second-Order | Intra-Particle Diffusion | |||||||
|---|---|---|---|---|---|---|---|---|---|
| qe mg/g | k1 1/min | R2 | qe mg/g | k2 g/mg min | R2 | C mg/g | ki mg/g min1/2 | R2 | |
| Dark | 22.7 | 0.9545 | 94.7 | 0.9995 | 74.7 | 0.9236 | |||
| Green | 22.1 | 0.9407 | 95.2 | 0.9997 | 76.2 | 0.8548 | |||
| Purple | 18.4 | 0.8282 | 94.3 | 0.9998 | 74.8 | 0.9608 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bello, R.; Rodríguez-Aguado, E.; Smith, V.A.; Grachev, D.; Castellón, E.R.; Bashkova, S. Ni-Doped Ordered Nanoporous Carbon Prepared from Chestnut Wood Tannins for the Removal and Photocatalytic Degradation of Methylene Blue. Nanomaterials 2022, 12, 1625. https://doi.org/10.3390/nano12101625
Bello R, Rodríguez-Aguado E, Smith VA, Grachev D, Castellón ER, Bashkova S. Ni-Doped Ordered Nanoporous Carbon Prepared from Chestnut Wood Tannins for the Removal and Photocatalytic Degradation of Methylene Blue. Nanomaterials. 2022; 12(10):1625. https://doi.org/10.3390/nano12101625
Chicago/Turabian StyleBello, Ruby, Elena Rodríguez-Aguado, Victoria A. Smith, Dmitry Grachev, Enrique Rodríguez Castellón, and Svetlana Bashkova. 2022. "Ni-Doped Ordered Nanoporous Carbon Prepared from Chestnut Wood Tannins for the Removal and Photocatalytic Degradation of Methylene Blue" Nanomaterials 12, no. 10: 1625. https://doi.org/10.3390/nano12101625