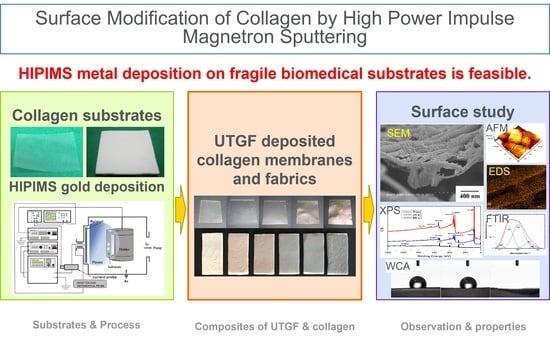
Nanoarchitectonics for Ultrathin Gold Films Deposited on Collagen Fabric by High-Power Impulse Magnetron Sputtering
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
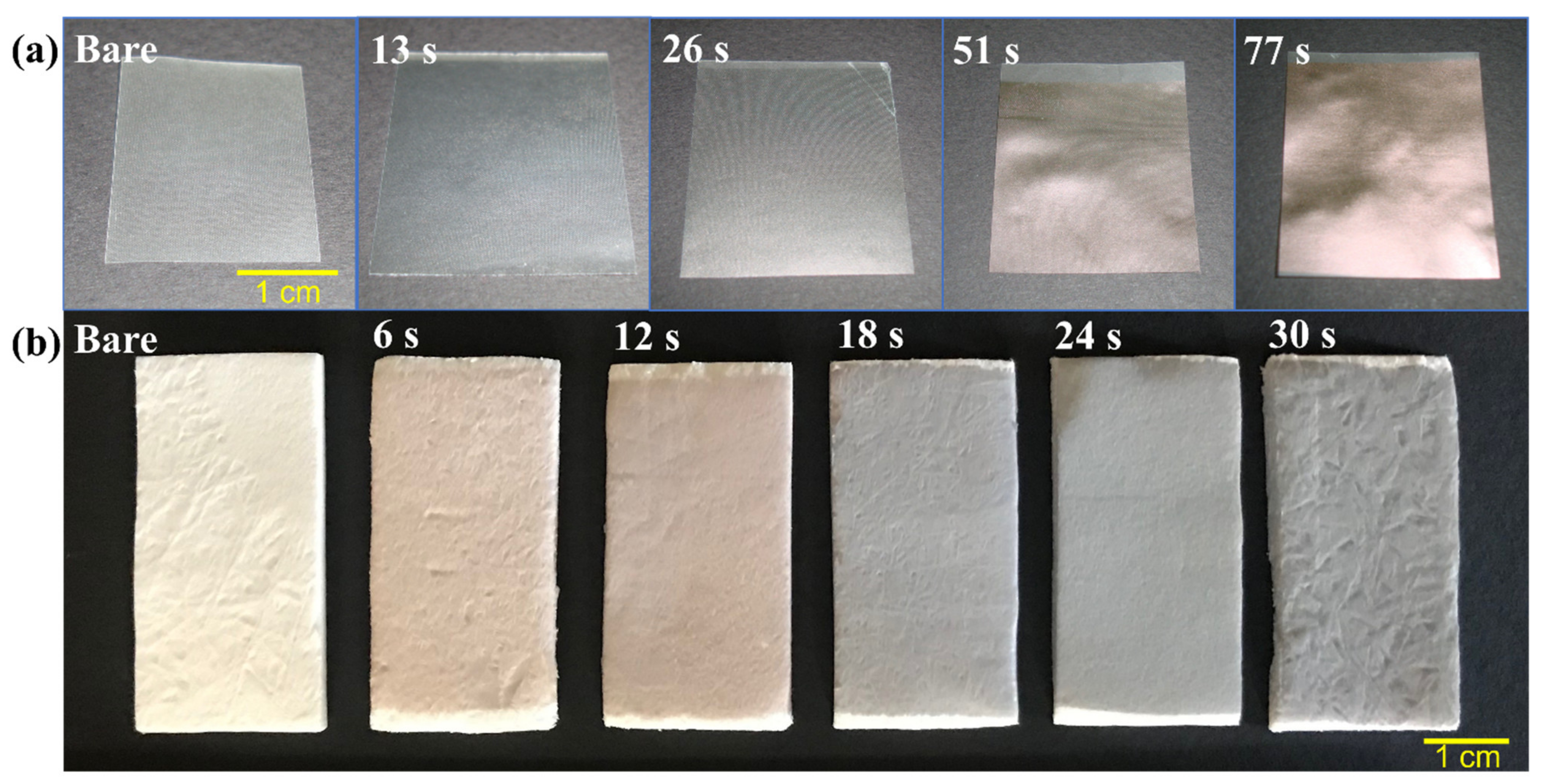
3.1. Gross Appearance of Untreated and UTGF-Deposited Collagen Substrates
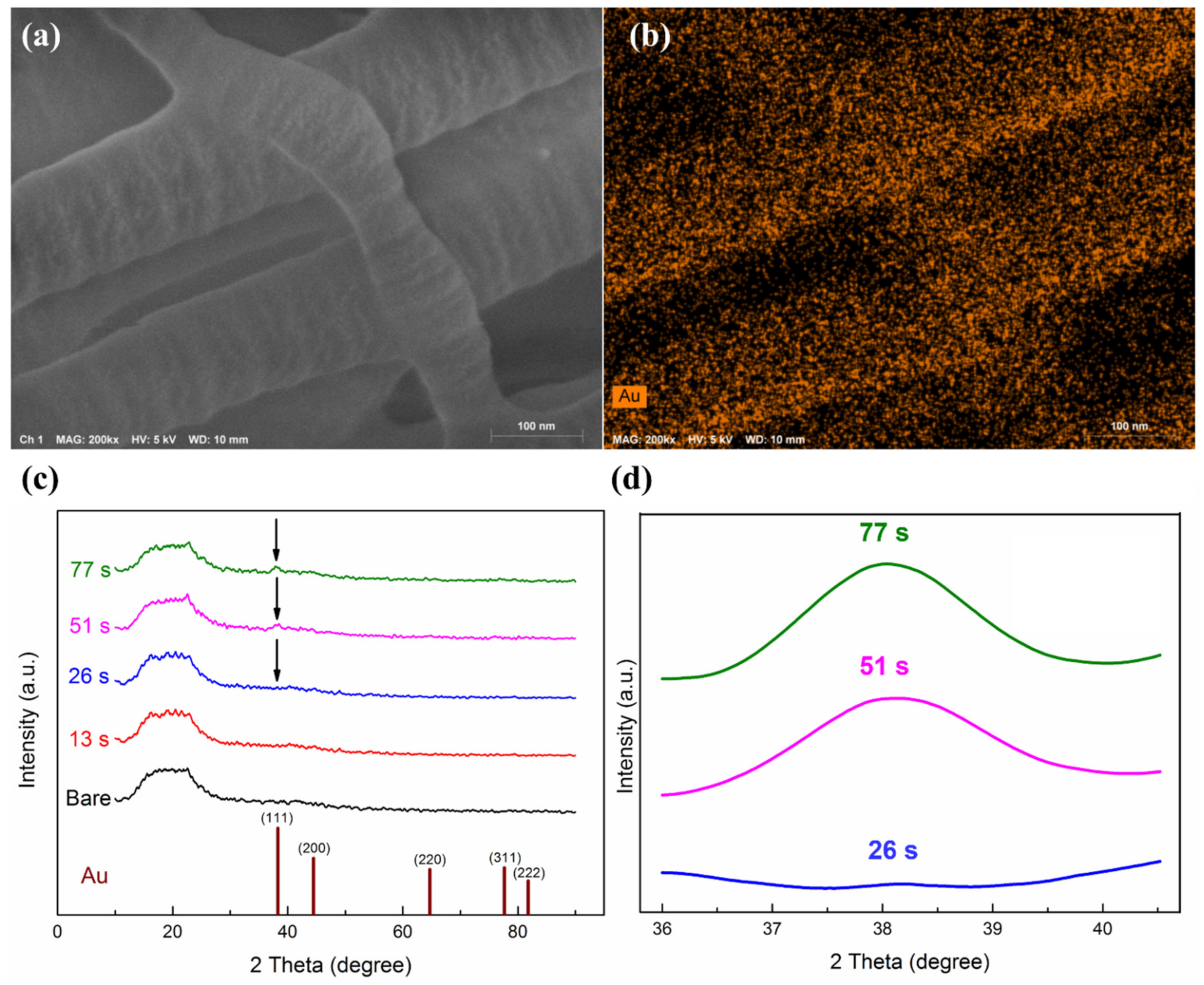
3.2. Surface Morphology and Microstructures of UTGF Deposited Specimens
3.3. Surface Roughness and Topography of UTGF-Deposited Specimens
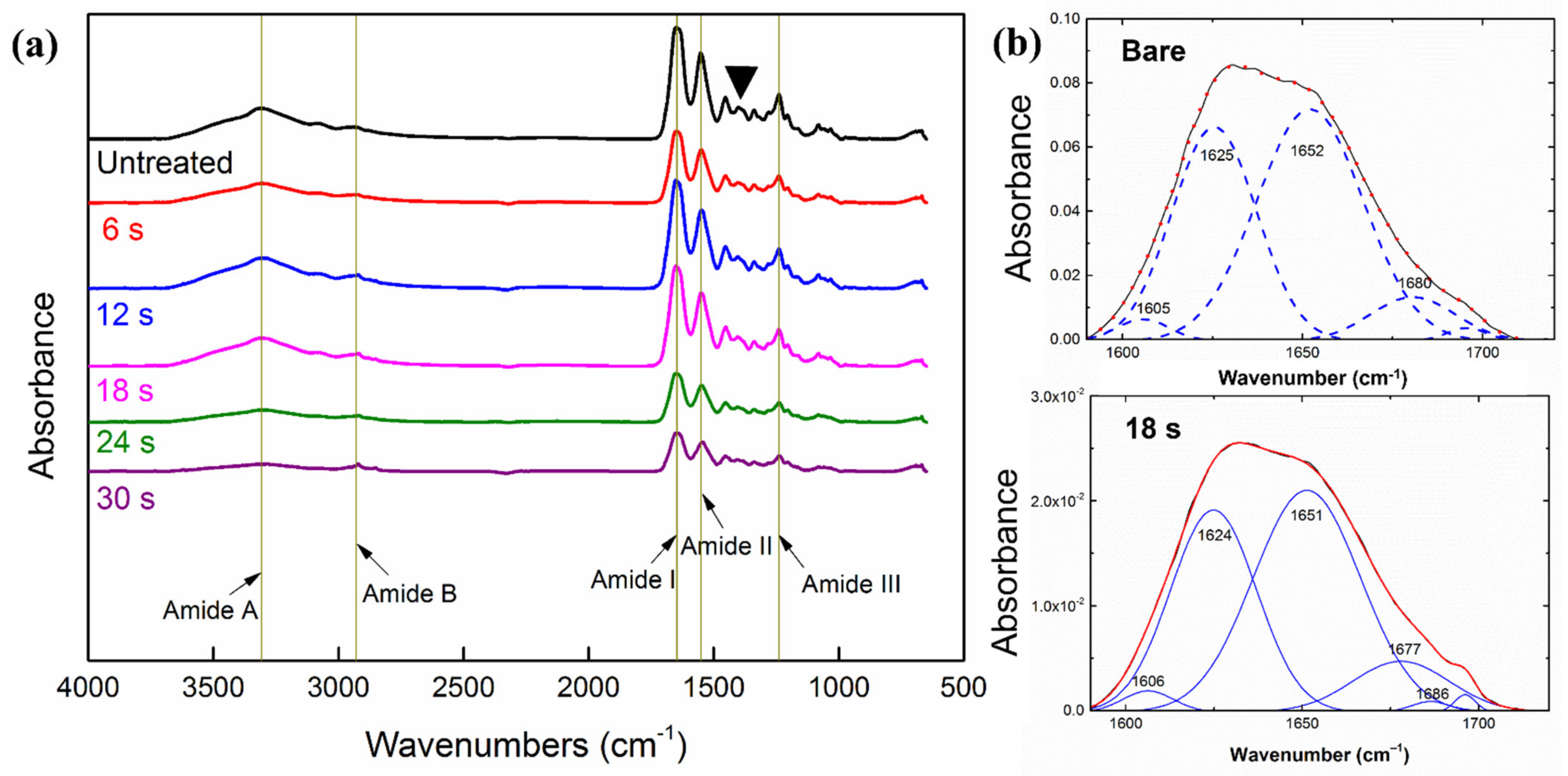
3.4. Surface Chemistry and Secondary Structure of Collagen
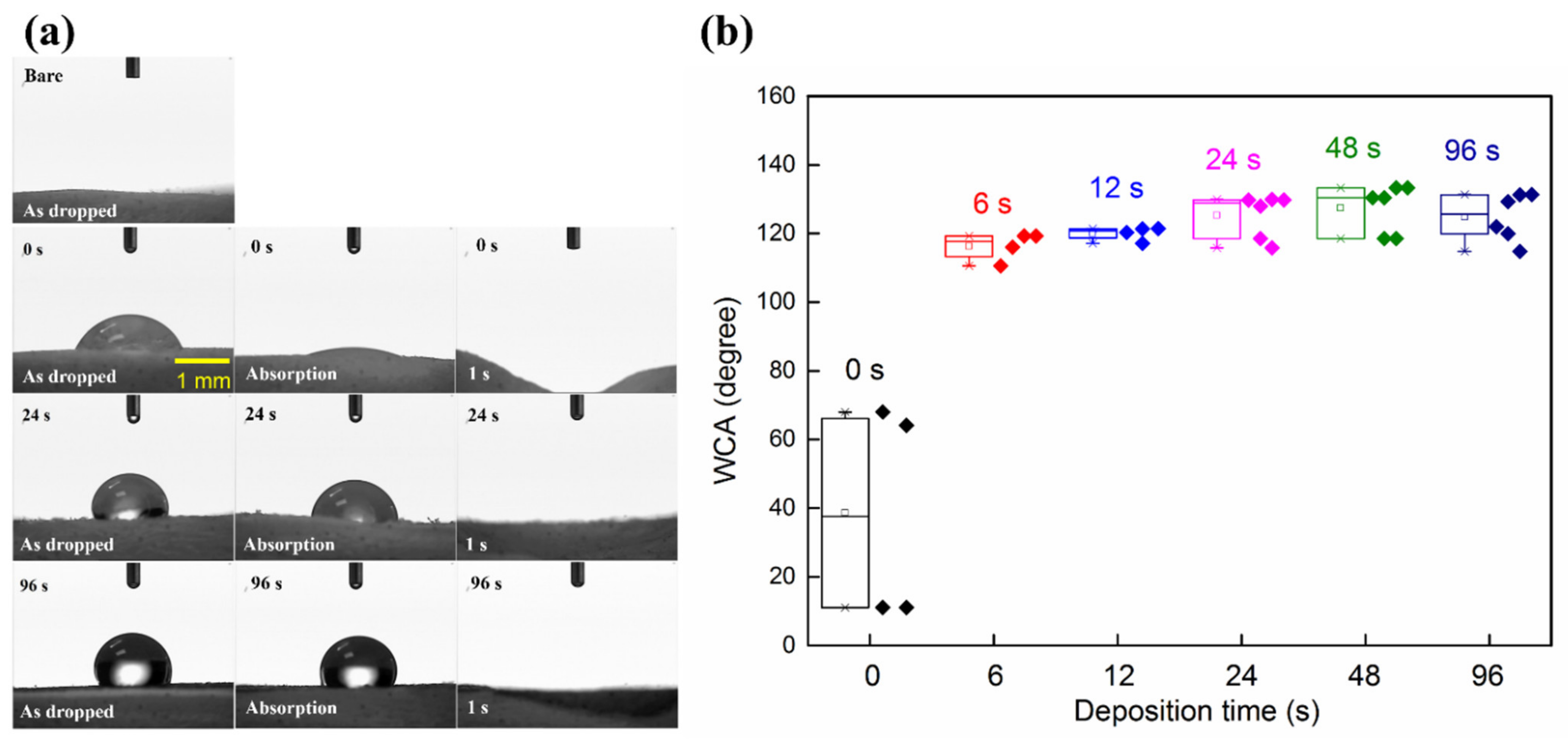
3.5. Surface Wettability
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Avila Rodríguez, M.I.; Rodríguez Barroso, L.G.; Sánchez, M.L. Collagen: A review on its sources and potential cosmetic applications. J. Cosmet. Dermatol. 2018, 17, 20–26. [Google Scholar] [CrossRef]
- Hsu, F.Y.; Hung, Y.S.; Liou, H.M.; Shen, C.H. Electrospun hyaluronate—Collagen nanofibrous matrix and the effects of varying the concentration of hyaluronate on the characteristics of foreskin fibroblast cells. Acta Biomater. 2010, 6, 2140–2147. [Google Scholar] [CrossRef]
- Huang, G.P.; Shanmugasundaram, S.; Masih, P.; Pandya, D.; Amara, S.; Collins, G.; Arinzeh, T.L. An investigation of common crosslinking agents on the stability of electrospun collagen scaffolds. J. Biomed. Mater. Res. A 2015, 103, 762–771. [Google Scholar] [CrossRef]
- Aktürk, Ö.; Keskin, D. Collagen/PEO/gold nanofibrous matrices for skin tissue engineering. Turk. J. Biol. 2016, 40, 380–398. [Google Scholar] [CrossRef]
- Yamane, K.; Mazaki, T.; Shiozaki, Y.; Yoshida, A.; Shinohara, K.; Nakamura, M.; Yoshida, Y.; Zhou, D.; Kitajima, T.; Tanaka, M.; et al. Collagen-binding hepatocyte growth factor (HGF) alone or with a gelatin- furfurylamine hydrogel enhances functional recovery in mice after spinal cord injury. Sci. Rep. 2018, 8, 917–928. [Google Scholar] [CrossRef] [Green Version]
- Mozdzen, L.C.; Vucetic, A.; Harley, B.A.C. Modifying the strength and strain concentration profile within collagen scaffolds using customizable arrays of poly-lactic acid fibers. J. Mech. Behav. Biomed. Mater. 2017, 66, 28–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodina, A.V.; Tenchurin, T.K.; Saprykin, V.P.; Shepelev, A.D.; Mamagulashvili, V.G.; Grigor’ev, T.E.; Lukanina, K.I.; Orekhov, A.S.; Moskaleva, E.Y.; Chvalun, S.N. Migration and proliferative activity of mesenchymal stem cells in 3D polylactide scaffolds depends on cell seeding technique and collagen modification. Bull. Exp. Biol. Med. 2016, 162, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Deeken, C.R.; Bachman, S.L.; Ramshaw, B.J.; Grant, S.A. Characterization of bionanocomposite scaffolds comprised of mercaptoethylamine-functionalized gold nanoparticles crosslinked to acellular porcine tissue. J. Mater. Sci. Mater. Med. 2012, 23, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, R.; Azhagiya Singam, E.R.; Vijaya Sundar, J.; Subramanian, V. Interaction of collagen like peptides with gold nanosurfaces: A molecular dynamics investigation. Phys. Chem. Chem. Phys. 2015, 17, 5172–5186. [Google Scholar] [CrossRef] [PubMed]
- Hung, H.S.; Chang, C.H.; Chang, C.J.; Tang, C.M.; Kao, W.C.; Lin, S.Z.; Hsieh, H.H.; Chu, M.Y.; Sun, W.S.; Hsu, S.H. In vitro study of a novel nanogold-collagen composite to enhance the mesenchymal stem cell behavior for vascular regeneration. PLoS ONE 2014, 9, e104019. [Google Scholar]
- Ehmann, H.M.A.; Breitwieser, D.; Winter, S.; Gspan, C.; Koraimann, G.; Maver, U.; Sega, M.; Köstler, S.; Stana-Kleinschek, K.; Spirk, S. Gold nanoparticles in the engineering of antibacterial and anticoagulant surfaces. Carbohydr. Polym. 2015, 117, 34–42. [Google Scholar] [CrossRef]
- Baptista, A.; Silva, F.; Porteiro, J.; Míguez, J.; Pinto, G. Sputtering physical vapour deposition (PVD) coatings: A critical review on process improvement and market trend demands. Coatings 2018, 8, 402–423. [Google Scholar] [CrossRef] [Green Version]
- Dreaden, E.C.; Alkilany, A.M.; Huang, X.; Murphy, C.J.; El-Sayed, M.A. The golden age: Gold nanoparticles for biomedicine. Chem. Soc. Rev. 2012, 41, 2740–2779. [Google Scholar] [CrossRef] [Green Version]
- Buehler, M.J. Nature designs tough collagen: Explaining the nanostructure of collagen fibrils. Proc. Natl. Acad. Sci. USA 2006, 103, 12285–12290. [Google Scholar] [CrossRef] [Green Version]
- Shukla, K.; Sugumaran, A.A.; Khan, I.; Ehiasarian, A.P.; Hovsepian, P.E. Low pressure plasma nitrided CoCrMo alloy utilising HIPIMS discharge for biomedical applications. J. Mech. Behav. Biomed. Mater. 2020, 111, 104004–104014. [Google Scholar] [CrossRef]
- Ganesan, R.; Akhavan, B.; Hiob, M.A.; McKenzie, D.R.; Weiss, A.S.; Bilek, M.M. HiPIMS carbon coatings show covalent protein binding that imparts enhanced hemocompatibility. Carbon 2018, 139, 118–128. [Google Scholar] [CrossRef]
- Tiron, V.; Ursu, E.L.; Cristea, D.; Munteanu, D.; Bulai, G.; Ceban, A.; Velicu, I.L. Overcoming the insulating materials limitation in HiPIMS: Ion-assisted deposition of DLC coatings using bipolar HiPIMS. Appl. Surf. Sci. 2019, 494, 871–879. [Google Scholar] [CrossRef]
- Lin, J.; Sproul, W.D.; Wei, R.; Chistyakov, R. Diamond like carbon films deposited by HiPIMS using oscillatory voltage pulses. Surf. Coat. Technol. 2014, 258, 1212–1222. [Google Scholar] [CrossRef]
- Santiago, J.A.; Fernández-Martínez, I.; Wennberg, A.; Molina-Aldareguia, J.M.; Castillo-Rodríguez, M.; Rojas, T.C.; Sánchez-López, J.C.; González, M.U.; García-Martín, J.M.; Li, H.; et al. Adhesion enhancement of DLC hard coatings by HiPIMS metal ion etching pretreatment. Surf. Coat. Technol. 2018, 349, 787–796. [Google Scholar] [CrossRef]
- Biswas, B.; Purandare, Y.; Sugumaran, A.; Khan, I.; Hovsepian, P.E. Effect of chamber pressure on defect generation and their influence on corrosion and tribological properties of HIPIMS deposited CrN/NbN coatings. Surf. Coat. Technol. 2018, 336, 84–91. [Google Scholar] [CrossRef]
- Ghailane, A.; Makha, M.; Larhlimi, H.; Alami, J. Design of hard coatings deposited by HiPIMS and dcMS. Mater. Lett. 2020, 280, 128540. [Google Scholar] [CrossRef]
- Silva Júnior, Z.S.; Botta, S.B.; Ana, P.A.; França, C.M.; Fernandes, K.P.; Mesquita-Ferrari, R.A.; Deana, A.; Bussadori, S.K. Effect of papain-based gel on type I collagen-spectroscopy applied for microstructural analysis. Sci. Rep. 2015, 23, 11448–11455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riaz, T.; Zeeshan, R.; Zarif, F.; Ilyas, K.; Muhammad, N.; Safi, S.Z.; Rahim, A.; Rizvi, S.A.A.; Rehman, I.U. FTIR analysis of natural and synthetic collagen. Appl. Spectrosc. Rev. 2018, 53, 703–746. [Google Scholar] [CrossRef]
- Petibois, C.; Gouspillou, G.; Wehbe, K.; Delage, J.P.; Déléris, G. Analysis of type I and IV collagens by FT-IR spectroscopy and imaging for a molecular investigation of skeletal muscle connective tissue. Anal. Bioanal. Chem. 2006, 386, 1961–1966. [Google Scholar] [CrossRef]
- Barth, A. Infrared spectroscopy of proteins. Biochim. Biophys. Acta Bioenerg. 2007, 1767, 1073–1101. [Google Scholar] [CrossRef] [Green Version]
- Harrell, C.R.; Djonov, V.; Fellabaum, C.; Volarevic, V. Risks of using sterilization by gamma radiation: The other side of the coin. Int. J. Med. Sci. 2018, 15, 274–279. [Google Scholar] [CrossRef] [Green Version]
- Kawabe, Y.; Ito, T.; Yoshida, H.; Moriwaki, H. Glowing gold nanoparticle coating restoring the lost property from bulk gold. Nanoscale 2019, 11, 3786–3793. [Google Scholar] [CrossRef]
- Bozec, L.; Odlyha, M. Thermal denaturation studies of collagen by microthermal analysis and atomic force microscopy. Biophys. J. 2011, 101, 228–236. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Chen, W.L.; Lin, S.J.; Jee, S.H.; Chen, Y.F.; Lin, L.C.; So, T.C.; Dong, C.Y. Investigating mechanisms of collagen thermal denaturation by high resolution second-harmonic generation imaging. Biophys. J. 2006, 91, 2620–2625. [Google Scholar] [CrossRef] [Green Version]
- Rochdi, A.; Foucat, L.; Renou, J.P. Effect of thermal denaturation on water–collagen interactions: NMR relaxation and differential scanning calorimetry analysis. Biopolymers 1999, 50, 690–696. [Google Scholar] [CrossRef]
- Markowicz, M.; Koellensperger, E.; Steffens, G.C.; Frentz, M.; Schrage, N.; Pallua, N. The impact of vacuum freeze-drying on collagen sponges after gas plasma sterilization. J. Biomater. Sci. Polym. Ed. 2006, 17, 61–75. [Google Scholar] [CrossRef]
- Anderton, C.R.; DelRio, F.W.; Bhadriraju, K.; Plant, A.L. Plant, The effect of high vacuum on the mechanical properties and bioactivity of collagen fibril matrices. Biointerphases 2013, 8, 2–13. [Google Scholar] [CrossRef] [Green Version]
- Shoulders, M.D.; Raines, R.T. Collagen structure and stability. Annu. Rev. Biochem. 2009, 78, 929–958. [Google Scholar] [CrossRef] [Green Version]
- Martin, P.J. Ion-enhanced adhesion of thin gold films. Gold Bull. 1986, 19, 102–116. [Google Scholar] [CrossRef] [Green Version]
- Graudejus, O.; Görrn, P.; Wagner, S. Controlling the morphology of gold films on poly(dimethylsiloxane). ACS Appl. Mater. Interfaces 2010, 2, 1927–1933. [Google Scholar] [CrossRef]
- Lin, J.; Shi, Y.; Men, Y.; Wang, X.; Ye, J.; Zhang, C. Mechanical roles in formation of oriented collagen fibers. Tissue Eng. Part B Rev. 2020, 26, 116–128. [Google Scholar] [CrossRef] [Green Version]
- Aragón-Sánchez, J.; Quintana-Marrero, Y.; Aragón-Hernández, C.; Hernández-Herero, M.J. ImageJ: A free, easy, and reliable method to measure leg ulcers using digital pictures. Int. J. Low. Extrem. Wounds 2017, 16, 269–273. [Google Scholar] [CrossRef]
- Collins, T.J. ImageJ for microscopy. BioTechniques 2007, 43, S25–S30. [Google Scholar] [CrossRef]
- Zarraoa, L.; González, M.U.; San Paulo, Á. Imaging low-dimensional nanostructures by very low voltage scanning electron microscopy: Ultra-shallow topography and depth-tunable material contrast. Sci. Rep. 2019, 9, 16263. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, K.; Odawara, A.; Nagata, A.; Ikeda, M.; Baba, Y.; Nakayama, S. A microcalorimeter EDS system suitable for low acceleration voltage analysis. Surf. Interface Anal. 2006, 38, 1646–1649. [Google Scholar] [CrossRef]
- Guiry, E.J.; Szpak, P. Quality control for modern bone collagen stable carbon and nitrogen isotope measurements. Methods Ecol. Evol. 2020, 11, 1049–1060. [Google Scholar] [CrossRef]
- Passiu, C.; Rossi, A.; Weinert, M.; Tysoe, W.; Spencer, N.D. Probing the outermost layer of thin gold films by XPS and density functional theory. Appl. Surf. Sci. 2020, 507, 145084–145089. [Google Scholar] [CrossRef]
- Zhang, L.J.; Feng, X.S.; Liu, H.G.; Qian, D.J.; Zhang, L.; Yu, X.L.; Cui, F.Z. Hydroxyapatite/collagen composite materials formation in simulated body fluid environment. Mater. Lett. 2004, 58, 719–722. [Google Scholar] [CrossRef]
- Häupl, K.; Lang, M.; Wissmann, P. X-ray Diffraction Investigations on Ultra-thin Gold Films. Surf. Interface Anal. 1986, 9, 27–30. [Google Scholar] [CrossRef]
- Yan, W.; Petkov, V.; Mahurin, S.M.; Overbury, S.H.; Dai, S. Powder XRD analysis and catalysis characterization of ultra-small gold nanoparticles deposited on titania-modified SBA-15. Catal. Commun. 2005, 6, 404–408. [Google Scholar] [CrossRef]
- Bonyár, A.; Molnár, L.M.; Harsányi, G. Localization factor: A new parameter for the quantitative characterization of surface structure with atomic force microscopy (AFM). Micron 2012, 43, 305–310. [Google Scholar] [CrossRef]
- Bonyár, A. AFM characterization of the shape of surface structures with localization factor. Micron 2016, 87, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Malinský, P.; Slepička, P.; Hnatowicz, V.; Švorčík, V. Early stages of growth of gold layers sputter deposited on glass and silicon substrates. Nanoscale Res. Lett. 2012, 7, 241. [Google Scholar] [CrossRef] [Green Version]
- Kajikawa, Y. Analysing surface roughness evolution in thin films. In Thin Film Growth, 1st ed.; Cao, Z., Ed.; Woodhead Publishing: Cambridge, UK, 2011; pp. 60–82. [Google Scholar]
- Talari, A.C.S.; Martinez, M.A.G.; Movasaghi, Z.; Rehman, S.; Rehman, I.U. Advances in Fourier transform infrared (FTIR) spectroscopy of biological tissues. Appl. Spectrosc. Rev. 2017, 52, 456–506. [Google Scholar] [CrossRef]
- Nair, R.S.; Ameer, J.M.; Alison, M.R.; Anilkumar, T.V. A gold nanoparticle coated porcine cholecyst-derived bioscaffold for cardiac tissue engineering. Colloids Surf. B 2017, 157, 130–137. [Google Scholar] [CrossRef]
- Belbachir, K.; Noreen, R.; Gouspillou, G.; Petibois, C. Collagen types analysis and differentiation by FTIR spectroscopy. Anal. Bioanal. Chem. 2009, 395, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Böse, O.; Kemnitz, E.; Lippitz, A.; Unger, W.E.S. C 1s and Au 4f7/2 referenced XPS binding energy data obtained with different aluminium oxides,-hydroxides and-fluorides. Fresenius J. Anal. Chem. 1997, 358, 175–179. [Google Scholar]
- Gross, T.; Ramm, M.; Sonntag, H.; Unger, W.; Weijers, H.M.; Adem, E.H. An XPS analysis of different SiO2 modifications employing a C 1s as well as an Au 4f7/2 static charge reference. Surf. Interface Anal. 1992, 18, 59–64. [Google Scholar] [CrossRef]
- Gelse, K.; Pöschl, E.; Aigner, T. Collagens—Structure, function, and biosynthesis. Adv. Drug Deliv. Rev. 2003, 55, 1531–1546. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Feng, Q.; Wang, W.; Cui, F. Chemical characteristics and cytocompatibility of collagen-based scaffold reinforced by chitin fibers for bone tissue engineering. J. Biomed. Mater. Res. Part B Appl. Biomater. 2006, 77, 219–226. [Google Scholar] [CrossRef]
- Wang, X.H.; Li, D.P.; Wang, W.J.; Feng, Q.L.; Cui, F.Z.; Xu, Y.X.; Song, X.H.; van der Werf, M. Crosslinked collagen/chitosan matrix for artificial livers. Biomaterials 2003, 24, 3213–3220. [Google Scholar] [CrossRef]
- Gogurla, N.; Mondal, S.P.; Sinha, A.K.; Katiyar, A.K.; Banerjee, W.; Kundu, S.C.; Ray, S.K. Transparent and flexible resistive switching memory devices with a very high ON/OFF ratio using gold nanoparticles embedded in a silk protein matrix. Nanotechnology 2013, 24, 345202. [Google Scholar] [CrossRef]
- Casaletto, M.P.; Longo, A.; Martorana, A.; Prestianni, A.; Venezia, A.M. Venezia, XPS study of supported gold catalysts: The role of Au0 and Au+δ species as active sites. Surf. Interface Anal. 2006, 38, 215–218. [Google Scholar] [CrossRef]
- Shchukarev, A.V.; Korolkov, D.V. XPS study of group IA carbonates. Cent. Eur. J. Chem. 2004, 2, 347–362. [Google Scholar] [CrossRef] [Green Version]
- Nohira, H.; Tsai, W.; Besling, W.; Young, E.; Petry, J.; Conard, T.; Vandervorst, W.; De Gendt, S.; Heyns, M.; Maes, J.; et al. Characterization of ALCVD-Al2O3 and ZrO2 layer using X-ray photoelectron spectroscopy. J. Non-Cryst. Solids. 2002, 303, 83–87. [Google Scholar] [CrossRef]
- Rignanese, G.M.; Pasquarello, A.; Charlier, J.C.; Gonze, X.; Car, R. Nitrogen incorporation at Si(001)−SiO2 interfaces: Relation between N 1s core-level shifts and microscopic structure. Phys. Rev. Lett. 1997, 79, 5174–5177. [Google Scholar] [CrossRef] [Green Version]
- Nagayama, G.; Zhang, D. Intermediate wetting state at nano/microstructured surfaces. Soft Matter. 2020, 16, 3514–3521. [Google Scholar] [CrossRef]
- Abdelsalam, M.E.; Bartlett, P.N.; Kelf, T.; Baumberg, J. Wetting of regularly structured gold surfaces. Langmuir 2005, 21, 1753–1757. [Google Scholar] [CrossRef]
- Dhar, A.; Zhao, Z.; Alford, T.L. Alford, Effect of different substrates on the wettability and electrical properties of Au thin films deposited by sputtering. JOM 2015, 67, 845–848. [Google Scholar] [CrossRef]
- Canning, J.; Tzoumis, N.; Beattie, J.K.; Gibson, B.C.; Ilagan, E. Water on Au sputtered films. Chem. Comm. 2014, 50, 9172–9175. [Google Scholar] [CrossRef]
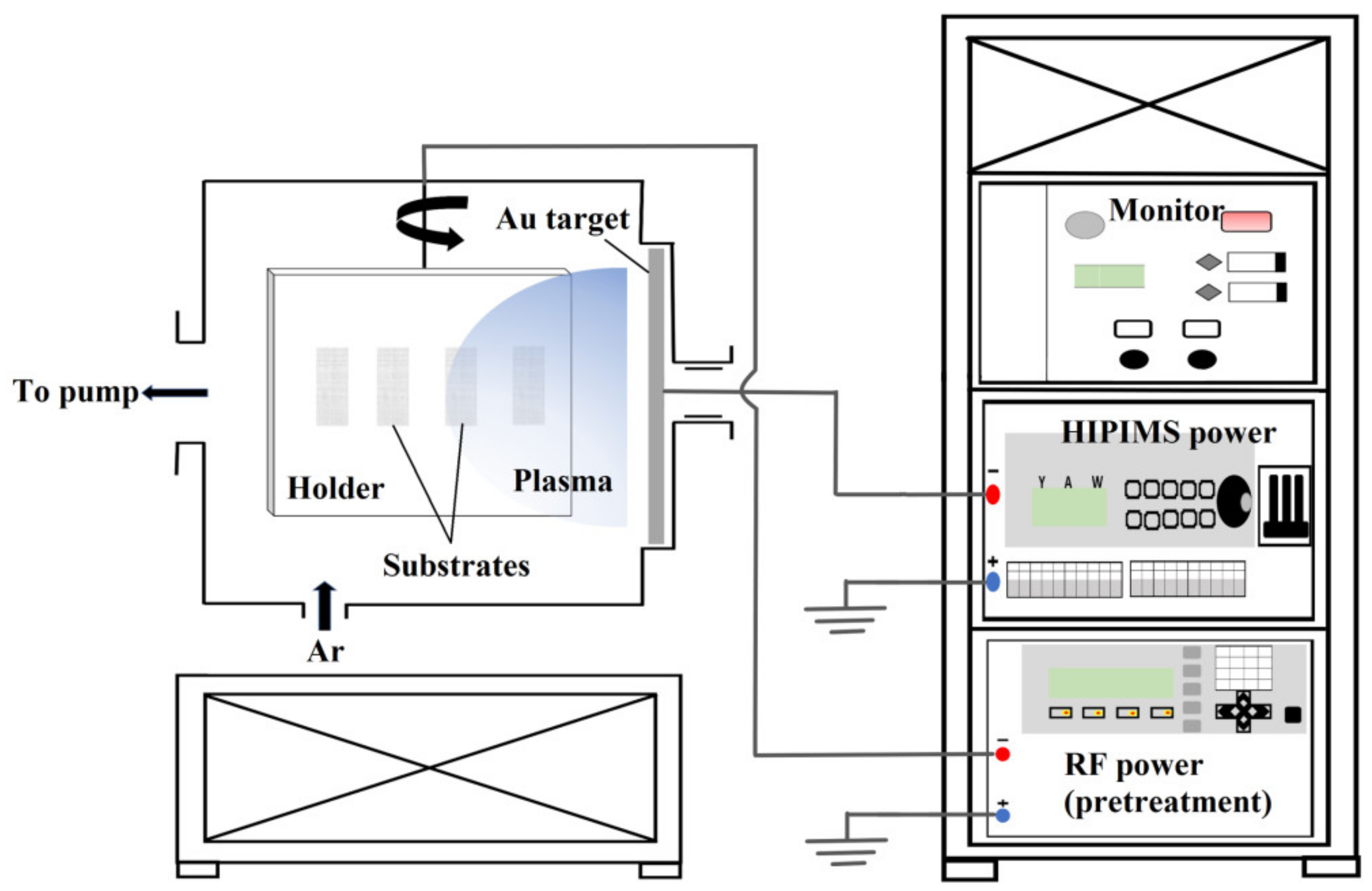


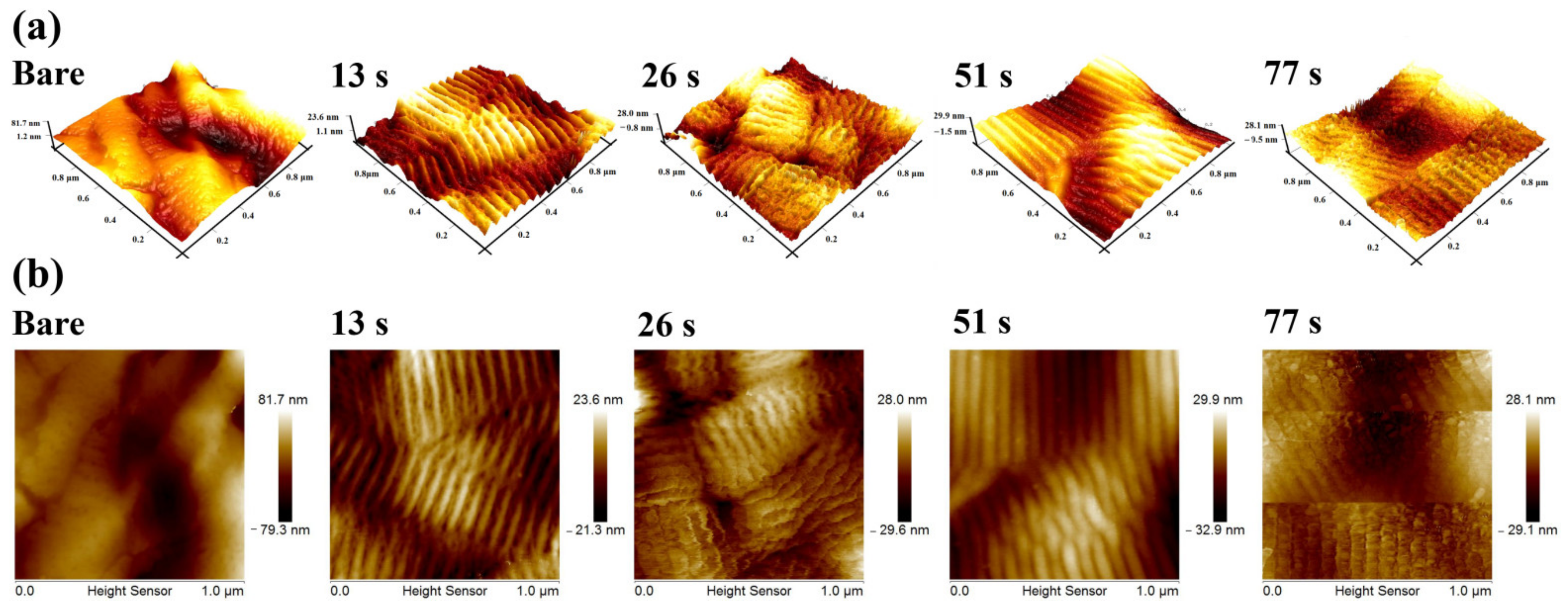

| Parameters | Value |
|---|---|
| Targe voltage (V) | 1391 |
| Peak target current (A) | 30 |
| Peak current density (A/cm2) | 0.106 |
| Average target power (W) | 133.1 |
| Peak targer power (W) | 41.7 |
| Peak power density (W/cm2) | 147.5 |
| Ton (µs) | 50 |
| Pulse frequency (Hz) | 100 |
| Ar flow rate (sccm) | 150 |
| Working pressure (Pa) | 0.54 |
| Deposition time (s) for substrate A | 13, 26, 51, 77 |
| Deposition time (s) for substrate B (FTIR) | 6, 12, 18, 24, 30 |
| Deposition time (s) for substrate B (WCA, XPS) | 0, 6, 12, 24, 48, 96 |
| Energy-Dispersive X-ray Spectroscopy (EDS) | ||||||
| UTGF Deposition Time (s) | Bare | 13 | 26 | 51 | 77 | |
| Atom (%) | ||||||
| C | 59.30 | 59.00 | 57.33 | 58.96 | 55.88 | |
| N | 14.30 | 17.17 | 18.54 | 17.59 | 16.05 | |
| O | 23.80 | 22.87 | 23.83 | 22.73 | 27.08 | |
| Au | 0.00 | 0.10 | 0.31 | 0.72 | 0.98 | |
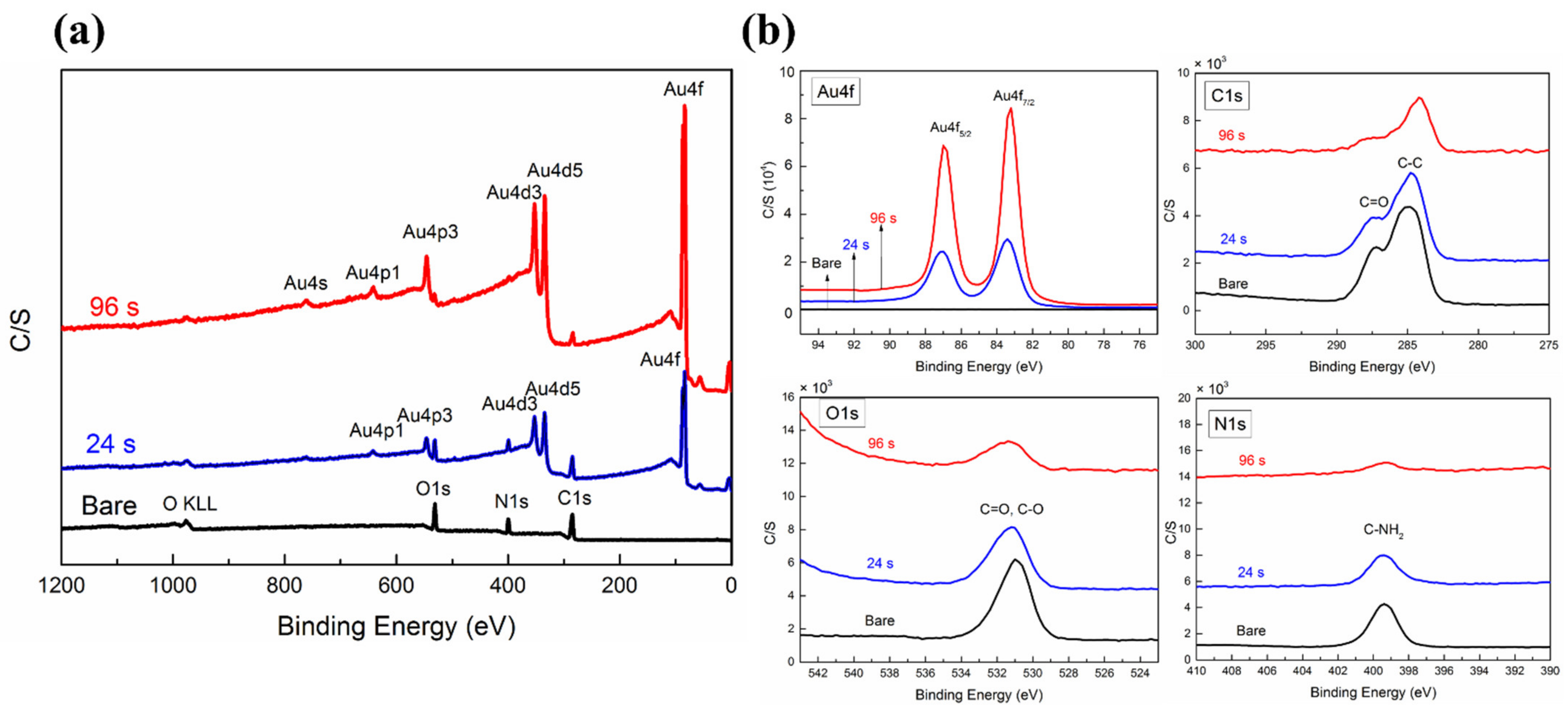
| X-ray Photoelectron Spectroscopy (XPS) | ||||||
| UTGF Deposition Time (s) | Bare | 6 | 12 | 24 | 48 | 96 |
| Atom (%) | ||||||
| C | 61.70 | 57.00 | 57.60 | 55.70 | 46.30 | 40.40 |
| N | 17.40 | 14.20 | 12.10 | 11.90 | 7.90 | 4.80 |
| O | 20.90 | 19.10 | 19.50 | 17.70 | 14.20 | 10.50 |
| Au | 0.00 | 9.70 | 10.80 | 14.70 | 31.50 | 44.30 |
| Deposition Time (s) * | 13 | 26 | 51 | 77 |
|---|---|---|---|---|
| Ra (nm) | 1.74 | 1.68 | 2.40 | 4.17 |
| Rmax (nm) | 5.46 | 4.98 | 11.08 | 11.70 |
| Film thickness (nm) | 3.99 | 5.10 | 7.98 | 10.37 |
| Deposition Time (s) | Appearance | UTGF Thickness (nm) |
|---|---|---|
| 13 |  | 3.99 |
| 26 |  | 5.10 |
| 51 |  | 7.98 |
| 77 |  | 10.37 |
| Peak Wavenumber (cm−1) | Functional Groups | Designed Bonding and Structure | References |
|---|---|---|---|
| 3301 | Amide A | NH stretching, secondary amine | [22] |
| 3093 | Overtone of Amide II | [23] | |
| 2960 | Amide B | CH2 stretching | [21] |
| 1630 | Amide I | CO stretching | [22] |
| 1547 | Amide II | NH bend/CN stretching | [22] |
| 1449 | Pyrrolidine rings | CH3 asymmetric bending | [48] |
| 1330–1343 | Pyrrolidine rings | CH2 twisting/wagging | [48] |
| 1239 | Amide III | NH bend/CN stretching | [22] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, S.-Y.; Hsieh, P.-Y.; Chung, C.-J.; Chou, C.-M.; He, J.-L. Nanoarchitectonics for Ultrathin Gold Films Deposited on Collagen Fabric by High-Power Impulse Magnetron Sputtering. Nanomaterials 2022, 12, 1627. https://doi.org/10.3390/nano12101627
Huang S-Y, Hsieh P-Y, Chung C-J, Chou C-M, He J-L. Nanoarchitectonics for Ultrathin Gold Films Deposited on Collagen Fabric by High-Power Impulse Magnetron Sputtering. Nanomaterials. 2022; 12(10):1627. https://doi.org/10.3390/nano12101627
Chicago/Turabian StyleHuang, Sheng-Yang, Ping-Yen Hsieh, Chi-Jen Chung, Chia-Man Chou, and Ju-Liang He. 2022. "Nanoarchitectonics for Ultrathin Gold Films Deposited on Collagen Fabric by High-Power Impulse Magnetron Sputtering" Nanomaterials 12, no. 10: 1627. https://doi.org/10.3390/nano12101627
APA StyleHuang, S.-Y., Hsieh, P.-Y., Chung, C.-J., Chou, C.-M., & He, J.-L. (2022). Nanoarchitectonics for Ultrathin Gold Films Deposited on Collagen Fabric by High-Power Impulse Magnetron Sputtering. Nanomaterials, 12(10), 1627. https://doi.org/10.3390/nano12101627