Mechanistic Investigation of the Formation of Nickel Nanocrystallites Embedded in Amorphous Silicon Nitride Nanocomposites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Single Source Precursor for SiNiN System
2.2. Conversion to Ni/Amorphous SiN Composite
2.3. Characterization
3. Results and Discussion
3.1. Chemical Structure of Ni-Modified PHPS
3.2. Chemical Reaction during in Situ Nano Structuring Process
- 480–800 °C: hydrochloric acid (HCl, m/z = 36, 38, Figure 2e).
3.3. Chemical Composition of Nanocomposites
3.4. Mechanistic Investigation of the in Situ Formation of Nanocomposites
4. Conclusions
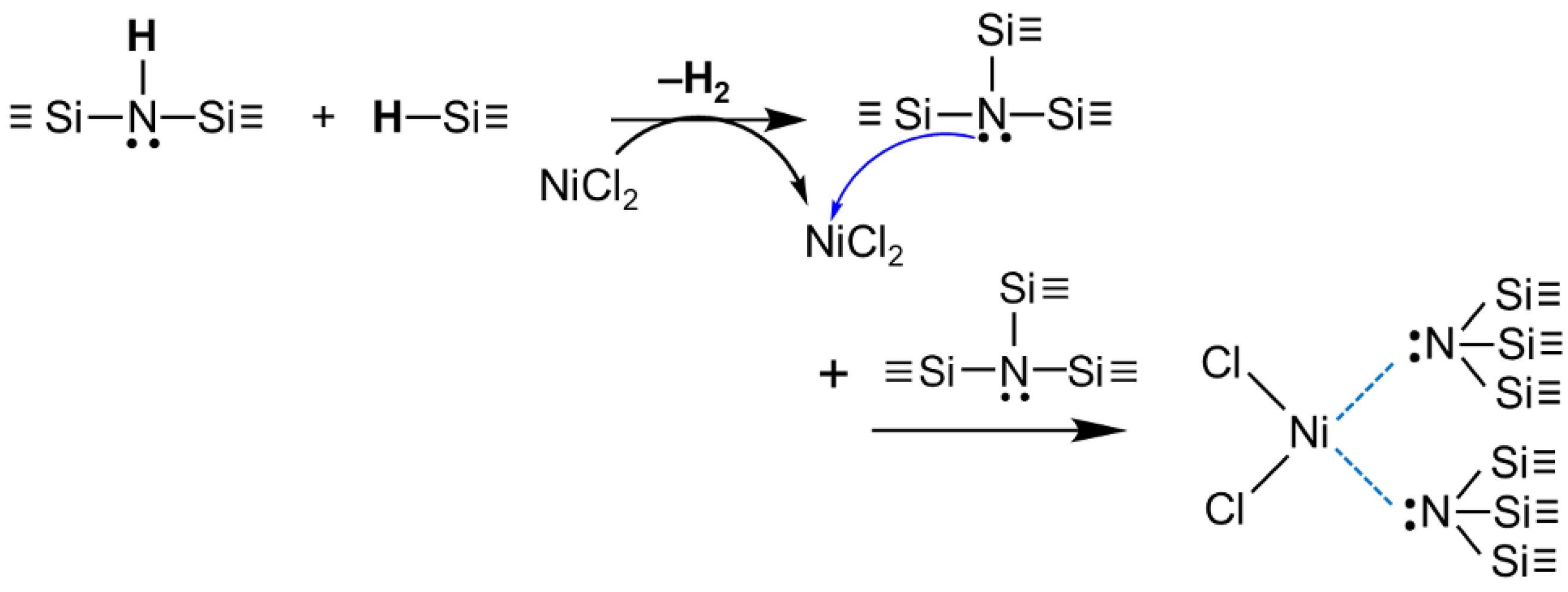
- ATR-FTIR spectroscopic analysis revealed that NiCl2 had high catalytic activity for dehydrocoupling reactions between Si-H and N-H of PHPS under the present precursor synthesis condition conducted in toluene at reflux (~110 °C), leading to the formation of ternary silylamino groups ((≡Si)3N:). Consequently, the ternary silylamino group coordinated the Ni(II) cation of NiCl2 to afford a complex such as the 4-coordinated Ni(II) complex.
- TG-MS and Raman spectroscopic analyses revealed that the Ni-N bond in Ni nitride species is intrinsically formed via the SN2 reaction of the preformed 4-coordinated Ni(II) complex at 200 °C: the nucleophilic attack of the N atom of the silylamino group on the center Ni(II) cation and simultaneous elimination of Cl– as a leaving group. Subsequently, the nucleophilic attack of the released Cl– on other electrophiles, such as Si center of PHPS moieties, would proceed accompanied by the evolution of monochlorosilane, while the N-bonded Ni species subsequently decomposed to give the Ni nitride species.
- XRD, ATR-FTIR, and Raman spectroscopic analyses revealed that Ni nanocrystallites started to form at temperatures as low as 200 to 300 °C through the decomposition reaction of the in situ formed Ni nitride species facilitated by H2, which was generated through the NiCl2-catalyzed dehydrocoupling reaction of PHPS. In addition, this NiCl2-catalyzed dehydrocoupling reaction of PHPS accelerated the polymer to the ceramic conversion of PHPS up to 400 °C.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marakatti, V.S.; Gaigneaux, E.M. Recent Advances in Heterogeneous Catalysis for Ammonia Synthesis. ChemCatChem 2020, 12, 5838–5857. [Google Scholar] [CrossRef]
- Aziz, M.A.A.; Jalil, A.A.; Triwahyono, S.; Ahmad, A. CO2 Methanation over Heterogeneous Catalysts: Recent Progress and Future Prospects. Green Chem. 2015, 17, 2647–2663. [Google Scholar] [CrossRef]
- Sudarsanam, P.; Zhong, R.; van Den Bosch, S.; Coman, S.M.; Parvulescu, V.I.; Sels, B.F. Functionalised Heterogeneous Catalysts for Sustainable Biomass Valorisation. Chem. Soc. Rev. 2018, 47, 8349–8402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gielen, D.; Boshell, F.; Saygin, D. Climate and Energy Challenges for Materials Science. Nat. Mater. 2016, 15, 117–120. [Google Scholar] [CrossRef]
- Colombo, P.; Mera, G.; Riedel, R.; Sorarù, G.D. Polymer-Derived Ceramics: 40 Years of Research and Innovation in Advanced Ceramics. J. Am. Ceram. Soc. 2010, 93, 1805–1837. [Google Scholar] [CrossRef]
- Iwamoto, Y.; Motz, G.; Ionescu, E.; Bernard, S. Preceramic Polymers as Precursors of Advanced Ceramics: The Polymer-Derived Ceramics (PDCs) Route. Encycl. Mater. Tech. Ceram. Glas. 2021, 1, 93–102. [Google Scholar] [CrossRef]
- Viard, A.; Fonblanc, D.; Lopez-Ferber, D.; Schmidt, M.; Lale, A.; Durif, C.; Balestrat, M.; Rossignol, F.; Weinmann, M.; Riedel, R.; et al. Polymer Derived Si–B–C–N Ceramics: 30 Years of Research. Adv. Eng. Mater. 2018, 20, 1–31. [Google Scholar] [CrossRef]
- Zaheer, M.; Hermannsdörfer, J.; Kretschmer, W.P.; Motz, G.; Kempe, R. Robust Heterogeneous Nickel Catalysts with Tailored Porosity for the Selective Hydrogenolysis of Aryl Ethers. ChemCatChem 2014, 6, 91–95. [Google Scholar] [CrossRef]
- Schumacher, D.; Wilhelm, M.; Rezwan, K. Porous SiOC Monoliths with Catalytiac Activity by in Situ Formation of Ni Nanoparticles in Solution-Based Freeze Casting. J. Am. Ceram. Soc. 2020, 103, 2991–3001. [Google Scholar] [CrossRef]
- Bazarjani, M.S.; Kleebe, H.J.; Müller, M.M.; Fasel, C.; Yazdi, M.B.; Gurlo, A.; Riedel, R. Nanoporous Silicon Oxycarbonitride Ceramics Derived from Polysilazanes in Situ Modified with Nickel Nanoparticles. Chem. Mater. 2011, 23, 4112–4123. [Google Scholar] [CrossRef]
- Forberg, D.; Schwob, T.; Kempe, R. Catalytic Condensation for the Formation of Polycyclic Heteroaromatic Compounds. Nat. Commun. 2018, 9, 1751. [Google Scholar] [CrossRef] [PubMed]
- Forberg, D.; Schwob, T.; Zaheer, M.; Friedrich, M.; Miyajima, N.; Kempe, R. Single-Catalyst High-Weight% Hydrogen Storage in an N-Heterocycle Synthesized from Lignin Hydrogenolysis Products and Ammonia. Nat. Commun. 2016, 7, 13201. [Google Scholar] [CrossRef] [PubMed]
- Glatz, G.; Schmalz, T.; Kraus, T.; Haarmann, F.; Motz, G.; Kempe, R. Copper-Containing SiCN Precursor Ceramics (Cu@SiCN) as Selective Hydrocarbon Oxidation Catalysts Using Air as an Oxidant. Chem. A Eur. J. 2010, 16, 4231–4238. [Google Scholar] [CrossRef] [PubMed]
- Zaheer, M.; Schmalz, T.; Motz, G.; Kempe, R. Polymer Derived Non-Oxide Ceramics Modified with Late Transition Metals. Chem. Soc. Rev. 2012, 41, 5102–5116. [Google Scholar] [CrossRef]
- Forberg, D.; Obenauf, J.; Friedrich, M.; Hühne, S.M.; Mader, W.; Motz, G.; Kempe, R. The Synthesis of Pyrroles via Acceptorless Dehydrogenative Condensation of Secondary Alcohols and 1,2-Amino Alcohols Mediated by a Robust and Reusable Catalyst Based on Nanometer-Sized Iridium Particles. Catal. Sci. Technol. 2014, 4, 4188–4192. [Google Scholar] [CrossRef]
- Hahn, G.; Ewert, J.K.; Denner, C.; Tilgner, D.; Kempe, R. A Reusable Mesoporous Nickel Nanocomposite Catalyst for the Selective Hydrogenation of Nitroarenes in the Presence of Sensitive Functional Groups. ChemCatChem 2016, 8, 2461–2465. [Google Scholar] [CrossRef]
- Kamperman, M.; Burns, A.; Weissgraeber, R.; van Vegten, N.; Warren, S.C.; Gruner, S.M.; Baiker, A.; Wiesner, U. Integrating Structure Control over Multiple Length Scales in Porous High Temperature Ceramics with Functional Platinum Nanoparticles. Nano Lett. 2009, 9, 2756–2762. [Google Scholar] [CrossRef]
- Sachau, S.M.; Zaheer, M.; Lale, A.; Friedrich, M.; Denner, C.E.; Demirci, U.B.; Bernard, S.; Motz, G.; Kempe, R. Micro-/Mesoporous Platinum–SiCN Nanocomposite Catalysts (Pt@SiCN): From Design to Catalytic Applications. Chem. A Eur. J. 2016, 22, 15508–15512. [Google Scholar] [CrossRef]
- Schwob, T.; Kempe, R. A Reusable Co Catalyst for the Selective Hydrogenation of Functionalized Nitroarenes and the Direct Synthesis of Imines and Benzimidazoles from Nitroarenes and Aldehydes. Angew. Chem. Int. Ed. 2016, 55, 15175–15179. [Google Scholar] [CrossRef]
- Bäumler, C.; Kempe, R. The Direct Synthesis of Imines, Benzimidazoles and Quinoxalines from Nitroarenes and Carbonyl Compounds by Selective Nitroarene Hydrogenation Employing a Reusable Iron Catalyst. Chem. A Eur. J. 2018, 24, 8989–8993. [Google Scholar] [CrossRef]
- Chen, L.; Qi, Z.; Zhang, S.; Su, J.; Somorjai, G.A. Catalytic Hydrogen Production from Methane: A Review on Recent Progress and Prospect. Catalysts 2020, 10, 858. [Google Scholar] [CrossRef]
- Frontera, P.; Macario, A.; Ferraro, M.; Antonucci, P.L. Supported Catalysts for CO2 Methanation: A Review. Catalysts 2017, 7, 59. [Google Scholar] [CrossRef]
- Kurzina, I.; Aires, F.J.C.S.; Bergeret, G.; Bertolini, J.C. Total Oxidation of Methane over Pd Catalysts Supported on Silicon Nitride: Influence of Support Nature. Chem. Eng. J. 2005, 107, 45–53. [Google Scholar] [CrossRef]
- Shang, R.; Sun, W.; Wang, Y.; Jin, G.Q.; Guo, X.Y. Silicon Nitride Supported Nickel Catalyst for Partial Oxidation of Methane to Syngas. Catal. Commun. 2008, 9, 2103–2106. [Google Scholar] [CrossRef]
- Aires, F.J.C.S.; Bertolini, J.C. On the Use of Silicon Nitride in Catalysis. Top. Catal. 2009, 52, 1492–1505. [Google Scholar] [CrossRef]
- Yan, L.; Liu, J.; Wang, X.; Ma, C.; Zhang, C.; Wang, H.; Wei, Y.; Wen, X.; Yang, Y.; Li, Y. Ru Catalysts Supported by Si3N4 for Fischer-Tropsch Synthesis. Appl. Surf. Sci. 2020, 526, 146631. [Google Scholar] [CrossRef]
- Lale, A.; Mallmann, M.D.; Tada, S.; Bruma, A.; Özkar, S.; Kumar, R.; Haneda, M.; Machado, R.A.F.; Iwamoto, Y.; Demirci, U.B.; et al. Highly Active, Robust and Reusable Micro-/Mesoporous TiN/Si3N4 Nanocomposite-Based Catalysts for Clean Energy: Understanding the Key Role of TiN Nanoclusters and Amorphous Si3N4 Matrix in the Performance of the Catalyst System. Appl. Catal. B Environ. 2020, 272, 118975. [Google Scholar] [CrossRef]
- Hullmann, D.; Wendt, G.; Ziegenbalg, G. Porous Silicon Nitride Materials as Basic Catalysts. Chem. Eng. Technol. 2001, 24, 147–150. [Google Scholar] [CrossRef]
- Lale, A.; Proust, V.; Bechelany, M.C.; Viard, A.; Malo, S.; Bernard, S. A Comprehensive Study on the Influence of the Polyorganosilazane Chemistry and Material Shape on the High Temperature Behavior of Titanium Nitride/Silicon Nitride Nanocomposites. J. Eur. Ceram. Soc. 2017, 37, 5167–5175. [Google Scholar] [CrossRef]
- Balestrat, M.; Lale, A.; Bezerra, A.V.A.; Proust, V.; Awin, E.W.; Machado, R.A.F.; Carles, P.; Kumar, R.; Gervais, C.; Bernard, S. In-Situ Synthesis and Characterization of Nanocomposites in the Si-Ti-N and Si-Ti-C Systems. Molecules 2020, 25, 5236. [Google Scholar] [CrossRef]
- Zhou, C.; Ott, A.; Ishikawa, R.; Ikuhara, Y.; Riedel, R.; Ionescu, E. Single-Source-Precursor Synthesis and High-Temperature Evolution of Novel Mesoporous SiVN(O)-Based Ceramic Nanocomposites. J. Eur. Ceram. Soc. 2020, 40, 6280–6287. [Google Scholar] [CrossRef]
- Tada, S.; Mallmann, M.D.; Takagi, H.; Iihama, J.; Asakuma, N.; Asaka, T.; Daiko, Y.; Honda, S.; Nishihora, R.K.; Machado, R.A.F.; et al. Low Temperature in Situ formation of Cobalt in Silicon Nitride toward Functional Nitride Nanocomposites. Chem. Commun. 2021, 57, 2057–2060. [Google Scholar] [CrossRef] [PubMed]
- Scardera, G.; Puzzer, T.; Conibeer, G.; Green, M.A. Fourier Transform Infrared Spectroscopy of Annealed Silicon-Rich Silicon Nitride Thin Films. J. Appl. Phys. 2008, 104, 104310. [Google Scholar] [CrossRef]
- Hasegawa, S.; Matsuda, M.; Kurata, Y. Si-H and N-H Vibrational Properties in Glow-Discharge Amorphous SiNx:H Films (0<x<1.55). Appl. Phys. Lett. 1990, 57, 2211–2213. [Google Scholar] [CrossRef] [Green Version]
- Clark, T.J.; Lee, K.; Manners, I. Transition-Metal-Catalyzed Dehydrocoupling: A Convenient Route to Bonds between Main-Group Elements. Chem.-A Eur. J. 2006, 12, 8634–8648. [Google Scholar] [CrossRef]
- Lucovsky, G.; Yang, J.; Chao, S.S.; Tyler, J.E.; Czubatyj, W. Nitrogen-Bonding Environments in Glow-Discharge Deposited a-Si:H Films. Phys. Rev. B 1983, 28, 3234–3240. [Google Scholar] [CrossRef]
- Yive, S.C.K.; Corriu, R.J.P.; Leclercq, D.; Mutin, P.H.; Vioux, A. Thermogravimetric Analysis/Mass Spectrometry Investigation of the Thermal Conversion of Organosilicon Precursors into Ceramics under Argon and Ammonia. 2. Poly(silazanes). Chem. Mater. 1992, 4, 1263–1271. [Google Scholar] [CrossRef]
- Yive, S.C.K.; Corriu, R.J.P.; Leclercq, D.; Mutin, P.H.; Vioux, A. Silicon Carbonitride from Polymeric Precursors: Thermal Cross-Linking and Pyrolysis of Oligosilazane Model Compounds. Chem. Mater. 1992, 4, 141–146. [Google Scholar] [CrossRef]
- Loaiza, L.C.; Monconduit, L.; Seznec, V. Siloxene: A Potential Layered Silicon Intercalation Anode for Na, Li and K Ion Batteries. J. Power Sources 2019, 417, 99–107. [Google Scholar] [CrossRef]
- Periasamy, S.; Venkidusamy, S.; Venkatesan, R.; Mayandi, J.; Pearce, J.; Selj, J.H.; Veerabahu, R. Micro-Raman Scattering of Nanoscale Silicon in Amorphous and Porous Silicon. Zeitschrift Phys. Chem. 2017, 231, 1585–1598. [Google Scholar] [CrossRef] [Green Version]
- Feshin, V.P.; Feshina, E.V. Nature of the Coordination Bond in the GeCl4-Trimethylamine Complex. Russ. J. Inorg. Chem. 2014, 59, 1157–1161. [Google Scholar] [CrossRef]
- Karki, B.; Freelon, B.; Rajapakse, M.; Musa, R.; Riyadh, S.S.M.; Morris, B.; Abu, U.O.; Yu, M.; Sumanasekera, G.U.; Jasinski, J.B. Strain-Induced Vibrational Properties of Few Layer Black Phosphorus and MoTe2 via Raman Spectroscopy. Nanotechnology 2020, 31, 425707. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Zou, Y.; Tao, L.; Chen, W.; Zhou, L.; Liu, Z.; Zhou, B.; Huang, G.; Lin, H.; Wang, S. Electrochemical Oxidation of 5-Hydroxymethylfurfural on Nickel Nitride/Carbon Nanosheets: Reaction Pathway Determined by In Situ Sum Frequency Generation Vibrational Spectroscopy. Angew. Chem. 2019, 131, 16042–16050. [Google Scholar] [CrossRef]
- Mueller, T.; Schwertheim, S.; Fahrner, W.R. Crystalline Silicon Surface Passivation by High-Frequency Plasma-Enhanced Chemical-Vapor-Deposited Nanocomposite Silicon Suboxides for Solar Cell Applications. J. Appl. Phys. 2010, 107, 014504. [Google Scholar] [CrossRef]
- Fang, C.M.; Sluiter, M.H.F.; Van Huis, M.A.; Zandbergen, H.W. Structural and Magnetic Properties of NiC x and NiN x (X = 0 to 13) Solid Solutions from First-Principles Calculations. Phys. Rev. B-Condens. Matter Mater. Phys. 2012, 86, 134114. [Google Scholar] [CrossRef] [Green Version]
- Neklyudov, I.M.; Morozov, A.N. Formation and Decay Kinetics of Nickel Nitrides Resulting from Nitrogen Ion Implantation. The Nickel-Nitrogen Phase Diagram. Phys. B Condens. Matter 2004, 350, 325–337. [Google Scholar] [CrossRef]
- Gage, S.H.; Trewyn, B.G.; Ciobanu, C.V.; Pylypenko, S.; Richards, R.M. Synthetic Advancements and Catalytic Applications of Nickel Nitride. Catal. Sci. Technol. 2016, 6, 4059–4076. [Google Scholar] [CrossRef]
- Baiker, A.; Maciejewski, M. Formation and Thermal Stability of Copper and Nickel Nitrides. J. Chem. Soc. Faraday Trans. Phys. Chem. Condens. Phases 1984, 80, 2331–2341. [Google Scholar] [CrossRef]






| Composition/wt% | Composition/at% | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| EDS (Ni/Si, Cl/Si Ratio) | Elemental Analysis /wt% | ||||||||
| Name | Si | Ni | Cl | C | N | O | Atomic ratio to Si | ||
| SiN200 | 71.58 | - | - | 1.01 | 23.65 | 0.33 | Si1C0.03N0.66O0.01 | ||
| SiN300 | 74.29 | - | - | 0.55 | 21.55 | 0.33 | Si1C0.02N0.58O0.01 | ||
| SiN400 | 76.98 | - | - | 1.08 | 18.70 | 0.40 | Si1C0.03N0.49O0.01 | ||
| Ni/SiN200 | 66.32 | 7.94 | 7.11 | 1.05 | 19.99 | 1.67 | Si1Ni0.06Cl0.08C0.04N0.60O0.04 | ||
| Ni/SiN300 | 66.48 | 10.62 | 3.62 | 0.96 | 17.44 | 1.44 | Si1Ni0.08Cl0.04C0.03N0.53O0.04 | ||
| Ni/SiN400 | 68.10 | 11.26 | 2.54 | 1.21 | 15.68 | 1.08 | Si1Ni0.08Cl0.03C0.04N0.46O0.03 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asakuma, N.; Tada, S.; Kawaguchi, E.; Terashima, M.; Honda, S.; Nishihora, R.K.; Carles, P.; Bernard, S.; Iwamoto, Y. Mechanistic Investigation of the Formation of Nickel Nanocrystallites Embedded in Amorphous Silicon Nitride Nanocomposites. Nanomaterials 2022, 12, 1644. https://doi.org/10.3390/nano12101644
Asakuma N, Tada S, Kawaguchi E, Terashima M, Honda S, Nishihora RK, Carles P, Bernard S, Iwamoto Y. Mechanistic Investigation of the Formation of Nickel Nanocrystallites Embedded in Amorphous Silicon Nitride Nanocomposites. Nanomaterials. 2022; 12(10):1644. https://doi.org/10.3390/nano12101644
Chicago/Turabian StyleAsakuma, Norifumi, Shotaro Tada, Erika Kawaguchi, Motoharu Terashima, Sawao Honda, Rafael Kenji Nishihora, Pierre Carles, Samuel Bernard, and Yuji Iwamoto. 2022. "Mechanistic Investigation of the Formation of Nickel Nanocrystallites Embedded in Amorphous Silicon Nitride Nanocomposites" Nanomaterials 12, no. 10: 1644. https://doi.org/10.3390/nano12101644
APA StyleAsakuma, N., Tada, S., Kawaguchi, E., Terashima, M., Honda, S., Nishihora, R. K., Carles, P., Bernard, S., & Iwamoto, Y. (2022). Mechanistic Investigation of the Formation of Nickel Nanocrystallites Embedded in Amorphous Silicon Nitride Nanocomposites. Nanomaterials, 12(10), 1644. https://doi.org/10.3390/nano12101644








