Synthesis of Titanium Nitride Nanoparticles by Pulsed Laser Ablation in Different Aqueous and Organic Solutions
Abstract
:1. Introduction
2. Materials and Methods
2.1. NPs Synthesis
2.2. NPs Characterization
2.3. EDX Data Processing
3. Results and Discussion
3.1. Synthesis of Nanoparticles
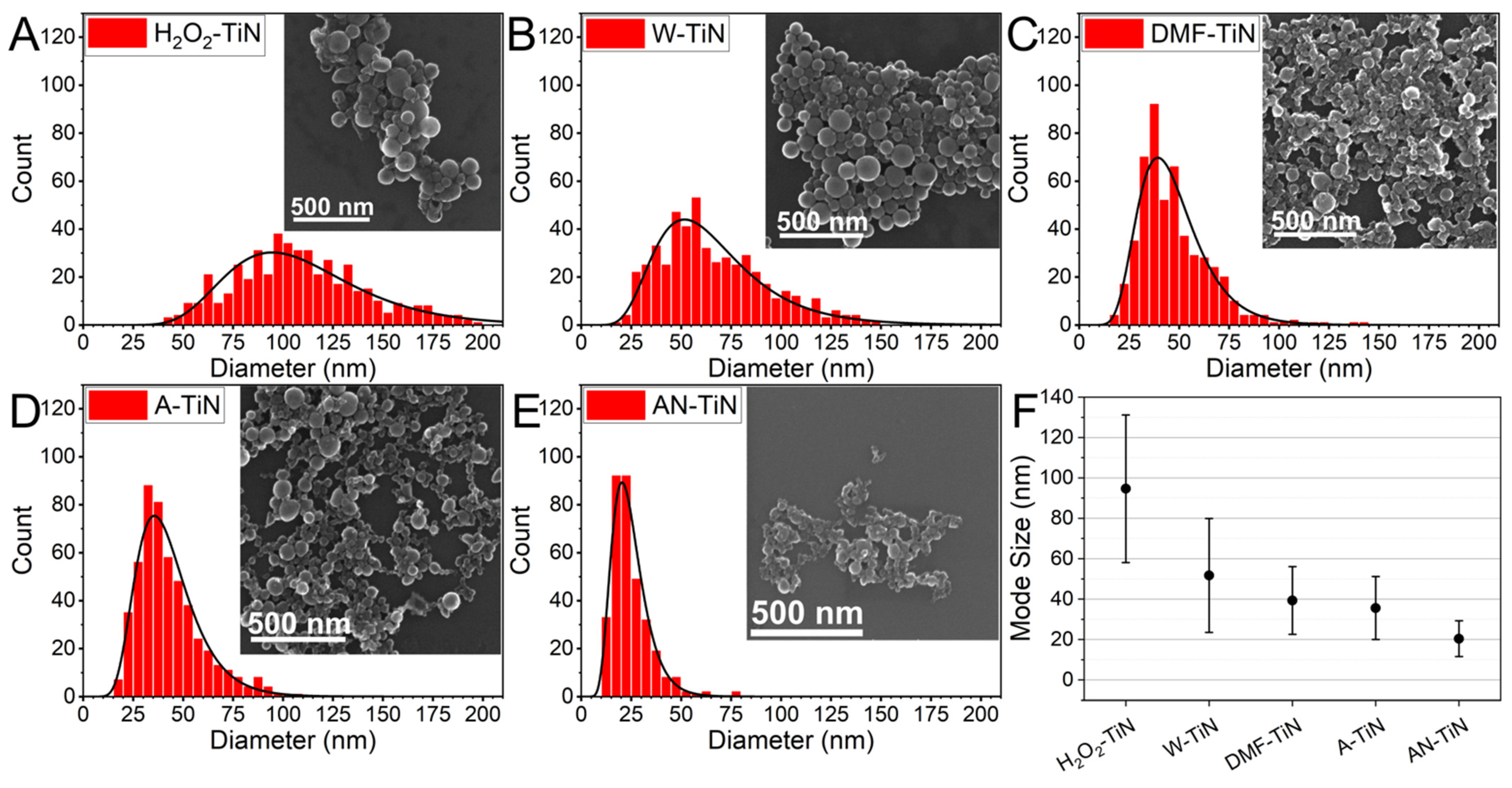
3.2. SEM Characterization
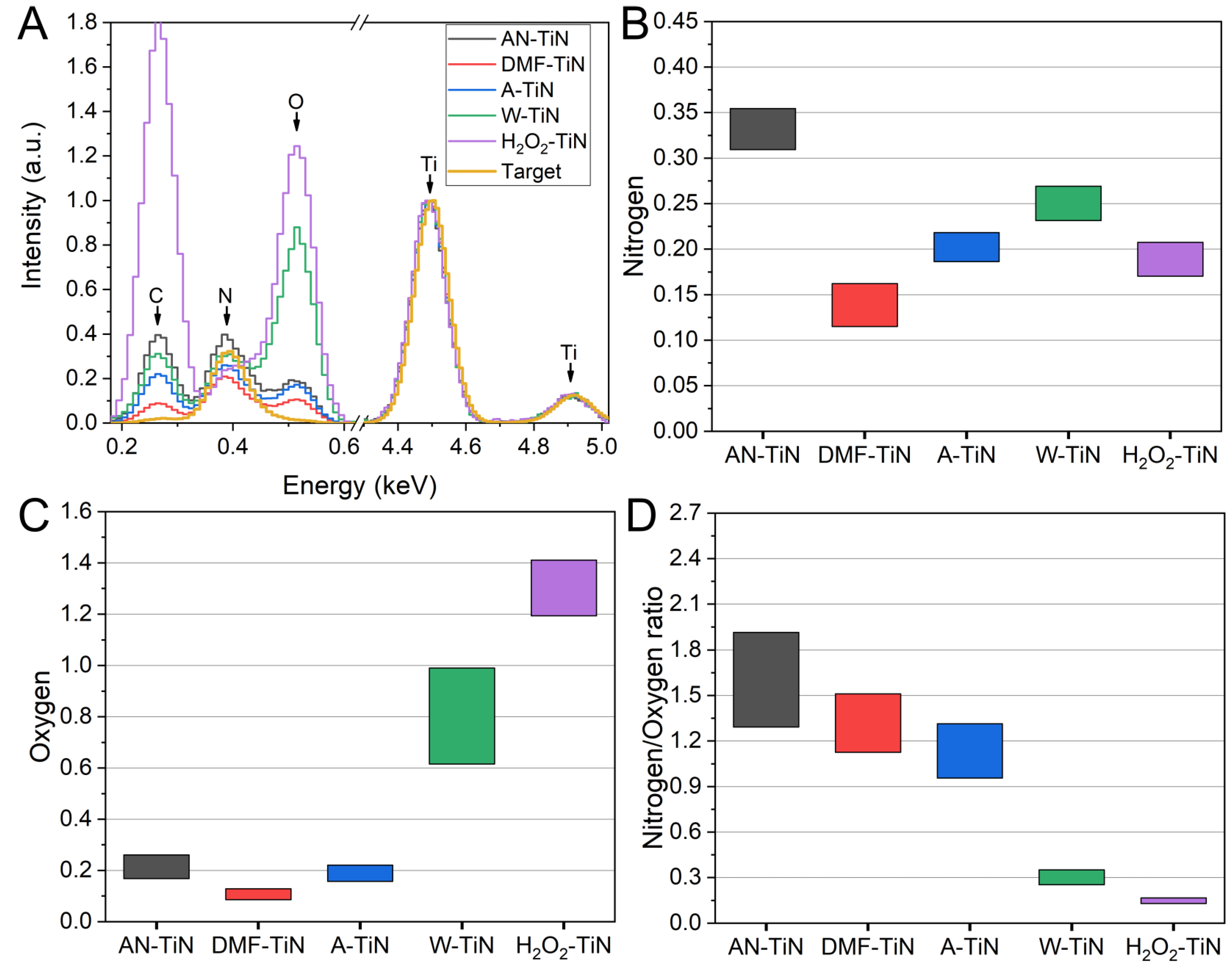
3.3. EDX Characterization
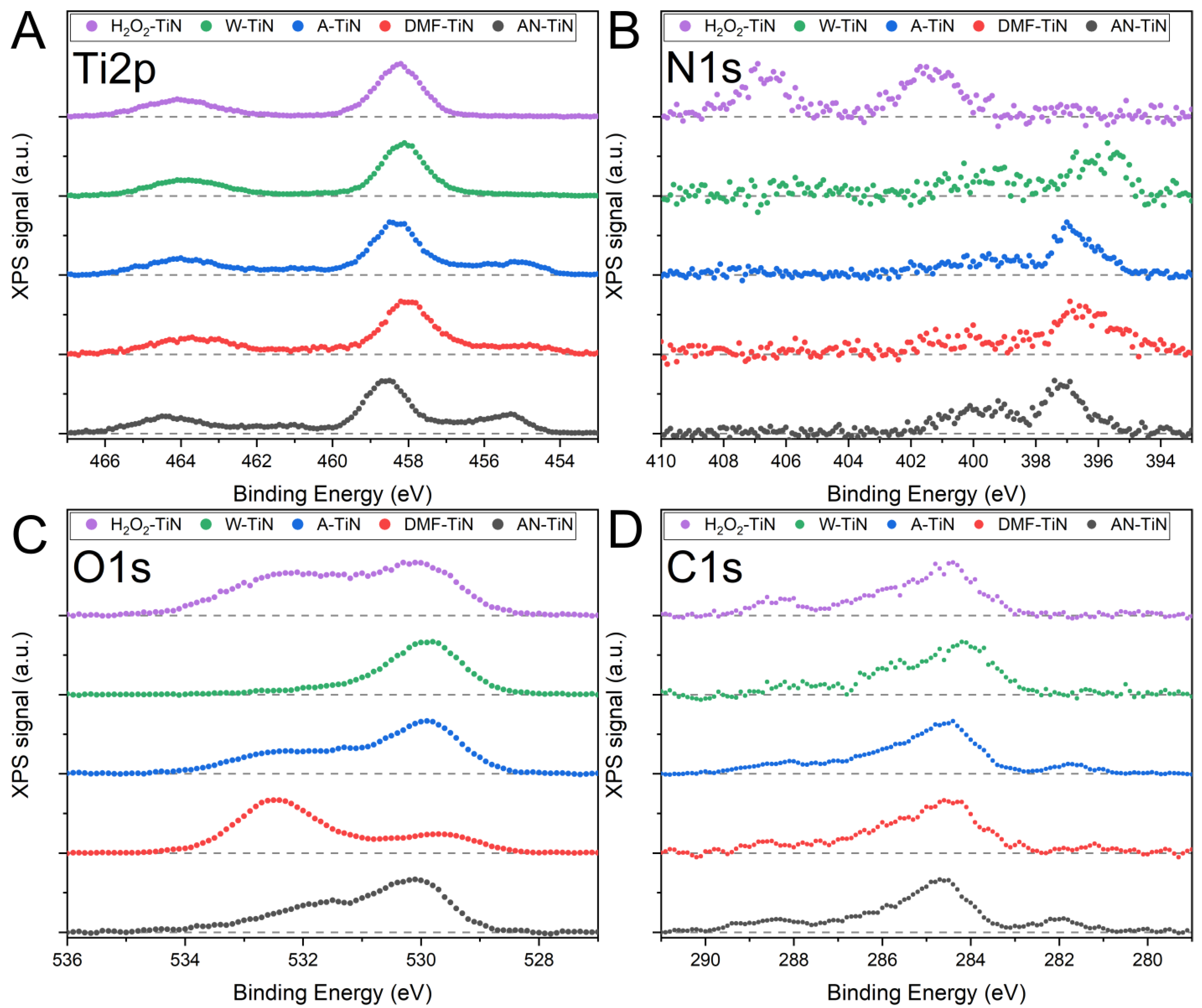
3.4. XPS Characterization
| Ti 2p | ||||||
|---|---|---|---|---|---|---|
| Bond | Sample | Peak Position, eV | Relative Peak Intensity, % | Reference | ||
| 2p3/2 | 2p1/2 | 2p3/2 | 2p1/2 | |||
| TiN | AN-TiN | 455.4 | 461 | 34 | 15 | [27,31,32,33,34,35] |
| DMF-TiN | 454.9 | 460.3 | 16 | 13 | ||
| A-TiN | 455.2 | 460.8 | 25 | 14 | ||
| W-TiN | (454.9) | (459.8) | (3) | (7) | ||
| H2O2-TiN | - | - | - | - | ||
| TiN, TiNO | AN-TiN | 457.1 | 463.3 | 20 | 8 | [31,32,33,34,36] |
| DMF-TiN | 456.8 | 462.8 | 22 | 12 | ||
| A-TiN | 457.1 | 463.1 | 19 | 9 | ||
| W-TiN | (456.9) | (462.7) | (9) | (6) | ||
| H2O2-TiN | - | - | - | - | ||
| TiO2 | AN-TiN | 458.6 | 464.4 | 100 | 26 | [33,37] |
| DMF-TiN | 458.1 | 464 | 100 | 26 | ||
| A-TiN | 458.4 | 464.2 | 100 | 27 | ||
| W-TiN | 458.2 | 464 | 100 | 29 | ||
| H2O2-TiN | 458.2 | 464.1 | 100 | 31 | ||
| N 1s | ||||||
| Bond | Sample | Peak position, eV | Relative peak intensity, % | Reference | ||
| TiN | AN-TiN | 397.1 | 100 | [31,32,33,35,36] | ||
| DMF-TiN | 396.4 | 100 | ||||
| A-TiN | 396.7 | 100 | ||||
| W-TiN | 395.9 | 100 | ||||
| H2O2-TiN | - | - | ||||
| TiNO | AN-TiN | 399.8 | 53 | [31,32,33,35,36] | ||
| DMF-TiN | 399.6 | 30 | ||||
| A-TiN | 399.7 | 35 | ||||
| W-TiN | 399.6 | 47 | ||||
| H2O2-TiN | - | - | ||||
| N2, NOx | AN-TiN | - | - | [33,38,39,40] | ||
| DMF-TiN | - | - | ||||
| A-TiN | - | - | ||||
| W-TiN | - | - | ||||
| H2O2-TiN | 401.4 | 406.7 | 100 | 90 | ||
| O 1s | ||||
| Bond | Sample | Peak Position, eV | Relative Peak Intensity, % | Reference |
| TiO2 | AN-TiN | 530.1 | 100 | [33,36] |
| DMF-TiN | 529.6 | 36 | ||
| A-TiN | 529.9 | 100 | ||
| W-TiN | 529.9 | 100 | ||
| H2O2-TiN | 530 | 100 | ||
| NO, OH− | AN-TiN | 531.6 | 58 | [33,37,39,43] |
| DMF-TiN | 531.6 | 29 | ||
| A-TiN | 531.2 | 21 | ||
| W-TiN | 531.2 | 19 | ||
| H2O2-TiN | 531.2 | 11 | ||
| H2O | AN-TiN | 533.2 | 11 | [33,37] |
| DMF-TiN | 532.6 | 100 | ||
| A-TiN | 532.3 | 43 | ||
| W-TiN | - | |||
| H2O2-TiN | 532.2 | 86 | ||
| C 1s | ||||
| TiC | AN-TiN | 282 | 25 | [41] |
| DMF-TiN | 281.2 | 13 | ||
| A-TiN | 281.7 | 23 | ||
| W-TiN | - | - | ||
| H2O2-TiN | - | - | ||
| CC | AN-TiN | 284.7 | 100 | [44] |
| DMF-TiN | 284.5 | 100 | ||
| A-TiN | 284.5 | 100 | ||
| W-TiN | 284.2 | 100 | ||
| H2O2-TiN | 284.5 | 100 | ||
| CO | AN-TiN | 286.2 | 36 | [44] |
| DMF-TiN | 286.1 | 46 | ||
| A-TiN | 285.7 | 60 | ||
| W-TiN | 285.8 | 53 | ||
| H2O2-TiN | 286.1 | 45 | ||
| C=O | AN-TiN | 288.5 | 25 | |
| DMF-TiN | 288.6 | 21 | ||
| A-TiN | 288.3 | 26 | [44] | |
| W-TiN | 287.9 | 21 | ||
| H2O2-TiN | 288.4 | 34 | ||
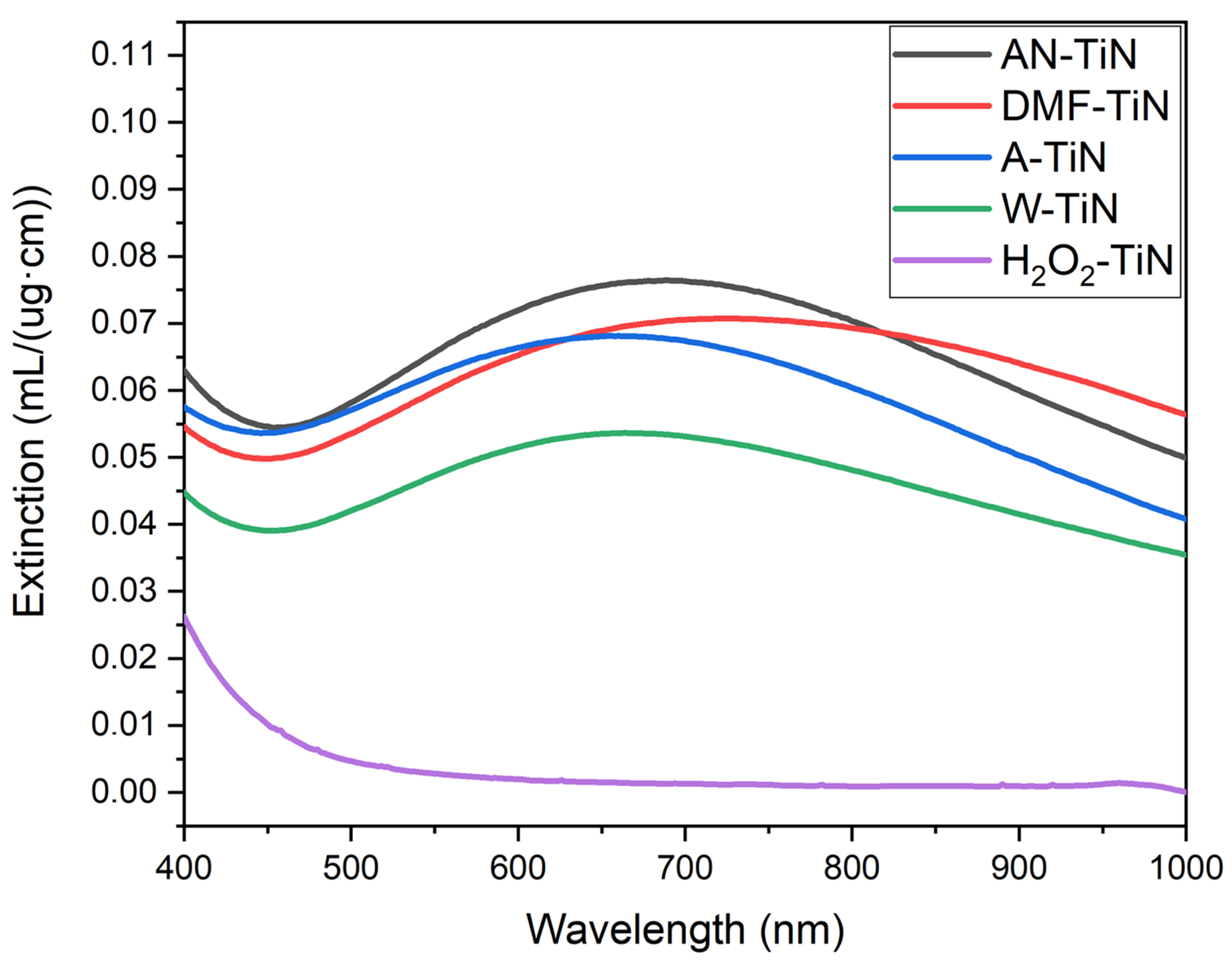
3.5. Optical Characterization
3.6. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Toth, L.E. Transition Metal Carbides and Nitrides; Margrave, J.L., Ed.; Academic Press: New York, NY, USA, 1971. [Google Scholar]
- Boltasseva, A.; Shalaev, V.M. All that glitters need not be gold. Science 2015, 347, 1308–1310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avasarala, B.; Haldar, P. Electrochemical oxidation behavior of titanium nitride based electrocatalysts under PEM fuel cell conditions. Electrochim. Acta 2010, 55, 9024–9034. [Google Scholar] [CrossRef]
- Patsalas, P.; Kalfagiannis, N.; Kassavetis, S. Optical Properties and Plasmonic Performance of Titanium Nitride. Materials 2015, 8, 3128–3154. [Google Scholar] [CrossRef] [Green Version]
- Guler, U.; Ndukaife, J.C.; Naik, G.V.; Nnanna, A.G.A.; Kildishev, A.V.; Shalaev, V.M.; Boltasseva, A. Local heating with lithographically fabricated plasmonic titanium nitride nanoparticles. Nano Lett. 2013, 13, 6078–6083. [Google Scholar] [CrossRef] [PubMed]
- Lalisse, A.; Tessier, G.; Plain, J.; Baffou, G. Plasmonic efficiencies of nanoparticles made of metal nitrides (TiN, ZrN) compared with gold. Sci. Rep. 2016, 6, 38647. [Google Scholar] [CrossRef]
- Wang, C.; Dai, C.; Hu, Z.; Li, H.; Yu, L.; Lin, H.; Bai, J.; Chen, Y. Photonic cancer nanomedicine using the near infrared-II biowindow enabled by biocompatible titanium nitride nanoplatforms. Nanoscale Horiz. 2019, 4, 415–425. [Google Scholar] [CrossRef]
- He, W.; Ai, K.; Jiang, C.; Li, Y.; Song, X.; Lu, L. Plasmonic titanium nitride nanoparticles for in vivo photoacoustic tomography imaging and photothermal cancer therapy. Biomaterials 2017, 132, 37–47. [Google Scholar] [CrossRef]
- Yang, X.; Li, C.; Yang, L.; Yan, Y.; Qian, Y. Reduction-nitridation synthesis of titanium nitride nanocrystals. J. Am. Ceram. Soc. 2003, 86, 206–208. [Google Scholar] [CrossRef]
- Zhang, H.; Li, F.; Jia, Q. Preparation of titanium nitride ultrafine powders by sol–gel and microwave carbothermal reduction nitridation methods. Ceram. Int. 2009, 35, 1071–1075. [Google Scholar] [CrossRef]
- Kumar, S.M.; Murugan, K.; Chandrasekhar, S.B.; Hebalkar, N.; Krishna, M.; Satyanarayana, B.S.; Madras, G. Synthesis and characterization of nano silicon and titanium nitride powders using atmospheric microwave plasma technique. J. Chem. Sci. 2012, 124, 557–563. [Google Scholar] [CrossRef] [Green Version]
- Tavares, J.; Coulombe, S.; Meunier, J.-L. Synthesis of cubic-structured monocrystalline titanium nitride nanoparticles by means of a dual plasma process. J. Phys. D. Appl. Phys. 2009, 42, 102001. [Google Scholar] [CrossRef]
- Fojtik, A.; Henglein, A. Laser Ablation of Films and Suspended Particles in a Solvent: Formation of Cluster and Colloid Solutions. Ber. Bunsenges. Für Phys. Chem. 1993, 97, 252. [Google Scholar] [CrossRef]
- Zhang, D.; Gökce, B.; Barcikowski, S. Laser Synthesis and Processing of Colloids: Fundamentals and Applications. Chem. Rev. 2017, 117, 3990–4103. [Google Scholar] [CrossRef] [PubMed]
- Kabashin, A.V.; Meunier, M. Synthesis of colloidal nanoparticles during femtosecond laser ablation of gold in water. J. Appl. Phys. 2003, 94, 7941. [Google Scholar] [CrossRef] [Green Version]
- Kögler, M.; Ryabchikov, Y.V.; Uusitalo, S.; Popov, A.; Popov, A.; Tselikov, G.; Välimaa, A.-L.; Al-Kattan, A.; Hiltunen, J.; Laitinen, R.; et al. Bare laser-synthesized Au-based nanoparticles as nondisturbing surface-enhanced Raman scattering probes for bacteria identification. J. Biophotonics 2018, 11, e201700225. [Google Scholar] [CrossRef] [Green Version]
- Al-Kattan, A.; Ryabchikov, Y.V.; Baati, T.; Chirvony, V.; Sánchez-Royo, J.F.; Sentis, M.; Braguer, D.; Timoshenko, V.Y.; Estève, M.-A.; Kabashin, A. V Ultrapure laser-synthesized Si nanoparticles with variable oxidation states for biomedical applications. J. Mater. Chem. B 2016, 4, 7852–7858. [Google Scholar] [CrossRef]
- Bulmahn, J.C.; Tikhonowski, G.; Popov, A.A.; Kuzmin, A.; Klimentov, S.M.; Kabashin, A.V.; Prasad, P.N. Laser-Ablative Synthesis of Stable Aqueous Solutions of Elemental Bismuth Nanoparticles for Multimodal Theranostic Applications. Nanomaterials 2020, 10, 1463. [Google Scholar] [CrossRef]
- Popov, A.A.; Tselikov, G.; Dumas, N.; Berard, C.; Metwally, K.; Jones, N.; Al-Kattan, A.; Larrat, B.; Braguer, D.; Mensah, S.; et al. Laser- synthesized TiN nanoparticles as promising plasmonic alternative for biomedical applications. Sci. Rep. 2019, 9, 1194. [Google Scholar] [CrossRef] [Green Version]
- Zelepukin, I.V.; Popov, A.A.; Shipunova, V.O.; Tikhonowski, G.V.; Mirkasymov, A.B.; Popova-Kuznetsova, E.A.; Klimentov, S.M.; Kabashin, A.V.; Deyev, S.M. Laser-synthesized TiN nanoparticles for biomedical applications: Evaluation of safety, biodistribution and pharmacokinetics. Mater. Sci. Eng. C 2021, 120, 111717. [Google Scholar] [CrossRef]
- Maldonado, M.E.; Das, A.; Gomes, A.S.L.; Popov, A.A.; Klimentov, S.M.; Kabashin, A.V. Nonlinear photoacoustic response of suspensions of laser-synthesized plasmonic titanium nitride nanoparticles. Opt. Lett. 2020, 45, 6695. [Google Scholar] [CrossRef]
- Gondal, M.A.; Qahtan, T.F.; Dastageer, M.A.; Saleh, T.A.; Maganda, Y.W.; Anjum, D.H. Effects of oxidizing medium on the composition, morphology and optical properties of copper oxide nanoparticles produced by pulsed laser ablation. Appl. Surf. Sci. 2013, 286, 149–155. [Google Scholar] [CrossRef]
- Tian, F.; Sun, J.; Yang, J.; Wu, P.; Wang, H.-L.; Du, X.-W. Preparation and photocatalytic properties of mixed-phase titania nanospheres by laser ablation. Mater. Lett. 2009, 63, 2384–2386. [Google Scholar] [CrossRef]
- Golightly, J.S.; Castleman, A.W. Analysis of Titanium Nanoparticles Created by Laser Irradiation under Liquid Environments. J. Phys. Chem. B 2006, 110, 19979–19984. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-H.; Chen, S.-Y.; Shen, P. C–H doped anatase nanospheres with disordered shell and planar defects synthesized by pulsed laser ablation of bulk Ti in tetraethyl orthosilicate. CrystEngComm 2014, 16, 2220. [Google Scholar] [CrossRef]
- Li, J.; Gao, L.; Sun, J.; Zhang, Q.; Guo, J.; Yan, D. Synthesis of nanocrystalline titanium nitride powders by direct nitridation of titanium oxide. J. Am. Ceram. Soc. 2001, 84, 3045–3047. [Google Scholar] [CrossRef]
- Alvarez Barragan, A.; Ilawe, N.V.; Zhong, L.; Wong, B.M.; Mangolini, L. A Non-Thermal Plasma Route to Plasmonic TiN Nanoparticles. J. Phys. Chem. C 2017, 121, 2316–2322. [Google Scholar] [CrossRef]
- Tikhonowski, G.V.; Popova-Kuznetsova, E.A.; Aleshchenko, Y.A.; Klimentov, S.M.; Kabashin, A.V.; Popov, A.A. Effect of Oxygen on Colloidal Stability of Titanium Nitride Nanoparticles Synthesized by Laser Ablation in Liquids. Bull. Lebedev Phys. Inst. 2021, 48, 216–220. [Google Scholar] [CrossRef]
- Amendola, V.; Meneghetti, M. What controls the composition and the structure of nanomaterials generated by laser ablation in liquid solution? Phys. Chem. Chem. Phys. 2013, 15, 3027–3046. [Google Scholar] [CrossRef]
- Logothetidis, S.; Meletis, E.I.; Stergioudis, G.; Adjaottor, A.A. Room temperature oxidation behavior of TiN thin films. Thin Solid Film. 1999, 338, 304–313. [Google Scholar] [CrossRef]
- Greczynski, G.; Primetzhofer, D.; Lu, J.; Hultman, L. Core-level spectra and binding energies of transition metal nitrides by non-destructive X-ray photoelectron spectroscopy through capping layers. Appl. Surf. Sci. 2017, 396, 347–358. [Google Scholar] [CrossRef] [Green Version]
- Jaeger, D.; Patscheider, J. A complete and self-consistent evaluation of XPS spectra of TiN. J. Electron Spectros. Relat. Phenom. 2012, 185, 523–534. [Google Scholar] [CrossRef]
- Saha, N.C.; Tompkins, H.G. Titanium nitride oxidation chemistry: An X-ray photoelectron spectroscopy study. J. Appl. Phys. 1992, 72, 3072–3079. [Google Scholar] [CrossRef]
- Porte, L.; Roux, L.; Hanus, J. Vacancy effects in the X-ray photoelectron spectra of TiNx. Phys. Rev. B 1983, 28, 3214–3224. [Google Scholar] [CrossRef]
- Vasile, M.J.; Emerson, A.B.; Baiocchi, F.A. The characterization of titanium nitride by X-ray photoelectron spectroscopy and Rutherford backscattering. J. Vac. Sci. Technol. A Vac. Surf. Film. 1990, 8, 99–105. [Google Scholar] [CrossRef]
- Rizzo, A.; Signore, M.A.; Mirenghi, L.; Di Luccio, T. Synthesis and characterization of titanium and zirconium oxynitride coatings. Thin Solid Film. 2009, 517, 5956–5964. [Google Scholar] [CrossRef]
- Zhu, L.; Lu, Q.; Lv, L.; Wang, Y.; Hu, Y.; Deng, Z.; Lou, Z.; Hou, Y.; Teng, F. Ligand-free rutile and anatase TiO2 nanocrystals as electron extraction layers for high performance inverted polymer solar cells. RSC Adv. 2017, 7, 20084–20092. [Google Scholar] [CrossRef] [Green Version]
- Shinn, N.D.; Tsang, K.-L. Strain-induced surface reactivity: Low temperature Cr/W(110) nitridation. J. Vac. Sci. Technol. A Vac. Surf. Film. 1991, 9, 1558–1562. [Google Scholar] [CrossRef]
- Milosev, I.; Strehblow, H.-H.; Navinsek, B.; Metikos-Hukovic, M. Electrochemical and thermal oxidation of TiN coatings studied by XPS. Surf. Interface Anal. 1995, 23, 529–539. [Google Scholar] [CrossRef]
- Wu, H.Z.; Chou, T.C.; Mishra, A.; Anderson, D.R.; Lampert, J.K.; Gujrathi, S.C. Characterization of titanium nitride thin films. Thin Solid Film. 1990, 191, 55–67. [Google Scholar] [CrossRef]
- Persson, I.; Näslund, L.Å.; Halim, J.; Barsoum, M.W.; Darakchieva, V.; Palisaitis, J.; Rosen, J.; Persson, P.O.Å. On the organization and thermal behavior of functional groups on Ti3C2 MXene surfaces in vacuum. 2D Mater. 2018, 5, 015002. [Google Scholar] [CrossRef]
- Bertóti, I.; Mohai, M.; Sullivan, J.L.; Saied, S.O. Surface characterisation of plasma-nitrided titanium: An XPS study. Appl. Surf. Sci. 1995, 84, 357–371. [Google Scholar] [CrossRef]
- Cheng, X.; Yu, X.; Xing, Z. Characterization and mechanism analysis of N doped TiO2 with visible light response and its enhanced visible activity. Appl. Surf. Sci. 2012, 258, 3244–3248. [Google Scholar] [CrossRef]
- Tang, L.; Wang, Y.; Li, Y.; Feng, H.; Lu, J.; Li, J. Preparation, Structure, and Electrochemical Properties of Reduced Graphene Sheet Films. Adv. Funct. Mater. 2009, 19, 2782–2789. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, C.; Liu, X.; Gu, F.; Jiang, H.; Shao, W.; Zhang, L.; He, Y. Synthesis and optical properties of TiO2 nanoparticles. Mater. Lett. 2007, 61, 79–83. [Google Scholar] [CrossRef]
- Sarycheva, A.; Makaryan, T.; Maleski, K.; Satheeshkumar, E.; Melikyan, A.; Minassian, H.; Yoshimura, M.; Gogotsi, Y. Two-Dimensional Titanium Carbide (MXene) as Surface-Enhanced Raman Scattering Substrate. J. Phys. Chem. C 2017, 121, 19983–19988. [Google Scholar] [CrossRef]
- Robinson, J.T.; Welsher, K.; Tabakman, S.M.; Sherlock, S.P.; Wang, H.; Luong, R.; Dai, H. High performance in vivo near-IR (>1 μm) imaging and photothermal cancer therapy with carbon nanotubes. Nano Res. 2010, 3, 779–793. [Google Scholar] [CrossRef] [Green Version]
- Robinson, J.T.; Tabakman, S.M.; Liang, Y.; Wang, H.; Sanchez Casalongue, H.; Vinh, D.; Dai, H. Ultrasmall Reduced Graphene Oxide with High Near-Infrared Absorbance for Photothermal Therapy. J. Am. Chem. Soc. 2011, 133, 6825–6831. [Google Scholar] [CrossRef]
- Cheng, L.; Liu, J.; Gu, X.; Gong, H.; Shi, X.; Liu, T.; Wang, C.; Wang, X.; Liu, G.; Xing, H.; et al. PEGylated WS2 Nanosheets as a Multifunctional Theranostic Agent for in vivo Dual-Modal CT/Photoacoustic Imaging Guided Photothermal Therapy. Adv. Mater. 2014, 26, 1886–1893. [Google Scholar] [CrossRef]
- Liu, T.; Wang, C.; Gu, X.; Gong, H.; Cheng, L.; Shi, X.; Feng, L.; Sun, B.; Liu, Z. Drug Delivery with PEGylated MoS2 Nano-sheets for Combined Photothermal and Chemotherapy of Cancer. Adv. Mater. 2014, 26, 3433–3440. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popov, A.A.; Tikhonowski, G.V.; Shakhov, P.V.; Popova-Kuznetsova, E.A.; Tselikov, G.I.; Romanov, R.I.; Markeev, A.M.; Klimentov, S.M.; Kabashin, A.V. Synthesis of Titanium Nitride Nanoparticles by Pulsed Laser Ablation in Different Aqueous and Organic Solutions. Nanomaterials 2022, 12, 1672. https://doi.org/10.3390/nano12101672
Popov AA, Tikhonowski GV, Shakhov PV, Popova-Kuznetsova EA, Tselikov GI, Romanov RI, Markeev AM, Klimentov SM, Kabashin AV. Synthesis of Titanium Nitride Nanoparticles by Pulsed Laser Ablation in Different Aqueous and Organic Solutions. Nanomaterials. 2022; 12(10):1672. https://doi.org/10.3390/nano12101672
Chicago/Turabian StylePopov, Anton A., Gleb V. Tikhonowski, Pavel V. Shakhov, Elena A. Popova-Kuznetsova, Gleb I. Tselikov, Roman I. Romanov, Andrey M. Markeev, Sergey M. Klimentov, and Andrei V. Kabashin. 2022. "Synthesis of Titanium Nitride Nanoparticles by Pulsed Laser Ablation in Different Aqueous and Organic Solutions" Nanomaterials 12, no. 10: 1672. https://doi.org/10.3390/nano12101672





