Characteristics and pH-Responsiveness of SDBS–Stabilized Crude Oil/Water Nanoemulsions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Nanoemulsion Formulation
2.3. Long-Term Stability of the Formed Nanoemulsions
2.4. Characteristics of the Formed Nanoemulsions
2.4.1. Droplet Size Measurements
2.4.2. Zeta Potential Measurements
2.4.3. Interfacial Tension Measurements
2.5. Rheology of the Formulated Nanoemulsions
2.6. Demulsification Induced by pH Alteration
3. Results and Discussion
3.1. Average Droplet Size and Size Distribution
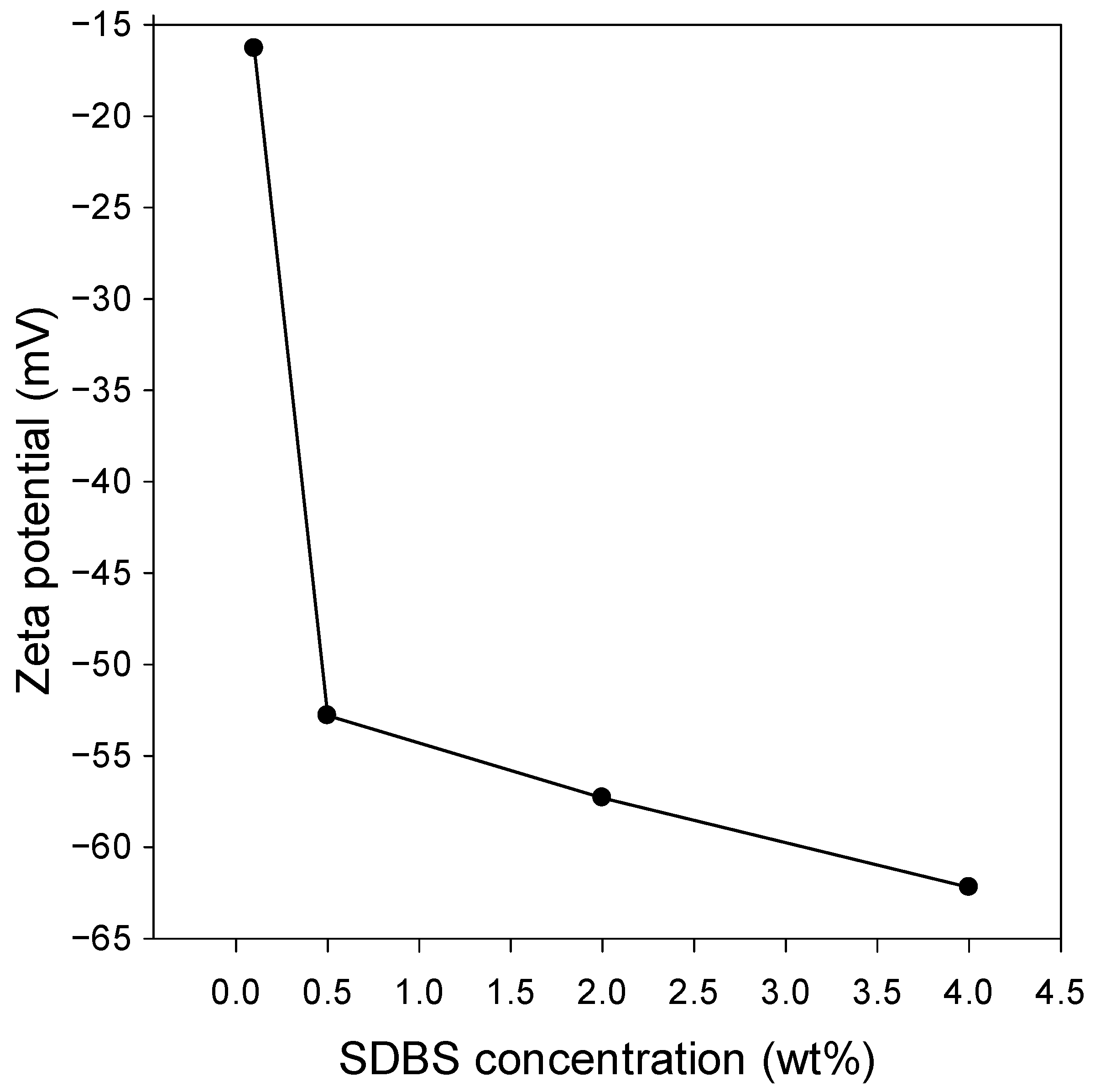
3.2. Zeta Potential
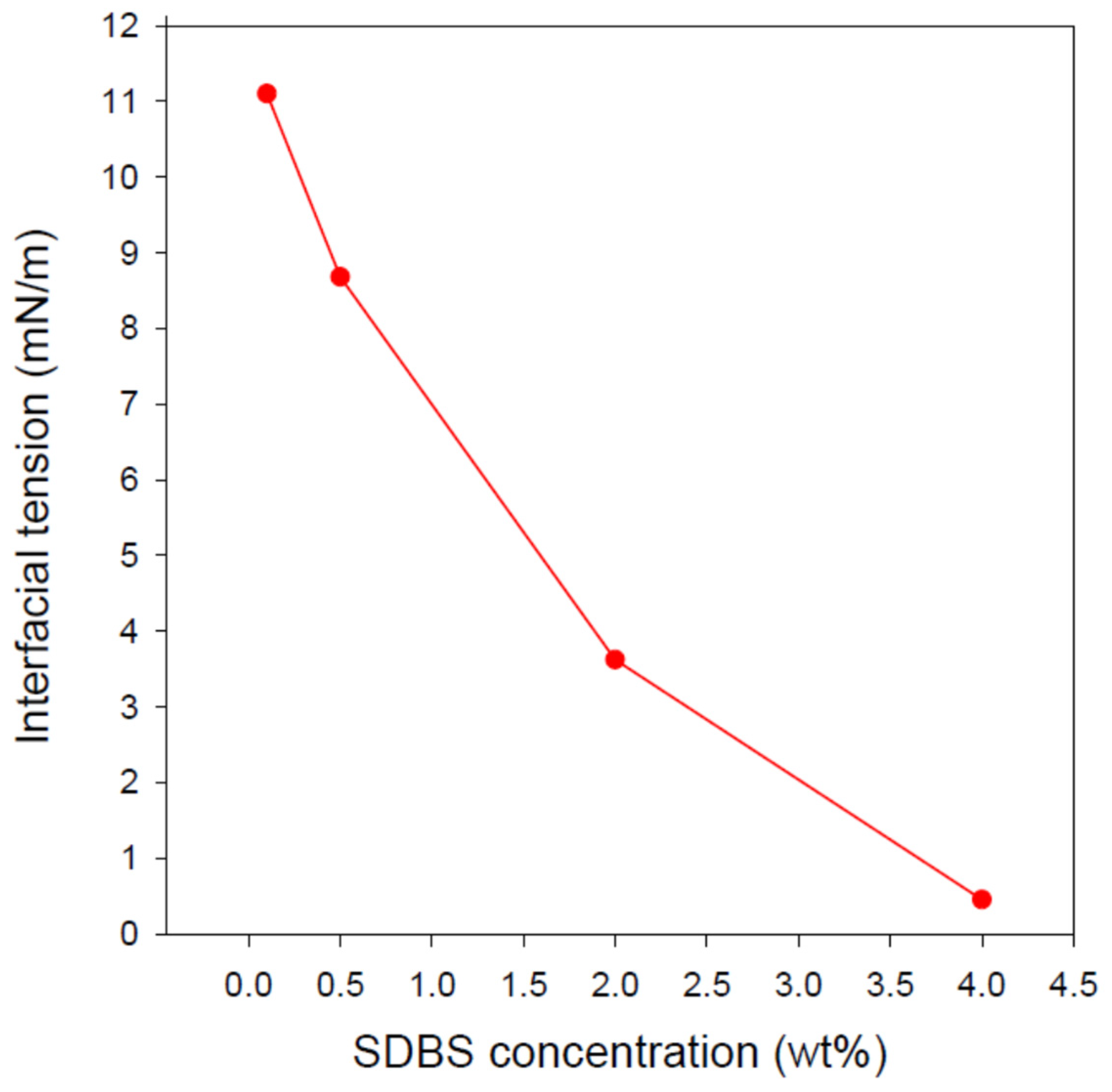
3.3. IFT between the Formulated Nanoemulsions and Diesel
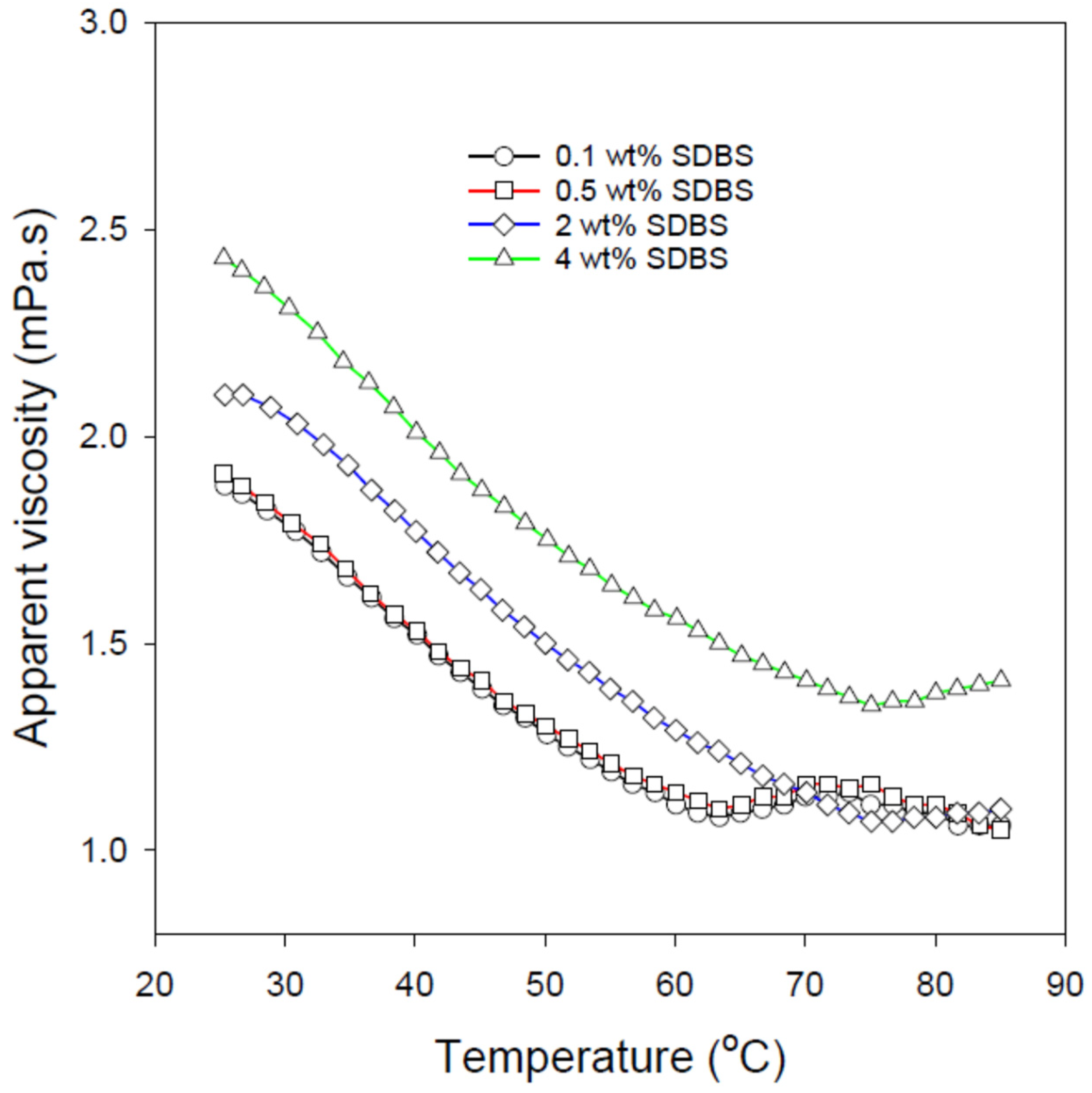
3.4. Rheological Studies
3.5. Emulsion Stability
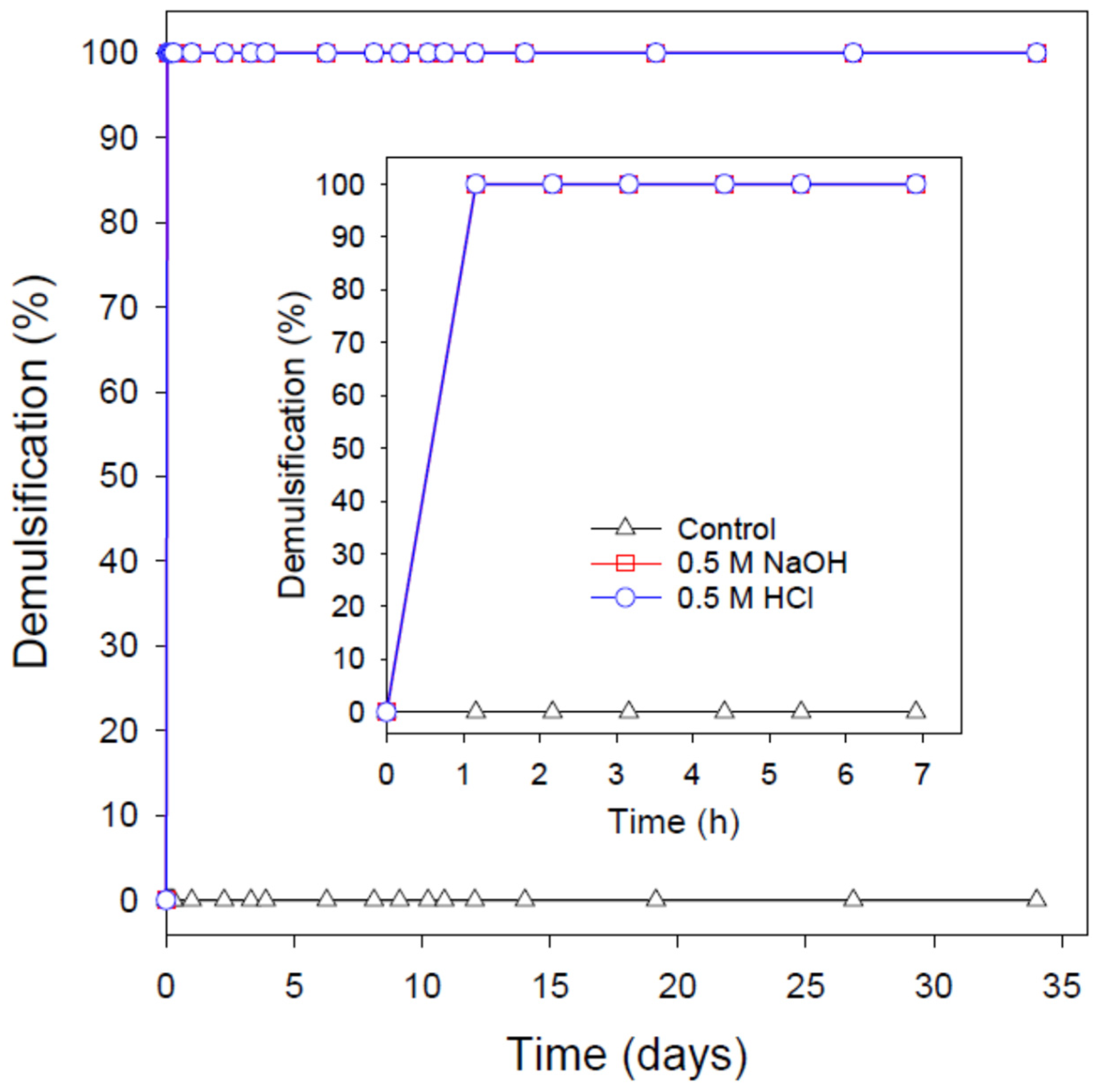
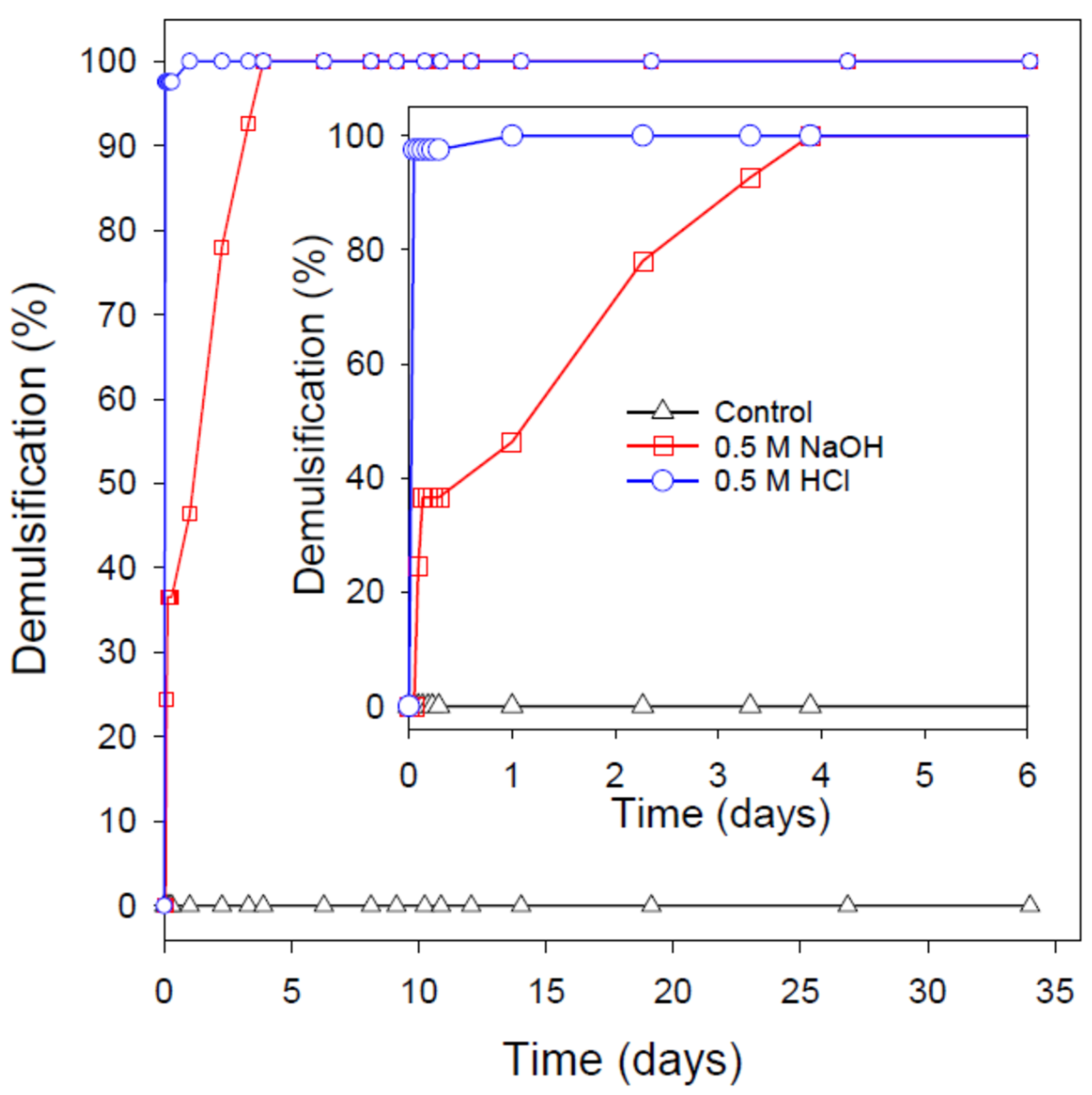
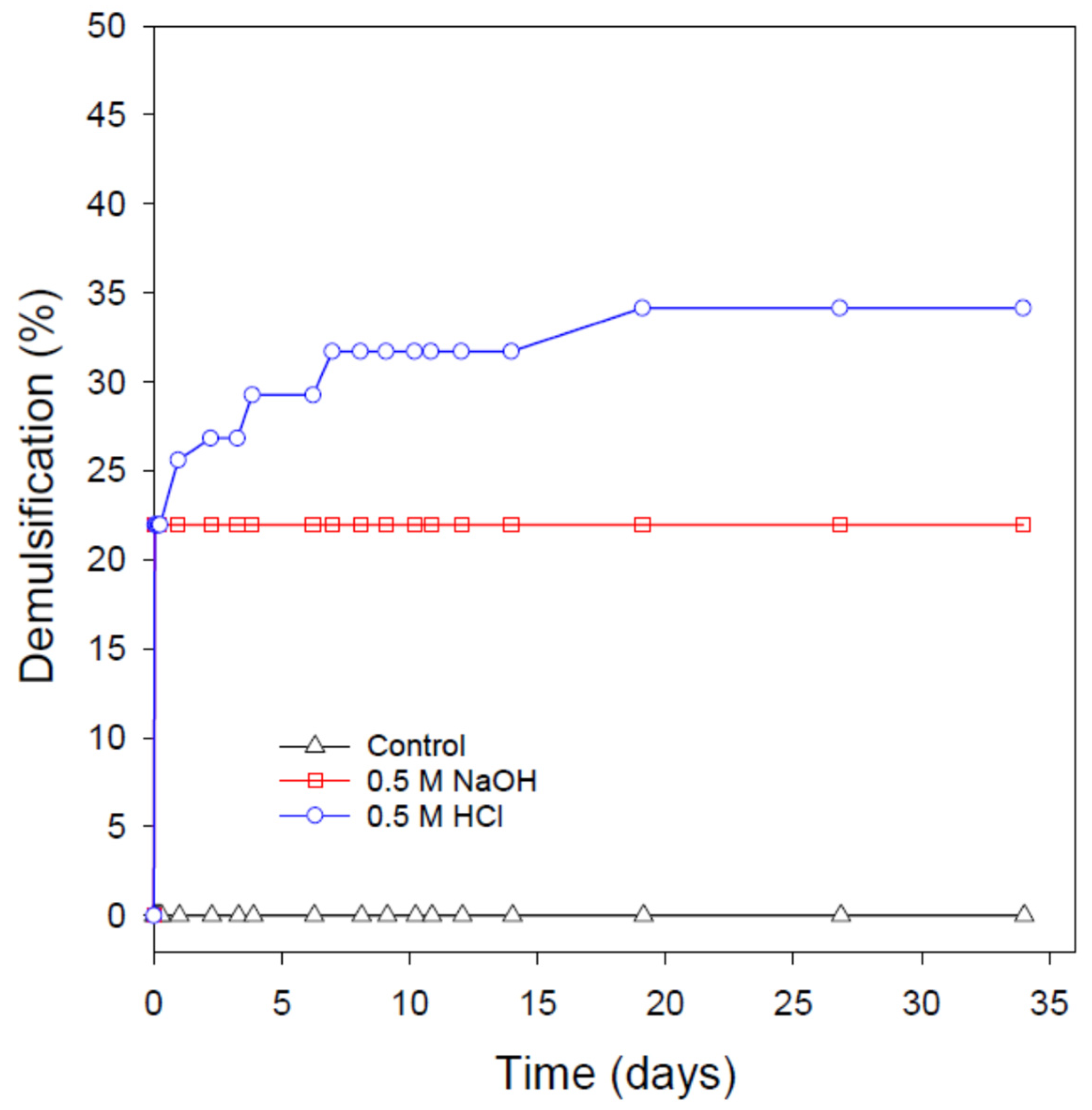
3.6. pH-Induced Destabilization of the Extremely Stable Nanoemulsions
4. Conclusions
Funding
Conflicts of Interest
References
- Kumar, N.; Verma, A.; Mandal, A. Formation, Characteristics and Oil Industry Applications of Nanoemulsions: A Review. J. Pet. Sci. Eng. 2021, 206, 109042. [Google Scholar] [CrossRef]
- Marzuki, N.H.C.; Wahab, R.A.; Hamid, M.A. An Overview of Nanoemulsion: Concepts of Development and Cosmeceutical Applications. Biotechnol. Biotechnol. Equip. 2019, 33, 779–797. [Google Scholar] [CrossRef] [Green Version]
- Onaizi, S.A.; Nasser, M.S.; Twaiq, F.A. Micellization and Interfacial Behavior of a Synthetic Surfactant-Biosurfactant Mixture. Colloids Surf. A Physicochem. Eng. Asp. 2012, 415, 388–393. [Google Scholar] [CrossRef]
- Onaizi, S.A. Enzymatic Treatment of Phenolic Wastewater: Effects of Salinity and Biosurfactant Addition; Society of Petroleum Engineers (SPE): Kuala Lumpur, Malaysia, March 2021. [Google Scholar] [CrossRef]
- Onaizi, S.A. Statistical Analyses of the Effect of Rhamnolipid Biosurfactant Addition on the Enzymatic Removal of Bisphenol A from Wastewater. Biocatal. Agric. Biotechnol. 2021, 32, 101929. [Google Scholar] [CrossRef]
- Onaizi, S.A. Effect of Salinity on the Characteristics, PH-Triggered Demulsification and Rheology of Crude Oil/Water Nanoemulsions. Sep. Purif. Technol. 2022, 281, 119956. [Google Scholar] [CrossRef]
- Onaizi, S.A.; He, L.; Middelberg, A.P.J. Rapid Screening of Surfactant and Biosurfactant Surface Cleaning Performance. Colloids Surf. B Biointerfaces 2009, 72, 68–74. [Google Scholar] [CrossRef]
- Alshabib, M.; Onaizi, S.A. A Review on Phenolic Wastewater Remediation Using Homogeneous and Heterogeneous Enzymatic Processes: Current Status and Potential Challenges. Sep. Purif. Technol. 2019, 219, 186–207. [Google Scholar] [CrossRef]
- He, L.; Malcolm, A.S.; Dimitrijev, M.; Onaizi, S.A.; Shen, H.H.; Holt, S.A.; Dexter, A.F.; Thomas, R.K.; Middelberg, A.P.J. Cooperative Tuneable Interactions between a Designed Peptide Biosurfactant and Positional Isomers of SDOBS at the Air Water Interface. Langmuir 2009, 25, 4021–4026. [Google Scholar] [CrossRef]
- Alshabib, M.; Onaizi, S.A. Enzymatic Remediation of Bisphenol A from Wastewaters: Effects of Biosurfactant, Anionic, Cationic, Nonionic, and Polymeric Additives. Water Air Soil Pollut. 2020, 231, 428. [Google Scholar] [CrossRef]
- Alshabib, M.; Onaizi, S.A. Effects of Surface Active Additives on the Enzymatic Treatment of Phenol and Its Derivatives: A Mini Review. Curr. Pollut. Rep. 2019, 5, 52–65. [Google Scholar] [CrossRef]
- Onaizi, S.A.; Alshabib, M. The Degradation of Bisphenol A by Laccase: Effect of Biosurfactant Addition on the Reaction Kinetics under Various Conditions. Sep. Purif. Technol. 2021, 257, 117785. [Google Scholar] [CrossRef]
- Jiao, J.; Burgess, D.J. Rheology and Stability of Water-in-Oil-in-Water Multiple Emulsions Containing Span 83 and Tween 80. AAPS J. 2003, 5, 7. [Google Scholar] [CrossRef] [PubMed]
- Onaizi, S.A.; Alsulaimani, M.; Al-Sakkaf, M.K.; Bahadi, S.A.; Mahmoud, M.; Alshami, A. Crude Oil/Water Nanoemulsions Stabilized by Biosurfactant: Stability and PH-Switchability. J. Pet. Sci. Eng. 2021, 198, 108173. [Google Scholar] [CrossRef]
- Kumar, N.; Mandal, A. Wettability Alteration of Sandstone Rock by Surfactant Stabilized Nanoemulsion for Enhanced Oil Recovery—A Mechanistic Study. Colloids Surf. A Physicochem. Eng. Asp. 2020, 601, 125043. [Google Scholar] [CrossRef]
- Al-Tayyar, N.A.; Youssef, A.M.; Al-Hindi, R.R. Edible Coatings and Antimicrobial Nanoemulsions for Enhancing Shelf Life and Reducing Foodborne Pathogens of Fruits and Vegetables: A Review. Sustain. Mater. Technol. 2020, 26, e00215. [Google Scholar] [CrossRef]
- Van Tran, V.; Loi Nguyen, T.; Moon, J.Y.; Lee, Y.C. Core-Shell Materials, Lipid Particles and Nanoemulsions, for Delivery of Active Anti-Oxidants in Cosmetics Applications: Challenges and Development Strategies. Chem. Eng. J. 2019, 368, 88–114. [Google Scholar] [CrossRef]
- Rai, V.K.; Mishra, N.; Yadav, K.S.; Yadav, N.P. Nanoemulsion as Pharmaceutical Carrier for Dermal and Transdermal Drug Delivery: Formulation Development, Stability Issues, Basic Considerations and Applications. J. Control. Release 2018, 270, 203–225. [Google Scholar] [CrossRef]
- Nirale, P.; Paul, A.; Yadav, K.S. Nanoemulsions for Targeting the Neurodegenerative Diseases: Alzheimer’s, Parkinson’s and Prion’s. Life Sci. 2020, 245, 117394. [Google Scholar] [CrossRef]
- Pal, N.; Mandal, A. Enhanced Oil Recovery Performance of Gemini Surfactant-Stabilized Nanoemulsions Functionalized with Partially Hydrolyzed Polymer/Silica Nanoparticles. Chem. Eng. Sci. 2020, 226, 115887. [Google Scholar] [CrossRef]
- Penny, G.; Zelenev, A.; Champagne, L.; Proppant, J.C. Proppant and Fluid Selection to Optimize Performance of Horizontal Shale Fracs. In Proceedings of the SPE Hydraulic Fracturing Technology Conference, The Woodlands, TX, USA, 6–8 February 2012. [Google Scholar] [CrossRef]
- Champagne, L.M.; Zhou, H.; Zelenev, A.S.; Lett, N. The Impact of Complex Nanofluid Composition on Enhancing Regained Permeability and Fluid Flowback from Tight Gas Formations and Propped Fractures. In Proceedings of the Proceedings-SPE International Symposium on Formation Damage Control, Lafayette, LA, USA, 6–7 February 2012; Volume 2, pp. 1013–1024. [Google Scholar]
- Braccalenti, E.; Del Gaudio, L.; Belloni, A.; Albonico, P.; Radaelli, E.; Bartosek, M.; Masserano, F. Nanoemulsion Flooding: The Journey to Field Begins; Society of Petroleum Engineers (SPE): Ravenna, Italy, March 2019; Paper Number: OMC-2019-0938. [Google Scholar]
- Braccalenti, E.; Del Gaudio, L.; Belloni, A.; Albonico, P.; Radaelli, E.; Bartosek, M. Enhancing Oil Recovery with Nanoemulsion Flooding; Society of Petroleum Engineers (SPE): Ravenna, Italy, March 2017; Paper Number: OMC-2017-819. [Google Scholar]
- Kumar, N.; Pal, N.; Mandal, A. Nanoemulsion Flooding for Enhanced Oil Recovery: Theoretical Concepts, Numerical Simulation and History Match. J. Pet. Sci. Eng. 2021, 202, 108579. [Google Scholar] [CrossRef]
- See, C.H.; Saphanuchart, W.; Nadarajan, S.; Lim, C.N. Nanoemulsion for Non-Aqueous Mud Removal in Wellbore. In Proceedings of the SPE/DGS Saudi Arabia Section Technical Symposium and Exhibition, Al-Khobar, Saudi Arabia, 4–7 April 2010. [Google Scholar] [CrossRef]
- Al-Anazi, M.S.; Al-Khaldi, M.H.; Fuseni, A.; Al-Marshad, K.M. Use of Nano-Emulsion Surfactants during Hydraulic Fracturing Treatments. In Proceedings of the Abu Dhabi International Petroleum Exhibition and Conference, Abu Dhabi, United Arab Emirates, 10–13 November 2014. [Google Scholar] [CrossRef]
- Luo, M.; Si, X.; Zhang, Y.; Yuan, Z.; Yang, D.; Gong, J. Performance Evaluation of Ater Cowntrol with Nanoemulsion as Prepad Fluid in Hydraulically Fracturing Tight Gas Formations. Energy Fuels 2017, 31, 3698–3707. [Google Scholar] [CrossRef]
- Kumar, S.; Mahto, V. Use of a Novel Surfactant to Prepare Oil-in-Water Emulsion of an Indian Heavy Crude Oil for Pipeline Transportation. Energy Fuels 2017, 31, 12010–12020. [Google Scholar] [CrossRef]
- Penny, G.; Pursley, J.T.; Holcomb, D. Microemulsion Additives Enable Optimized Formation Damage Repair and Prevention. In Proceedings of the SPE International Symposium and Exhibition on Formation Damage Control, Doha, Qatar, 20–22 January 2014. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, F.J. Cause Analysis and Solutions of Water Blocking Damage in Cracked/Non-Cracked Tight Sandstone Gas Reservoirs. Pet. Sci. 2021, 18, 219–233. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, Y.; Chen, G.; Gai, Z. Application of Nanoparticles in Enhanced Oil Recovery: A Critical Review of Recent Progress. Energies 2017, 10, 345. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Zhang, W.; Tang, Y.; Li, B.; Song, Z.; Hou, J. Formation Damage during Alkaline-Surfactant-Polymer Flooding in the Sanan-5 Block of the Daqing Oilfield, China. J. Nat. Gas Sci. Eng. 2016, 35, 826–835. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, J. Process, Mechanism and Impacts of Scale Formation in Alkaline Flooding by a Variable Porosity and Permeability Model. Acta Mech. Sin. Xuebao 2016, 32, 406–421. [Google Scholar] [CrossRef] [Green Version]
- Onaizi, S.A. Demulsification of Crude Oil/Water Nanoemulsions Stabilized by Rhamnolipid Biosurfactant Using Enzymes and PH-Swing. Sep. Purif. Technol. 2021, 259, 118060. [Google Scholar] [CrossRef]
- Al-Sakkaf, M.K.; Onaizi, S.A. Rheology, Characteristics, Stability, and PH-Responsiveness of Biosurfactant-Stabilized Crude Oil/Water Nanoemulsions. Fuel 2022, 307, 121845. [Google Scholar] [CrossRef]
- Onaizi, S.A. Effect of Oil/Water Ratio on Rheological Behavior, Droplet Size, Zeta Potential, Long-Term Stability, and Acid-Induced Demulsification of Crude Oil/Water Nanoemulsions. J. Pet. Sci. Eng. 2022, 209, 109857. [Google Scholar] [CrossRef]
- Bethell, D.; Fessey, R.E.; Namwindwa, E.; Roberts, D.W. The Hydrolysis of C12 Primary Alkyl Sulfates in Concentrated Aqueous Solutions. Part 1. General Features, Kinetic Form and Mode of Catalysis in Sodium Dodecyl Sulfate Hydrolysis. J. Chem. Soc. Perkin Trans. 2001, 2, 1489–1495. [Google Scholar] [CrossRef]
- Motsavage, V.A.; Kostenbauder, H.B. The Influence of the State of Aggregation on the Specific Acid-Catalyzed Hydrolysis of Sodium Dodecyl Sulfate. J. Colloid Sci. 1963, 18, 603–615. [Google Scholar] [CrossRef]
- Nakagaki, M.; Yokoyama, S. Acid-catalyzed Hydrolysis of Sodium Dodecyl Sulfate. J. Pharm. Sci. 1985, 74, 1047–1052. [Google Scholar] [CrossRef] [PubMed]
- Komaiko, J.; McClements, D.J. Low-Energy Formation of Edible Nanoemulsions by Spontaneous Emulsification: Factors Influencing Particle Size. J. Food Eng. 2015, 146, 122–128. [Google Scholar] [CrossRef]
- Kumar, S.; Mahto, V. Emulsification of Indian Heavy Crude Oil Using a Novel Surfactant for Pipeline Transportation. Pet. Sci. 2017, 14, 372–382. [Google Scholar] [CrossRef] [Green Version]
- Kumar, N.; Mandal, A. Experimental Investigation of PEG 6000/Tween 40/SiO2 NPs Stabilized Nanoemulsion Properties: A Versatile Oil Recovery Approach. J. Mol. Liq. 2020, 319, 114087. [Google Scholar] [CrossRef]
- Jadhav, A.J.; Holkar, C.R.; Karekar, S.E.; Pinjari, D.V.; Pandit, A.B. Ultrasound Assisted Manufacturing of Paraffin Wax Nanoemulsions: Process Optimization. Ultrason. Sonochem. 2015, 23, 201–207. [Google Scholar] [CrossRef]
- Kumar, N.; Mandal, A. Oil-in-Water Nanoemulsion Stabilized by Polymeric Surfactant: Characterization and Properties Evaluation for Enhanced Oil Recovery. Eur. Polym. J. 2018, 109, 265–276. [Google Scholar] [CrossRef]
- Kumar, N.; Mandal, A. Surfactant Stabilized Oil-in-Water Nanoemulsion: Stability, Interfacial Tension, and Rheology Study for Enhanced Oil Recovery Application. Energy Fuels 2018, 32, 6452–6466. [Google Scholar] [CrossRef]
- Liu, W.; Sun, D.; Li, C.; Liu, Q.; Xu, J. Formation and Stability of Paraffin Oil-in-Water Nano-Emulsions Prepared by the Emulsion Inversion Point Method. J. Colloid Interface Sci. 2006, 303, 557–563. [Google Scholar] [CrossRef]
- Onaizi, S.A.; Nasser, M.S.; Al-Lagtah, N.M.A. Self-Assembly of a Surfactin Nanolayer at Solid–Liquid and Air–Liquid Interfaces. Eur. Biophys. J. 2016, 45, 331–339. [Google Scholar] [CrossRef]
- Onaizi, S.A.; Nasser, M.S.; Al-Lagtah, N.M.A. Adsorption of an Anionic Surfactant at Air-Liquid and Different Solid-Liquid Interfaces from Solutions Containing High Counter-Ion Concentration. Colloid Polym. Sci. 2015, 293, 507–516. [Google Scholar] [CrossRef]
- Onaizi, S.A.; Malcolm, A.S.; He, L.; Middelberg, A.P.J. Directed Disassembly of an Interfacial Rubisco Protein Network. Langmuir 2007, 23, 6336–6341. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Onaizi, S.A.; Dimitrijev-Dwyer, M.; Malcolm, A.S.; Shen, H.H.; Dong, C.; Holt, S.A.; Thomas, R.K.; Middelberg, A.P.J. Comparison of Positional Surfactant Isomers for Displacement of Rubisco Protein from the Air-Water Interface. J. Colloid Interface Sci. 2011, 360, 617–622. [Google Scholar] [CrossRef] [PubMed]
- Onaizi, S.A.; Nasser, M.S.; Al-Lagtah, N.M.A. Benchmarking the Self-Assembly of Surfactin Biosurfactant at the Liquid–Air Interface to Those of Synthetic Surfactants. J. Surfactants Deterg. 2016, 19, 645–652. [Google Scholar] [CrossRef] [Green Version]
- Onaizi, S.A. Dynamic Surface Tension and Adsorption Mechanism of Surfactin Biosurfactant at the Air–Water Interface. Eur. Biophys. J. 2018, 47, 631–640. [Google Scholar] [CrossRef]
- Jia, H.; Wu, H.; Wei, X.; Han, Y.; Wang, Q.; Song, J.; Dai, J.; Yan, H.; Liu, D. Investigation on the Effects of AlOOH Nanoparticles on Sodium Dodecylbenzenesulfonate Stabilized o/w Emulsion Stability for EOR. Colloids Surfaces A Physicochem. Eng. Asp. 2020, 603, 125278. [Google Scholar] [CrossRef]
- Kumar, S.; Mahto, V. Emulsification of Indian Heavy Crude Oil in Water for Its Efficient Transportation through Offshore Pipelines. Chem. Eng. Res. Des. 2016, 115, 34–43. [Google Scholar] [CrossRef]
- Almarouf, H.S.; Nasser, M.S.; Al-Marri, M.J.; Khraisheh, M.; Onaizi, S.A. Demulsification of Stable Emulsions from Produced Water Using a Phase Separator with Inclined Parallel Arc Coalescing Plates. J. Pet. Sci. Eng. 2015, 135, 16–21. [Google Scholar] [CrossRef]
- Ha, J.W.; Yang, S.M. Breakup of a Multiple Emulsion Drop in a Uniform Electric Field. J. Colloid Interface Sci. 1999, 213, 92–100. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Wang, J.; Wang, J. Effects of Mixed Anionic-Cationic Surfactants and Alcohol on Solubilization of Water-in-Oil Microemulsions. J. Dispers. Sci. Technol. 1999, 20, 993–1007. [Google Scholar] [CrossRef]
- Marchese, J.; Ochoa, N.A.; Pagliero, C.; Almandoz, C. Pilot-Scale Ultrafiltration of an Emulsified Oil Wastewater. Environ. Sci. Technol. 2000, 34, 2990–2996. [Google Scholar] [CrossRef]
- Opawale, F.O.; Burgess, D.J. Influence of Interfacial Rheological Properties of Mixed Emulsifier Films on the Stability of Water-in-Oil-in-Water Emulsions. J. Pharm. Pharmacol. 1998, 50, 965–973. [Google Scholar] [CrossRef] [PubMed]
- Long, X.; He, N.; He, Y.; Jiang, J.; Wu, T. Biosurfactant Surfactin with PH-Regulated Emulsification Activity for Efficient Oil Separation When Used as Emulsifier. Bioresour. Technol. 2017, 241, 1027–1035. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zu, Y.; Zhu, J.; Jin, M.; Cui, T.; Long, X. Application of Biosurfactant Surfactin as a PH-Switchable Biodemulsifier for Efficient Oil Recovery from Waste Crude Oil. Chemosphere 2019, 240, 124946. [Google Scholar] [CrossRef] [PubMed]
- Dorigon, L.; Ruiz de Almeida da Frota, J.P.; Kreutz, J.C.; Mello Giona, R.; Pereira Moisés, M.; Bail, A. Synthesis and Characterization of Mesoporous Silica-Coated Magnetite Containing Cetyltrimethylammonium Bromide and Evaluation on the Adsorption of Sodium Dodecylbenzenesulfonate. Appl. Surf. Sci. 2017, 420, 954–962. [Google Scholar] [CrossRef]
- Ren, X.; Zhang, Y. Switching Pickering Emulsion Stabilized by Chitosan-SDS Complexes through Ion Competition. Colloids Surfaces A Physicochem. Eng. Asp. 2020, 587, 124316. [Google Scholar] [CrossRef]
- Liu, K.; Jiang, J.; Cui, Z.; Binks, B.P. PH-Responsive Pickering Emulsions Stabilized by Silica Nanoparticles in Combination with a Conventional Zwitterionic Surfactant. Langmuir 2017, 33, 2296–2305. [Google Scholar] [CrossRef]
- Lin, Q.; Liu, K.H.; Cui, Z.G.; Pei, X.M.; Jiang, J.Z.; Song, B.L. PH-Responsive Pickering Foams Stabilized by Silica Nanoparticles in Combination with Trace Amount of Dodecyl Dimethyl Carboxyl Betaine. Colloids Surfaces A Physicochem. Eng. Asp. 2018, 544, 44–52. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Onaizi, S.A. Characteristics and pH-Responsiveness of SDBS–Stabilized Crude Oil/Water Nanoemulsions. Nanomaterials 2022, 12, 1673. https://doi.org/10.3390/nano12101673
Onaizi SA. Characteristics and pH-Responsiveness of SDBS–Stabilized Crude Oil/Water Nanoemulsions. Nanomaterials. 2022; 12(10):1673. https://doi.org/10.3390/nano12101673
Chicago/Turabian StyleOnaizi, Sagheer A. 2022. "Characteristics and pH-Responsiveness of SDBS–Stabilized Crude Oil/Water Nanoemulsions" Nanomaterials 12, no. 10: 1673. https://doi.org/10.3390/nano12101673





