Theoretical Study on Electronic, Magnetic and Optical Properties of Non-Metal Atoms Adsorbed onto Germanium Carbide
Abstract
:1. Introduction
2. Computational Methods and Theoretics
3. Results and Discussion
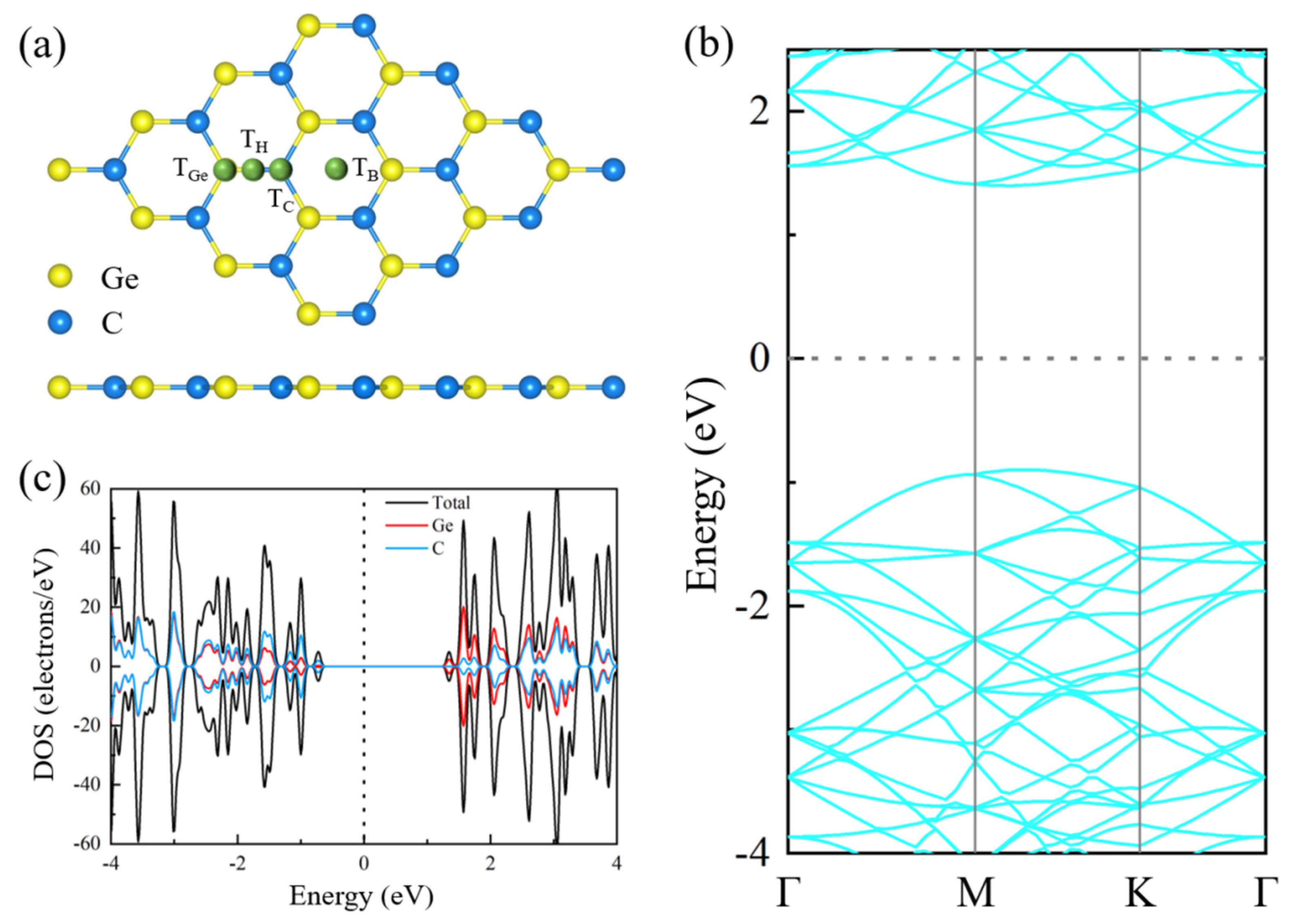
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Katsnelson, M.I.; Grigorieva, I.V.; Dubonos, S.V.; Firsov, A.A. Two-dimensional gas of massless Dirac fermions in graphene. Nature 2005, 438, 197–200. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Kaner, R.B. Graphene-based materials. Science 2008, 320, 1170–1171. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurthy, M.; Drucker, J.S.; Challa, A. Epitaxial growth and characterization of Ge1-xCx alloys on Si (100). J. Appl. Phys. 1995, 78, 7070–7073. [Google Scholar] [CrossRef]
- Mélinon, P.; Masenelli, B.; Tournus, F.; Perez, A. Playing with carbon and silicon at the nanoscale. Nat. Mater. 2007, 6, 479–490. [Google Scholar] [CrossRef]
- Pino-Rios, R.; Chigo-Anota, E.; Shakerzadeh, E.; Cardenas-Jiron, G. B12N12 cluster as a collector of noble gases: A quantum chemical study. Phys. E. 2020, 115, 113697. [Google Scholar] [CrossRef]
- Miró, P.; Audiffred, M.; Heine, T. An atlas of two-dimensional materials. Chem. Soc. Rev. 2014, 43, 6537–6554. [Google Scholar] [CrossRef]
- Cui, Z.; Wang, M.J.; Lyu, N.; Zhang, S.; Ding, Y.C.; Bai, K.F. Electronic, magnetism and optical properties of transition metals adsorbed puckered arsenene. Superlattices Microstruct. 2021, 152, 106852. [Google Scholar] [CrossRef]
- Sun, M.; Tang, W.; Li, S.; Chou, J.-P.; Hu, A.; Schwingenschlögl, U. Molecular doping of blue phosphorene: A first-principles investigation. J. Phys. Condens. Matter 2019, 32, 055501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Q.H.; Kalantar-Zadeh, K.; Kis, A.; Coleman, J.N.; Strano, M.S. Electronics and optoelectronics of two-dimensional transition metal dichalcogenides. Nat. Nanotechnol. 2012, 7, 699–712. [Google Scholar] [CrossRef]
- Cui, Z.; Wang, X.; Ding, Y.C.; Li, E.L.; Bai, K.F.; Zheng, J.S.; Liu, T. Adsorption of CO, NH3, NO, and NO2 on pristine and defective g-GaN: Improved gas sensing and functionalization. Appl. Surf. Sci. 2020, 530, 147275. [Google Scholar] [CrossRef]
- Sun, M.L.; Chou, J.P.; Ren, Q.; Zhao, Y.; Yu, J.; Tang, W. Tunable Schottky barrier in van der Waals heterostructures of graphene and g-GaN. Appl. Phys. Lett. 2017, 110, 173105. [Google Scholar] [CrossRef]
- Ferdous, N.; Islam, S.; Park, J.; Hashimoto, A. Tunable electronic properties in stanene and two dimensional silicon-carbide heterobilayer: A first principles investigation. AIP Adv. 2019, 9, 025120. [Google Scholar] [CrossRef] [Green Version]
- Ravindra, P.; Rerat, M.; Darrigan, C.; Causa, M. A theoretical study of stability, electronic, and optical properties of GeC and SnC. J. Appl. Phys. 2000, 88, 6462–6466. [Google Scholar]
- Ghojavand, A.; Hashemifar, S.J.; Ahmadpour, M.T.; Shapeev, A.V.; Alhaji, A.; Hassanzada, Q. Ab initio analysis of structural and electronic properties and excitonic optical responses of eight Ge-based 2D materials. J. Appl. Phys. 2020, 127, 214301. [Google Scholar] [CrossRef]
- Li, D.; Li, S.; Zhong, C.; He, J. Tuning magnetism at the two-dimensional limit: A theoretical perspective. Nanoscale 2021, 13, 19812–19827. [Google Scholar] [CrossRef]
- Yuan, J.; Cai, Y.; Shen, L.; Xiao, Y.; Ren, J.-C.; Wang, A.; Feng, Y.P.; Yan, X. One-dimensional thermoelectrics induced by Rashba spin-orbit coupling in two-dimensional BiSb monolayer. Nano Energy 2018, 52, 163–170. [Google Scholar] [CrossRef]
- Komsa, H.; Kotakoski, J.; Kurasch, S.; Lehtinen, O.; Kaise, U.; Krasheninnikov, A. Two-dimensional transition metal dichalcogenides under electron irradiation: Defect production and doping. Phys. Rev. Lett. 2012, 109, 035503. [Google Scholar] [CrossRef]
- Sun, M.L.; Schwingenschlögl, U. Unique omnidirectional negative Poisson’s ratio in δ-phase carbon monocalclgenides. J. Phys. Chem. C 2021, 125, 4133–4138. [Google Scholar] [CrossRef]
- Bratschitsch, R. Optoelectronic devices: Monolayer diodes light up. Nat. Nanotechnol. 2014, 9, 247–248. [Google Scholar] [CrossRef]
- Sun, M.L.; Yi, L.; Yuan, Y.; Schwingenschlögl, U. Ultrahigh carrier mobility in the two dimensional semiconductors B8Si4, B8Ge4, and B8Sn4. Chem. Mater. 2021, 33, 6475–6483. [Google Scholar] [CrossRef]
- Sun, M.L.; Schwingenschlögl, U. δ-CS: A direct-band-gap semiconductor combining auxeticity, ferroelasticity, and potential for high-efficiency solar cells. Phys. Rev. Appl. 2020, 14, 044015. [Google Scholar] [CrossRef]
- Pospischil, A.; Furchi, M.M.; Mueller, T. Solar-energy conversion and light emission in an atomic monolayer p–n diode. Nat. Nanotechnol. 2014, 9, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Schwingenschlögl, U. B2P6: A Two-Dimensional Anisotropic Janus Material with Potential in Photocatalytic Water Splitting and Metal-Ion Batteries. Chem. Mater. 2020, 32, 4795–4800. [Google Scholar] [CrossRef]
- Luo, Y.; Ren, C.; Xu, Y.; Yu, J.; Wang, S.; Sun, M.L. A first principles investigation on the structural, mechanical, electronic, and catalytic properties of biphenylene. Sci. Rep. 2021, 11, 19008. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Ren, C.D.; Tian, H.Y.; Yu, J.; Sun, M. MoS2/ZnO van der Waals heterostructure as a high-efficiency water splitting photocatalyst: A first-principles study. Phys. Chem. Chem. Phys. 2018, 20, 13394–13399. [Google Scholar] [CrossRef]
- Cui, Z.; Bai, K.; Ding, Y.; Wang, X.; Li, E.; Zheng, J.; Wang, S. Electronic and optical properties of janus MoSSe and ZnO vdWs heterostructures. Superlattices Microstruct. 2020, 140, 106445. [Google Scholar] [CrossRef]
- Sun, S.Y.; Hussain, T.; Zhang, W.; Karton, A. Blue phosphorene monolayers as potential nano sensors for volatile organic compounds under point defects. Appl. Surf. Sci. 2019, 486, 52–57. [Google Scholar] [CrossRef]
- Kooti, M.; Keshtkar, S.; Askarieh, M.; Rashidi, A. Progress toward a novel methane gas sensor based on SnO2 nanorods-nanoporous graphene hybrid. Sens. Actuator B Chem. 2019, 281, 96–106. [Google Scholar] [CrossRef]
- Abdullah, Q.; Yam, F.; Hassan, Z.; Bououdina, M. Hydrogen gas sensing performance of GaN nanowires-based sensor at low operating temperature. Sens. Actuators B Chem. 2014, 204, 497–506. [Google Scholar] [CrossRef]
- Peng, Q.; Liang, C.; Ji, W.; De, S. A first-principles study of the mechanical properties of g-GeC. Mech. Mater. 2013, 64, 135–141. [Google Scholar] [CrossRef]
- Pan, L.; Liu, H.; Wen, Y.; Tan, X.; Lv, H.; Shi, J.; Tang, X. First-principles study of monolayer and bilayer honeycomb structures of group-IV elements and their binary compounds. Phys. Lett. A 2010, 375, 614–619. [Google Scholar] [CrossRef]
- Gao, X.; Shen, Y.; Ma, Y.; Wu, S.; Zhou, Z. A water splitting photocatalysis: Blue phosphorus/g-GeC van der Waals heterostructure. Appl. Phys. Lett. 2019, 114, 093902. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, H.; Zhang, Y. Transition metal doped graphene-like germanium carbide: Screening of high performance electrocatalysts for oxygen reduction, oxygen evolution, or hydrogen evolution. Colloids Surf. A Physicochem. Eng. Asp. 2021, 630, 127628. [Google Scholar] [CrossRef]
- Guilhon, I.; Teles, L.K.; Marques, M.; Pela, R.; Bechstedt, F. Influence of structure and thermodynamic stability on electronic properties of two-dimensional SiC, SiGe, and GeC alloys. Phys. Rev. B 2015, 92, 075435. [Google Scholar] [CrossRef]
- Zhao, Q.; Xiong, Z.; Qin, Z.; Chen, L.; Wu, N.; Li, X. Tuning magnetism of monolayer GaN by vacancy and nonmagnetic chemical doping. J. Phys. Chem. Solids 2015, 91, 1–6. [Google Scholar] [CrossRef]
- Cui, Z.; Luo, Y.; Yu, J.; Xu, Y. Tuning the electronic properties of MoSi2N4 by molecular doping: A first principles investigation. Phys. E Low-Dimens. Syst. Nanostruct. 2021, 134, 114873. [Google Scholar] [CrossRef]
- Sun, M.L.; Ren, Q.Q.; Zhao, Y.M.; Chou, J.P.; Yu, J.; Tang, W.C. Electronic and magnetic properties of 4D series transition metal substituted graphene: A first-principles study. Carbon 2017, 120, 265–273. [Google Scholar] [CrossRef]
- Santos, E.; Ayuela, A.; Sanchez-Portal, D. First-principles study of substitutional metal impurities in graphene: Structural, electronic and magnetic properties. New J. Phys. 2010, 12, 053012. [Google Scholar] [CrossRef] [Green Version]
- Sun, M.; Tang, W.; Ren, Q.; Wang, S.-K.; Yu, J.; Du, Y. A first-principles study of light non-metallic atom substituted blue phosphorene. Appl. Surf. Sci. 2015, 356, 110–114. [Google Scholar] [CrossRef]
- Sahin, H.; Cahangirov, S.; Topsakal, M.; Bekaroglu, E.; Akturk, E.; Senger, R.T.; Ciraci, S. Honolayer honeycomb structures of group-IV elements and III–V binary compounds: First-principles calculations. Phys. Rev. B 2009, 80, 155453. [Google Scholar] [CrossRef] [Green Version]
- Tuan, V.V.; Nguyen, T.T.; Duy, P.; Hoat, D.M.; Nguyen, T.T.B.; Tong, H.D.; Hoi, B.D.; Nguyen, C.V.; Phuc, H.V.; Nguyen, N.H. Surface functionalization of GeC monolayer with F and Cl: Electronic and optical properties. Superlattices Microstruct. 2020, 137, 106359. [Google Scholar]
- Behzad, S. Direct to indirect band gap transition in two-dimensional germanium carbide through Si substitution. Results Phys. 2019, 13, 102306. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 1169. [Google Scholar] [CrossRef]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef] [Green Version]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104–154119. [Google Scholar] [CrossRef] [Green Version]
- Hybertsen, M.S.; Louie, S.G. Electron correlation in semiconductors and insulators: Band gaps and quasiparticle energies. Phys. Rev. B 1986, 34, 5390. [Google Scholar] [CrossRef]
- Toll, J.S. Causality and the Dispersion Relation: Logical Foundations. Phys. Rev. 1956, 104, 1760. [Google Scholar] [CrossRef]
- Fox, A.M. Optical Properties of Solids; Oxford University Press: Oxford, UK, 2001; Volume 3. [Google Scholar]
- Tang, W.; Sun, M.; Yu, J.; Chou, J.-P. Magnetism in non-metal atoms adsorbed graphene-like gallium nitride monolayers. Appl. Surf. Sci. 2018, 427, 609–612. [Google Scholar] [CrossRef]
- Henkelman, G.; Arnaldsson, A.; Jónsson, H. A fast and robust algorithm for Bader decomposition of charge density. Comput. Mater. Sci. 2005, 36, 354–360. [Google Scholar] [CrossRef]
- Sanville, E.; Kenny, S.D.; Smith, R.; Henkelman, G. Improved grid-based algorithm for Bader charge allocation. J. Comput. Chem. 2007, 28, 899–908. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Cui, Z. Electronic, Magnetic, and Optical Performances of Non-Metals Doped Silicon Carbide. Front. Chem. 2022, 10, 898174. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Zhang, G.; Zhang, Y.-W. Layer-dependent Band Alignment and Work Function of Few-Layer Phosphorene. Sci. Rep. 2014, 4, 6677. [Google Scholar] [CrossRef]
- Yu, Y.-J.; Zhao, Y.; Ryu, S.; Brus, L.E.; Kim, K.S.; Kim, P. Tuning the Graphene Work Function by Electric Field Effect. Nano Lett. 2009, 9, 3430–3434. [Google Scholar] [CrossRef] [Green Version]
- Jiao, N.; He, C.; Zhang, C.X.; Peng, X.; Zhang, K.W.; Sun, L.Z. Modulation effect of hydrogen and fluorine decoration on the surface work function of BN sheets. AIP Adv. 2012, 2, 022125. [Google Scholar] [CrossRef]
- Soo, H.C.; Zhang, S.L.; Woo, C.Y. Layer-number-dependent work function of MoS2 nanoflakes. J. Korean Phys. Soc. 2014, 64, 1550–1555. [Google Scholar]
- The AM 1.5G Spectrum was Taken from the NREL Website. Available online: http://rredc.nrel.gov/solar/spectra/am1.5 (accessed on 1 January 2003).






| Adsorption Atom | Adsorption Site | Ead (eV) | C (e) | Mtotal (μB) | Eg (eV) | d (Å) |
|---|---|---|---|---|---|---|
| H | TC | −3.252 | +0.071 | 0 | 0 | 1.11 |
| B | TH | −5.416 | +0.674 | 0 | 0 | 1.11 |
| C | TH | −9.343 | −0.633 | 0 | 0.71 | 0.54 |
| N | TH | −7.929 | −1.249 | 1 | 0.28 | 0.91 |
| O | TH | −5.973 | −0.955 | 0 | 1.83 | 1.19 |
| F | TGe | −4.583 | −0.713 | 0.55 | 0 | 1.82 |
| P | TC | −4.089 | −0.316 | 1 | 0 | 1.62 |
| S | TH | −4.092 | −0.400 | 0 | 1.90 | 1.81 |
| Cl | TGe | −2.933 | −0.555 | 0.55 | 0 | 2.26 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Cui, Z. Theoretical Study on Electronic, Magnetic and Optical Properties of Non-Metal Atoms Adsorbed onto Germanium Carbide. Nanomaterials 2022, 12, 1712. https://doi.org/10.3390/nano12101712
Zhang L, Cui Z. Theoretical Study on Electronic, Magnetic and Optical Properties of Non-Metal Atoms Adsorbed onto Germanium Carbide. Nanomaterials. 2022; 12(10):1712. https://doi.org/10.3390/nano12101712
Chicago/Turabian StyleZhang, Lin, and Zhen Cui. 2022. "Theoretical Study on Electronic, Magnetic and Optical Properties of Non-Metal Atoms Adsorbed onto Germanium Carbide" Nanomaterials 12, no. 10: 1712. https://doi.org/10.3390/nano12101712





