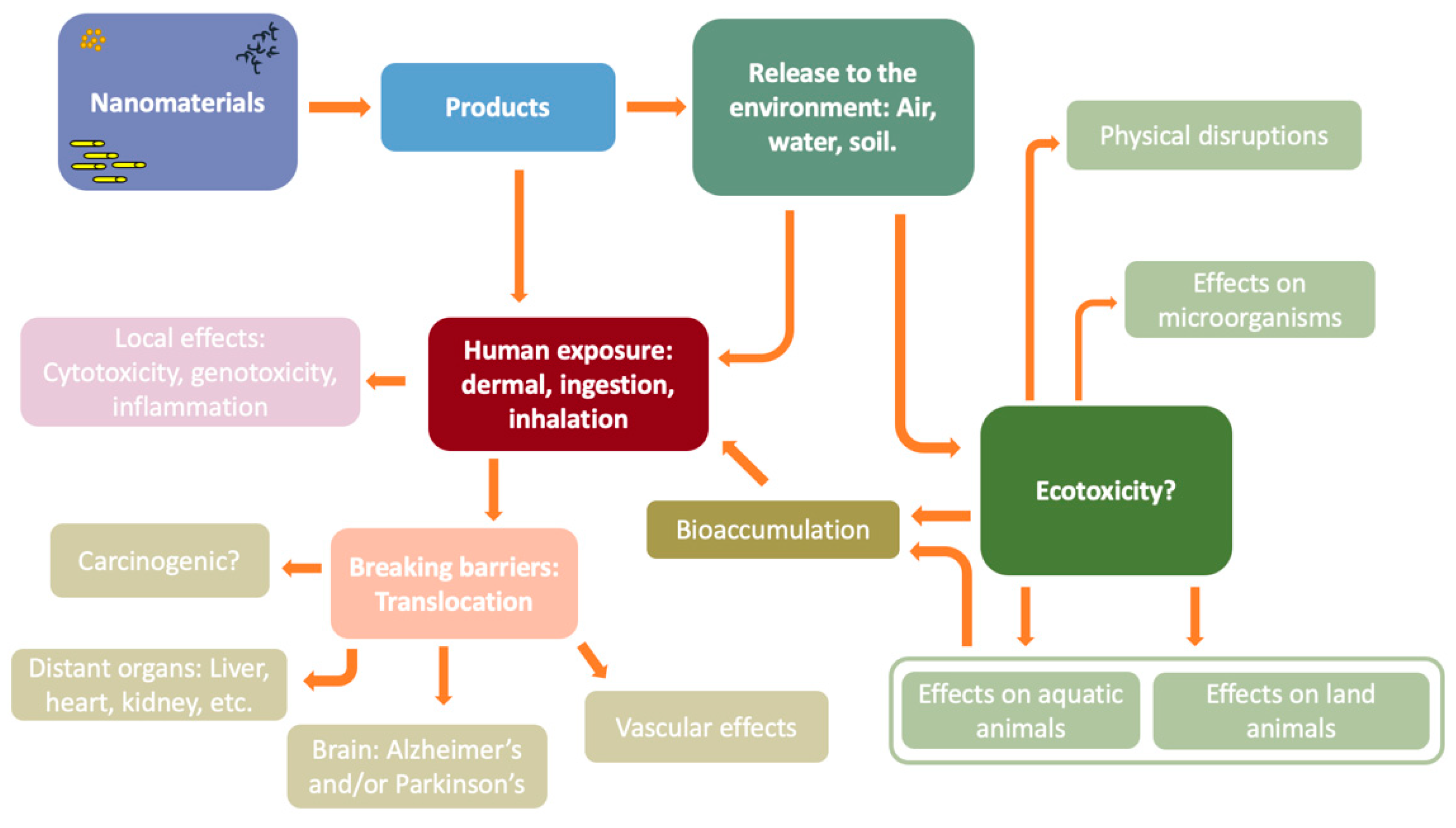
Nanosafety: An Evolving Concept to Bring the Safest Possible Nanomaterials to Society and Environment
Abstract
:1. Introduction
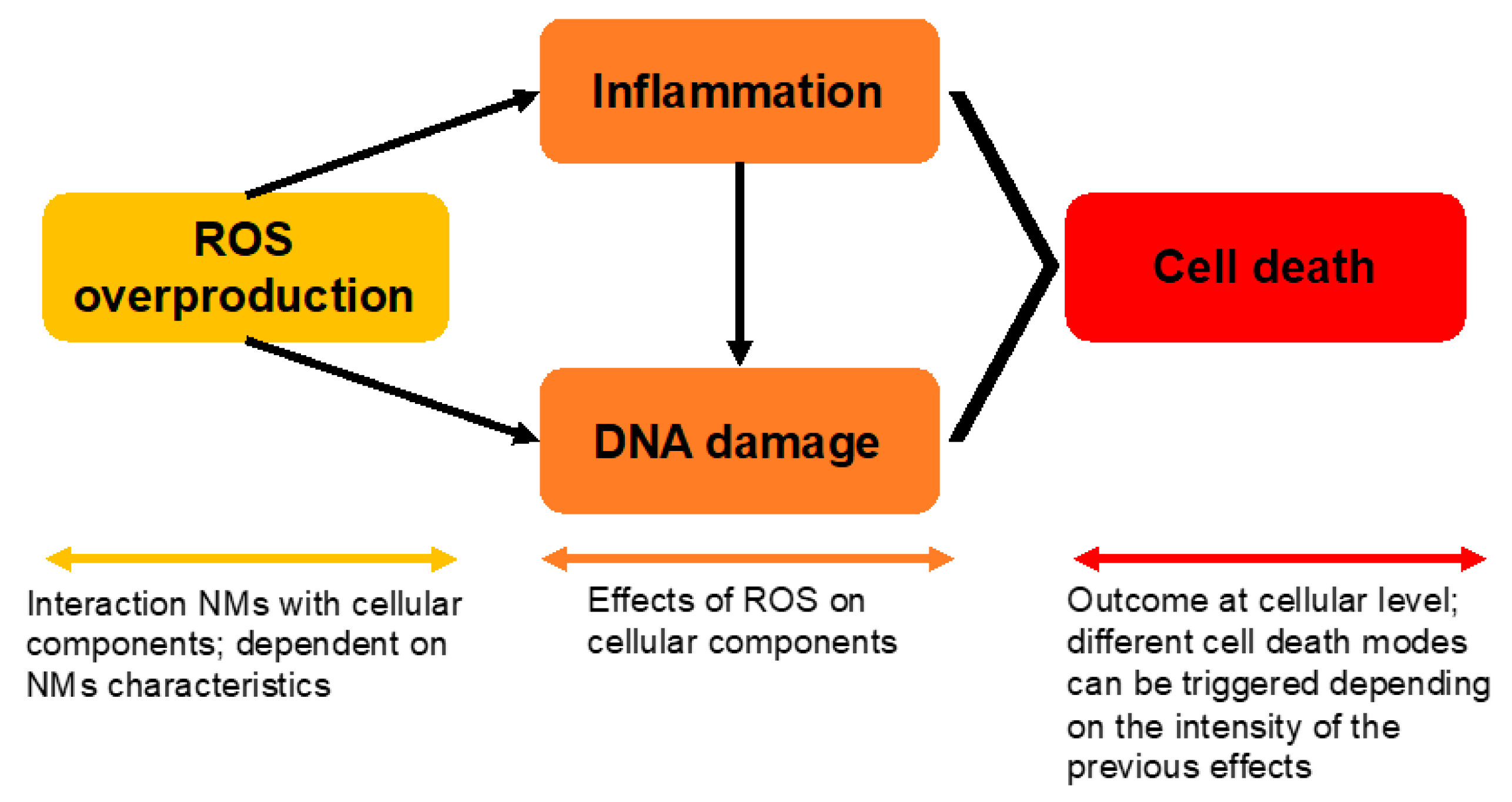
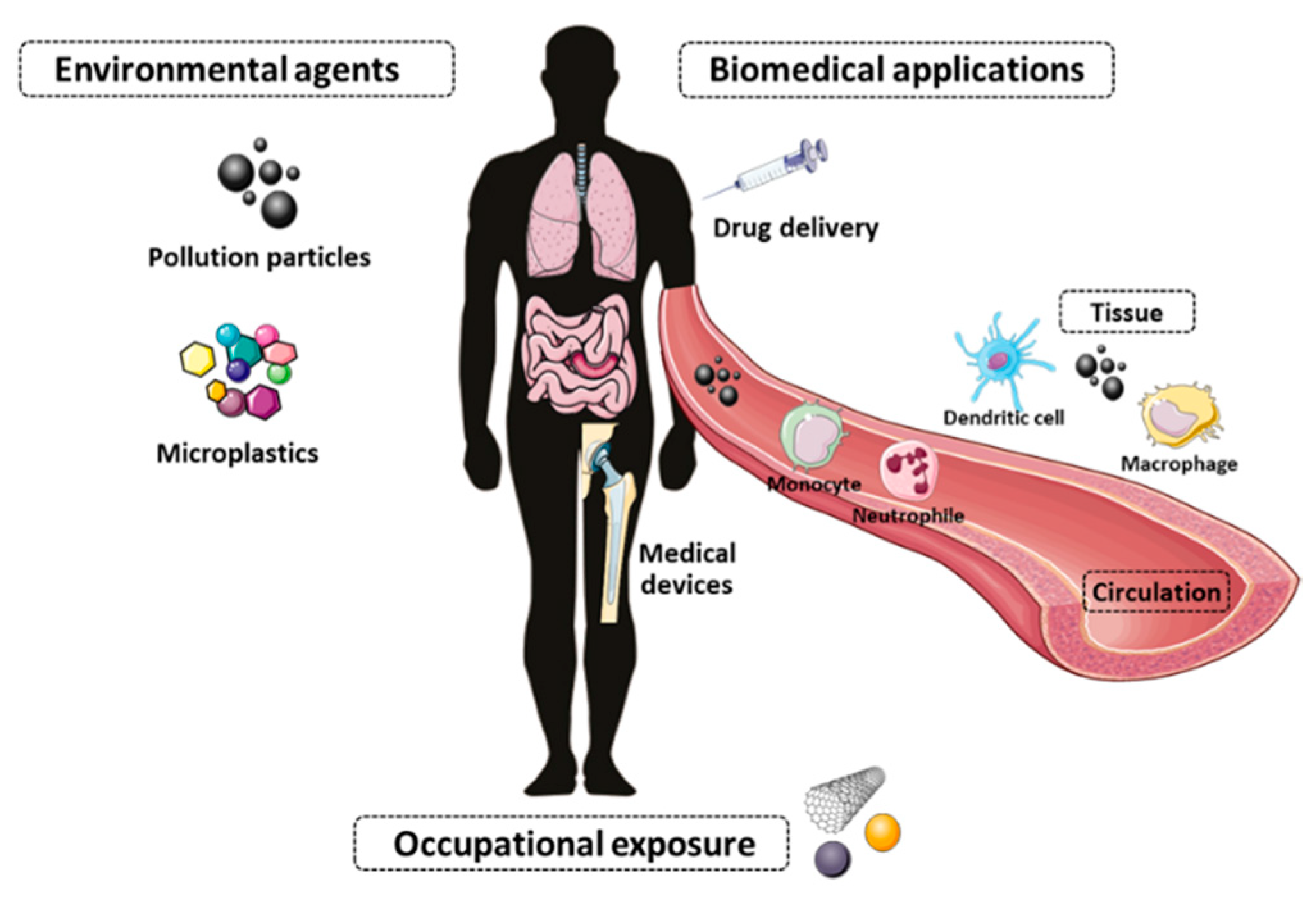
2. Nanotoxicology
3. Immunotoxicity
4. Genotoxicity and Epigenetics
4.1. Nanogenotoxicity
4.2. Nanoepigenetics
5. Advanced Models for In Vitro Testing
5.1. 3D Cultures
5.2. Organs-on-Chips
5.3. Multiple-Organs-on-Chips
5.4. Sensor Integration with Microphysiological Models
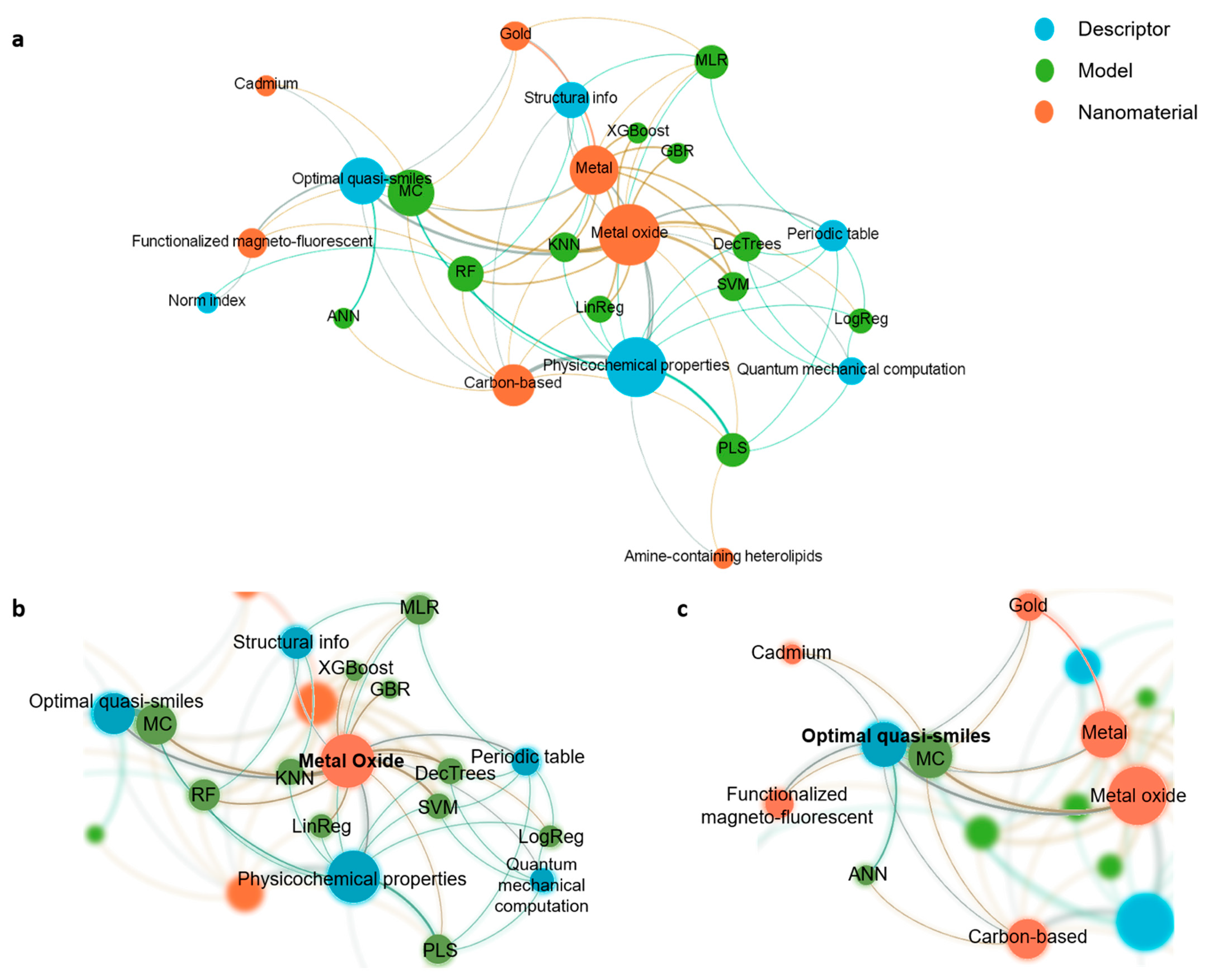
6. In Silico Tools in Nanotoxicology
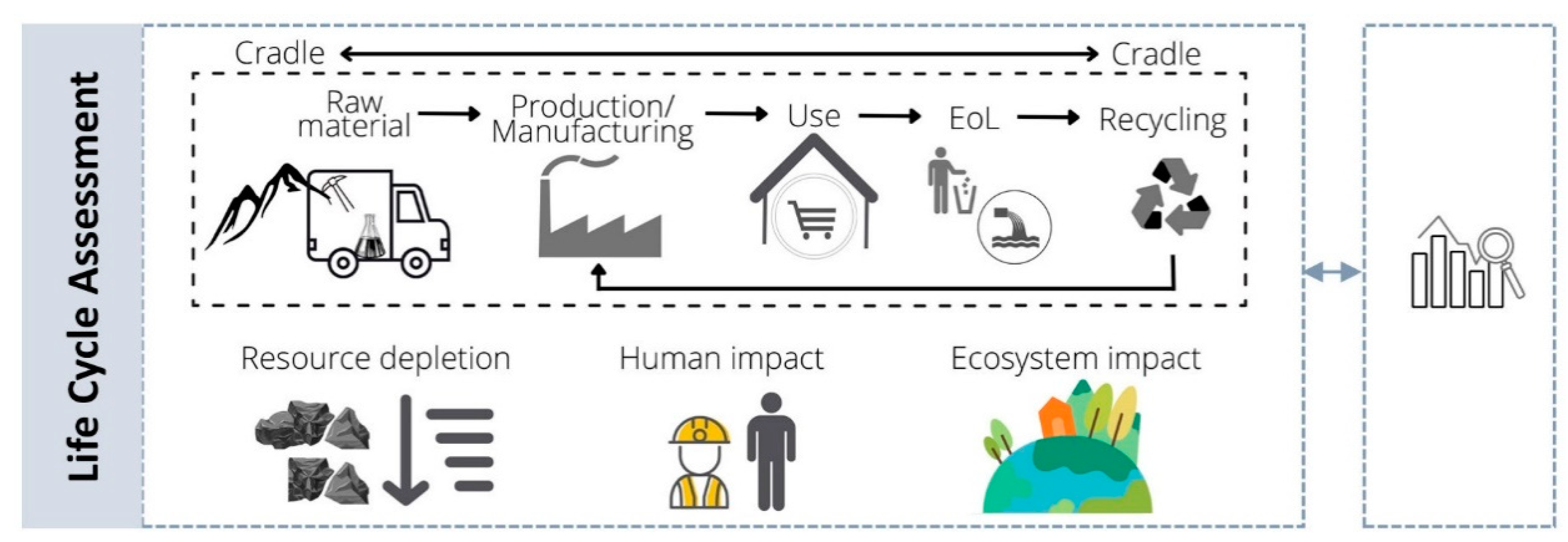
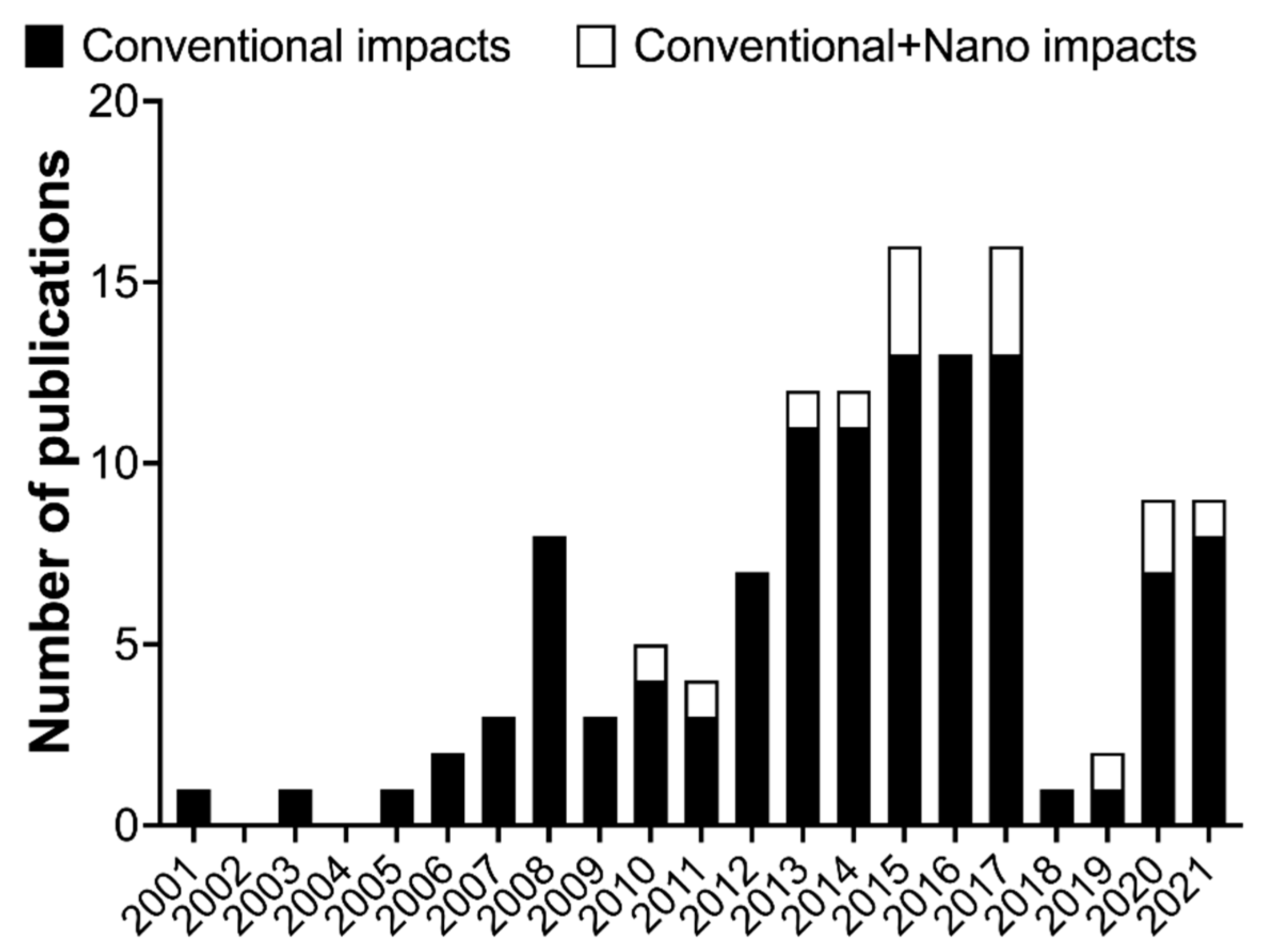
7. Life Cycle Assessment and Nanosafety
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tripathi, G.K. Engineered Nanomaterials And Their Properties: A Review. Biosci. Biotechnol. Res. Commun. 2019, 12, 764–771. [Google Scholar] [CrossRef]
- Wu, Q.; Miao, W.-S.; Zhang, Y.-D.; Gao, H.-J.; Hui, D. Mechanical properties of nanomaterials: A review. Nanotechnol. Rev. 2020, 9, 259–273. [Google Scholar] [CrossRef]
- Schaming, D.; Remita, H. Nanotechnology: From the ancient time to nowadays. Found. Chem. 2015, 17, 187–205. [Google Scholar] [CrossRef]
- Feynman, R.P. Plenty of Room at the Bottom. Available online: https://web.archive.org/web/20170105015142/http:/www.its.caltech.edu/~feynman/plenty.html (accessed on 15 April 2022).
- Taniguchi, N. On the Basic Concept of “Nano-Technology”. In Proceedings of the International Conference on Production Engineering, Part II, Society of Precision Engineering, Tokyo, Japan, 26–29 August 1974. [Google Scholar]
- Roduner, E. Size matters: Why nanomaterials are different. Chem. Soc. Rev. 2006, 35, 583–592. [Google Scholar] [CrossRef]
- Subhan, A.; Choudhury, K.P.; Neogi, N. Advances with Molecular Nanomaterials in Industrial Manufacturing Applications. Nanomanufacturing 2021, 1, 8. [Google Scholar] [CrossRef]
- National Institute for Occupational Safety and Health. Approaches to Safe Nanotechnology Managing the Health and Safety Concerns Associated with Engineered Nanomaterials; National Institute for Occupational Safety and Health: Washington, DC, USA, 2009.
- Zielińska, A.; Costa, B.; Ferreira, M.V.; Miguéis, D.; Louros, J.M.S.; Durazzo, A.; Lucarini, M.; Eder, P.; Chaud, M.V.; Morsink, M.; et al. Nanotoxicology and Nanosafety: Safety-By-Design and Testing at a Glance. Int. J. Environ. Res. Public Health 2020, 17, 4657. [Google Scholar] [CrossRef]
- Leslie, H.A.; van Velzen, M.J.; Brandsma, S.H.; Vethaak, A.D.; Garcia-Vallejo, J.J.; Lamoree, M.H. Discovery and quantification of plastic particle pollution in human blood. Environ. Int. 2022, 163, 107199. [Google Scholar] [CrossRef]
- Nemmar, A.; Hoet, P.; Vanquickenborne, B.; Dinsdale, D.; Thomeer, M.; Hoylaerts, M.; Vanbilloen, H.; Mortelmans, L.; Nemery, B. Passage of Inhaled Particles Into the Blood Circulation in Humans. Circulation 2002, 105, 411–414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raftis, J.B.; Miller, M.R. Nanoparticle translocation and multi-organ toxicity: A particularly small problem. Nano Today 2019, 26, 8–12. [Google Scholar] [CrossRef]
- Oberdörster, G.; Elder, A.; Rinderknecht, A. Nanoparticles and the Brain: Cause for Concern? J. Nanosci. Nanotechnol. 2009, 9, 4996–5007. [Google Scholar] [CrossRef] [Green Version]
- Calderón-Garcidueñas, L.; Pérez-Calatayud, Á.A.; González-Maciel, A.; Reynoso-Robles, R.; Silva-Pereyra, H.G.; Ramos-Morales, A.; Torres-Jardón, R.; Soberanes-Cerino, C.D.J.; Carrillo-Esper, R.; Briones-Garduño, J.C.; et al. Environmental Nanoparticles Reach Human Fetal Brains. Biomedicines 2022, 10, 410. [Google Scholar] [CrossRef] [PubMed]
- Piccardo, M.; Renzi, M.; Terlizzi, A. Nanoplastics in the oceans: Theory, experimental evidence and real world. Mar. Pollut. Bull. 2020, 157, 111317. [Google Scholar] [CrossRef] [PubMed]
- Kugiya, H. Hoovering the Ocean. Available online: https://www.washingtonpost.com/climate-solutions/2020/05/13/climate-solutions-plastics/ (accessed on 15 April 2022).
- Carrington, D. Microplastics Found in Human Blood for First Time. Available online: https://www.theguardian.com/environment/2022/mar/24/microplastics-found-in-human-blood-for-first-time (accessed on 10 April 2022).
- Salem, S.; Hammad, E.; Mohamed, A.; El-Dougdoug, W. A Comprehensive Review of Nanomaterials: Types, Synthesis, Characterization, and Applications. Biointerface Res. Appl. Chem. 2022, 13, 41. [Google Scholar] [CrossRef]
- Part, F.; Berge, N.; Baran, P.; Stringfellow, A.; Sun, W.; Bartelt-Hunt, S.; Mitrano, D.; Li, L.; Hennebert, P.; Quicker, P.; et al. A review of the fate of engineered nanomaterials in municipal solid waste streams. Waste Manag. 2018, 75, 427–449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inshakova, E.; Inshakov, O. World market for nanomaterials: Structure and trends. In Proceedings of the MATEC Web of Conferences; International Conference on Modern Trends in Manufacturing Technologies and Equipment (ICMTMTE 2017), Sevastopol, Russia, 11–15 September 2017; 2017; Volume 129, p. 02013. [Google Scholar] [CrossRef]
- Research and Markets Global Nanotechnology Market 2018–2024: Market Is Expected to Exceed US$ 125 Billion. Available online: https://www.prnewswire.com/news-releases/global-nanotechnology-market-2018-2024-market-is-expected-to-exceed-us-125-billion-300641054.html (accessed on 15 April 2022).
- StatNano Nanotechnology Patents of 2018 at the USPTO and EPO through the Lens of Statistics. Available online: https://statnano.com/news/65159 (accessed on 14 April 2022).
- Gochfeld, M. Chronologic History of Occupational Medicine. J. Occup. Environ. Med. 2005, 47, 96–114. [Google Scholar] [CrossRef]
- Sousa, M.; Arezes, P.; Silva, F. Occupational exposure to incidental nanoparticles: A review on control banding. J. Physics: Conf. Ser. 2021, 1953, 012008. [Google Scholar] [CrossRef]
- Suhendra, E.; Chang, C.-H.; Hou, W.-C.; Hsieh, Y.-C. A Review on the Environmental Fate Models for Predicting the Distribution of Engineered Nanomaterials in Surface Waters. Int. J. Mol. Sci. 2020, 21, 4554. [Google Scholar] [CrossRef]
- Valic, M.S.; Halim, M.; Schimmer, P.; Zheng, G. Guidelines for the experimental design of pharmacokinetic studies with nanomaterials in preclinical animal models. J. Control. Release 2020, 323, 83–101. [Google Scholar] [CrossRef]
- Kathawala, M.H.; Xiong, S.; Richards, M.; Ng, K.W.; George, S.; Loo, S.C.J. Emerging In Vitro Models for Safety Screening of High-Volume Production Nanomaterials under Environmentally Relevant Exposure Conditions. Small 2013, 9, 1504–1520. [Google Scholar] [CrossRef]
- Oberlintner, A.; Likozar, B.; Novak, U. Hydrophobic functionalization reactions of structured cellulose nanomaterials: Mechanisms, kinetics and in silico multi-scale models. Carbohydr. Polym. 2021, 259, 117742. [Google Scholar] [CrossRef]
- Oberdörster, G. Safety Assessment for Nanotechnology and Nanomedicine: Concepts of Nanotoxicology. J. Intern. Med. 2010, 267, 89–105. [Google Scholar] [CrossRef] [PubMed]
- Gatoo, M.A.; Naseem, S.; Arfat, M.Y.; Dar, A.M.; Qasim, K.; Zubair, S. Physicochemical Properties of Nanomaterials: Implication in Associated Toxic Manifestations. BioMed Res. Int. 2014, 2014, 498420. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Singh, S.; Shanker, R.; Dhawan, A. Chapter 1. Nanotoxicology: Challenges for Biologists. In Issues in Toxicology; Royal Society of Chemistry: London, UK, 2018; pp. 1–16. [Google Scholar] [CrossRef]
- Hull, M.; Kennedy, A.J.; Detzel, C.; Vikesland, P.; Chappell, M.A. Moving beyond Mass: The Unmet Need to Consider Dose Metrics in Environmental Nanotoxicology Studies. Environ. Sci. Technol. 2012, 46, 10881–10882. [Google Scholar] [CrossRef]
- Duffin, R.; Tran, L.; Brown, D.; Stone, V.; Donaldson, K. Proinflammogenic Effects of Low-Toxicity and Metal Nanoparticles In Vivo and In Vitro: Highlighting the Role of Particle Surface Area and Surface Reactivity. Inhal. Toxicol. 2007, 19, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Hennig, F.; Quass, U.; Hellack, B.; Küpper, M.; Kuhlbusch, T.; Stafoggia, M.; Hoffmann, B. Ultrafine and Fine Particle Number and Surface Area Concentrations and Daily Cause-Specific Mortality in the Ruhr Area, Germany, 2009–2014. Environ. Health Perspect. 2018, 126, 027008. [Google Scholar] [CrossRef]
- Monteiller, C.; Tran, L.; MacNee, W.; Faux, S.; Jones, A.; Miller, B.; Donaldson, K. The pro-inflammatory effects of low-toxicity low-solubility particles, nanoparticles and fine particles, on epithelial cells in vitro: The role of surface area. Occup. Environ. Med. 2007, 64, 609–615. [Google Scholar] [CrossRef] [Green Version]
- Sager, T.M.; Castranova, V. Surface area of particle administered versus mass in determining the pulmonary toxicity of ultrafine and fine carbon black: Comparison to ultrafine titanium dioxide. Part. Fibre Toxicol. 2009, 6, 15. [Google Scholar] [CrossRef]
- Noël, A.; Truchon, G.; Cloutier, Y.; Charbonneau, M.; Maghni, K.; Tardif, R. Mass or total surface area with aerosol size distribution as exposure metrics for inflammatory, cytotoxic and oxidative lung responses in rats exposed to titanium dioxide nanoparticles. Toxicol. Ind. Health 2017, 33, 351–364. [Google Scholar] [CrossRef]
- Teeguarden, J.G.; Hinderliter, P.M.; Orr, G.; Thrall, B.D.; Pounds, J.G. Particokinetics In Vitro: Dosimetry Considerations for In Vitro Nanoparticle Toxicity Assessments. Toxicol. Sci. 2007, 95, 300–312. [Google Scholar] [CrossRef]
- Braakhuis, H.M.; Cassee, F.R.; Fokkens, P.H.; De La Fonteyne, L.J.; Oomen, A.G.; Krystek, P.; De Jong, W.H.; Van Loveren, H.; Park, M.V. Identification of the appropriate dose metric for pulmonary inflammation of silver nanoparticles in an inhalation toxicity study. Nanotoxicology 2016, 10, 63–73. [Google Scholar] [CrossRef]
- Jeong, J.; Han, Y.; Poland, C.A.; Cho, W.-S. Response-metrics for acute lung inflammation pattern by cobalt-based nanoparticles. Part. Fibre Toxicol. 2015, 12, 13. [Google Scholar] [CrossRef] [Green Version]
- Cohen, J.M.; Teeguarden, J.G.; Demokritou, P. An integrated approach for the in vitro dosimetry of engineered nanomaterials. Part. Fibre Toxicol. 2014, 11, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rischitor, G.; Parracino, M.; La Spina, R.; Urbán, P.; Ojea-Jiménez, I.; Bellido, E.; Valsesia, A.; Gioria, S.; Capomaccio, R.; Kinsner-Ovaskainen, A.; et al. Quantification of the cellular dose and characterization of nanoparticle transport during in vitro testing. Part. Fibre Toxicol. 2016, 13, 47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shabbir, S.; Kulyar, M.F.-E.; Bhutta, Z.A.; Boruah, P.; Asif, M. Toxicological Consequences of Titanium Dioxide Nanoparticles (TiO2NPs) and Their Jeopardy to Human Population. BioNanoScience 2021, 11, 621–632. [Google Scholar] [CrossRef] [PubMed]
- Vlastou, E.; Gazouli, M.; Ploussi, A.; Platoni, K.; Efstathopoulos, E.P. Nanoparticles: Nanotoxicity Aspects. In Journal of Physics: Conference Series, Proceedings of the International Conference on Bio-Medical Instrumentation and Related Engineering and Physical Sciences (BIOMEP 2017), Athens, Greece, 12–13 October 2017; Institute of Physics Publishing: Athens, Greece, 2017; Volume 931. [Google Scholar]
- Xiong, P.; Huang, X.; Ye, N.; Lu, Q.; Zhang, G.; Peng, S.; Wang, H.; Liu, Y. Cytotoxicity of Metal-Based Nanoparticles: From Mechanisms and Methods of Evaluation to Pathological Manifestations. Adv. Sci. 2022, 2106049. [Google Scholar] [CrossRef]
- Moore, T.L.; Urban, D.; Rodriguez-Lorenzo, L.; Milosevic, A.; Crippa, F.; Spuch-Calvar, M.; Balog, S.; Rothen-Rutishauser, B.; Lattuada, M.; Petri-Fink, A. Nanoparticle administration method in cell culture alters particle-cell interaction. Sci. Rep. 2019, 9, 900. [Google Scholar] [CrossRef]
- European Commission Environment and Health. (HORIZON-HLTH-2021-ENVHLTH-02). 2021. Available online: https://ec.europa.eu/info/funding-tenders/opportunities/portal/screen/opportunities/topic-details/horizon-hlth-2021-envhlth-02-02;callCode=HORIZON-HLTH-2021-ENVHLTH-02;freeTextSearchKeyword=;matchWholeText=true;typeCodes=1;statusCodes=31094501,31094502,31094503;programmePeriod=null;programCcm2Id=null;programDivisionCode=null;focusAreaCode=null;destination=null;mission=null;geographicalZonesCode=null;programmeDivisionProspect=null;startDateLte=null;startDateGte=null;crossCuttingPriori-ty-Code=null;cpvCode=null;performanceOfDelivery=null;sortQuery=sortStatus;orderBy=asc;onlyTenders=false;topicListKey=callTopicSearchTableState (accessed on 17 April 2022).
- Hubal, E.A.C.; Wetmore, B.A.; Wambaugh, J.; El-Masri, H.; Sobus, J.; Bahadori, T. Advancing internal exposure and physiologically-based toxicokinetic modeling for 21st-century risk assessments. J. Expo. Sci. Environ. Epidemiol. 2018, 29, 11–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skalska, J.; Dąbrowska-Bouta, B.; Strużyńska, L. Oxidative stress in rat brain but not in liver following oral administration of a low dose of nanoparticulate silver. Food Chem. Toxicol. 2016, 97, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Tentschert, J.; Laux, P.; Jungnickel, H.; Brunner, J.; Estrela-Lopis, I.; Merker, C.; Meijer, J.; Ernst, H.; Ma-Hock, L.; Keller, J.; et al. Organ burden of inhaled nanoceria in a 2-year low-dose exposure study: Dump or depot? Nanotoxicology 2020, 14, 554–576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Comfort, K.K.; Braydich-Stolle, L.K.; Maurer, E.I.; Hussain, S.M. Less Is More: Long-Term in Vitro Exposure to Low Levels of Silver Nanoparticles Provides New Insights for Nanomaterial Evaluation. ACS Nano 2014, 8, 3260–3271. [Google Scholar] [CrossRef]
- Llewellyn, S.V.; Conway, G.E.; Zanoni, I.; Jørgensen, A.K.; Shah, U.-K.; Seleci, D.A.; Keller, J.G.; Kim, J.W.; Wohlleben, W.; Jensen, K.A.; et al. Understanding the impact of more realistic low-dose, prolonged engineered nanomaterial exposure on genotoxicity using 3D models of the human liver. J. Nanobiotechnology 2021, 19, 193. [Google Scholar] [CrossRef] [PubMed]
- Torres, A.; Dalzon, B.; Collin-Faure, V.; Rabilloud, T. Repeated vs. Acute Exposure of RAW264.7 Mouse Macrophages to Silica Nanoparticles: A Bioaccumulation and Functional Change Study. Nanomaterials 2020, 10, 215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsatsakis, A.M.; Docea, A.O.; Tsitsimpikou, C. New challenges in risk assessment of chemicals when simulating real exposure scenarios; simultaneous multi-chemicals’ low dose exposure. Food Chem. Toxicol. 2016, 96, 174–176. [Google Scholar] [CrossRef] [PubMed]
- Vilas-Boas, V.; Vinken, M. Hepatotoxicity induced by nanomaterials: Mechanisms and in vitro models. Arch. Toxicol. 2021, 95, 27–52. [Google Scholar] [CrossRef]
- Hussain, Z.; Thu, H.E.; Elsayed, I.; Abourehab, M.A.; Khan, S.; Sohail, M.; Sarfraz, R.M.; Farooq, M.A. Nano-scaled materials may induce severe neurotoxicity upon chronic exposure to brain tissues: A critical appraisal and recent updates on predisposing factors, underlying mechanism, and future prospects. J. Control. Release 2020, 328, 873–894. [Google Scholar] [CrossRef] [PubMed]
- Teng, C.; Jiang, C.; Gao, S.; Liu, X.; Zhai, S. Fetotoxicity of Nanoparticles: Causes and Mechanisms. Nanomaterials 2021, 11, 791. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.; Leite, P.E.; Falagan-Lotsch, P.; Benetti, F.; Micheletti, C.; Budtz, H.; Jacobsen, N.R.; Lisboa-Filho, P.; Rocha, L.; Kühnel, D.; et al. Challenges on the toxicological predictions of engineered nanoparticles. NanoImpact 2017, 8, 59–72. [Google Scholar] [CrossRef]
- Shatkin, J.A. The Future in Nanosafety. Nano Lett. 2020, 20, 1479–1480. [Google Scholar] [CrossRef] [Green Version]
- Irvine, D.J.; Swartz, M.A.; Szeto, G. Engineering synthetic vaccines using cues from natural immunity. Nat. Mater. 2013, 12, 978–990. [Google Scholar] [CrossRef] [Green Version]
- Kyriakides, T.R.; Raj, A.; Tseng, T.H.; Xiao, H.; Nguyen, R.; Mohammed, F.S.; Halder, S.S.; Xu, M.; Wu, M.J.; Bao, S.; et al. Biocompatibility of nanomaterials and their immunological properties. Biomed. Mater. 2021, 16, 042005. [Google Scholar] [CrossRef]
- Lebre, F.; Hearnden, C.H.; Lavelle, E.C. Modulation of Immune Responses by Particulate Materials. Adv. Mater. 2016, 28, 5525–5541. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Wolf, N.; Lavelle, E.C. Promotion of trained innate immunity by nanoparticles. Semin. Immunol. 2021, 56, 101542. [Google Scholar] [CrossRef] [PubMed]
- Burnett, R.; Chen, H.; Szyszkowicz, M.; Fann, N.; Hubbell, B.; Pope, C.A., 3rd; Apte, J.S.; Brauer, M.; Cohen, A.; Weichenthal, S.; et al. Global estimates of mortality associated with long-term exposure to outdoor fine particulate matter. Proc. Natl. Acad. Sci. USA 2018, 115, 9592–9597. [Google Scholar] [CrossRef] [Green Version]
- Vohra, K.; Vodonos, A.; Schwartz, J.; Marais, E.A.; Sulprizio, M.P.; Mickley, L.J. Global mortality from outdoor fine particle pollution generated by fossil fuel combustion: Results from GEOS-Chem. Environ. Res. 2021, 195, 110754. [Google Scholar] [CrossRef]
- Shears, R.K.; Jacques, L.C.; Naylor, G.; Miyashita, L.; Khandaker, S.; Lebre, F.; Lavelle, E.C.; Grigg, J.; French, N.; Neill, D.R.; et al. Exposure to diesel exhaust particles increases susceptibility to invasive pneumococcal disease. J. Allergy Clin. Immunol. 2020, 145, 1272–1284.e6. [Google Scholar] [CrossRef]
- ISO/TR 10993-22:2017; Biological Evaluation of Medical Devices—Part 22: Guidance on Nanomaterials. International Organization for Standardization: Geneva, Switzerland, 2017.
- Burrell, R. Human responses to bacterial endotoxin. Circ. Shock 1994, 43, 137–153. [Google Scholar]
- Copeland, S.; Warren, H.S.; Lowry, S.F.; Calvano, S.E.; Remick, D. Acute Inflammatory Response to Endotoxin in Mice and Humans. Clin. Vaccine Immunol. 2005, 12, 60–67. [Google Scholar] [CrossRef] [Green Version]
- Ma, P.; Liu, H.-T.; Wei, P.; Xu, Q.-S.; Bai, X.-F.; Du, Y.-G.; Yu, C. Chitosan oligosaccharides inhibit LPS-induced over-expression of IL-6 and TNF-α in RAW264.7 macrophage cells through blockade of mitogen-activated protein kinase (MAPK) and PI3K/Akt signaling pathways. Carbohydr. Polym. 2011, 84, 1391–1398. [Google Scholar] [CrossRef]
- Park, J.; Babensee, J.E. Differential functional effects of biomaterials on dendritic cell maturation. Acta Biomater. 2012, 8, 3606–3617. [Google Scholar] [CrossRef] [Green Version]
- Bueter, C.L.; Lee, C.K.; Wang, J.P.; Ostroff, G.R.; Specht, C.A.; Levitz, S.M. Spectrum and Mechanisms of Inflammasome Activation by Chitosan. J. Immunol. 2014, 192, 5943–5951. [Google Scholar] [CrossRef] [Green Version]
- Gorbet, M.B.; Sefton, M.V. Endotoxin: The uninvited guest. Biomaterials 2005, 26, 6811–6817. [Google Scholar] [CrossRef] [PubMed]
- Fujita, Y.; Nabetani, T. Iron sulfate inhibits Limulus activity by induction of structural and qualitative changes in lipid A. J. Appl. Microbiol. 2014, 116, 89–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, J.; Clippinger, A.J.; Briglia, C.F.; Casey, W.; Coleman, K.; Fritsch, A.; Hartung, T.; Maouyo, D.; Muller, T.; Reich, J.; et al. Using the monocyte activation test as a stand-alone release test for medical devices. ALTEX 2021, 38, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Mangini, M.; Verde, A.; Boraschi, D.; Puntes, V.F.; Italiani, P.; De Luca, A.C. Interaction of nanoparticles with endotoxin Importance in nanosafety testing and exploitation for endotoxin binding. Nanotoxicology 2021, 15, 558–576. [Google Scholar] [CrossRef]
- Lin, A.; Giuliano, C.J.; Palladino, A.; John, K.M.; Abramowicz, C.; Yuan, M.L.; Sausville, E.L.; Lukow, D.A.; Liu, L.; Chait, A.R.; et al. Off-target toxicity is a common mechanism of action of cancer drugs undergoing clinical trials. Sci. Transl. Med. 2019, 11, eaaw8412. [Google Scholar] [CrossRef]
- Chabner, B.A. NCI-60 Cell Line Screening: A Radical Departure in its Time. JNCI: J. Natl. Cancer Inst. 2016, 108, djv388. [Google Scholar] [CrossRef] [Green Version]
- Roma-Rodrigues, C.; Mendes, R.; Baptista, P.V.; Fernandes, A.R. Targeting Tumor Microenvironment for Cancer Therapy. Int. J. Mol. Sci. 2019, 20, 840. [Google Scholar] [CrossRef] [Green Version]
- Paul, S.M.; Mytelka, D.; Dunwiddie, C.T.; Persinger, C.C.; Munos, B.H.; Lindborg, S.R.; Schacht, A.L. How to improve R&D productivity: The pharmaceutical industry’s grand challenge. Nat. Rev. Drug Discov. 2010, 9, 203–214. [Google Scholar] [CrossRef]
- Shih, H.-P.; Zhang, X.; Aronov, A.M. Drug discovery effectiveness from the standpoint of therapeutic mechanisms and indications. Nat. Rev. Drug Discov. 2017, 17, 19–33. [Google Scholar] [CrossRef]
- Chanput, W.; Peters, V.; Wichers, H. THP-1 and U937 Cells. In The Impact of Food Bioactives on Health; Springer International Publishing: Cham, Switzerland, 2015; pp. 147–159. [Google Scholar]
- Tedesco, S.; De Majo, F.; Kim, J.; Trenti, A.; Trevisi, L.; Fadini, G.P.; Bolego, C.; Zandstra, P.W.; Cignarella, A.; Vitiello, L. Convenience versus Biological Significance: Are PMA-Differentiated THP-1 Cells a Reliable Substitute for Blood-Derived Macrophages When Studying in Vitro Polarization? Front. Pharmacol. 2018, 9, 71. [Google Scholar] [CrossRef] [Green Version]
- Netea, M.G.; Domínguez-Andrés, J.; Barreiro, L.B.; Chavakis, T.; Divangahi, M.; Fuchs, E.; Joosten, L.A.B.; Van Der Meer, J.W.M.; Mhlanga, M.M.; Mulder, W.J.M.; et al. Defining trained immunity and its role in health and disease. Nat. Rev. Immunol. 2020, 20, 375–388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riksen, N.P.; Netea, M.G. Immunometabolic control of trained immunity. Mol. Asp. Med. 2021, 77, 100897. [Google Scholar] [CrossRef] [PubMed]
- Kleinnijenhuis, J.; Quintin, J.; Preijers, F.; Joosten, L.A.B.; Ifrim, D.C.; Saeed, S.; Jacobs, C.; van Loenhout, J.; de Jong, D.; Stunnenberg, H.G.; et al. Bacille Calmette-Guérin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc. Natl. Acad. Sci. USA 2012, 109, 17537–17542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schrum, J.E.; Crabtree, J.N.; Dobbs, K.R.; Kiritsy, M.C.; Reed, G.W.; Gazzinelli, R.T.; Netea, M.G.; Kazura, J.W.; Dent, A.E.; Fitzgerald, K.A.; et al. Cutting Edge: Plasmodium falciparum Induces Trained Innate Immunity. J. Immunol. 2018, 200, 1243–1248. [Google Scholar] [CrossRef] [Green Version]
- Bekkering, S.; Quintin, J.; Joosten, L.A.; van der Meer, J.W.; Netea, M.G.; Riksen, N.P. Oxidized Low-Density Lipoprotein Induces Long-Term Proinflammatory Cytokine Production and Foam Cell Formation via Epigenetic Reprogramming of Monocytes. Arter. Thromb. Vasc. Biol. 2014, 34, 1731–1738. [Google Scholar] [CrossRef]
- Christ, A.; Günther, P.; Lauterbach, M.A.; Duewell, P.; Biswas, D.; Pelka, K.; Scholz, C.-J.; Oosting, M.; Haendler, K.; Baßler, K.; et al. Western Diet Triggers NLRP3-Dependent Innate Immune Reprogramming. Cell 2018, 172, 162–175.e14. [Google Scholar] [CrossRef] [Green Version]
- Lebre, F.; Boland, J.B.; Gouveia, P.; Gorman, A.L.; Lundahl, M.L.E.; Lynch, R.I.; O’Brien, F.J.; Coleman, J.; Lavelle, E.C. Pristine graphene induces innate immune training. Nanoscale 2020, 12, 11192–11200. [Google Scholar] [CrossRef]
- Swartzwelter, B.J.; Barbero, F.; Verde, A.; Mangini, M.; Pirozzi, M.; De Luca, A.C.; Puntes, V.F.; Leite, L.C.C.; Italiani, P.; Boraschi, D. Gold Nanoparticles Modulate BCG-Induced Innate Immune Memory in Human Monocytes by Shifting the Memory Response towards Tolerance. Cells 2020, 9, 284. [Google Scholar] [CrossRef] [Green Version]
- Shukla, R.K.; Badiye, A.; Vajpayee, K.; Kapoor, N. Genotoxic Potential of Nanoparticles: Structural and Functional Modifications in DNA. Front. Genet. 2021, 12, 728250. [Google Scholar] [CrossRef]
- Doak, S.; Manshian, B.; Jenkins, G.; Singh, N. In vitro genotoxicity testing strategy for nanomaterials and the adaptation of current OECD guidelines. Mutat. Res. Toxicol. Environ. Mutagen. 2012, 745, 104–111. [Google Scholar] [CrossRef]
- Dusinska, M.; Tulinska, J.; El Yamani, N.; Kuricova, M.; Liskova, A.; Rollerova, E.; Rundén-Pran, E.; Smolkova, B. Immunotoxicity, genotoxicity and epigenetic toxicity of nanomaterials: New strategies for toxicity testing? Food Chem. Toxicol. 2017, 109, 797–811. [Google Scholar] [CrossRef] [PubMed]
- Evans, S.J.; Clift, M.J.D.; Singh, N.; Wills, J.W.; Hondow, N.; Wilkinson, T.S.; Burgum, M.J.; Brown, A.P.; Jenkins, G.J.; Doak, S.H. In vitro detection of in vitro secondary mechanisms of genotoxicity induced by engineered nanomaterials. Part. Fibre Toxicol. 2019, 16, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samadian, H.; Salami, M.S.; Jaymand, M.; Azarnezhad, A.; Najafi, M.; Barabadi, H.; Ahmadi, A. Genotoxicity assessment of carbon-based nanomaterials; Have their unique physicochemical properties made them double-edged swords? Mutat. Res. Rev. Mutat. Res. 2020, 783, 108296. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, K.; Poland, C.; Schins, R.P.F. Possible genotoxic mechanisms of nanoparticles: Criteria for improved test strategies. Nanotoxicology 2010, 4, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Elespuru, R.; Pfuhler, S.; Aardema, M.J.; Chen, T.; Doak, S.H.; Doherty, A.; Farabaugh, C.S.; Kenny, J.; Manjanatha, M.; Mahadevan, B.; et al. Genotoxicity Assessment of Nanomaterials: Recommendations on Best Practices, Assays, and Methods. Toxicol. Sci. 2018, 164, 391–416. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, H.L.; Di Bucchianico, S.; Collins, A.; Dusinska, M. Can the comet assay be used reliably to detect nanoparticle-induced genotoxicity? Environ. Mol. Mutagen. 2015, 56, 82–96. [Google Scholar] [CrossRef]
- Warheit, D.B.; Donner, E.M. Rationale of genotoxicity testing of nanomaterials: Regulatory requirements and appropriateness of available OECD test guidelines. Nanotoxicology 2010, 4, 409–413. [Google Scholar] [CrossRef]
- Ali, D.; Falodah, F.A.; Almutairi, B.; Alkahtani, S.; Alarifi, S. Assessment of DNA damage and oxidative stress in juvenile Channa punctatus (Bloch) after exposure to multi-walled carbon nanotubes. Environ. Toxicol. 2020, 35, 359–367. [Google Scholar] [CrossRef]
- Boran, H.; Şaffak, S. Comparison of Dissolved Nickel and Nickel Nanoparticles Toxicity in Larval Zebrafish in Terms of Gene Expression and DNA Damage. Arch. Environ. Contam. Toxicol. 2018, 74, 193–202. [Google Scholar] [CrossRef]
- Carmona, E.R.; Escobar, B.; Vales, G.; Marcos, R. Genotoxic testing of titanium dioxide anatase nanoparticles using the wing-spot test and the comet assay in Drosophila. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2015, 778, 12–21. [Google Scholar] [CrossRef]
- Chatterjee, N.; Eom, H.J.; Choi, J. Effects of silver nanoparticles on oxidative DNA damage-repair as a function of p38 MAPK status: A comparative approach using human Jurkat T cells and the nematode Caenorhabditis elegans. Environ. Mol. Mutagen. 2014, 55, 122–133. [Google Scholar] [CrossRef] [PubMed]
- Butler, K.S.; Peeler, D.J.; Casey, B.J.; Dair, B.J.; Elespuru, R.K. Silver nanoparticles: Correlating nanoparticle size and cellular uptake with genotoxicity. Mutagenesis 2015, 30, 577–591. [Google Scholar] [CrossRef] [PubMed]
- Nelson, B.C.; Wright, C.W.; Ibuki, Y.; Moreno-Villanueva, M.; Karlsson, H.L.; Hendriks, G.; Sims, C.M.; Singh, N.; Doak, S.H. Emerging metrology for high-throughput nanomaterial genotoxicology. Mutagenesis 2017, 32, 215–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karlsson, H.L.; Gliga, A.R.; Calléja, F.M.; Gonçalves, C.S.; Wallinder, I.O.; Vrieling, H.; Fadeel, B.; Hendriks, G. Mechanism-based genotoxicity screening of metal oxide nanoparticles using the ToxTracker panel of reporter cell lines. Part. Fibre Toxicol. 2014, 11, 41. [Google Scholar] [CrossRef] [Green Version]
- Collins, A.R.; Annangi, B.; Rubio, L.; Marcos, R.; Dorn, M.; Merker, C.; Estrela-Lopis, I.; Cimpan, M.R.; Ibrahim, M.; Cimpan, E.; et al. High throughput toxicity screening and intracellular detection of nanomaterials. WIREs Nanomed. Nanobiotechnol. 2017, 9, e1413. [Google Scholar] [CrossRef]
- Bobyk, L.; Tarantini, A.; Beal, D.; Veronesi, G.; Kieffer, I.; Motellier, S.; Valsami-Jones, E.; Lynch, I.; Jouneau, P.-H.; Pernet-Gallay, K.; et al. Toxicity and chemical transformation of silver nanoparticles in A549 lung cells: Dose-rate-dependent genotoxic impact. Environ. Sci. Nano 2021, 8, 806–821. [Google Scholar] [CrossRef]
- Biola-Clier, M.; Beal, D.; Caillat, S.; Libert, S.; Armand, L.; Herlin-Boime, N.; Sauvaigo, S.; Douki, T.; Carriere, M. Comparison of the DNA damage response in BEAS-2B and A549 cells exposed to titanium dioxide nanoparticles. Mutagenesis 2017, 32, 161–172. [Google Scholar] [CrossRef] [Green Version]
- Armand, L.; Tarantini, A.; Beal, D.; Biola-Clier, M.; Bobyk, L.; Sorieul, S.; Pernet-Gallay, K.; Marie-Desvergne, C.; Lynch, I.; Herlin-Boime, N.; et al. Long-term exposure of A549 cells to titanium dioxide nanoparticles induces DNA damage and sensitizes cells towards genotoxic agents. Nanotoxicology 2016, 10, 913–923. [Google Scholar] [CrossRef]
- Toprani, S.M.; Bitounis, D.; Huang, Q.; Oliveira, N.; Ng, K.W.; Tay, C.Y.; Nagel, Z.D.; Demokritou, P. High-Throughput Screening Platform for Nanoparticle-Mediated Alterations of DNA Repair Capacity. ACS Nano 2021, 15, 4728–4746. [Google Scholar] [CrossRef]
- Singh, N.; Nelson, B.C.; Scanlan, L.D.; Coskun, E.; Jaruga, P.; Doak, S.H. Exposure to Engineered Nanomaterials: Impact on DNA Repair Pathways. Int. J. Mol. Sci. 2017, 18, 1515. [Google Scholar] [CrossRef] [Green Version]
- Piao, M.J.; Kim, K.C.; Choi, J.-Y.; Choi, J.; Hyun, J.W. Silver nanoparticles down-regulate Nrf2-mediated 8-oxoguanine DNA glycosylase 1 through inactivation of extracellular regulated kinase and protein kinase B in human Chang liver cells. Toxicol. Lett. 2011, 207, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.K.; Asharani, P.V.; Hande, M.P. Enhanced Genotoxicity of Silver Nanoparticles in DNA Repair Deficient Mammalian Cells. Front. Genet. 2012, 3, 104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bitounis, D.; Huang, Q.; Toprani, S.M.; Setyawati, M.I.; Oliveira, N.; Wu, Z.; Tay, C.Y.; Ng, K.W.; Nagel, Z.D.; Demokritou, P. Printer center nanoparticles alter the DNA repair capacity of human bronchial airway epithelial cells. NanoImpact 2022, 25, 100379. [Google Scholar] [CrossRef] [PubMed]
- Ecasati, L.; Sendra, R.; Esibilia, V.; Ecelotti, F. Endocrine disrupters: The new players able to affect the epigenome. Front. Cell Dev. Biol. 2015, 3, 37. [Google Scholar] [CrossRef]
- Norouzitallab, P.; Baruah, K.; Vanrompay, D.; Bossier, P. Can epigenetics translate environmental cues into phenotypes? Sci. Total Environ. 2019, 647, 1281–1293. [Google Scholar] [CrossRef] [PubMed]
- Bowers, E.C.; McCullough, S.D. Linking the Epigenome with Exposure Effects and Susceptibility: The Epigenetic Seed and Soil Model. Toxicol. Sci. 2017, 155, 302–314. [Google Scholar] [CrossRef] [Green Version]
- Thomson, J.P.; Moggs, J.G.; Wolf, C.R.; Meehan, R.R. Epigenetic profiles as defined signatures of xenobiotic exposure. Mutat. Res. Toxicol. Environ. Mutagen. 2014, 764–765, 3–9. [Google Scholar] [CrossRef]
- Ren, N.; Atyah, M.; Chen, W.-Y.; Zhou, C. The various aspects of genetic and epigenetic toxicology: Testing methods and clinical applications. J. Transl. Med. 2017, 15, 110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chatterjee, N.; Yang, J.; Choi, J. Differential genotoxic and epigenotoxic effects of graphene family nanomaterials (GFNs) in human bronchial epithelial cells. Mutat. Res. Toxicol. Environ. Mutagen. 2016, 798, 1–10. [Google Scholar] [CrossRef]
- Ghosh, M.; Öner, D.; Poels, K.; Tabish, A.M.; Vlaanderen, J.; Pronk, A.; Kuijpers, E.; Lan, Q.; Vermeulen, R.; Bekaert, B.; et al. Changes in DNA methylation induced by multi-walled carbon nanotube exposure in the workplace. Nanotoxicology 2017, 11, 1195–1210. [Google Scholar] [CrossRef]
- Ghosh, M.; Öner, D.; Duca, R.-C.; Bekaert, B.; Vanoirbeek, J.; Godderis, L.; Hoet, P.H. Single-walled and multi-walled carbon nanotubes induce sequence-specific epigenetic alterations in 16 HBE cells. Oncotarget 2018, 9, 20351–20365. [Google Scholar] [CrossRef]
- Gong, C.; Tao, G.; Yang, L.; Liu, J.; Liu, Q.; Li, W.; Zhuang, Z. Methylation of PARP-1 promoter involved in the regulation of nano-SiO2-induced decrease of PARP-1 mRNA expression. Toxicol. Lett. 2012, 209, 264–269. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, M.; Zhang, J.; Ma, J.; Gao, M.; Zhang, Z.; Xu, Y.; Liu, S. Genome-Wide DNA Methylation Variations upon Exposure to Engineered Nanomaterials and Their Implications in Nanosafety Assessment. Adv. Mater. 2017, 29, 1604580. [Google Scholar] [CrossRef] [PubMed]
- Stapleton, P.A.; Hathaway, Q.A.; Nichols, C.E.; Abukabda, A.B.; Pinti, M.V.; Shepherd, D.L.; McBride, C.R.; Yi, J.; Castranova, V.C.; Hollander, J.M.; et al. Maternal engineered nanomaterial inhalation during gestation alters the fetal transcriptome. Part. Fibre Toxicol. 2018, 15, 3. [Google Scholar] [CrossRef]
- Cuvier, O.; Fierz, B. Dynamic chromatin technologies: From individual molecules to epigenomic regulation in cells. Nat. Rev. Genet. 2017, 18, 457–472. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Lee, H.; Lee, H.; Lee, H. Dopant-Dependent Toxicity of CeO2 Nanoparticles Is Associated with Dynamic Changes in H3K4me3 and H3K27me3 and Transcriptional Activation of NRF2 Gene in HaCaT Human Keratinocytes. Int. J. Mol. Sci. 2021, 22, 3087. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, V.; Ray, P.; Sachdeva, G.; Bhatt, P. Biocompatible arsenic trioxide nanoparticles induce cell cycle arrest by p21WAF1/CIP1 expression via epigenetic remodeling in LNCaP and PC3 cell lines. Life Sci. 2016, 148, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Ma, N.; Zhou, H.; Wang, Q.; Zhang, H.; Wang, P.; Hou, H.; Wen, H.; Gao, F. Zinc oxide nanoparticles-induced epigenetic change and G2/M arrest are associated with apoptosis in human epidermal keratinocytes. Int. J. Nanomed. 2016, 11, 3859–3874. [Google Scholar] [CrossRef] [Green Version]
- Shvedova, A.A.; Yanamala, N.; Kisin, E.R.; Khailullin, T.O.; Birch, M.E.; Fatkhutdinova, L. Integrated Analysis of Dysregulated ncRNA and mRNA Expression Profiles in Humans Exposed to Carbon Nanotubes. PLoS ONE 2016, 11, e0150628. [Google Scholar] [CrossRef]
- Liu, L.; Jia, Y.; Zhang, X.; Chen, S.; Wang, S.; Zhu, J.; Zheng, L.; Chen, Z.; Huang, L. Identification of the function and regulatory network of circ_009773 in DNA damage induced by nanoparticles of neodymium oxide. Toxicol. Vitr. 2022, 78, 105271. [Google Scholar] [CrossRef]
- Eom, H.-J.; Chatterjee, N.; Lee, J.; Choi, J. Integrated mRNA and micro RNA profiling reveals epigenetic mechanism of differential sensitivity of Jurkat T cells to AgNPs and Ag ions. Toxicol. Lett. 2014, 229, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Chappell, G.; Pogribny, I.P.; Guyton, K.Z.; Rusyn, I. Epigenetic alterations induced by genotoxic occupational and environmental human chemical carcinogens: A systematic literature review. Mutat. Res. Mutat. Res. 2016, 768, 27–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.; Meng, P.; Cheng, S.; Jiang, X.; Zhang, J.; Qin, X.; Tang, Q.; Bai, L.; Zou, Z.; Chen, C. Pregnancy exposure to carbon black nanoparticles induced neurobehavioral deficits that are associated with altered m6A modification in offspring. NeuroToxicology 2020, 81, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Chu, C.; Su, X.; Zhang, N.; Zhou, L.; Zhang, M.; Yang, S.; Shi, L.; Zhao, B.; Niu, Y.; et al. N6-methyladenosine-dependent primary microRNA-126 processing activated PI3K-AKT-mTOR pathway drove the development of pulmonary fibrosis induced by nanoscale carbon black particles in rats. Nanotoxicology 2020, 14, 1–20. [Google Scholar] [CrossRef]
- Oh, J.-H.; Son, M.-Y.; Choi, M.-S.; Kim, S.; Choi, A.-Y.; Lee, H.-A.; Kim, K.-S.; Kim, J.; Song, C.W.; Yoon, S. Integrative analysis of genes and miRNA alterations in human embryonic stem cells-derived neural cells after exposure to silver nanoparticles. Toxicol. Appl. Pharmacol. 2016, 299, 8–23. [Google Scholar] [CrossRef]
- Srivastava, A.K.; Yadav, S.S.; Mishra, S.; Yadav, S.K.; Parmar, D.; Yadav, S. A combined microRNA and proteome profiling to investigate the effect of ZnO nanoparticles on neuronal cells. Nanotoxicology 2020, 14, 757–773. [Google Scholar] [CrossRef]
- Wu, T.; Li, Y.; Liang, X.; Liu, X.; Tang, M. Identification of potential circRNA-miRNA-mRNA regulatory networks in response to graphene quantum dots in microglia by microarray analysis. Ecotoxicol. Environ. Saf. 2020, 208, 111672. [Google Scholar] [CrossRef]
- Rossnerova, A.; Honkova, K.; Chvojkova, I.; Pelclova, D.; Zdimal, V.; Hubacek, J.; Lischkova, L.; Vlckova, S.; Ondracek, J.; Dvorackova, S.; et al. Individual DNA Methylation Pattern Shifts in Nanoparticles-Exposed Workers Analyzed in Four Consecutive Years. Int. J. Mol. Sci. 2021, 22, 7834. [Google Scholar] [CrossRef]
- Liou, S.-H.; Wu, W.-T.; Liao, H.-Y.; Chen, C.-Y.; Tsai, C.-Y.; Jung, W.-T.; Lee, H.-L. Global DNA methylation and oxidative stress biomarkers in workers exposed to metal oxide nanoparticles. J. Hazard. Mater. 2017, 331, 329–335. [Google Scholar] [CrossRef]
- Rossnerova, A.; Honkova, K.; Pelclova, D.; Zdimal, V.; Hubacek, J.A.; Chvojkova, I.; Vrbova, K.; Rossner, J.P.; Topinka, J.; Vlckova, S.; et al. DNA Methylation Profiles in a Group of Workers Occupationally Exposed to Nanoparticles. Int. J. Mol. Sci. 2020, 21, 2420. [Google Scholar] [CrossRef] [Green Version]
- Bicho, R.C.; Scott-Fordsmand, J.J.; Amorim, M.J.B. Multigenerational Exposure to WCCo Nanomaterials—Epigenetics in the Soil Invertebrate Enchytraeus crypticus. Nanomaterials 2020, 10, 836. [Google Scholar] [CrossRef]
- Zhou, W.; Tian, D.; He, J.; Yan, X.; Zhao, J.; Yuan, X.; Peng, S. Prolonged exposure to carbon nanoparticles induced methylome remodeling and gene expression in zebrafish heart. J. Appl. Toxicol. 2019, 39, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Lin, W.; Lin, B.; Wu, K.; Fan, H.; Yu, Y. Persistent DNA methylation changes in zebrafish following graphene quantum dots exposure in surface chemistry-dependent manner. Ecotoxicol. Environ. Saf. 2019, 169, 370–375. [Google Scholar] [CrossRef] [PubMed]
- Wamucho, A.; Heffley, A.; Tsyusko, O.V. Epigenetic effects induced by silver nanoparticles in Caenorhabditis elegans after multigenerational exposure. Sci. Total Environ. 2020, 725, 138523. [Google Scholar] [CrossRef]
- Kohl, Y.; Rundén-Pran, E.; Mariussen, E.; Hesler, M.; El Yamani, N.; Longhin, E.M.; Dusinska, M. Genotoxicity of Nanomaterials: Advanced In Vitro Models and High Throughput Methods for Human Hazard Assessment—A Review. Nanomaterials 2020, 10, 1911. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Li, S.; Chen, J. Modeling better in vitro models for the prediction of nanoparticle toxicity: A review. Toxicol. Mech. Methods 2021, 31, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Tirumala, M.G.; Anchi, P.; Raja, S.; Rachamalla, M.; Godugu, C. Novel Methods and Approaches for Safety Evaluation of Nanoparticle Formulations: A Focus Towards In Vitro Models and Adverse Outcome Pathways. Front. Pharmacol. 2021, 12, 2157. [Google Scholar] [CrossRef] [PubMed]
- Mahto, S.K.; Charwat, V.; Ertl, P.; Rothen-Rutishauser, B.; Rhee, S.W.; Sznitman, J. Microfluidic platforms for advanced risk assessments of nanomaterials. Nanotoxicology 2015, 9, 381–395. [Google Scholar] [CrossRef]
- Wick, P.; Chortarea, S.; Guenat, O.T.; Roesslein, M.; Stucki, J.D.; Hirn, S.; Fink, A.; Rothen-Rutishauser, B. In vitro-ex vivo model systems for nanosafety assessment. Eur. J. Nanomed. 2015, 7, 169–179. [Google Scholar] [CrossRef] [Green Version]
- Ashammakhi, N.; Darabi, M.A.; Çelebi-Saltik, B.; Tutar, R.; Hartel, M.C.; Lee, J.; Hussein, S.M.; Goudie, M.J.; Cornelius, M.B.; Dokmeci, M.R.; et al. Microphysiological Systems: Next Generation Systems for Assessing Toxicity and Therapeutic Effects of Nanomaterials. Small Methods 2020, 4, 1900589. [Google Scholar] [CrossRef]
- Joseph, X.; Akhil, V.; Arathi, A.; Mohanan, P. Comprehensive Development in Organ-On-A-Chip Technology. J. Pharm. Sci. 2022, 111, 18–31. [Google Scholar] [CrossRef] [PubMed]
- Donoghue, L.; Nguyen, K.; Graham, C.; Sethu, P. Tissue Chips and Microphysiological Systems for Disease Modeling and Drug Testing. Micromachines 2021, 12, 139. [Google Scholar] [CrossRef] [PubMed]
- Fröhlich, E. Comparison of conventional and advanced in vitro models in the toxicity testing of nanoparticles. Artif. Cells, Nanomedicine, Biotechnol. 2018, 46, 1091–1107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- E Watson, D.; Hunziker, R.; Wikswo, J.P. Fitting tissue chips and microphysiological systems into the grand scheme of medicine, biology, pharmacology, and toxicology. Exp. Biol. Med. 2017, 242, 1559–1572. [Google Scholar] [CrossRef] [Green Version]
- Prasad, M.; Kumar, R.; Buragohain, L.; Kumari, A.; Ghosh, M. Organoid Technology: A Reliable Developmental Biology Tool for Organ-Specific Nanotoxicity Evaluation. Front. Cell Dev. Biol. 2021, 9, 2445. [Google Scholar] [CrossRef]
- Sanches, P.L.; Geaquinto, L.R.D.O.; Cruz, R.; Schuck, D.C.; Lorencini, M.; Granjeiro, J.M.; Ribeiro, A.R.L. Toxicity Evaluation of TiO2 Nanoparticles on the 3D Skin Model: A Systematic Review. Front. Bioeng. Biotechnol. 2020, 8, 575. [Google Scholar] [CrossRef]
- Lee, E.M.; Singh, D.; Singh, D.; Choi, S.M.; Zo, S.M.; Park, S.J.; Han, S.S. Novel Alginate-Gelatin Hybrid Nanoparticle for Drug Delivery and Tissue Engineering Applications. J. Nanomater. 2014, 2014, 147. [Google Scholar] [CrossRef]
- Doak, S.H.; Clift, M.J.D.; Costa, A.; Delmaar, C.; Gosens, I.; Halappanavar, S.; Kelly, S.; Pejinenburg, W.J.G.M.; Rothen-Rutishauser, B.; Schins, R.P.F.; et al. The Road to Achieving the European Commission’s Chemicals Strategy for Nanomaterial Sustainability—A PATROLS Perspective on New Approach Methodologies. Small 2022, 18, 2200231. [Google Scholar] [CrossRef]
- Kermanizadeh, A.; Valli, J.; Sanchez, K.; Hutter, S.; Pawlowska, A.; Whyte, G.; Moritz, W.; Stone, V. Particulate and drug-induced toxicity assessed in novel quadruple cell human primary hepatic disease models of steatosis and pre-fibrotic NASH. Arch. Toxicol. 2022, 96, 287–303. [Google Scholar] [CrossRef]
- Vanderburgh, J.; Sterling, J.A.; Guelcher, S.A. 3D Printing of Tissue Engineered Constructs for In Vitro Modeling of Disease Progression and Drug Screening. Ann. Biomed. Eng. 2017, 45, 164–179. [Google Scholar] [CrossRef] [Green Version]
- VijayaVenkataRaman, S.; Yan, W.-C.; Lu, W.F.; Wang, C.-H.; Fuh, J.Y.H. 3D bioprinting of tissues and organs for regenerative medicine. Adv. Drug Deliv. Rev. 2018, 132, 296–332. [Google Scholar] [CrossRef]
- Ahmed, H.M.; Teixeira, L.S.M. New Endeavors of (Micro)Tissue Engineering: Cells Tissues Organs on-Chip and Communication Thereof. Cells Tissues Organs 2022, 211, 88–102. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Park, S.E.; Huh, D.D. Organ-on-a-chip technology for nanoparticle research. Nano Converg. 2021, 8, 20. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.X.Z.; Radisic, M. Organ-on-a-chip platforms for evaluation of environmental nanoparticle toxicity. Bioact. Mater. 2021, 6, 2801–2819. [Google Scholar] [CrossRef] [PubMed]
- Huh, D.; Matthews, B.D.; Mammoto, A.; Montoya-Zavala, M.; Hsin, H.Y.; Ingber, D.E. Reconstituting Organ-Level Lung Functions on a Chip. Science 2010, 328, 1662–1668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahn, S.; Ardoña, H.A.M.; Lind, J.U.; Eweje, F.; Kim, S.L.; Gonzalez, G.M.; Liu, Q.; Zimmerman, J.F.; Pyrgiotakis, G.; Zhang, Z.; et al. Mussel-inspired 3D fiber scaffolds for heart-on-a-chip toxicity studies of engineered nanomaterials. Anal. Bioanal. Chem. 2018, 410, 6141–6154. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hu, C.; Chang, Q.; Deng, Q.; Yang, X.; Wu, Y. Study of the neurotoxicity of indoor airborne nanoparticles based on a 3D human blood-brain barrier chip. Environ. Int. 2020, 143, 105598. [Google Scholar] [CrossRef]
- Xu, C.; Zhang, M.; Chen, W.; Jiang, L.; Chen, C.; Qin, J. Assessment of Air Pollutant PM2.5 Pulmonary Exposure Using a 3D Lung-on-Chip Model. ACS Biomater. Sci. Eng. 2020, 6, 3081–3090. [Google Scholar] [CrossRef]
- Schuller, P.; Rothbauer, M.; Kratz, S.R.; Höll, G.; Taus, P.; Schinnerl, M.; Genser, J.; Bastús, N.G.; Moriones, O.H.; Puntes, V.; et al. A lab-on-a-chip system with an embedded porous membrane-based impedance biosensor array for nanoparticle risk assessment on placental Bewo trophoblast cells. Sens. Actuators B Chem. 2020, 312, 127946. [Google Scholar] [CrossRef]
- Yin, F.; Zhu, Y.; Zhang, M.; Yu, H.; Chen, W.; Qin, J. A 3D human placenta-on-a-chip model to probe nanoparticle exposure at the placental barrier. Toxicol. Vitr. 2018, 54, 105–113. [Google Scholar] [CrossRef]
- Kohl, Y.; Biehl, M.; Spring, S.; Hesler, M.; Ogourtsov, V.; Todorovic, M.; Owen, J.; Elje, E.; Kopecka, K.; Moriones, O.H.; et al. Microfluidic In Vitro Platform for (Nano)Safety and (Nano)Drug Efficiency Screening. Small 2021, 17, e2006012. [Google Scholar] [CrossRef] [PubMed]
- Bendre, A.; Bhat, M.P.; Lee, K.-H.; Altalhi, T.; Alruqi, M.A.; Kurkuri, M. Recent developments in microfluidic technology for synthesis and toxicity-efficiency studies of biomedical nanomaterials. Mater. Today Adv. 2022, 13, 100205. [Google Scholar] [CrossRef]
- Li, M.; Gong, J.; Gao, L.; Zou, T.; Kang, J.; Xu, H. Advanced human developmental toxicity and teratogenicity assessment using human organoid models. Ecotoxicol. Environ. Saf. 2022, 235, 113429. [Google Scholar] [CrossRef] [PubMed]
- Clarke, G.A.; Hartse, B.X.; Asli, A.E.N.; Taghavimehr, M.; Hashemi, N.; Shirsavar, M.A.; Montazami, R.; Alimoradi, N.; Nasirian, V.; Ouedraogo, L.J.; et al. Advancement of Sensor Integrated Organ-on-Chip Devices. Sensors 2021, 21, 1367. [Google Scholar] [CrossRef]
- Zoio, P.; Lopes-Ventura, S.; Oliva, A. Barrier-on-a-Chip with a Modular Architecture and Integrated Sensors for Real-Time Measurement of Biological Barrier Function. Micromachines 2021, 12, 816. [Google Scholar] [CrossRef]
- Alexander, F.; Eggert, S.; Wiest, J. A novel lab-on-a-chip platform for spheroid metabolism monitoring. Cytotechnology 2018, 70, 375–386. [Google Scholar] [CrossRef]
- Shaegh, S.A.M.; De Ferrari, F.; Zhang, Y.S.; Nabavinia, M.; Mohammad, N.B.; Ryan, J.; Pourmand, A.; Laukaitis, E.; Sadeghian, R.B.; Nadhman, A.; et al. A microfluidic optical platform for real-time monitoring of pH and oxygen in microfluidic bioreactors and organ-on-chip devices. Biomicrofluidics 2016, 10, 044111. [Google Scholar] [CrossRef]
- Azizgolshani, H.; Coppeta, J.R.; Vedula, E.M.; Marr, E.E.; Cain, B.P.; Luu, R.J.; Lech, M.P.; Kann, S.H.; Mulhern, T.J.; Tandon, V.; et al. High-throughput organ-on-chip platform with integrated programmable fluid flow and real-time sensing for complex tissue models in drug development workflows. Lab a Chip 2021, 21, 1454–1474. [Google Scholar] [CrossRef]
- Fuchs, S.; Johansson, S.; Tjell, A.; Werr, G.; Mayr, T.; Tenje, M. In-Line Analysis of Organ-on-Chip Systems with Sensors: Integration, Fabrication, Challenges, and Potential. ACS Biomater. Sci. Eng. 2021, 7, 2926–2948. [Google Scholar] [CrossRef]
- Ehrlich, A.; Tsytkin-Kirschenzweig, S.; Ioannidis, K.; Ayyash, M.; Riu, A.; Note, R.; Ouedraogo, G.; Vanfleteren, J.; Cohen, M.; Nahmias, Y. Microphysiological flux balance platform unravels the dynamics of drug induced steatosis. Lab Chip 2018, 18, 2510–2522. [Google Scholar] [CrossRef]
- Weltin, A.; Hammer, S.; Noor, F.; Kaminski, Y.; Kieninger, J.; Urban, G.A. Accessing 3D microtissue metabolism: Lactate and oxygen monitoring in hepatocyte spheroids. Biosens. Bioelectron. 2017, 87, 941–948. [Google Scholar] [CrossRef] [PubMed]
- Dornhof, J.; Kieninger, J.; Muralidharan, H.; Maurer, J.; Urban, G.A.; Weltin, A. Microfluidic organ-on-chip system for multi-analyte monitoring of metabolites in 3D cell cultures. Lab Chip 2022, 22, 225–239. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Patel, D.; Kwa, T.; Haque, A.; Matharu, Z.; Stybayeva, G.; Gao, Y.; Diehl, A.M.; Revzin, A. Liver injury-on-a-chip: Microfluidic co-cultures with integrated biosensors for monitoring liver cell signaling during injury. Lab Chip 2015, 15, 4467–4478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.S.; Aleman, J.; Shin, S.R.; Kilic, T.; Kim, D.; Shaegh, S.A.M.; Massa, S.; Riahi, R.; Chae, S.; Hu, N.; et al. Multisensor-integrated organs-on-chips platform for automated and continual in situ monitoring of organoid behaviors. Proc. Natl. Acad. Sci. USA 2017, 114, E2293–E2302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glieberman, A.L.; Pope, B.D.; Zimmerman, J.F.; Liu, Q.; Ferrier, J.P.; Kenty, J.H.R.; Schrell, A.M.; Mukhitov, N.; Shores, K.L.; Tepole, A.B.; et al. Synchronized stimulation and continuous insulin sensing in a microfluidic human Islet on a Chip designed for scalable manufacturing. Lab Chip 2019, 19, 2993–3010. [Google Scholar] [CrossRef]
- Roy, K. Advances in QSAR Modeling. Applications in Pharmaceutical, Chemical, Food, Agricultural and Environmental Sciences; Springer: Cham, Switzerland, 2017; Volume 555, p. 39. [Google Scholar]
- Hitaoka, S.; Chuman, H. Revisiting the Hansch–Fujita approach and development of a fundamental QSAR. J. Pestic. Sci. 2013, 38, 60–67. [Google Scholar] [CrossRef] [Green Version]
- Winter, R.; Montanari, F.; Noé, F.; Clevert, D.-A. Learning continuous and data-driven molecular descriptors by translating equivalent chemical representations. Chem. Sci. 2019, 10, 1692–1701. [Google Scholar] [CrossRef] [Green Version]
- Lima, T.S.M.; Souza, W.; Geaquinto, L.R.O.; Sanches, P.L.; Stepień, E.L.; Meneses, J.; Gortari, E.F.-D.; Meisner-Kober, N.; Himly, M.; Granjeiro, J.M.; et al. Nanomaterial Exposure, Extracellular Vesicle Biogenesis and Adverse Cellular Outcomes: A Scoping Review. Nanomaterials 2022, 12, 1231. [Google Scholar] [CrossRef]
- Huang, H.-J.; A Kraevaya, O.; I Voronov, I.; A Troshin, P.; Hsu, S.-H. Fullerene Derivatives as Lung Cancer Cell Inhibitors: Investigation of Potential Descriptors Using QSAR Approaches. Int. J. Nanomed. 2020, 15, 2485–2499. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Zhai, H.; She, X.; Si, H. Quantitative Structure-activity Relationships; Studying the Toxicity of Metal Nanoparticles. Curr. Top. Med. Chem. 2020, 20, 2506–2517. [Google Scholar] [CrossRef]
- Roy, J.; Roy, K. Modeling and mechanistic understanding of cytotoxicity of metal oxide nanoparticles (MeOxNPs) to Escherichia coli: Categorization and data gap filling for untested metal oxides. Nanotoxicology 2022, 1–13. [Google Scholar] [CrossRef]
- Wang, S.; Yan, X.; Su, G.; Yan, B. Cytotoxicity Induction by the Oxidative Reactivity of Nanoparticles Revealed by a Combinatorial GNP Library with Diverse Redox Properties. Molecules 2021, 26, 3630. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Wang, Z.; Vijver, M.G.; Peijnenburg, W.J. Probing nano-QSAR to assess the interactions between carbon nanoparticles and a SARS-CoV-2 RNA fragment. Ecotoxicol. Environ. Saf. 2021, 219, 112357. [Google Scholar] [CrossRef] [PubMed]
- Dhumal, D.M.; Patil, P.D.; Kulkarni, R.V.; Akamanchi, K.G. Experimentally Validated QSAR Model for Surface PKaPrediction of Heterolipids Having Potential as Delivery Materials for Nucleic Acid Therapeutics. ACS Omega 2020, 5, 32023–32031. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, X.; Xu, S.; Zheng, H.; Zhang, L.; Chen, J.; Hong, H.; Kusko, R.; Li, R. Quantitative Structure–Activity Relationship Models for Predicting Inflammatory Potential of Metal Oxide Nanoparticles. Environ. Health Perspect. 2020, 128, 067010. [Google Scholar] [CrossRef]
- Shi, H.; Pan, Y.; Yang, F.; Cao, J.; Tan, X.; Yuan, B.; Jiang, J. Nano-SAR Modeling for Predicting the Cytotoxicity of Metal Oxide Nanoparticles to PaCa2. Molecules 2021, 26, 2188. [Google Scholar] [CrossRef]
- Gousiadou, C.; Robinson, R.L.M.; Kotzabasaki, M.; Doganis, P.; A Wilkins, T.; Jia, X.; Sarimveis, H.; Harper, S.L. Machine learning predictions of concentration-specific aggregate hazard scores of inorganic nanomaterials in embryonic zebrafish. Nanotoxicology 2021, 15, 446–476. [Google Scholar]
- Liu, G.; Yan, X.; Sedykh, A.; Pan, X.; Zhao, X.; Yan, B.; Zhu, H. Analysis of model PM2.5-induced inflammation and cytotoxicity by the combination of a virtual carbon nanoparticle library and computational modeling. Ecotoxicol. Environ. Saf. 2020, 191, 110216. [Google Scholar] [CrossRef]
- Qi, R.; Pan, Y.; Cao, J.; Jia, Z.; Jiang, J. The cytotoxicity of nanomaterials: Modeling multiple human cells uptake of functionalized magneto-fluorescent nanoparticles via nano-QSAR. Chemosphere 2020, 249, 126175. [Google Scholar] [CrossRef]
- Toropova, A.P.; Toropov, A.A.; Leszczynska, D.; Leszczynski, J. Application of quasi-SMILES to the model of gold-nanoparticles uptake in A549 cells. Comput. Biol. Med. 2021, 136, 104720. [Google Scholar] [CrossRef]
- Toropov, A.A.; Toropova, A.P. The system of self-consistent models for the uptake of nanoparticles in PaCa2 cancer cells. Nanotoxicology 2021, 15, 995–1004. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, S.; Toropova, A.P.; Toropov, A.A. Correlation intensity index: Mathematical modeling of cytotoxicity of metal oxide nanoparticles. Nanotoxicology 2020, 14, 1118–1126. [Google Scholar] [CrossRef] [PubMed]
- Toropov, A.A.; Toropova, A.P. Quasi-SMILES as a basis for the development of models for the toxicity of ZnO nanoparticles. Sci. Total Environ. 2021, 772, 145532. [Google Scholar] [CrossRef] [PubMed]
- Toropova, A.P.; Toropov, A.A.; Leszczynski, J.; Sizochenko, N. Using quasi-SMILES for the predictive modeling of the safety of 574 metal oxide nanoparticles measured in different experimental conditions. Environ. Toxicol. Pharmacol. 2021, 86, 103665. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Kumar, A. Unswerving modeling of hepatotoxicity of cadmium containing quantum dots using amalgamation of quasiSMILES, index of ideality of correlation, and consensus modeling. Nanotoxicology 2021, 15, 1199–1214. [Google Scholar] [CrossRef]
- Fjodorova, N.; Novič, M.; Venko, K.; Drgan, V.; Rasulev, B.; Saçan, M.T.; Erdem, S.S.; Tugcu, G.; Toropova, A.P.; Toropov, A.A. How fullerene derivatives (FDs) act on therapeutically important targets associated with diabetic diseases. Comput. Struct. Biotechnol. J. 2022, 20, 913–924. [Google Scholar] [CrossRef]
- Bunmahotama, W.; Vijver, M.G.; Peijnenburg, W. Development of a Quasi–Quantitative Structure–Activity Relationship Model for Prediction of the Immobilization Response of Daphnia magna Exposed to Metal-Based Nanomaterials. Environ. Toxicol. Chem. 2022, 41, 1439–1450. [Google Scholar] [CrossRef]
- Wilkinson, M.D.; Dumontier, M.; Aalbersberg, I.J.; Appleton, G.; Axton, M.; Baak, A.; Blomberg, N.; Boiten, J.W.; da Silva Santos, L.B.; Bourne, P.E.; et al. The FAIR Guiding Principles for scientific data management and stewardship. Sci. Data 2016, 3, 160018. [Google Scholar] [CrossRef] [Green Version]
- Jeliazkova, N.; Chomenidis, C.; Doganis, P.; Fadeel, B.; Grafström, R.; Hardy, B.; Hastings, J.; Hegi, M.; Jeliazkov, V.; Kochev, N.; et al. The eNanoMapper database for nanomaterial safety information. Beilstein J. Nanotechnol. 2015, 6, 1609–1634. [Google Scholar] [CrossRef] [Green Version]
- COM(2019) 640 Final; Communication from the Commission to the European Parliament, the European Council, the Council, the European Economic and Social Committee and the Committee of the Regions the European Green Deal. European Commission: Brussels, Belgium, 2019.
- COM(2020) 98 Final; Communication from the Commission to the European Parliament, The Council, The European Economic and Social Committee and The Committee of The Regions A New Circular Economy Action Plan For a Cleaner and More Competitive Europe. European Commission: Brussels, Belgium, 2020.
- Transforming Our World: The 2030 Agenda for Sustainable Development. Available online: https://sdgs.un.org/2030agenda (accessed on 26 April 2022).
- Gottardo, S.; Mech, A.; Drbohlavová, J.; Małyska, A.; Bøwadt, S.; Sintes, J.R.; Rauscher, H. Towards safe and sustainable innovation in nanotechnology: State-of-play for smart nanomaterials. NanoImpact 2021, 21, 100297. [Google Scholar] [CrossRef]
- Friedersdorf, L.E.; Bjorkland, R.; Klaper, R.; Sayes, C.; Wiesner, M.R. Fifteen years of nanoEHS research advances science and fosters a vibrant community. Nat. Nanotechnol. 2019, 14, 996–998. [Google Scholar] [CrossRef]
- Dekkers, S.; Wijnhoven, S.W.; Braakhuis, H.M.; Soeteman-Hernandez, L.G.; Sips, A.J.; Tavernaro, I.; Kraegeloh, A.; Noorlander, C.W. Safe-by-Design part I: Proposal for nanospecific human health safety aspects needed along the innovation process. NanoImpact 2020, 18, 100227. [Google Scholar] [CrossRef]
- Shandilya, N.; Marcoulaki, E.; Barruetabeña, L.; Llopis, I.R.; Noorlander, C.; Jiménez, A.S.; Oudart, Y.; Puelles, R.C.; Pérez-Fernández, M.; Falk, A.; et al. Perspective on a risk-based roadmap towards the implementation of the safe innovation approach for industry. NanoImpact 2020, 20, 100258. [Google Scholar] [CrossRef]
- Pomar-Portillo, V.; Park, B.; Crossley, A.; Vázquez-Campos, S. Nanosafety research in Europe—Towards a focus on nano-enabled products. NanoImpact 2021, 22, 100323. [Google Scholar] [CrossRef] [PubMed]
- Tsalidis, G.A.; Soeteman-Hernández, L.G.; Noorlander, C.W.; Saedy, S.; van Ommen, J.R.; Vijver, M.G.; Korevaar, G. Safe-and-Sustainable-by-Design Framework Based on a Prospective Life Cycle Assessment: Lessons Learned from a Nano-Titanium Dioxide Case Study. Int. J. Environ. Res. Public Health 2022, 19, 4241. [Google Scholar] [CrossRef]
- Jantunen, A.P.K.; Gottardo, S.; Rasmussen, K.; Crutzen, H.P. An inventory of ready-to-use and publicly available tools for the safety assessment of nanomaterials. NanoImpact 2018, 12, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Soeteman-Hernandez, L.G.; Apostolova, M.D.; Bekker, C.; Dekkers, S.; Grafström, R.C.; Groenewold, M.; Handzhiyski, Y.; Herbeck-Engel, P.; Hoehener, K.; Karagkiozaki, V.; et al. Safe innovation approach: Towards an agile system for dealing with innovations. Mater. Today Commun. 2019, 20, 100548. [Google Scholar] [CrossRef]
- Jiménez, A.S.; Puelles, R.; Perez-Fernandez, M.; Barruetabeña, L.; Jacobsen, N.R.; Suarez-Merino, B.; Micheletti, C.; Manier, N.; Salieri, B.; Hischier, R.; et al. Safe(r) by design guidelines for the nanotechnology industry. NanoImpact 2022, 25, 100385. [Google Scholar] [CrossRef]
- No. 96. ENV/JM/MONO(2020)36/REV1; Moving Towards a Safe(r) Innovation Approach (SIA) for More Sustainable Nanomaterials and Nano-Enabled Products. Series on the Safety of Manufactured Nanomaterials, Organisation for Economic Co-operation and Development (OECD): Paris, France, 2020.
- Keijer, T.; Bakker, V.; Slootweg, J.C. Circular chemistry to enable a circular economy. Nat. Chem. 2019, 11, 190–195. [Google Scholar] [CrossRef]
- Furxhi, I.; Perucca, M.; Blosi, M.; de Ipiña, J.L.; Oliveira, J.; Murphy, F.; Costa, A.L. ASINA Project: Towards a Methodological Data-Driven Sustainable and Safe-by-Design Approach for the Development of Nanomaterials. Front. Bioeng. Biotechnol. 2022, 9, 805096. [Google Scholar] [CrossRef]
- Salieri, B.; Barruetabeña, L.; Rodríguez-Llopis, I.; Jacobsen, N.R.; Manier, N.; Trouiller, B.; Chapon, V.; Hadrup, N.; Jiménez, A.S.; Micheletti, C.; et al. Integrative approach in a safe by design context combining risk, life cycle and socio-economic assessment for safer and sustainable nanomaterials. NanoImpact 2021, 23, 100335. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, A.S.; Puelles, R.; Pérez-Fernández, M.; Gómez-Fernández, P.; Barruetabeña, L.; Jacobsen, N.R.; Suarez-Merino, B.; Micheletti, C.; Manier, N.; Trouiller, B.; et al. Safe(r) by design implementation in the nanotechnology industry. NanoImpact 2020, 20, 100267. [Google Scholar] [CrossRef]
- Schaumann, G.E.; Philippe, A.; Bundschuh, M.; Metreveli, G.; Klitzke, S.; Rakcheev, D.; Grün, A.; Kumahor, S.K.; Kühn, M.; Baumann, T.; et al. Understanding the fate and biological effects of Ag- and TiO2-nanoparticles in the environment: The quest for advanced analytics and interdisciplinary concepts. Sci. Total Environ. 2014, 535, 3–19. [Google Scholar] [CrossRef]
- Nowack, B.; Baalousha, M.; Bornhöft, N.; Chaudhry, Q.; Cornelis, G.; Cotterill, J.; Gondikas, A.; Hassellöv, M.; Lead, J.; Mitrano, D.M.; et al. Progress towards the Validation of Modeled Environmental Concentrations of Engineered Nanomaterials by Analytical Measurements. Environ. Sci. Nano 2015, 2, 421–428. [Google Scholar] [CrossRef]
- Mitrano, D.; Nowack, B. The need for a life-cycle based aging paradigm for nanomaterials: Importance of real-world test systems to identify realistic particle transformations. Nanotechnology 2017, 28, 072001. [Google Scholar] [CrossRef]
- Cai, X.; Liu, X.; Jiang, J.; Gao, M.; Wang, W.; Zheng, H.; Xu, S.; Li, R. Molecular Mechanisms, Characterization Methods, and Utilities of Nanoparticle Biotransformation in Nanosafety Assessments. Small 2020, 16, e1907663. [Google Scholar] [CrossRef] [PubMed]
- Nowack, B.; Mitrano, D.M. Procedures for the production and use of synthetically aged and product released nanomaterials for further environmental and ecotoxicity testing. NanoImpact 2018, 10, 70–80. [Google Scholar] [CrossRef]
- Mitrano, D.M.; Motellier, S.; Clavaguera, S.; Nowack, B. Review of nanomaterial aging and transformations through the life cycle of nano-enhanced products. Environ. Int. 2015, 77, 132–147. [Google Scholar] [CrossRef] [Green Version]
- Klaine, S.J.; Koelmans, A.A.; Horne, N.; Carley, S.; Handy, R.D.; Kapustka, L.; Nowack, B.; von der Kammer, F. Paradigms to assess the environmental impact of manufactured nanomaterials. Environ. Toxicol. Chem. 2012, 31, 3–14. [Google Scholar] [CrossRef]
- Hischier, R.; Walser, T. Life cycle assessment of engineered nanomaterials: State of the art and strategies to overcome existing gaps. Sci. Total Environ. 2012, 425, 271–282. [Google Scholar] [CrossRef]
- Hischier, R.; Nowack, B.; Gottschalk, F.; Hincapie, I.; Steinfeldt, M.; Som, C. Life cycle assessment of façade coating systems containing manufactured nanomaterials. J. Nanoparticle Res. 2015, 17, 68. [Google Scholar] [CrossRef]
- Salieri, B.; Turner, D.A.; Nowack, B.; Hischier, R. Life Cycle Assessment of Manufactured Nanomaterials: Where Are We? NanoImpact 2018, 10, 108–120. [Google Scholar] [CrossRef]
- García Calvo, J.L.; Pérez, G.; Carballosa, P.; Erkizia, E.; Gaitero, J.J.; Guerrero, A. The effect of nanoparticles on the self-healing capacity of high performance concrete. In Nanotechnology in Eco-Efficient Construction: Materials, Processes and Applications; Woodhead Publishing: Shaston, UK, 2018; pp. 43–67. ISBN 9780081026410. [Google Scholar]
- Nizam, N.U.M.; Hanafiah, M.M.; Woon, K.S. A Content Review of Life Cycle Assessment of Nanomaterials: Current Practices, Challenges, and Future Prospects. Nanomaterials 2021, 11, 3324. [Google Scholar] [CrossRef] [PubMed]
- Chong, W.C.; Chung, Y.T.; Teow, Y.H.; Zain, M.M.; Mahmoudi, E.; Mohammad, A.W. Environmental impact of nanomaterials in composite membranes: Life cycle assessment of algal membrane photoreactor using polyvinylidene fluoride—Composite membrane. J. Clean. Prod. 2018, 202, 591–600. [Google Scholar] [CrossRef]
- Fernandes, S.; Esteves Da Silva, J.C.; Pinto Da Silva, L. Life Cycle Assessment of the Sustainability of Enhancing the Photodegradation Activity of TiO2 with Metal-Doping. Materials 2020, 13, 1487. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, S.; da Silva, J.C.E.; da Silva, L.P. Comparative life cycle assessment of high-yield synthesis routes for carbon dots. NanoImpact 2021, 23, 100332. [Google Scholar] [CrossRef]
- Pallas, G.; Vijver, M.G.; Peijnenburg, W.J.G.M.; Guinée, J. Life cycle assessment of emerging technologies at the lab scale: The case of nanowire-based solar cells. J. Ind. Ecol. 2020, 24, 193–204. [Google Scholar] [CrossRef]
- Pallas, G.; Vijver, M.G.; Peijnenburg, W.J.G.M.; Guinée, J. Ex ante life cycle assessment of GaAs/Si nanowire–based tandem solar cells: A benchmark for industrialization. Int. J. Life Cycle Assess. 2020, 25, 1767–1782. [Google Scholar] [CrossRef]
- Beloin-Saint-Pierre, D.; Hischier, R. Towards a more environmentally sustainable production of graphene-based materials. Int. J. Life Cycle Assess. 2021, 26, 327–343. [Google Scholar] [CrossRef]
- Garas, G.; Sayed, A.M.; Bakhoum, E.S.H. Application of nano waste particles in concrete for sustainable construction: A comparative study. Int. J. Sustain. Eng. 2021, 14, 2041–2047. [Google Scholar] [CrossRef]
- Lin, S.; Ng, S.-F.; Ong, W.-J. Life cycle assessment of environmental impacts associated with oxidative desulfurization of diesel fuels catalyzed by metal-free reduced graphene oxide. Environ. Pollut. 2021, 288, 117677. [Google Scholar] [CrossRef] [PubMed]
- Mio, A.; Bertagna, S.; Cozzarini, L.; Laurini, E.; Bucci, V.; Marinò, A.; Fermeglia, M. Multiscale modelling techniques in life cycle assessment: Application to nanostructured polymer systems in the maritime industry. Sustain. Mater. Technol. 2021, 29, e00327. [Google Scholar] [CrossRef]
- Musino, D.; Rosset, A.; Lelong, C.; Luche, S.; Bergé, V.; Brochard, G.; Plumail, M.; Trouiller, B.; Auger, A.; Guiot, A.; et al. CNC/AgNP hybrids as safer-by-design biocides in paints. Environ. Sci. Nano 2021, 8, 3673–3684. [Google Scholar] [CrossRef]
- Ghomi, E.R.; Khosravi, F.; Ardahaei, A.S.; Dai, Y.; Neisiany, R.; Foroughi, F.; Wu, M.; Das, O.; Ramakrishna, S. The Life Cycle Assessment for Polylactic Acid (PLA) to Make It a Low-Carbon Material. Polymers 2021, 13, 1854. [Google Scholar] [CrossRef]
- Ponnusamy, P.G.; Mani, S. Life cycle assessment of manufacturing cellulose nanofibril-reinforced chitosan composite films for packaging applications. Int. J. Life Cycle Assess. 2022, 27, 380–394. [Google Scholar] [CrossRef]
- Gonzalez, M.N.G.; Quiroga-Flores, R.; Börjesson, P. Life cycle assessment of a nanomaterial-based adsorbent developed on lab scale for cadmium removal: Comparison of the impacts of production, use and recycling. Clean. Environ. Syst. 2022, 4, 100071. [Google Scholar] [CrossRef]
- Beloin-Saint-Pierre, D.; Turner, D.A.; Salieri, B.; Haarman, A.; Hischier, R. How Suitable Is LCA for Nanotechnology Assessment? Overview of Current Methodological Pitfalls and Potential Solutions. Int. J. Life Cycle Assess. 2018, 23, 191–196. [Google Scholar] [CrossRef]
- Organisation for Economic Co-operation and Development (OECD). Nanomaterials in Waste Streams; OECD: Paris, France, 2016; ISBN 9789264240612. [Google Scholar]
- Zahra, Z.; Habib, Z.; Hyun, S.; Sajid, M. Nanowaste: Another Future Waste, Its Sources, Release Mechanism, and Removal Strategies in the Environment. Sustainability 2022, 14, 2041. [Google Scholar] [CrossRef]
- Brar, S.K.; Verma, M.; Tyagi, R.; Surampalli, R. Engineered nanoparticles in wastewater and wastewater sludge—Evidence and impacts. Waste Manag. 2010, 30, 504–520. [Google Scholar] [CrossRef]
- Gottschalk, F.; Nowack, B. The release of engineered nanomaterials to the environment. J. Environ. Monit. 2011, 13, 1145–1155. [Google Scholar] [CrossRef]
- Part, F.; Zecha, G.; Causon, T.; Sinner, E.-K.; Huber-Humer, M. Current limitations and challenges in nanowaste detection, characterisation and monitoring. Waste Manag. 2015, 43, 407–420. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Keller, A.A.; Cervantes-Avilés, P.; Nelson, J. Fast Multielement Quantification of Nanoparticles in Wastewater and Sludge Using Single-Particle ICP-MS. ACS ES&T Water 2021, 1, 205–213. [Google Scholar] [CrossRef]
- Thonemann, N.; Schulte, A.; Maga, D. How to Conduct Prospective Life Cycle Assessment for Emerging Technologies? A Systematic Review and Methodological Guidance. Sustainability 2020, 12, 1192. [Google Scholar] [CrossRef] [Green Version]
- Gottschalk, F.; Scholz, R.W.; Nowack, B. Probabilistic material flow modeling for assessing the environmental exposure to compounds: Methodology and an application to engineered nano-TiO2 particles. Environ. Model. Softw. 2010, 25, 320–332. [Google Scholar] [CrossRef]
- Adam, V.; Caballero-Guzman, A.; Nowack, B. Considering the forms of released engineered nanomaterials in probabilistic material flow analysis. Environ. Pollut. 2018, 243, 17–27. [Google Scholar] [CrossRef]
- Zheng, Y.; Nowack, B. Size-Specific, Dynamic, Probabilistic Material Flow Analysis of Titanium Dioxide Releases into the Environment. Environ. Sci. Technol. 2021, 55, 2392–2402. [Google Scholar] [CrossRef] [PubMed]
- Adam, V.; Wu, Q.; Nowack, B. Integrated dynamic probabilistic material flow analysis of engineered materials in all European countries. NanoImpact 2021, 22, 100312. [Google Scholar] [CrossRef] [PubMed]
- Hischier, R. Framework for LCI modelling of releases of manufactured nanomaterials along their life cycle. Int. J. Life Cycle Assess. 2014, 19, 838–849. [Google Scholar] [CrossRef] [Green Version]
- Gilbertson, L.M.; Wender, B.A.; Zimmerman, J.B.; Eckelman, M.J. Coordinating modeling and experimental research of engineered nanomaterials to improve life cycle assessment studies. Environ. Sci. Nano 2015, 2, 669–682. [Google Scholar] [CrossRef]
- No. 57 ENV/JM/MONO(2015)30; Guidance Manual Towards The Integration of Risk Assessment into Life Cycle Assessment of Nano-Enabled Applications. Series on the Safety of Manufactured Nanomaterials, Organisation for Economic Co-operation and Development (OECD): Paris, France, 2015.
- Salieri, B.; Hischier, R.; Quik, J.T.; Jolliet, O. Fate modelling of nanoparticle releases in LCA: An integrative approach towards “USEtox4Nano”. J. Clean. Prod. 2019, 206, 701–712. [Google Scholar] [CrossRef]
- Fantke, P.; Aylward, L.; Bare, J.; Chiu, W.A.; Dodson, R.; Dwyer, R.; Ernstoff, A.; Howard, B.; Jantunen, M.; Jolliet, O.; et al. Advancements in Life Cycle Human Exposure and Toxicity Characterization. Environ. Health Perspect. 2018, 126, 125001. [Google Scholar] [CrossRef]
- Salieri, B.; Kaiser, J.-P.; Rösslein, M.; Nowack, B.; Hischier, R.; Wick, P. Relative potency factor approach enables the use of in vitro information for estimation of human effect factors for nanoparticle toxicity in life-cycle impact assessment. Nanotoxicology 2020, 14, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Meesters, J.A.J.; Peijnenburg, W.J.G.M.; Hendriks, A.J.; Van De Meent, D.; Quik, J.T.K. A model sensitivity analysis to determine the most important physicochemical properties driving environmental fate and exposure of engineered nanoparticles. Environ. Sci. Nano 2019, 6, 2049–2060. [Google Scholar] [CrossRef]
- Wigger, H.; Nowack, B. Material-specific properties applied to an environmental risk assessment of engineered nanomaterials—Implications on grouping and read-across concepts. Nanotoxicology 2019, 13, 623–643. [Google Scholar] [CrossRef] [PubMed]
- Hetherington, A.C.; Borrion, A.L.; Griffiths, O.G.; McManus, M.C. Use of LCA as a development tool within early research: Challenges and issues across different sectors. Int. J. Life Cycle Assess. 2014, 19, 130–143. [Google Scholar] [CrossRef] [Green Version]
- Adrianto, L.R.; van der Hulst, M.K.; Tokaya, J.P.; Arvidsson, R.; Blanco, C.F.; Caldeira, C.; Guillén-Gonsálbez, G.; Sala, S.; Steubing, B.; Buyle, M.; et al. How can LCA include prospective elements to assess emerging technologies and system transitions? The 76th LCA Discussion Forum on Life Cycle Assessment, 19 November 2020. Int. J. Life Cycle Assess. 2021, 26, 1541–1544. [Google Scholar] [CrossRef]




| Conventional Methodology | Observed Interference | Proposed Solution | |
|---|---|---|---|
| Cause | Result/Interpretation | ||
| MTT reduction LDH leakage WST reduction | NM optical density; NM aggregation in cell medium | Falsely increased viability | Sample centrifugation after cell lysis |
| NM redox activity | Falsely decreased viability | None | |
| ELISA (cytokine release) | Protein adsorption to NMs | Falsely decreased cytokine production | Add serum proteins to NM suspension |
| Comet assay | Interference enzyme activity | Falsely decreased genotoxicity | None |
| ROS quantification (H2DCF-DA) | NM redox activity | Falsely increased ROS levels | None |
| NMs quench fluorescence; NMs scatter emitted fluorescence | Falsely decreased ROS levels | Sample centrifugation after cell lysis | |
| Genotoxicity Marker | Assays | References |
|---|---|---|
| Gene mutation | Bacterial reverse mutation (Ames test) | OECD TG 471 |
| In vitro mammalian mutagenicity assay: mouse lymphoma (L5178Y) TK+/-assay | OECD TG 490 | |
| In vitro mammalian mutagenicity assay: HPRT assay | OECD TG 476 | |
| In vivo gene mutation assay (transgenic rodent somatic and germ cell gene mutation) | OECD TG 488 | |
| Chromosomal damage assays | In vitro chromosomal aberration assay | OECD TG 473 |
| In vitro MN assay | OECD TG 487 | |
| In vivo (mammalian bone marrow) chromosomal aberration test | OECD TG 475 | |
| In vivo MN assay (mammalian erythrocyte MN) | OECD TG 474 | |
| DNA damage (strand-break and DNA adduct) | In vitro comet assay Modified in vitro comet assay with DNA repair enzymes (e.g., OGG1, FPG) | JaCVAM EURL-ECVAM/ICCVAM [94,99] |
| In vivo (mammalian alkaline) comet assay | OECD TG 489 | |
| DNA damage (DNA adduct) | HPLC/MS; ELISA | [104,109,110] |
| DNA damage response and repair | The γH2AX and 53BP1 foci count assay | [108,111] |
| Multiplex array for DNA repair activity | [109,110] | |
| FM-HCR assay | [112] |
| Epigenetic Endpoints | Specific Epigenetic Markers | Analytical Methods | References |
|---|---|---|---|
| DNA methylation | Global DNA methylation screening (5mc, 5hmC, 6mA, etc.) | HPLC/MS, ELISA, methylation-sensitive comet assay, pyrosequencing (repetitive sequences LINE-1 or Alu) | [121,122,123,124] |
| Gene-specific promoter methylation | Methylation-specific PCR | [121,125] | |
| Differentially methylated regions (whole-genome sequencing) | MPS, DNA methylation-specific microarrays, MeDIP followed by sequencing | [121,126] | |
| Histone modification | Whole genome (specific histone marker) | ChIP with DNA microarray, ChIP-Seq, ChIP-Chip | [127,128] |
| Gene-specific histone (specific) modification | ChIP-qPCR | [128,129] | |
| Global histone modification markers | HPLC/MS, ELISA, immunostaining, immunoblotting | [130,131] | |
| Noncoding RNAs | Whole genome | RNA-seq, microarray | [132,133] |
| Gene-specific | qPCR | [134] |
| Advanced Cell Models | Cell Types | Nanomaterial Exposure Conditions | Sensorization | Toxicological Assays | Key Biological Outcomes |
|---|---|---|---|---|---|
| Heart microphysiological system | NRVMs | TiO2 NPs at 10 and 100 μg·mL−1 and Ag NPs at 50 μg·mL−1 | Electrical sensors | LDH assay, MTT assay | The high-dose exposure of TiO2 NPs (100 μg·mL−1) demonstrated impaired contractile function and damaged tissue structure after 48 h of exposure. Ag NP exposure caused cytotoxicity [169] |
| Blood–brain barrier on a chip | HAs and HUVECs | INPM exposure at 0, 5, 10, 20, and 40 μg·mL−1 | - | ROS detection assay, CCK8 assay | The INPM could potentially activate several inflammatory pathways that directly damage brain structures and further lead to neurological diseases [170] |
| Liver on a chip | PRHs | 10 nm Fe3O4 NPs | - | - | Perfusion of Fe3O4 NPs results in the reduction in albumin and urea production, indicating potential liver injury [167] |
| Lung on a chip | HPAEpiC, HUVECs, and THP-1 | PM2.5 exposure at 200 and 400 μg·mL−1 | - | Immunofluorescence staining assay, FITC-dextran permeability assay, ELISA | A low concentration of PM2.5 causes limited cytotoxicity, but a higher concentration of PM2.5 (>200 μg·mL−1) could significantly increase the ROS generation, apoptosis, and inflammation responses of epithelial cells and endothelial cells on the barrier and attachments of monocytes to the vessels [171] |
| BEAS-2B and HUVECs | CSE at 10, 20, and 50 μg·mL−1 | - | RT-PCR, ELISA, Western blotting | Lung on a chip enables the study of nanoparticle adsorption during various breathing frequencies, puff profiles of smoking, breath-holding patterns during inhalation and exhalation, and particle deposition in the lungs and the respiratory tract [154] | |
| Placenta barrier on a chip | BeWo | 20 nm SiO2 and TiO2 NPs, and 80 nm ZnO NPs for 24 h | Membrane-bound impedance sensor array | ROS detection assay | SiO2 and TiO2 NPs induced no loss in barrier integrity. In contrast, ZnO NPs displayed severe acute cytotoxicity already after 4 h [172] |
| BeWo and HUVECs | TiO2 NPs exposure at 50 and 200 μg·mL−1 | - | Immunofluorescence staining assay, ROS detection assay | Gradually increased cell death with increasing concentrations of NPs, thereby potentially leading to placental membrane rupture [173] | |
| Gut/liver on a chip | Caco-2, HT29-MTX + HepG2, C3A | 50 nm carboxylated PS NPs | - | AST assay | Gut/liver chip model demonstrates compounding effects of interorgan crosstalk between gut and the liver in facilitating NP toxicity [167] |
| Lung/liver/kidney on a chip | A549 + HepG2 + TH-1 | Ag, Au-PEG, TiO2, and SiO2-FITC NPs | TEER measurements | Live/dead assay | The interconnection of the different modules aims at the simulation of whole-body exposure and response. SiO2-FITC NPs showed a cytotoxic effect on TH-1 after 12 h, which could be due to the interaction of NPs with cancerous cells releasing a substance that may have induced a cytotoxic effect [174,175] |
| Nanomaterials | Descriptors | Models 1 | Main Goal |
|---|---|---|---|
| FD | 204 molecular descriptors generated from the QSAR analyzing tools of BIOVIA Discovery Studio | LinReg | Predict the physicochemical properties of FDs that promote their cytotoxic effects/anticancer activity [193] |
| Metal NPs | 24 physicochemical descriptors and toxicity data | MLR | Predict the toxicity and design the structures of metal NPs with low toxicity [194] |
| Metal oxide NPs | 61 periodic table descriptors | MLR | Predict and investigate the essential descriptors responsible for the cytotoxicity of metal oxide NPs on E. coli cells under different conditions [195] |
| Gold NPs | Structural information (i.e., Dragon descriptors) of the surface ligands | MLR | Predict possible relationships between the oxidative reactivity of gold NPs and their cytotoxicity [196] |
| Carbon NPs | Physicochemical descriptors (molecular weight, overall surface area, volume, specific surface area, and sum of degrees) | Orthogonal PLS regression | Predict the interaction between carbon NPs and SARS-CoV-2 RNA fragments [197] |
| Amine-containing heterolipid NPs | 116 physicochemical descriptors | PLS regression coupled with stepwise forward algorithm | Predict the pKa of the amine-containing heterolipid NPs [198] |
| Metal oxide NPs | Quantum-mechanical computations (such as molecular geometries), physicochemical descriptors (such as zeta-potential in water), and periodic table descriptors (such as electronegativity of each atom) | PLS regression, DecTrees, SVM, and logReg | Predict the inflammatory potential of metal oxide NPs [199] |
| Functionalized magneto-fluorescent NPs | Norm index descriptors (describing the structural characteristics of the involved NPs) | RF | Predict the cellular uptake of functionalized magneto-fluorescent NPs to PaCa2 cells. Provide guidance for the design and manufacture of safer nanomaterials [200] |
| Metal and metal oxide NPs | Structural information (such as core structure and material type), supported by physicochemical descriptors (such as zeta potential and average agglomerate size in media) | DecTrees, GBR, KNN, LinReg, RF, SVM, and XGBoost | Predict the cytotoxicity of metal and metal oxide NPs in zebrafish embryos [201] |
| Virtual carbon NP library | 126 nanodescriptors (such as electronegativity of each atom) | KNN and RF | Predict cytotoxicity and inflammatory responses induced by PM2.5 [202] |
| Functionalized magneto-fluorescent NPs | Improved optimal quasi-SMILES-based descriptors | MC | Predict the cellular uptake of functionalized magneto-fluorescent NPs to PaCa2 and HUVEC cell lines [203] |
| Gold NPs | Optimal quasi-SMILES-based descriptors | MC | Predict the cellular uptake of gold NPs to A549 cells [204] |
| Functionalized magneto-fluorescent NPs | Optimal quasi-SMILES-based descriptors | MC | Develop self-consistent predictive models for the cellular uptake of functionalized magneto-fluorescent NPs to PaCa2 cells [205] |
| Metal oxide NPs | Optimal quasi-SMILES-based descriptors | MC | Predict the cell viability of different cell lines when exposed to metal oxide NPs [206] |
| ZnO NPs | Optimal quasi-SMILES-based descriptors | MC | Predict the toxicity of ZnO NPs in rats via intraperitoneal injections [207] |
| Metal oxide NPs | Optimal quasi-SMILES-based descriptors | MC | Predict the cell viability (expressed in %) and cytotoxicity (categorized as true or false) of different cell lines when exposed to 7 types of metal oxide NPs [208] |
| Cadmium QDs | Optimal quasi-SMILES-based descriptors | MC | Predict hepatic cell viability when exposed to cadmium QDs [209] |
| FDs | Structural information (such as polarizability), optimal quasi-SMILES-based descriptors, and physicochemical properties (obtained from Data Warrior) | MC and CPANN | Predict the binding score activity for 169 FDs related to 5 proteins classified as antidiabetes targets [210] |
| Metal-based nanomaterials | Optimal quasi-SMILES-based descriptors | MC | Predict the response of Daphnia magna when exposed to metal-based nanomaterials [211] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lebre, F.; Chatterjee, N.; Costa, S.; Fernández-de-Gortari, E.; Lopes, C.; Meneses, J.; Ortiz, L.; Ribeiro, A.R.; Vilas-Boas, V.; Alfaro-Moreno, E. Nanosafety: An Evolving Concept to Bring the Safest Possible Nanomaterials to Society and Environment. Nanomaterials 2022, 12, 1810. https://doi.org/10.3390/nano12111810
Lebre F, Chatterjee N, Costa S, Fernández-de-Gortari E, Lopes C, Meneses J, Ortiz L, Ribeiro AR, Vilas-Boas V, Alfaro-Moreno E. Nanosafety: An Evolving Concept to Bring the Safest Possible Nanomaterials to Society and Environment. Nanomaterials. 2022; 12(11):1810. https://doi.org/10.3390/nano12111810
Chicago/Turabian StyleLebre, Filipa, Nivedita Chatterjee, Samantha Costa, Eli Fernández-de-Gortari, Carla Lopes, João Meneses, Luís Ortiz, Ana R. Ribeiro, Vânia Vilas-Boas, and Ernesto Alfaro-Moreno. 2022. "Nanosafety: An Evolving Concept to Bring the Safest Possible Nanomaterials to Society and Environment" Nanomaterials 12, no. 11: 1810. https://doi.org/10.3390/nano12111810
APA StyleLebre, F., Chatterjee, N., Costa, S., Fernández-de-Gortari, E., Lopes, C., Meneses, J., Ortiz, L., Ribeiro, A. R., Vilas-Boas, V., & Alfaro-Moreno, E. (2022). Nanosafety: An Evolving Concept to Bring the Safest Possible Nanomaterials to Society and Environment. Nanomaterials, 12(11), 1810. https://doi.org/10.3390/nano12111810











