A Critical Review of the Antimicrobial and Antibiofilm Activities of Green-Synthesized Plant-Based Metallic Nanoparticles
Abstract
:1. Introduction
2. Microbial Origins and Antimicrobial Resistance of Bacterial and Fungal Infections
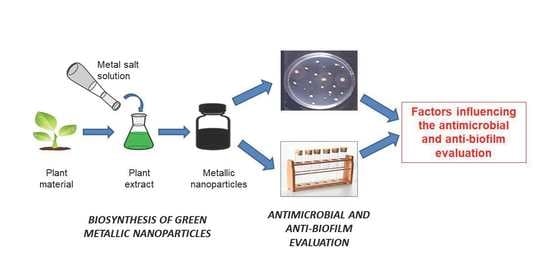
3. General Background on Antimicrobial and Anti-Biofilm Metallic Nanoparticles Green-Synthesized
3.1. Green Synthesis of MNPs
3.1.1. Plant-Mediated Synthesis of Silver, Gold and Zinc Oxide Nanoparticles
3.1.2. Synthesis of Platinum and Palladium Nanoparticles Using Plant Extracts
3.1.3. Biosynthesis of Other Green Metallic Nanoparticles
3.2. Antimicrobial Activities of Green-Synthesized MNPs
3.2.1. Silver Nanoparticles
| Plant Type | Part Used | Operative Conditions for Synthesis | NP Characteristics (Shape and Size) | Microbiological Analyzes (Operative Conditions) | Refs. | ||
|---|---|---|---|---|---|---|---|
| Methods, Incubation Temperature, Incubation Time, pH, Inoculum Density, Positive Control | Tested Bacteria and Fungi | MIC, ZOI or PI * | |||||
| Lysiloma acapulcensis | Roots | Silver nitrate 1 mM/plant extract 2% (1:1 v/v) Room temperature 2 min pH NM | Spherical 1.2–62 nm | Diffusion 37 °C 24 h pH NM Inoculum NM No control | E. coli ATCC 25922 P. aeruginosa ATCC 27853 S. aureus ATCC 49476 | 18 15 16 mm | [87] |
| Perilla frutescens | Leaves | Silver nitrate 2 mM/plant extract 10% (9:1 v/v) 50 °C 2 h pH NM | Spherical, rhombic, triangle, and rod 25.7 nm | Diffusion 37 °C 24 h pH NM Inoculum NM Streptomycin ** | E. coli B. substilis S. aureus | 14 12 10 mm | [149] |
| Ocimum canum | Leaves | Silver nitrate 1 mM/plant extract 10% (9:1 v/v) 80 °C 15 min pH NM | Spherical 6.1–32.1 nm | Diffusion 28 °C 24 h pH NM Inoculum NM No control | E. coli | 25 mm | [150] |
| Piper longum | Catkin | Silver nitrate 1 mM/plant extract 10% (5:1 v/v) Room temperature 2 h pH NM | Spherical 10–42 nm | Diffusion 37 °C 24 h pH NM Inoculum NM No control | B. cereus MTCC 1272 E. coli MTCC 1687 K. pneumoniae MTCC 530 Proteus mirabilis MTCC 425 P. aeruginosa MTCC 1688 S. typhi MTCC 531 S. aureus MTCC 96 | 12 13 14 15 11 12 11 mm | [139] |
| Diospyros malabarica | Fruits | Silver nitrate 1 mM/plant extract 20% (9:1 v/v) 25 °C 1 h pH NM | Spherical 17.4 nm | Diffusion 37 °C 24 h pH NM Inoculum NM Streptomycin 10 µg Tetracycline 30 µg Chloramphenicol 30 µg | E. coli S. aureus | 13 12 mm | [151] |
| Pyrenacantha grandiflora | Tuber | Silver nitrate 1 mM/plant extract 0.1% (1:1 v/v) Room temperature Incubation time NM pH NM | Spherical 3–25 nm | Dilution 37 °C 24 h pH NM Inoculum NM No control | E. coli K. pneumoniae S. aureus | 0.8 0.8 0.8 µg/mL | [152] |
| Carissa carandas | Leaves | Silver nitrate 1 mM/plant extract 10% (9:1 v/v) 60 °C 1 h pH 7.2 | Spherical 30 nm | Diffusion 37 °C 24 h pH NM 1 × 108 CFU/mL No control | S. typhi Enterococcus faecalis Shigella flexneri Citrobacter spp. Gonococci spp. | 12 16 24 14 21 mm | [153] |
| Solanum tricobatum | Leaves | Silver nitrate 1 mM/plant extract 1.5% (1:10 v/v) 37 °C 24–48 h pH NM | Irregular 26.5 nm | Diffusion 35 °C 18 h pH NM Inoculum NM No control | S. aureus P. aeruginosa E. coli K. pneumoniae | 30 12 14 18 mm | [154] |
| Melissa officinalis | Leaves | Silver nitrate 5 mM/plant extract 25% (1:2 v/v) 25 °C 1 h pH NM | Spherical 12 nm | Diffusion 36 °C 24 h pH NM Inoculum NM No control | E. coli S. aureus | 12 13 mm | [155] |
| Piper betle | Leaves | Silver nitrate 1 mM/plant extract 10% (10:1 v/v) Room temperature 24 h pH NM | Spherical Size NM | Diffusion 30 °C 24 h pH NM Inoculum NM No control | B. subtilis Klebsiella planticola | 14 13 mm | [156] |
| Rosa canina | Fruits | Silver nitrate 1 mM/plant extract% NM (5:1 v/v) 85 °C Incubation time NM pH NM | Spherical 13–21 nm | Dilution 37 °C 24 h pH NM 2.4 × 107 CFU/mL No control | Bacillus cereus E. coli ATCC 10536 S. aureus ATCC 6538 P. aeruginosa ATCC 9027 Enterococcus hirae ATCC 10541 Legionella pneumophila ATCC 33152 | 32 256 256 128 256 16 µg/mL | [157] |
| Dilution 25 °C 48 h pH NM 2.4 × 107 CFU/mL No control | C. albicans | 128 μg/mL | |||||
| Fagonia indica | Callus | Silver nitrate 4 mM/plant extract 2% (1:1 v/v) 20 °C 3 h pH NM | Cubic Size NM | Diffusion 37 °C 24 h pH NM 1 × 108 CFU/mL Ciprofloxacin ** | E. coli ATCC 23716 S. typhi ATCC 35664 Shigella sonnei ATCC 29930 Citrobacteramalonaticus ATCC 25405 | 12 13 13 12 mm | [158] |
| Barleria longiflora | Leaves | Silver nitrate 1 mM/plant extract 20% (9:1 v/v) Temperature NM Incubation time NM pH NM | Spherical 2.4 ± 0.5 nm | Diffusion 37 °C 24 h pH NM Inoculum NM Chloramphenicol ** | Enterococcus spp. Streptococcus spp. Bacillus megaterium Pseudomonas putida P. aeruginosa S. aureus | 18 16 15 17 18 14.5 mm | [159] |
| Ipomoea batatas | Outer peels | Silver nitrate 1 mM/plant extract 40% (10:1 v/v) 55 °C 24 h pH NM | Shape NM Size NM | Diffusion Temperature NM Incubation time NM pH NM Inoculum NM No control | Enterococcus feacium DB 01 S. enteritica KCCM 11806 Listeria monocytogenes ATCC 19111 B. cereus KCTC 3624 S. aureus ATCC 13565 | 10 11 11 11 0 mm | [160] |
| Oedera genistifolia | Leaves | Silver nitrate 0.1 mM/plant extract 20% (9:1 v/v) Room temperature 1 h pH NM | Spherical 10–60 nm | Dilution 37 °C 24 h pH NM 1 × 108 CFU/mL Ciprofloxacin ** | Enterobacter cloacae ATCC 13047 Listeria ivanovic ATCC 19119 Streptococcus uberis ATCC 700407 S. aureus ATCC 29213 Vibrio spp. Mycobacterium smergatis ATCC 19420 | 0.5 1 0.5 0.5 0.25 0.25 mg/mL | [161] |
| Derris trifoliate | Seeds | Silver nitrate 1 mM/plant extract 20% (20:1 v/v) Temperature NM Incubation time NM pH NM | Spherical 16 ± 5 nm | Diffusion NM 24 h pH NM Inoculum NM No control | E. coli MTCC 723 K. pneumoniae MTCC 109 P. aeruginosa MTCC 424 S. aureus MTCC 96 | 19.5 20 36 0 mm | [162] |
| Ficus krishnae | Stem bark | Silver nitrate 1 mM/plant extract 5% (1:1 v/v) 37 °C 24 h pH NM | Spherical 160–260 nm | Diffusion 37 °C 24 h pH NM Inoculum NM No control | E. coli MTCC 45 S. typhimurium MTCC 98 S. aureus ATCC 29122 | 18 13 12 mm | [163] |
| Psidium guajava | Leaves | Silver nitrate 10 mM/plant extract 2% (10:1 v/v) 70 °C 1 h pH NM | Spherical 96 ± 4 nm | Diffusion 37 ± 2 °C 48 h pH NM 1–2 × 105 CFU/mL No control | C. albicans ATCC 10231 | 14.2 mm | [164] |
| Citrus limon | Leaves | Silver nitrate 2 mM/plant extract 20% (9:1 v/v) 25 °C 1 h pH NM | Spherical 8–15 nm | Diffusion Temperature NM 18–24 h pH NM Inoculum size NM No control | Fusarium oxysporium Alternaria brassicicola | 15 10 mm | [165] |
| Chaenomeles sinensis | Fruits | Silver nitrate 1 mM/plant extract 10% (ratio NM) 80 °C 65 min pH NM | Spherical 5–20 nm | Diffusion 37 °C 24 h pH NM Inoculum NM Neomycin ** | E. coli S. aureus | 14 10 mm | [166] |
| Persicaria odorata | Leaves | Silver nitrate 1 mM/plant extract 2% (10:1 v/v) 25 °C 24 h pH NM | Spherical 11 ± 3 nm | Dilution 37 °C 18 h pH NM 1 × 106 CFU/mL No control | S. epidermidis ATCC 12228 MRSA ATCC 43300 | 3-LR *** 6-LR | [167] |
| Citrus reticulata | Peels | Silver nitrate 1 mM/plant extract 21.8% (1:1 v/v) Temperature NM Incubation time NM pH NM | Spherical 45 nm | Dilution 37 °C 48 h pH NM 1 × 105 CFU/mL No control | Desulfovibrio spp. | 3-LR | [168] |
| Cuccuma longa | Rhizome | Silver nitrate 1 mM/plant extract 6.8% (4:1 v/v) Temperature NM 24 h pH NM | Spherical 18 nm | Dilution 37 °C 24 h pH NM 1 × 108–109 CFU/mL No control | E. coli Listeria monocytogenes | 4-LR 4-LR | [169] |
| Plant Type | Part Used | Operative Conditions for Synthesis | NP Characteristics (Shape and Size) | Microbiological Analyzes (Operative Conditions) | Refs. | ||
|---|---|---|---|---|---|---|---|
| Methods, Incubation Temperature, Incubation Time, pH, Inoculum Density, Positive Control | Tested Bacteria and Fungi | MIC, ZOI or PI * | |||||
| Punica granatum | Peel | Silver nitrate */plant extract 5% (ratioNM) Temperature NM Incubation time NM pH NM | Spherical 32–85 nm | Microtiter plate 37 °C 24 h pH NM 1.5 × 108 CFU/mL No control | P. aeruginosa ATCC 10662 | 89.6% | [170] |
| Artemisia scoporia | NM | Silver nitrate 1000 mM/plant extract 10% (20:1 v/v) Temperature NM 24 h pH NM | Spherical 10–80 nm | Microtiter plate 37 °C 24 h pH NM 1.5 × 108 CFU/mL No control | S. aureus | 6.25 µg/mL | [171] |
| Prosopis juliflora | Leaves | Silver nitrate 1 mM/plant extract 10% (9.5:0.5 v/v) 25 °C 40 min pH NM | Spherical 10–20 nm | Congo red agar plate 37 °C 24–48 h pH NM Inoculum size NM No control | B. substilis P. aeruginosa | NM NM | [172] |
| Malva sylvestris | Leaves | Silver nitrate 1 mM/plant extract 20% (10:0.4 v/v) Temperature NM Incubation time NM pH NM | Spherical 10–50 nm | Dilution 37 °C 40 h pH NM 1 × 108 CFU/mL No control | P. aeruginosa 48 P. aeruginosa B 52 | 62.5 62.5 μg/mL | [173] |
| Cannabis sativa | Stem | Silver nitrate 1 mM/plant extract 10% (1:1 v/v) Temperature NM Incubation time NM pH NM | Spherical 20–40 nm | Microtriter plate 37 °C 24 h pH NM 2–5 × 106 CFU/mL No control | P. aeruginosa PA01 E. coli UTI89 S. epidermidis | 6.25 12.5 50 µg/mL | [174] |
| Rhodiola rosea | Rhizome | Silver nitrate 5 mM/plant extract 10% (2:8 v/v) 90 °C 10 min pH NM | Spherical 15–30 nm | Dilution 37 °C 24 h pH NM 1–2 × 106 CFU/mLNo control | P. aeruginosa E. coli | 50 100 µg/mL | [131] |
| Flacourtia indica | Leaves | Silver nitrate 1 mM/plant extract 10% (1:1 v/v) 70 °C Incubation time NM pH NM | Spherical 45.9–64.9 nm | Congo red 37 °C 24 h pH NM Inoculum size NM No control | Acinetobacter baumannii SAB5 P. aeruginosa ETPS11 K. pneumoniae SKP7 P. mirabilis PPM8 E. coli ETEC12 | 80 80 80 80 80 μg/mL | [116] |
| Dodonaea viscosa | Leaves | Silver nitrate 1 mM/plant extract 10% (ratio NM) Temperature NM 18 h pH NM | Spherical 40–55 nm | Crystal violet assay 37 °C 24 h pH NM 1 × 107 CFU/mL No control | C. albicans Candida tropicalis Candida glabrata | 80 80 80% | [175] |
| Piper betle | Leaves | Silver nitrate 1 mM/plant extract 5% (19:1 v/v) 37 °C 6 h pH NM | Spherical 156.4 nm | Microtiter plate 18 °C Temperature NM pH NM Inoculum size NM No control | Serratia marcescens Proteus mirabilis | 71 69% | [176] |
| Pedalium murex | Seed | Silver nitrate 1 mM/plant extract 5% (49:1 v/v) Temperature NM 20 min pH NM | Hexagonal 20–30 nm | Microtiter plate 37 °C 24 h pH NM Inoculum size NM No control | Enterococcus faecalis S. aureus Shigella sonnei P. aeruginosa | 64 62 50 54% | [177] |
| Solanum nigrum | Fruit | Silver nitrate 1 mM/plant extract 10% (50:1 v/v) Temperature NM10 min pH NM | Spherical 10–20 nm | Microtiter plate 37 °C 24 h pH 7.2 1 × 108 CFU/mL No control | Bacillus pumulis Enterococcus faecalis Proteus vulgaris Vibrio parahaemolyticus | 92 84 74 62% | [178] |
| Eucalyptus globulus | Leaves | Silver nitrate 1 mM/plant extract 20% (4:1 v/v) 60 °C 30 min pH 8 | Spherical 18 nm | Microtitre plate 37 °C 24 h pH NM 1 × 107 CFU/mL No control | P. aeruginosa S. aureus | 95 90% | [179] |
| Allophylus cobbe | Leaves | Silver nitrate 5 mM/plant extract 20% (10:1 v/v) 60 °C 6 h pH 8 | Spherical 2–10 nm | Microtitre plate 37 °C 4 h pH NM 1 × 106 CFU/mL No control | P. aeruginosa Shigella flexneri S. aureus Streptococcus pneumoniae | 90 90 60 75% | [180] |
| Cinnamomum aromaticum | NM | NM NM NM NM | Spherical 15–50 nm | Dilution Temperature NM 24 h pH NM 1 × 106 CFU/mL No control | Streptococcus agalactiae ATCC 27956 | 4 μg/mL | [181] |
| Prunica granatum | Leaves | Silver nitrate 1.5 mM/plant extract 5% (1:1 v/v) 31.4 °C 20 min pH NM | Spherical 37.5 nm | Congo red agar 37 °C 24 h pH NM Inoculum size NM No control | P. aeruginosa S. aureus | 45 28% | [182] |
| Terminalia catappa | Leaves | Silver nitrate 10 mM/plant extract 5% (1:1 v/v) 30 °C 20 min pH NM | Spherical 3.5–10.1 nm | Microtiter plate 37 °C 24 h pH NM 1 × 107 CFU/mL No control | P. aeruginosa S. aureus | 73.7 69.6% | [183] |
| Microtiter plate 37 °C 24 h pH NM 5 × 106 CFU/mL No control | C. albicans | 63.6% | |||||
3.2.2. Gold Nanoparticles
| Plant Type | Part Used | Operative Conditions for Synthesis | NP Characteristics (Shape and Size) | Microbiological Analyzes (Operative Conditions) | Refs. | ||
|---|---|---|---|---|---|---|---|
| Methods, Incubation Temperature, Incubation Time, pH, Inoculum Density, Positive Control | Tested Bacteria and Fungi | MIC, DOI or PI * | |||||
| Piper betle | Leaves | Gold (III) chloride 1 mM/plant extract 1% (10:1 v/v) 30 °C 24 h pH NM | Spherical Size NM | Diffusion 30 °C 24 h pH NM Inoculum size NM No control | B. subtilis Klebsiella planticola | 13 14 mm | [156] |
| Musa acuminata | Flowers | Chloroauric acid 1 mM/plant extract 25% (9:1 v/v) Room temperature 30 min pH NM | Spherical 10–16 nm | Diffusion Temperature NM 24 h pH NMInoculum size NM Streptomycin 10 μg | S. aureus Enterococcus faecalis E. coli S. typhi P. aeruginosa Proteus mirabilis | 0 11 7 9 9 8 mm | [192] |
| Zingiber officinale | Roots | Chloroauric acid 1 mM/plant extract 1% (2:1 v/v) 50 °C 24 h pH NM | Hexagonal 10–20 nm | Diffusion 37 °C 24 h pH NM 1.5 × 108 CFU/mL No control | S. aureus E. coli K. pneumoniae | 14 11 17 mm | [193] |
| Areca catechu | Nut | Chloroauric acid 1 mM/plant extract 5% (10:1 v/v) 80 °C 1 h pH NM | Spherical 14 nm | Diffusion 37 °C 24 h pH NM Inoculum size NM No control | S. aureus E. coli | 12 14 mm | [194] |
| Momordica cochinchinensis | Rhizome | Chloroauric acid 0.01 mM/plant extract 10% (2:1 v/v) Room temperature 24 h pH NM | Spherical 16 ± 2 nm | Diffusion 37 ± 1 °C 24 h pH NM 1 × 108 CFU/mL Streptomycin 100 µg/mL | S. aureus E. coli B. subtilis P. aeruginosa | 19 22 19 24 mm | [195] |
| Plumeria alba | Flowers | Chloroauric acid 1 mM/plant extract 5% (5:2 v/v) Room temperature 4 h pH NM | Spherical 15 nm | Diffusion 37 °C 24 h pH NM Inoculum size NM No control | E. coli | 16 mm | [196] |
| Coleus forskohlii | Root | Chloroauric acid 0.1 mM/plant extract 8% (1:1 v/v) Room temperature 2 h pH 13 | Spherical 5 nm | Diffusion 37 °C 24–48 h pH NM Inoculum size NM Tetracyclin 30 μg/mL | Proteus vulgaris Micrococcus luteus | 18 14 mm | [197] |
| Euphorbia wallichii | Leaves | Chloroauric acid 1 mM/plant extract 5% (1:10 v/v) 30 °C 24 h pH NM | Hexagonal 8 nm | Dilution 34 °C 24 h pH NM Inoculum size NM Streptomycin ** | E. coli S. aureus Bacillus pumilus P. aeruginosa K. pneumonia | 21 15 21 17 17 mm | [198] |
| Coleus aromaticus | Leaves | Chloroauric acid 1 mM/plant extract 30% (1:1 v/v) 100 °C 30 min pH NM | Triangular 20 nm | Diffusion 37 °C 24 h pH NM 1 × 108 CFU/mL No control | S. epidermidis E. coli | 22 27 mm | [199] |
| Origanum vulgare | Leaves | Chloroauric acid 1 mM/plant extract 10% (10:1 v/v) 85 °C 1 min pH NM | Spherical 52 nm | Diffusion 37 °C 24 h pH NM 1 × 108 CFU/mL No control | Salmonella enteritidis ATCC 13076 E. coli ATCC 25922 Listeria monocytogenes ATCC 13932 S. aureus ATCC 6538 C. albicans ATCC 10231 | 10 8 10 21 28 mm | [200] |
| Perilla frutescens | Leaves | Chloroauric acid 1 mM/plant extract 10% (1:10 v/v) 30 °C 10 min pH NM | Triangular 50 nm | Diffusion 37 °C 24 h pH NM Inoculum size NM No control | E. coli B. subtilis S. aureus | 14 10 10 mm | [201] |
| Parkia roxburghii | Leaves | Chloroauric acid 1 mM/plant extract 1% (1:1 v/v) 30 °C 12 h pH NM | Quasi-spherical 5–25 nm | Diffusion 37 °C 24 h pH NM Inoculum size NM No control | S. aureus E. coli | NM NM | [202] |
| Cibotium barometz | Roots | Chloroauric acid 1 mM/plant extract 5% (20:1 v/v) 80 °C Incubation time NM pH NM | Spherical 23 nm | Diffusion 37 °C 24 h pH NM Inoculum size NM Neomycin 30 µg | E. coli ATCC 10798 S. aureus ATCC 6538 Salmonella enterica ATCC 13076 P. aeruginosa ATCC 10145 | 16 17 13 12 mm | [203] |
| Mangifera indica | Seed | Chloroauric acid 1 mM/plant extract 10% (6:4 v/v) 80 °C 1 h pH NM | Spherical 50 nm | Diffusion 37 °C 24–48 h pH NM 1 × 108 CFU/mL No control | E. coli S. aureus | 25 25 μg/mL | [204] |
| Rhodiola rosea | Rhizome | Chloroauric acid 1 mM/plant extract 10% (10:1 v/v) 80 °C 30 min pH NM | Spherical 13–17 nm | Diffusion 37 °C 24 h pH NM Inoculum size NM No control | S. aureus ATCC 29213 E. coli ATCC 25922 | 15 12 mm | [131] |
| Amomum villosum | Fruit | Chloroauric acid 1 mM/plant extract 10% (10:1 v/v) 100 °C 60 min pH NM | Spherical 5–10 nm | Diffusion 37 °C 24 h pH NM Inoculum size NM Neomycin ** | S. aureus E. coli | 15 15 mm | [205] |
| Syzygium cumini | Seed | Chloroauric acid 1 mM/plant extract 2% (1:2 v/v) 90 °C 1 h pH NM | Spherical 13–30 nm | Diffusion 32 °C 24 h pH NM1 × 104 CFU/mL Gentamicin ** | E. coli B. subtilis S. aureus | 30 33 29 mm | [206] |
| Hovenia dulcis | Fruit | Chloroauric acid 1 mM/plant extract 2.5% (5:1 v/v) 80 °C 10 min pH NM | Spherical and hexagonal 20 nm | Diffusion 37 °C 24 h pH NM Inoculum size NM Ciprofloxacin 100 µg | E. coli S. aureus | 18 19 mm | [207] |
| Inonotus obliquus | Leaves | Chloroauric acid 1 mM/plant extract 5% (19:1 v/v) Room temperature 30 min pH NM | Spherical 23 nm | Diffusion 37 °C 24 h pH NM Inoculum size NM No control | B. subtilis S. aureus E. coli | 12 16 14 mm | [208] |
| Gloriosa superba | Leaves | Chloroauric acid 1 mM/plant extract 10% (20:1 v/v) 50–60 °C 10 min pH 5.2 | Triangular and spherical 20 nm | Diffusion 37 °C 24 h pH NM 1.5 × 108 CFU/mL Ampicillin 30 µg | B. subtilis ATCC 6633 E. coli MTCC 40 | 6.3 5.3 mm | [209] |
3.2.3. Zinc Oxide Nanoparticles
3.2.4. Platinum Nanoparticles
3.2.5. Palladium Nanoparticles
3.2.6. Other Green Metallic Nanoparticles
4. Methods for Testing Antibacterial and Antifungal Activities of Green MNPs
4.1. Analytical Techniques: Diffusion and Dilution Susceptibility Testing Methods
4.2. Factors Influencing the Evaluation of Antimicrobial Activities of Metallic Nanoparticles
4.2.1. Types of Bacterial and Fungal Species and Strains
4.2.2. Inoculum Density
4.2.3. Agar Depth and Spacing of Impregnated Discs
4.2.4. Timing of Disc Application
4.2.5. Temperature and Time of Incubation
4.2.6. Size and Shape of Nanoparticles
4.2.7. Zeta Potential of Nanoparticles
4.2.8. pH of Culture Media
4.2.9. Antibiotic and Antifungal Reference Standards
4.2.10. Synergistic Activity of Nanoparticles with Antimicrobial Substances
5. Methods for Testing Anti-Biofilm Activities of Green Metallic Nanoparticles
5.1. Microplate Assays
5.2. Factors Influencing the Evaluation of Antibiofilm Activities of Metallic Nanoparticles
5.2.1. Storage Conditions
5.2.2. Type of Microorganisms
5.2.3. Inoculum Density
5.2.4. Culture Medium
5.2.5. Type of Microplates
5.2.6. Time and Temperature of Incubation
5.2.7. Washing, Fixation and Staining Steps
6. Conclusions and Perspective
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- National Nanotechnology Initiative. What Is Nanotechnology? National Nanotechnology Initiative: Alexandria, VA, USA, 2000.
- Ijaz, I.; Gilani, E.; Nazir, A.; Bukhari, A. Detail Review on Chemical, Physical and Green Synthesis, Classification, Characterizations and Applications of Nanoparticles. Green Chem. Lett. Rev. 2020, 13, 59–81. [Google Scholar] [CrossRef]
- Iravani, S.; Korbekandi, H.; Mirmohammadi, S.V.; Zolfaghari, B. Synthesis of Silver Nanoparticles: Chemical, Physical and Biological Methods. Res. Pharm. Sci. 2014, 9, 385–406. [Google Scholar] [PubMed]
- Gudikandula, K.; Charya Maringanti, S. Synthesis of Silver Nanoparticles by Chemical and Biological Methods and Their Antimicrobial Properties. J. Exp. Nanosci. 2016, 11, 714–721. [Google Scholar] [CrossRef]
- De Marco, B.A.; Rechelo, B.S.; Tótoli, E.G.; Kogawa, A.C.; Salgado, H.R.N. Evolution of Green Chemistry and Its Multidimensional Impacts: A Review. Saudi Pharm. J. 2019, 27, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hurst, G.A. Systems Thinking Approaches for International Green Chemistry Education. Curr. Opin. Green Sustain. Chem. 2020, 21, 93–97. [Google Scholar] [CrossRef]
- Iravani, S. Bacteria in Nanoparticle Synthesis: Current Status and Future Prospects. Int. Sch. Res. Not. 2014, 2014, 359316. [Google Scholar] [CrossRef] [Green Version]
- Shah, M.; Fawcett, D.; Sharma, S.; Tripathy, S.K.; Poinern, G.E.J. Green Synthesis of Metallic Nanoparticles via Biological Entities. Materials 2015, 8, 7278–7308. [Google Scholar] [CrossRef] [Green Version]
- Kambale, E.K.; Nkanga, C.I.; Mutonkole, B.I.; Bapolisi, A.M.; Tassa, D.O.; Liesse, J.I.; Krause, R.W.M.; Memvanga, P.B. Green Synthesis of Antimicrobial Silver Nanoparticles Using Aqueous Leaf Extracts from Three Congolese Plant Species (Brillantaisia patula, Crossopteryx febrifuga and Senna siamea). Heliyon 2020, 6, e04493. [Google Scholar] [CrossRef]
- Vanlalveni, C.; Lallianrawna, S.; Biswas, A.; Selvaraj, M.; Changmai, B.; Rokhum, S.L. Green Synthesis of Silver Nanoparticles Using Plant Extracts and their Antimicrobial Activities: A Review of Recent Literature. RSC Adv. 2021, 11, 2804–2837. [Google Scholar] [CrossRef]
- Rónavári, A.; Igaz, N.; Adamecz, D.I.; Szerencsés, B.; Molnar, C.; Kónya, Z.; Pfeiffer, I.; Kiricsi, M. Green Silver and Gold Nanoparticles: Biological Synthesis Approaches and Potentials for Biomedical Applications. Molecules 2021, 26, 844. [Google Scholar] [CrossRef]
- Roy, A.; Bulut, O.; Some, S.; Mandal, A.K.; Yilmaz, M.D. Green Synthesis of Silver Nanoparticles: Biomolecule-Nanoparticle Organizations Targeting Antimicrobial Activity. RSC Adv. 2019, 9, 2673–2702. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.; Gautam, P.K.; Verma, A.; Singh, V.; Shivapriya, P.M.; Shivalkar, S.; Sahoo, A.K.; Samanta, S.K. Green Synthesis of Metallic Nanoparticles as Effective Alternatives to Treat Antibiotics Resistant Bacterial Infections: A Review. Biotechnol. Rep. 2020, 25, e00427. [Google Scholar] [CrossRef] [PubMed]
- Dikshit, P.K.; Kumar, J.; Das, A.K.; Sadhu, S.; Sharma, S.; Singh, S.; Gupta, P.K.; Kim, B.S. Green Synthesis of Metallic Nanoparticles: Applications and Limitations. Catalysts 2021, 11, 902. [Google Scholar] [CrossRef]
- Mohanta, Y.K.; Biswas, K.; Jena, S.K.; Hashem, A.; Abd_Allah, E.F.; Mohanta, T.K. Anti-Biofilm and Antibacterial Activities of Silver Nanoparticles Synthesized by the Reducing Activity of Phytoconstituents Present in the Indian Medicinal Plants. Front. Microbiol. 2020, 11, 1143. [Google Scholar] [CrossRef]
- Shkodenko, L.; Kassirov, I. Metal Oxide Nanoparticles Against Bacterial Biofilms: Perspectives and Limitations. Microorganisms 2020, 8, 1545. [Google Scholar] [CrossRef]
- Ahmed, S.; Ahmad, M.; Swami, B.L.; Ikram, S. A Review on Plants Extract Mediated Synthesis of Silver Nanoparticles for Antimicrobial Applications: A Green Expertise. J. Adv. Res. 2016, 7, 17–28. [Google Scholar] [CrossRef] [Green Version]
- Singh, J.; Dutta, T.; Kim, K.H.; Rawat, M.; Samddar, P.; Kumar, P. ‘Green’ Synthesis of Metals and Their Oxide Nanoparticles: Applications for Environmental Remediation. J. Nanobiotechnol. 2018, 16, 84. [Google Scholar] [CrossRef]
- Khan, Z.U.H.; Sadiq, H.M.; Shah, N.S.; Khan, A.U.; Muhammad, N.; Hassan, S.U.; Tahir, K.; Safi, S.Z.; Khan, F.U.; Imran, M.; et al. Greener Synthesis of Zinc Oxide Nanoparticles Using Trianthema Portulacastrum Extract and Evaluation of Its Photocatalytic and Biological Applications. J. Photochem. Photobiol. B Biol. 2019, 192, 147–157. [Google Scholar] [CrossRef]
- Perugu, S.; Nagati, V.; Bhanoori, M. Green Synthesis of Silver Nanoparticles Using Leaf Extract of Medicinally Potent Plant Saraca Indica: A Novel Study. Appl. Nanosci. 2016, 6, 747–753. [Google Scholar] [CrossRef] [Green Version]
- Das, R.K.; Pachapur, V.L.; Lonappan, L.; Naghdi, M.; Pulicharla, R.; Maiti, S.; Cledon, M.; Dalila, L.M.A.; Sarma, S.J.; Brar, S.K. Biological Synthesis of Metallic Nanoparticles: Plants, Animals and Microbial Aspects. Nanotechnol. Environ. Eng. 2017, 2, 18. [Google Scholar] [CrossRef] [Green Version]
- Shaikh, S.; Nazam, N.; Rizvi, S.M.D.; Ahmad, K.; Baig, M.H.; Lee, E.J.; Choi, I. Mechanistic Insights into the Antimicrobial Actions of Metallic Nanoparticles and Their Implications for Multidrug Resistance. Int. J. Mol. Sci. 2019, 20, 2468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rana, A.; Yadav, K.; Jagadevan, S. A Comprehensive Review on Green Synthesis of Nature-Inspired Metal Nanoparticles: Mechanism, Application and Toxicity. J. Clean. Prod. 2020, 272, 122880. [Google Scholar] [CrossRef]
- Naikoo, G.A.; Mustaqeem, M.; Hassan, I.U.; Awan, T.; Arshad, F.; Salim, H.; Qurashi, A. Bioinspired and Green Synthesis of Nanoparticles from Plant Extracts with Antiviral and Antimicrobial Properties: A Critical Review. J. Saudi Chem. Soc. 2021, 25, 101304. [Google Scholar] [CrossRef]
- Kamran, U.; Bhatti, H.N.; Iqbal, M.; Nazir, A. Green Synthesis of Metal Nanoparticles and Their Applications in Different Fields: A Review. Z. Phys. Chem. 2019, 233, 1325–1349. [Google Scholar] [CrossRef]
- Shyam, A.; Chandran, S.S.; George, B.; E, S. Plant Mediated Synthesis of AgNPs and Its Applications: An Overview. Inorg. Nano-Met. Chem. 2021, 51, 1646–1662. [Google Scholar] [CrossRef]
- Martín, R.; Miquel, S.; Ulmer, J.; Kechaou, N.; Langella, P.; Bermúdez-Humarán, L.G. Role of Commensal and Probiotic Bacteria in Human Health: A Focus on Inflammatory Bowel Disease. Microb. Cell Fact. 2013, 12, 71. [Google Scholar] [CrossRef] [Green Version]
- Proença, J.T.; Barral, D.C.; Gordo, I. Commensal-to-Pathogen Transition: One-Single Transposon Insertion Results in Two Pathoadaptive Traits in Escherichia Coli-Macrophage Interaction. Sci. Rep. 2017, 7, 4504. [Google Scholar] [CrossRef]
- Dueker, M.E.; French, S.; O’Mullan, G.D. Comparison of Bacterial Diversity in Air and Water of a Major Urban Center. Front. Microbiol. 2018, 9, 2868. [Google Scholar] [CrossRef]
- Marinari, S.; Mancinelli, R.; Campiglia, E.; Grego, S. Chemical and Biological Indicators of Soil Quality in Organic and Conventional Farming Systems in Central Italy. Ecol. Indic. 2006, 6, 701–711. [Google Scholar] [CrossRef]
- Casadevall, A.; Pirofski, L.A. Host-Pathogen Interactions: Basic Concepts of Microbial Commensalism, Colonization, Infection, and Disease. Infect. Immun. 2000, 68, 6511–6518. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Antimicrobial Resistance. 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 30 December 2021).
- Gao, L.; Wang, H.; Zheng, B.; Huang, F. Combating Antibiotic Resistance: Current Strategies for the Discovery of Novel Antibacterial Materials Based on Macrocycle Supramolecular Chemistry. Giant 2021, 7, 100066. [Google Scholar] [CrossRef]
- Friedman, N.D.; Temkin, E.; Carmeli, Y. The Negative Impact of Antibiotic Resistance. Clin. Microbiol. Infect. 2016, 22, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Panghal, M.; Kadyan, S.; Chaudhary, U.; Yadav, J.P. Green Silver Nanoparticles of Phyllanthus amarus: As an Antibacterial Agent against Multi Drug Resistant Clinical Isolates of Pseudomonas aeruginosa. J. Nanobiotechnol. 2014, 12, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, L.Y.; Ying, G.G.; Liu, Y.S.; Su, H.C.; Chen, J.; Liu, S.S.; Zhao, J.L. Discharge of Swine Wastes Risks Water Quality and Food Safety: Antibiotics and Antibiotic Resistance Genes from Swine Sources to the Receiving Environments. Environ. Int. 2016, 92–93, 210–219. [Google Scholar] [CrossRef]
- Zhu, Y.G.; Zhao, Y.; Li, B.; Huang, C.L.; Zhang, S.Y.; Yu, S.; Chen, Y.S.; Zhang, T.; Gillings, M.R.; Su, J.Q. Continental-Scale Pollution of Estuaries with Antibiotic Resistance Genes. Nat. Microbiol. 2017, 2, 270. [Google Scholar] [CrossRef]
- Zhuang, M.; Achmon, Y.; Cao, Y.; Liang, X.; Chen, L.; Wang, H.; Siame, B.A.; Leung, K.Y. Distribution of Antibiotic Resistance Genes in the Environment. Environ. Pollut. 2021, 285, 117402. [Google Scholar] [CrossRef]
- Su, H.C.; Liu, Y.S.; Pan, C.G.; Chen, J.; He, L.Y.; Ying, G.G. Persistence of Antibiotic Resistance Genes and Bacterial Community Changes in Drinking Water Treatment System: From Drinking Water Source to Tap Water. Sci. Total Environ. 2018, 616–617, 453–461. [Google Scholar] [CrossRef]
- Szekeres, E.; Chiriac, C.M.; Baricz, A.; Szőke-Nagy, T.; Lung, I.; Soran, M.L.; Rudi, K.; Dragos, N.; Coman, C. Investigating Antibiotics, Antibiotic Resistance Genes, and Microbial Contaminants in Groundwater in Relation to the Proximity of Urban Areas. Environ. Pollut. 2018, 236, 734–744. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, S.; Liu, X.; Chen, J.; Han, M.; Wang, Z.; Guo, W. Profiles of Antibiotic Resistance Genes in an Inland Salt-Lake Ebinur Lake, Xinjiang, China: The Relationship with Antibiotics, Environmental Factors, and Microbial Communities. Ecotoxicol. Environ. Saf. 2021, 221, 112427. [Google Scholar] [CrossRef]
- Urra, J.; Alkorta, I.; Mijangos, I.; Epelde, L.; Garbisu, C. Application of Sewage Sludge to Agricultural Soil Increases the Abundance of Antibiotic Resistance Genes without Altering the Composition of Prokaryotic Communities. Sci. Total Environ. 2019, 647, 1410–1420. [Google Scholar] [CrossRef]
- Hu, J.; Zhao, F.; Zhang, X.X.; Li, K.; Li, C.; Ye, L.; Li, M. Metagenomic Profiling of ARGs in Airborne Particulate Matters during a Severe Smog Event. Sci. Total Environ. 2018, 615, 1332–1340. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.; Lu, X.; Rensing, C.; Friman, V.P.; Geisen, S.; Chen, Z.; Yu, Z.; Wei, Z.; Zhou, S.; Zhu, Y. Hyperthermophilic Composting Accelerates the Removal of Antibiotic Resistance Genes and Mobile Genetic Elements in Sewage Sludge. Environ. Sci. Technol. 2018, 52, 266–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wimalasena, S.H.P.; Pathirana, H.N.K.; De Silvia, S.H.; Sugaya, E.; Nakai, T.; Heo, G.-J. Antibiotic Resistance and Virulence- Associated Gene Profile of Edwardsiella Tarda Isolated from Cultured Fish in Japan. Turk. J. Fish. Aquat. Sci. 2011, 19, 51–57. [Google Scholar] [CrossRef]
- Yakimov, A.; Bakhlanova, I.; Baitin, D. Targeting Evolution of Antibiotic Resistance by SOS Response Inhibition. Comput. Struct. Biotechnol. J. 2021, 19, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Smitha, M.S.; Singh, S.P. The Role of Nanotechnology in Combating Multi-Drug Resistant Bacteria. J. Nanosci. Nanotechnol. 2014, 14, 4745–4756. [Google Scholar] [CrossRef] [PubMed]
- Ventola, C.L. The Antibiotic Resistance Crisis: Causes and Threats: Part 1: Causes and Threats. Pharm. Ther. 2015, 40, 277–283. [Google Scholar]
- World Health Organization. Tripartite and UNEP Support OHHLEP’s Definition of “One Health”. 2021. Available online: https://www.who.int/news/item/01-12-2021-tripartite-and-unep-support-ohhlep-s-definition-of-one-health (accessed on 30 December 2021).
- Centers for Disease Control and Prevention. One Health, One Health Basics. 2018. Available online: https://www.cdc.gov/onehealth/basics/index.html (accessed on 30 December 2021).
- Iyamba, J.M.L.; Seil, M.; Devleeschouwer, M.; Kikuni, N.B.T.; Dehaye, J.P. Study of the Formation of a Biofilm by Clinical Strains of Staphylococcus Aureus. Biofouling 2011, 27, 811–821. [Google Scholar] [CrossRef]
- Watnick, P.; Kolter, R. Biofilm, City of Microbes. J. Bacteriol. 2000, 182, 2675–2679. [Google Scholar] [CrossRef] [Green Version]
- Flemming, H.C.; Wingender, J. The Biofilm Matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef]
- Kassinger, S.J.; van Hoek, M.L. Biofilm Architecture: An Emerging Synthetic Biology Target. Synth. Syst. Biotechnol. 2020, 5, 1–10. [Google Scholar] [CrossRef]
- Ciofu, O.; Tolker-Nielsen, T. Tolerance and Resistance of Pseudomonas Aeruginosa biofilms to Antimicrobial Agents—How P. Aeruginosa Can Escape Antibiotics. Front. Microbiol. 2019, 10, 913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khatoon, Z.; McTiernan, C.D.; Suuronen, E.J.; Mah, T.F.; Alarcon, E.I. Bacterial Biofilm Formation on Implantable Devices and Approaches to Its Treatment and Prevention. Heliyon 2018, 4, e01067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Toole, G.A. Microtiter Dish Biofilm Formation Assay. J. Vis. Exp. 2010, 47, 2437. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, R.; Santhakumari, S.; Poonguzhali, P.; Geetha, M.; Dyavaiah, M.; Xiangmin, L. Bacterial Biofilm Inhibition: A Focused Review on Recent Therapeutic Strategies for Combating the Biofilm Mediated Infections. Front. Microbiol. 2021, 12, 676458. [Google Scholar] [CrossRef]
- Zhang, C.; Li, B.; Tang, J.Y.; Wang, X.L.; Qin, Z.; Feng, X.Q. Experimental and Theoretical Studies on the Morphogenesis of Bacterial Biofilms. Soft Matter 2017, 13, 7389–7397. [Google Scholar] [CrossRef]
- Toyofuku, M.; Inaba, T.; Kiyokawa, T.; Obana, N.; Yawata, Y.; Nomura, N. Environmental Factors That Shape Biofilm Formation. Biosci. Biotechnol. Biochem. 2016, 80, 7–12. [Google Scholar] [CrossRef]
- Van den Driessche, F.; Brackman, G.; Swimberghe, R.; Rigole, P.; Coenye, T. Screening a Repurposing Library for Potentiators of Antibiotics against Staphylococcus Aureus Biofilms. Int. J. Antimicrob. Agents 2017, 49, 315–320. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Healthcare-Associated Infections (HAIs), HAI Data. 2018. Available online: https://www.cdc.gov/hai/data/index.html (accessed on 30 December 2021).
- World Health Organization. Establishment of National Laboratory-Based Surveillance of Antimicrobial Resistance; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- European Centre for Disease Prevention and Control. Healthcare-Associated Infections. 2021. Available online: https://www.ecdc.europa.eu/en/healthcare-associated-infections (accessed on 30 December 2021).
- World Health Organization. Newborns: Improving Survival and Well-Being. 2020. Available online: https://www.who.int/news-room/fact-sheets/detail/newborns-reducing-mortality (accessed on 30 December 2021).
- Iravani, S. Green Synthesis of Metal Nanoparticles Using Plants. Green Chem. 2011, 13, 2638–2650. [Google Scholar] [CrossRef]
- Khan, S.; Singh, S.; Gaikwad, S.; Nawani, N.; Junnarkar, M.; Pawar, S.V. Optimization of Process Parameters for the Synthesis of Silver Nanoparticles from Piper Betle Leaf Aqueous Extract, and Evaluation of Their Antiphytofungal Activity. Environ. Sci. Pollut. Res. Int. 2020, 27, 27221–27233. [Google Scholar] [CrossRef]
- Patra, J.K.; Baek, K.H. Green Nanobiotechnology: Factors Affecting Synthesis and Characterization Techniques. J. Nanomater. 2014, 2014, 417305. [Google Scholar] [CrossRef] [Green Version]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, Applications and Toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Al-Shabib, N.A.; Husain, F.M.; Ahmed, F.; Khan, R.A.; Ahmad, I.; Alsharaeh, E.; Khan, M.S.; Hussain, A.; Rehman, M.T.; Yusuf, M.; et al. Biogenic Synthesis of Zinc Oxide Nanostructures from Nigella sativa Seed: Prospective Role as Food Packaging Material Inhibiting Broad-Spectrum Quorum Sensing and Biofilm. Sci. Rep. 2016, 6, srep36761. [Google Scholar] [CrossRef] [Green Version]
- Sulaiman, G.M.; Mohammed, W.H.; Marzoog, T.R.; Al-Amiery, A.A.A.; Kadhum, A.A.H.; Mohamad, A.B. Green Synthesis, Antimicrobial and Cytotoxic Effects of Silver Nanoparticles Using Eucalyptus Chapmaniana Leaves Extract. Asian Pac. J. Trop. Biomed. 2013, 3, 58–63. [Google Scholar] [CrossRef] [Green Version]
- Sukhanova, A.; Bozrova, S.; Sokolov, P.; Berestovoy, M.; Karaulov, A.; Nabiev, I. Dependence of Nanoparticle Toxicity on Their Physical and Chemical Properties. Nanoscale Res. Lett. 2018, 13, 44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Lima, R.; Seabra, A.B.; Durán, N. Silver Nanoparticles: A Brief Review of Cytotoxicity and Genotoxicity of Chemically and Biogenically Synthesized Nanoparticles. J. Appl. Toxicol. 2012, 32, 867–879. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.U.; Yuan, Q.; Ul, Z.; Khan, H.; Ahmad, A.; Khan, F.U.; Tahir, K.; Shakeel, M.; Ullah, S. An Eco-Benign Synthesis of AgNPs Using Aqueous Extract of Longan Fruit Peel: Antiproliferative Response against Human Breast Cancer Cell Line MCF-7, Antioxidant and Photocatalytic Deprivation of Methylene Blue. J. Photochem. Photobiol. B Biol. 2018, 183, 367–373. [Google Scholar] [CrossRef]
- Dobrucka, R. Biofabrication of Platinum Nanoparticles Using Fumariae Herba Extract and Their Catalytic Properties. Saudi J. Biol. Sci. 2019, 26, 31–37. [Google Scholar] [CrossRef]
- Roy, N.; Gaur, A.; Jain, A.; Bhattacharya, S.; Rani, V. Green Synthesis of Silver Nanoparticles: An Approach to Overcome Toxicity. Environ. Toxicol. Pharmacol. 2013, 36, 807–812. [Google Scholar] [CrossRef]
- Akhtar, M.S.; Panwar, J.; Yun, Y.S. Biogenic Synthesis of Metallic Nanoparticles by Plant Extracts. ACS Sustain. Chem. Eng. 2013, 1, 591–602. [Google Scholar] [CrossRef]
- Raji, P.; Samrot, A.V.; Keerthana, D.; Karishma, S. Antibacterial Activity of Alkaloids, Flavonoids, Saponins and Tannins Mediated Green Synthesised Silver Nanoparticles Against Pseudomonas Aeruginosa and Bacillus Subtilis. J. Clust. Sci. 2019, 30, 881–895. [Google Scholar] [CrossRef]
- Koul, B.; Poonia, A.K.; Yadav, D.; Jin, J.O. Microbe-Mediated Biosynthesis of Nanoparticles: Applications and Future Prospects. Biomolecules 2021, 11, 886. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xu, H.; Chen, Z.S.; Chen, G. Biosynthesis of Nanoparticles by Microorganisms and Their Applications. J. Nanomater. 2011, 2011, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Gericke, M.; Pinches, A. Microbial Production of Gold Nanoparticles. Gold Bull. 2006, 39, 22–28. [Google Scholar] [CrossRef] [Green Version]
- Makarov, V.V.; Makarova, S.S.; Love, A.J.; Sinitsyna, O.V.; Dudnik, A.O.; Yaminsky, I.V.; Taliansky, M.E.; Kalinina, N.O. Biosynthesis of Stable Iron Oxide Nanoparticles in Aqueous Extracts of Hordeum Vulgare and Rumex Acetosa Plants. Langmuir 2014, 30, 5982–5988. [Google Scholar] [CrossRef] [PubMed]
- Quintero-quiroz, C.; Acevedo, N.; Zapata-giraldo, J.; Botero, L.E.; Quintero, J.; Zárate-triviño, D.; Saldarriaga, J.; Pérez, V.Z. Optimization of Silver Nanoparticle Synthesis by Chemical Reduction and Evaluation of Its Antimicrobial and Toxic Activity. Biomater. Res. 2019, 23, 27. [Google Scholar] [CrossRef] [PubMed]
- Bhadra, P.; Mitra, M.K.; Das, G.C.; Dey, R.; Mukherjee, S. Interaction of Chitosan Capped ZnO Nanorods with Escherichia Coli. Mater. Sci. Eng. C 2011, 31, 929–937. [Google Scholar] [CrossRef]
- Jalal, R.; Goharshadi, E.K.; Abareshi, M.; Moosavi, M.; Yousefi, A.; Nancarrow, P. ZnO Nanofluids: Green Synthesis, Characterization, and Antibacterial Activity. Mater. Chem. Phys. 2010, 121, 198–201. [Google Scholar] [CrossRef]
- Premanathan, M.; Karthikeyan, K.; Jeyasubramanian, K.; Manivannan, G. Selective Toxicity of ZnO Nanoparticles toward Gram-Positive Bacteria and Cancer Cells by Apoptosis through Lipid Peroxidation. Nanomedecine 2011, 7, 184–192. [Google Scholar] [CrossRef]
- Garibo, D.; Borbón-Nuñez, H.; De León, D.J.N.; Mendoa, E.G.; Estrada, I. Green Synthesis of Silver Nanoparticles Using Lysiloma Acapulcensis Exhibit High-Antimicrobial Activity. Sci. Rep. 2020, 10, 7. [Google Scholar] [CrossRef]
- Griffith, M.; Udekwu, K.I.; Gkotzis, S.; Mah, T.-F.; Alarcon, E.I. Anti-Microbiological and Anti-Infective Activities of Silver. In Silver Nanoparticle Applications; Engineering Materials; Springer Nature: Cham, Switzerland, 2015; pp. 127–146. [Google Scholar]
- Chernousova, S.; Epple, M. Silver as Antibacterial Agent: Ion, Nanoparticle, and Metal. Angew. Chem. Int. Ed. 2013, 52, 1636–1653. [Google Scholar] [CrossRef]
- El-Sherbiny, I.M.; Salih, E. Green Synthesis of Metallic Nanoparticles Using Biopolymers and Plant Extracts: Synthesis, Characterization and Their Applications. In Green Metal Nanoparticles; Scrivener Publishing LLC: Beverly, MA, USA, 2018; pp. 293–319. [Google Scholar] [CrossRef]
- Abdel-Shafy, H.I.; Mansour, M.S.M. Green Synthesis of Metallic Nanoparticles from Natural Resources and Food Waste and Their Environmental Application. In Green Metal Nanoparticles; Scrivener Publishing LLC: Beverly, MA, USA, 2018; pp. 321–386. [Google Scholar] [CrossRef]
- Ganesh Kumar, V.; Dinesh Gokavarapu, S.; Rajeswari, A.; Stalin Dhas, T.; Karthick, V.; Kapadia, Z.; Shrestha, T.; Barathy, I.A.; Roy, A.; Sinha, S. Facile Green Synthesis of Gold Nanoparticles Using Leaf Extract of Antidiabetic Potent Cassia auriculata. Colloids Surf. B Biointerfaces 2011, 87, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Raouf, N.; Al-Enazi, N.M.; Ibraheem, I.B.M. Green Biosynthesis of Gold Nanoparticles Using Galaxaura elongata and Characterization of Their Antibacterial Activity. Arab. J. Chem. 2017, 10, S3029–S3039. [Google Scholar] [CrossRef] [Green Version]
- Siddiqi, K.S.; Husen, A. Green Synthesis, Characterization and Uses of Palladium/Platinum Nanoparticles. Nanoscale Res. Lett. 2016, 11, 482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saif, S.; Tahir, A.; Chen, Y. Green Synthesis of Iron Nanoparticles and Their Environmental Applications and Implications. Nanomaterials 2016, 6, 209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bharath, B.; Sasidharan, S.; Bhamidipati, S.K.; Saudagar, P. Green-Synthesized FeSO4 Nanoparticles Exhibit Antibacterial and Cytotoxic Activity by DNA Degradation. Curr. Pharm. Biotechnol. 2020, 21, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Sabouri, Z.; Akbari, A.; Hosseini, H.A.; Darroudi, M. Facile Green Synthesis of NiO Nanoparticles and Investigation of Dye Degradation and Cytotoxicity Effects. J. Mol. Struct. 2018, 1173, 931–936. [Google Scholar] [CrossRef]
- Chen, G.; Li, J. Synthesis of In2O3 Nanoparticles via a Green and Solvent-Free Method. Green Process. Synth. 2016, 5, 389–394. [Google Scholar] [CrossRef]
- Sudhasree, S.; Banu, A.S.; Brindha, P.; Kurian, G.A. Synthesis of Nickel Nanoparticles by Chemical and Green Route and Their Comparison in Respect to Biological Effect and Toxicity. Toxicol. Environ. Chem. 2014, 96, 743–754. [Google Scholar] [CrossRef]
- Elango, G.; Roopan, S.M.; Al-dhabi, N.A.; Arasu, V.; Damodharan, K.I.; Elumalai, K. Cocos Nucifera Coir-Mediated Green Synthesis of Pd NPs and Its Investigation against Larvae and Agricultural Pest. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1581–1587. [Google Scholar] [CrossRef] [Green Version]
- Miri, A.; Sarani, M.; Hashemzadeh, A.; Mardani, Z.; Darroudi, M. Biosynthesis and Cytotoxic Activity of Lead Oxide Nanoparticles. Green Chem. Lett. Rev. 2018, 11, 567–572. [Google Scholar] [CrossRef]
- Unuofin, J.O.; Oladipo, A.O.; Msagati, T.A.M.; Lebelo, S.L.; Meddows-Taylor, S.; More, G.K. Novel Silver-Platinum Bimetallic Nanoalloy Synthesized from Vernonia mespilifolia Extract: Antioxidant, Antimicrobial, and Cytotoxic Activities. Arab. J. Chem. 2020, 13, 6639–6648. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Sajjadi, M.; Dadashi, J.; Ghafuri, H. Pd-Based Nanoparticles: Plant-Assisted Biosynthesis, Characterization, Mechanism, Stability, Catalytic and Antimicrobial Activities. Adv. Colloid Interface Sci. 2020, 276, 102103. [Google Scholar] [CrossRef] [PubMed]
- Veisi, H.; Zohrabi, A.; Kamangar, S.A.; Karmakar, B.; Saremi, S.G.; Varmira, K.; Hamelian, M. Green Synthesis of Pd/Fe3O4 Nanoparticles Using Chamomile Extract as Highly Active and Recyclable Catalyst for Suzuki Coupling Reaction. J. Organomet. Chem. 2021, 951, 122005. [Google Scholar] [CrossRef]
- Sofalgar, P.; Sabbaghan, M.; Naimi-Jamal, M.R. Green Fabrication of 2D Fe3SO4/Mg(OH)2 and 2D Fe3O4/MgO Nanocomposites Using [OMIM] Br Ionic Liquid and Comparing Catalytic Activity with Green Metrics. Polycycl. Aromat. Compd. 2021, 41, 1180–1199. [Google Scholar] [CrossRef]
- Virkutyte, J.; Varma, R.S. Green Synthesis of Metal Nanoparticles: Biodegradable Polymers and Enzymes in Stabilization and Surface Functionalization. Chem. Sci. 2011, 2, 837–846. [Google Scholar] [CrossRef]
- Norrrahim, M.N.F.; Nurazzi, N.M.; Jenol, M.A.; Farid, M.A.A.; Janudin, N.; Ujang, F.A.; Yasim-Anuar, T.A.T.; Syed Najmuddin, S.U.F.; Ilyas, R.A. Emerging Development of Nanocellulose as an Antimicrobial Material: An Overview. Mater. Adv. 2021, 2, 3538–3551. [Google Scholar] [CrossRef]
- Nehra, P.; Chauhan, R.P. Eco-Friendly Nanocellulose and Its Biomedical Applications: Current Status and Future Prospect. J. Biomater. Sci. Polym. Ed. 2021, 32, 112–149. [Google Scholar] [CrossRef]
- Medina-Ramirez, I.; Bashir, S.; Luo, Z.; Liu, J.L. Green Synthesis and Characterization of Polymer-Stabilized Silver Nanoparticles. Colloids Surf. B Biointerfaces 2009, 73, 185–191. [Google Scholar] [CrossRef]
- Phan, T.T.V.; Phan, D.T.; Cao, X.T.; Huynh, T.C.; Oh, J. Roles of Chitosan in Green Synthesis of Metal Nanoparticles for Biomedical Applications. Nanomaterials 2021, 11, 273. [Google Scholar] [CrossRef]
- Parmar, A.; Kaur, G.; Kapil, S.; Sharma, V.; Sharma, S. Biogenic PLGA-Zinc Oxide Nanocomposite as Versatile Tool for Enhanced Photocatalytic and Antibacterial Activity. Appl. Nanosci. 2019, 9, 2001–2016. [Google Scholar] [CrossRef]
- Chandran, S.; Ravichandran, V.; Chandran, S.; Chemmanda, J.; Chandarshekar, B. Biosynthesis of PVA Encapsulated Silver Nanoparticles. J. Appl. Res. Technol. 2016, 14, 319–324. [Google Scholar] [CrossRef] [Green Version]
- Darroudi, M.; Ahmad, M.B.; Abdullah, A.H.; Ibrahim, N.A. Green Synthesis and Characterization of Gelatin-Based and Sugar-Reduced Silver Nanoparticles. Int. J. Nanomed. 2011, 6, 569–574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia, R.; Stevanovic, T.; Berthier, J.; Njamen, G.; Tolnai, B.; Achim, A. Cellulose, Nanocellulose, and Antimicrobial Materials for the Manufacture of Disposable Face Masks: A Review. BioResources 2021, 16, 4321–4353. [Google Scholar] [CrossRef]
- Kupnik, K.; Primožič, M.; Kokol, V.; Leitgeb, M. Nanocellulose in Drug Delivery and Antimicrobially Active Materials. Polymers 2020, 12, 2825. [Google Scholar] [CrossRef]
- Ahmad, F.; Taj, M.B.; Ramzan, M.; Raheel, A.; Shabbir, S. Flacourtia indica Based Biogenic Nanoparticles: Development, Characterization, and Bioactivity Flacourtia Indica Based Biogenic Nanoparticles: Development, Characterization, and Bioactivity against Wound Associated Pathogens. Mater. Res. Express 2020, 7, 015026. [Google Scholar] [CrossRef]
- Tortorella, S.; Buratti, V.V.; Maturi, M.; Sambri, L.; Franchini, M.C.; Locatelli, E. Surface-Modified Nanocellulose for Application in Biomedical Engineering and Nanomedicine: A Review. Int. J. Nanomed. 2020, 15, 9909–9937. [Google Scholar] [CrossRef]
- Garza-Cervantes, J.A.; Mendiola-Garza, G.; de Melo, E.M.; Dugmore, T.I.J.; Matharu, A.S.; Morones-Ramirez, J.R. Antimicrobial Activity of a Silver-Microfibrillated Cellulose Biocomposite against Susceptible and Resistant Bacteria. Sci. Rep. 2020, 10, 9. [Google Scholar] [CrossRef]
- Oun, A.A.; Shankar, S.; Rhim, J.W. Multifunctional Nanocellulose/Metal and Metal Oxide Nanoparticle Hybrid Nanomaterials. Crit. Rev. Food Sci. Nutr. 2020, 60, 435–460. [Google Scholar] [CrossRef]
- Mocanu, A.; Isopencu, G.; Busuioc, C.; Popa, O.M.; Dietrich, P.; Socaciu-Siebert, L. Bacterial Cellulose Films with ZnO Nanoparticles and Propolis Extracts: Synergistic Antimicrobial Effect. Sci. Rep. 2019, 9, 17687. [Google Scholar] [CrossRef]
- Razavi, R.; Molaei, R.; Moradi, M.; Tajik, H.; Ezati, P.; Shafipour, A. Biosynthesis of Metallic Nanoparticles Using Mulberry Fruit (Morus Alba L.) Extract for the Preparation of Antimicrobial Nanocellulose Film. Appl. Nanosci. 2020, 10, 465–476. [Google Scholar] [CrossRef]
- Mourdikoudis, S.; Pallares, R.M.; Thanh, N.T.K. Characterization Techniques for Nanoparticles: Comparison and Complementarity upon Studying Nanoparticle Properties. Nanoscale 2018, 10, 12871–12934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thema, F.T.; Manikandan, E.; Dhlamini, M.S.; Maaza, M. Green Synthesis of ZnO Nanoparticles via Agathosma Betulina Natural Extract. Mater. Lett. 2015, 161, 124–127. [Google Scholar] [CrossRef]
- Matinise, N.; Fuku, X.G.; Kaviyarasu, K.; Mayedwa, N.; Maaza, M. ZnO Nanoparticles via Moringa Oleifera Green Synthesis: Physical Properties & Mechanism of Formation. Appl. Surf. Sci. 2017, 406, 339–347. [Google Scholar] [CrossRef]
- Lomelí-Rosales, D.A.; Zamudio-Ojeda, K.; Reyes-Maldonado, O.K.; López-Reyes, M.E.; Basulto-Padilla, G.C.; Lopez-Naranjo, E.J.; Zuñiga-Mayo, V.M.; Velázquez-Juarez, G. Green Synthesis of Gold and Silver Nanoparticles Using Leaf Extract of Capsicum chinense Plant. Molecules 2022, 27, 1692. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Henríquez, L.; Alfaro-Aguilar, K.; Ugalde-álvarez, J.; Vega-Fernández, L.; de Oca-Vásquez, G.M.; Vega-Baudrit, J.R. Green Synthesis of Gold and Silver Nanoparticles from Plant Extracts and Their Possible Applications as Antimicrobial Agents in the Agricultural Area. Nanomaterials 2020, 10, 1763. [Google Scholar] [CrossRef]
- Zhang, T.; Dang, M.; Zhang, W.; Lin, X. Gold Nanoparticles Synthesized from Euphorbia Fischeriana Root by Green Route Method Alleviates the Isoprenaline Hydrochloride Induced Myocardial Infarction in Rats. J. Photochem. Photobiol. B 2020, 202, 111705. [Google Scholar] [CrossRef]
- Siddiqi, K.S.; Rahman, A.; Husen, A. Properties of Zinc Oxide Nanoparticles and Their Activity Against Microbes. Nanoscale Reseach Lett. 2018, 13, 141. [Google Scholar] [CrossRef]
- Baptista, P.V.; McCusker, M.P.; Carvalho, A.; Ferreira, D.A.; Mohan, N.M.; Martins, M.; Fernandes, A.R. Nano-Strategies to Fight Multidrug Resistant Bacteria—“A Battle of the Titans”. Front. Microbiol. 2018, 9, 1441. [Google Scholar] [CrossRef] [Green Version]
- Rudramurthy, G.R.; Swamy, M.K.; Sinniah, U.R.; Ghasemzadeh, A. Nanoparticles: Alternatives against Drug-Resistant Pathogenic Microbes. Molecules 2016, 21, 836. [Google Scholar] [CrossRef]
- Singh, P.; Pandit, S.; Beshay, M.; Mokkapati, V.R.S.S.; Olsson, M.E.; Sultan, A.; Mackevica, A.; Mateiu, V.; Lütken, H.; Daugaard, A.E.; et al. Anti-Biofilm Effects of Gold and Silver Nanoparticles Synthesized by the Rhodiola Rosea Rhizome Extracts. Artif. Cells Nanomed. Biotechnol. 2018, 46 (Suppl. 3), 5886–5899. [Google Scholar] [CrossRef] [Green Version]
- Majeed, M.; Hakeem, K.R.; Rehman, R.U. Synergistic Effect of Plant Extract Coupled Silver Nanoparticles in Various Therapeutic Applications—Present Insights and Bottlenecks. Chemosphere 2022, 288, 132527. [Google Scholar] [CrossRef] [PubMed]
- Akintelu, S.A.; Folorunso, A.S.; Folorunso, F.A.; Oyebamiji, A.K. Green Synthesis of Copper Oxide Nanoparticles for Biomedical Application and Environmental Remediation. Heliyon 2020, 6, e04508. [Google Scholar] [CrossRef] [PubMed]
- Arif, R.; Uddin, R. A Review on Recent Developments in the Biosynthesis of Silver Nanoparticles and Its Biomedical Applications. Med. Devices Sens. 2021, 4, e10158. [Google Scholar] [CrossRef]
- Naqvi, S.Z.H.; Kiran, U.; Ali, M.I.; Jamal, A.; Hameed, A.; Ahmed, S.; Ali, N. Combined Efficacy of Biologically Synthesized Silver Nanoparticles and Different Antibiotics against Multidrug-Resistant Bacteria. Int. J. Nanomed. 2013, 8, 3187–3195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salar, R.K.; Sharma, P.; Kumar, N. Enhanced Antibacterial Activity of Streptomycin against Some Human Pathogens Using Green Synthesized Silver Nanoparticles. Resour. Technol. 2015, 1, 106–115. [Google Scholar] [CrossRef] [Green Version]
- Nag, S.; Biswas, A.; Chattopadhyay, D.; Bhattacharyya, M. Protein-Stabilized Silver Nanoparticles Encapsulating Gentamycin for the Therapy of Bacterial Biofilm Infections. Nanomedicine 2021, 16, 801–818. [Google Scholar] [CrossRef]
- Krishnaraj, C.; Ramachandran, R.; Mohan, K.; Kalaichelvan, P.T. Optimization for Rapid Synthesis of Silver Nanoparticles and Its Effect on Phytopathogenic Fungi. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 93, 95–99. [Google Scholar] [CrossRef]
- Huang, W.; Yan, M.; Duan, H.; Bi, Y.; Cheng, X.; Yu, H. Synergistic Antifungal Activity of Green Synthesized Silver Nanoparticles and Epoxiconazole against Setosphaeria turcica. J. Nanomater. 2020, 2020, 9535432. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.S.; Lytton-Jean, A.K.R.; Hurst, S.J.; Mirkin, C.A. Silver Nanoparticle—Oligonucleotide Conjugates Based on DNA with Triple Cyclic Disulfide Moieties. Nano Lett. 2007, 7, 2112–2115. [Google Scholar] [CrossRef] [Green Version]
- Tokareva, I.; Hutter, E. Hybridization of Oligonucleotide-Modified Silver and Gold Nanoparticles in Aqueous Dispersions and on Gold Films. J. Am. Chem. Soc. 2004, 126, 15784–15789. [Google Scholar] [CrossRef]
- Vidal, B.C.; Deivaraj, T.C.; Yang, J.; Too, H.P.; Chow, G.M.; Gan, L.M.; Lee, J.Y. Stability and Hybridization-Driven Aggregation of Silver Nanoparticle-Oligonucleotide Conjugates. New J. Chem. 2005, 29, 812–816. [Google Scholar] [CrossRef]
- Barbinta-Patrascu, M.E.; Bunghez, I.R.; Iordache, S.M.; Badea, N.; Fierascu, R.C.; Ion, R.M. Antioxidant Properties of Biohybrids Based on Liposomes and Sage Silver Nanoparticles. J. Nanosci. Nanotechnol. 2013, 13, 2051–2060. [Google Scholar] [CrossRef] [PubMed]
- Celebioglu, A.; Topuz, F.; Yildiz, Z.I.; Uyar, T. One-Step Green Synthesis of Antibacterial Silver Nanoparticles Embedded in Electrospun Cyclodextrin Nanofibers. Carbohydr. Polym. 2019, 207, 471–479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, A.; Briffa, S.M.; Swingler, S.; Gibson, H.; Kannappan, V.; Adamus, G.; Kowalczuk, M.; Martin, C.; Radecka, I. Synthesis of Silver Nanoparticles Using Curcumin-Cyclodextrins Loaded into Bacterial Cellulose-Based Hydrogels for Wound Dressing Applications. Biomacromolecules 2020, 21, 1802–1811. [Google Scholar] [CrossRef]
- Rafique, M.; Sadaf, I.; Rafique, M.S.; Tahir, M.B. A Review on Green Synthesis of Silver Nanoparticles and Their Applications. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1272–1291. [Google Scholar] [CrossRef]
- Ameta, R.K.; Shankar, K.R.; Man, S. Plant Extract: An Effective Medium for Synthesis of Metal Nanoparticles. SF J. Nanochem. Nanotechnol. 2018, 1, 1008. [Google Scholar]
- Deshmukh, A.R.; Gupta, A.; Kim, B.S. Ultrasound Assisted Green Synthesis of Silver and Iron Oxide Nanoparticles Using Fenugreek Seed Extract and Their Enhanced Antibacterial and Antioxidant Activities. Biomed Res. Int. 2019, 2019, 1714358. [Google Scholar] [CrossRef]
- Reddy, N.V.; Li, H.; Hou, T.; Bethu, M.S.; Ren, Z.; Zhang, Z. Phytosynthesis of Silver Nanoparticles Using Perilla Frutescens Leaf Extract: Characterization and Evaluation of Antibacterial, Antioxidant, and Anticancer Activities. Int. J. Nanomed. 2021, 16, 15–29. [Google Scholar] [CrossRef]
- Tailor, G.; Yadav, B.L.; Chaudhary, J.; Joshi, M.; Suvalka, C. Green Synthesis of Silver Nanoparticles Using Ocimum Canum and Their Anti-Bacterial Activity. Biochem. Biophys. Rep. 2020, 24, 100848. [Google Scholar] [CrossRef]
- Bharadwaj, K.K.; Rabha, B.; Sarkar, T.; Gogoi, S.K.; Kakati, N.; Baishya, D. Green Synthesis of Silver Nanoparticles Using Diospyros Malabarica Fruit Extract and Assessments of Their Antimicrobial, Anticancer and Catalytic Reduction of 4-Nitrophenol (4-NP). Nanomaterials 2021, 11, 1999. [Google Scholar] [CrossRef]
- Murei, A.; Pillay, K.; Govender, P.; Thovhogi, N.; Gitari, W.M.; Samie, A. Synthesis, Characterization and in Vitro Antibacterial Evaluation of Pyrenacantha Grandiflora Conjugated Silver Nanoparticles. Nanomaterials 2021, 11, 1568. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Hano, C.; Nath, G.; Sharma, B. Green Biosynthesis of Silver Nanoparticles Using Leaf Extract of Carissa carandas L. and Their Antioxidant and Antimicrobial Activity against Human Pathogenic Bacteria. Biomolecules 2021, 11, 299. [Google Scholar] [CrossRef] [PubMed]
- Ravindran, D.; Ramanathan, S.; Arunachalam, K.; Jeyaraj, G.P.; Shunmugiah, K.P. Phytosynthesized Silver Nanoparticles as Antiquorum Sensing and Antibiofilm Agent against the Nosocomial Pathogen Serratia Marcescens: An in Vitro Study. J Appl Microbiol. 2018, 124, 1425–1440. [Google Scholar] [CrossRef] [PubMed]
- Ruíz-Baltazar, Á.D.J.; Reyes-López, S.Y.; Larrañaga, D.; Estévez, M.; Pérez, R. Green Synthesis of Silver Nanoparticles Using a Melissa Officinalis Leaf Extract with Antibacterial Properties. Results Phys. 2017, 7, 2639–2643. [Google Scholar] [CrossRef]
- Lagashetty, A.; Ganiger, S.K. Synthesis, Characterization and Antibacterial Study of Ag–Au Bi-Metallic Nanocomposite by Bioreduction Using Piper Betle Leaf Extract. Heliyon 2019, 5, e02794. [Google Scholar] [CrossRef] [Green Version]
- Gulbagca, F.; Ozdemir, S.; Gulcan, M.; Sen, F. Synthesis and Characterization of Rosa canina-Mediated Biogenic Silver Nanoparticles for Anti-Oxidant, Antibacterial, Antifungal, and DNA Cleavage Activities. Heliyon 2019, 5, e02980. [Google Scholar] [CrossRef] [Green Version]
- Adil, M.; Khan, T.; Aasim, M.; Khan, A.A.; Ashraf, M. Evaluation of the Antibacterial Potential of Silver Nanoparticles Synthesized through the Interaction of Antibiotic and Aqueous Callus Extract of Fagonia indica. AMB Express 2019, 9, 75. [Google Scholar] [CrossRef]
- Cittrarasu, V.; Balasubramanian, B.; Park, S.; Maluventhan, V.; Kaul, T.; Liu, W.C. Biological Mediated Ag Nanoparticles from Barleria longiflora for Antimicrobial Activity and Photocatalytic Degradation Using Methylene Blue Biological Mediated Ag Nanoparticles from Barleria longiflora for Antimicrobial Activity and Photocatalytic Degrad. Artif. Cells Nanomed. Biotechnol. 2019, 47, 2424–2430. [Google Scholar] [CrossRef] [Green Version]
- Das, G.; Patra, J.K.; Basavegowda, N.; Vishnuprasad, C.N.; Shin, H. Comparative Study on Antidiabetic, Cytotoxicity, Antioxidant and Antibacterial Properties of Biosynthesized Silver Nanoparticles Using Outer Peels of Two Varieties of Ipomoea Batatas (L.) Lam. Int. J. Nanomed. 2019, 14, 4741–4754. [Google Scholar] [CrossRef] [Green Version]
- Okaiyeto, K.; Ojemaye, M.O.; Hoppe, H.; Mabinya, L.V.; Okoh, A.I. Phytofabrication of Silver/Silver Chloride Oedera genistifolia: Characterization and Antibacterial Potential. Molecules 2019, 24, 4382. [Google Scholar] [CrossRef] [Green Version]
- Cyril, N.; George, J.B.; Joseph, L.; Raghavamenon, A.C.; Sylas, V.P. Assessment of Antioxidant, Antibacterial and Anti-Proliferative (Lung Cancer Cell Line Akan549) Activities of Green Synthesized Silver Nanoparticles from Derris trifoliata. Toxicol. Res. 2019, 8, 297–308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanjikar, A.P.; Hugar, A.L.; Londonkar, R.L. Characterization of Phyto-Nanoparticles from Ficus Krishnae for Their Antibacterial and Anticancer Activities. Drug Dev. Ind. Pharm. 2018, 44, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Suwan, T.; Khongkhunthian, S.; Okonogi, S. Antifungal Activity of Polymeric Micelles of Silver Nanoparticles Prepared from Psidium guajava aqueous Extract. Drug Disc. Ther. 2019, 13, 62–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vankar, P.S.; Shukla, D.; Silk, Á.C.Á. Biosynthesis of Silver Nanoparticles Using Lemon Leaves Extract and Its Application for Antimicrobial Finish on Fabric. Appl. Nanosci. 2012, 2, 163–168. [Google Scholar] [CrossRef] [Green Version]
- Oh, K.H.; Soshnikova, V.; Markus, J.; Kim, Y.J.; Chul, S.; Singh, P.; Castro-Aceituno, V.; Ahn, S.; Hyun, D.; Shim, Y.J.; et al. Biosynthesized Gold and Silver Nanoparticles by Aqueous Fruit Extract of Chaenomeles Sinensis and Screening of Their Biomedical Activities. Artif. Cells Nanomed. Biotechnol. 2018, 46, 599–606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lubis, A.F.; Malek, N.A.N.N.; Sani, S.N.; Jemon, K. Biogenic Synthesis of Silver Nanoparticles Using Persicaria Odorata Leaf Extract: Antibacterial, Cytocompatibility, and in Vitro Wound Healing Evaluation. Particuology 2022, 70, 10–19. [Google Scholar] [CrossRef]
- Ituen, E.; Ekemini, E.; Yuanhua, L.; Singh, A. Green Synthesis of Citrus Reticulata Peels Extract Silver Nanoparticles and Characterization of Structural, Biocide and Anticorrosion Properties. J. Mol. Struct. 2020, 1207, 127819. [Google Scholar] [CrossRef]
- Alsammarraie, F.K.; Wang, W.; Zhou, P.; Mustapha, A.; Lin, M. Green Synthesis of Silver Nanoparticles Using Turmeric Extracts and Investigation of Their Antibacterial Activities. Colloids Surf. B Biointerfaces 2018, 171, 398–405. [Google Scholar] [CrossRef]
- Habibipour, R.; Moradi-Haghgou, L.; Farmany, A. Green Synthesis of AgNPs@PPE and Its Pseudomonas Aeruginosa Biofilm Formation Activity Compared to Pomegranate Peel Extract. Int. J. Nanomed. 2019, 14, 6891–6899. [Google Scholar] [CrossRef] [Green Version]
- Moulavi, P.; Noorbazargan, H. Antibiofilm Effect of Green Engineered Silver Nanoparticles Fabricated from Artemisia scoporia Extract on the Expression of IcaA and IcaR Genes against Multidrug-Resistant Staphylococcus aureus Bacterial Isolates. J. Basic Microbiol. 2019, 59, 701–712. [Google Scholar] [CrossRef]
- Arya, G.; Kumari, R.M.; Gupta, N.; Kumar, A.; Chandra, R.; Nimesh, S. Green Synthesis of Silver Nanoparticles Using Prosopis Juliflora Bark Extract: Reaction Optimization, Antimicrobial and Catalytic Activities. Artif. Cells Nanomed. Biotechnol. 2018, 46, 985–993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feizi, S.; Taghipour, E.; Ghadam, P.; Mohammadi, P. Antifungal, Antibacterial, Antibiofilm and Colorimetric Sensing of Toxic Metals Activities of Eco Friendly, Economical Synthesized Ag/AgCl Nanoparticles Using Malva Sylvestris Leaf Extracts. Microb. Pathog. 2018, 125, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Pandit, S.; Garnæs, J.; Tunjic, S.; Mokkapati, V.R.S.S.; Sultan, A.; Thygesen, A.; Mackevica, A.; Mateiu, R.V.; Daugaard, A.E.; et al. Green Synthesis of Gold and Silver Nanoparticles from Cannabis sativa (Industrial Hemp) and Their Capacity for Biofilm Inhibition. Int. J. Nanomed. 2018, 13, 3571–3591. [Google Scholar] [CrossRef] [PubMed]
- Muthamil, S.; Amsa, V.; Boopathi, D.; Pandian, S.K.; Balamurugan, K.; Pandian, S.K. Green Synthesized Silver Nanoparticles Demonstrating Enhanced in Vitro and in Vivo Antibiofilm Activity against Candida spp. J. Basic Microbiol. 2018, 58, 343–357. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, R.; Vigneshwari, L.; Rajavel, T.; Durgadevi, R.; Kannappan, A.; Balamurugan, K.; Pandima Devi, K.; Veera Ravi, A. Biogenic Synthesis of Silver Nanoparticles Using Piper Betle Aqueous Extract and Evaluation of Its Anti-Quorum Sensing and Antibiofilm Potential against Uropathogens with Cytotoxic Effects: An In Vitro and In Vivo Approach. Environ. Sci. Pollut. Res. 2018, 25, 10538–10554. [Google Scholar] [CrossRef]
- Ishwarya, R.; Vaseeharan, B.; Anuradha, R.; Rekha, R.; Govindarajan, M.; Alharbi, N.S.; Kadaikunnan, S.; Khaled, J.M.; Benelli, G. Eco-Friendly Fabrication of Ag Nanostructures Using the Seed Extract of Pedalium murex, an Ancient Indian Medicinal Plant: Histopathological Effects on the Zika Virus Vector Aedes Aegypti and Inhibition of Biofilm-Forming Pathogenic Bacteria. J. Photochem. Photobiol. B Biol. 2017, 174, 133–143. [Google Scholar] [CrossRef]
- Malaikozhundan, B.; Vijayakumar, S.; Vaseeharan, B.; Jenifer, A.A.; Chitra, P.; Prabhu, N.M.; Kannapiran, E. Two Potential Uses for Silver Nanoparticles Coated with Solanum nigrum Unripe Fruit Extract: Biofilm Inhibition and Photodegradation of Dye Effluent. Microb. Pathog. 2017, 111, 316–324. [Google Scholar] [CrossRef]
- Ali, K.; Ahmed, B.; Dwivedi, S.; Saquib, Q.; Al-Khedhairy, A.A. Microwave Accelerated Green Synthesis of Stable Silver Nanoparticles with Eucalyptus Globulus Leaf Extract and Their Antibacterial and Antibiofilm Activity on Clinical Isolates. PLoS ONE 2015, 10, e0131178. [Google Scholar] [CrossRef]
- Gurunathan, S.; Han, J.W.; Kwon, D.; Kim, J. Enhanced Antibacterial and Anti-Biofilm Activities of Silver Nanoparticles against Gram-Negative and Gram-Positive Bacteria. Nanoscale Res. Lett. 2014, 9, 373. [Google Scholar] [CrossRef] [Green Version]
- Abdel-aziz, M.S.; Shaheen, M.S.; El-nekeety, A.A. Antioxidant and Antibacterial Activity of Silver Nanoparticles Biosynthesized Using Chenopodium murale Leaf Extract. J. Saudi Chem. Soc. 2013, 18, 356–363. [Google Scholar] [CrossRef] [Green Version]
- Singhal, M.; Chatterjee, S.; Kumar, A.; Syed, A.; Bahkali, A.H.; Gupta, N.; Nimesh, S. Molecules Exploring the Antibacterial and Antibiofilm Efficacy of Silver Nanoparticles Biosynthesized Using Punica granatum Leaves. Molecules 2021, 26, 5762. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.A.; Kalam, A.; Al-Sehemi, A.G.; Alomary, M.N.; Alyahya, S.; Kashif Aziz, M.; Srivastava, S.; Alghamdi, S.; Almalki, H.D.; Adil, S.F.; et al. Counteraction of Biofilm Formation and Antimicrobial Potential of Terminalia catappa Functionalized Silver Nanoparticles against Candida albicans and Multidrug-Resistant Gram-Negative and Gram-Positive Bacteria. Antibiotics 2021, 10, 725. [Google Scholar] [CrossRef] [PubMed]
- Ben Haddada, M.; Gerometta, E.; Chawech, R.; Sorres, J.; Bialecki, A.; Pesnel, S.; Spadavecchia, J.; Morel, A.L. Assessment of Antioxidant and Dermoprotective Activities of Gold Nanoparticles as Safe Cosmetic Ingredient. Colloids Surf. B Biointerfaces 2020, 189, 110855. [Google Scholar] [CrossRef] [PubMed]
- Bahrulolum, H.; Nooraei, S.; Javanshir, N.; Tarrahimofrad, H.; Mirbagheri, V.S.; Easton, A.J.; Ahmadian, G. Green Synthesis of Metal Nanoparticles Using Microorganisms and Their Application in the Agrifood Sector. J. Nanobiotechnol. 2021, 19, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Lahtinen, E.; Kukkonen, E.; Kinnunen, V.; Lahtinen, M.; Kinnunen, K.; Suvanto, S.; Vaïsänen, A.; Haukka, M. Gold Nanoparticles on 3D-Printed Filters: From Waste to Catalysts. ACS Omega 2019, 4, 16891–16898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anwar, Y.; Ullah, I.; Ul-Islam, M.; Alghamdi, K.M.; Khalil, A.; Kamal, T. Adopting a Green Method for the Synthesis of Gold Nanoparticles on Cotton Cloth for Antimicrobial and Environmental Applications. Arab. J. Chem. 2021, 14, 103327. [Google Scholar] [CrossRef]
- Zhang, Y.; Shareena Dasari, T.P.; Deng, H.; Yu, H. Antimicrobial Activity of Gold Nanoparticles and Ionic Gold. J. Environ. Sci. Health Part C Environ. Carcinog. Ecotoxicol. Rev. 2015, 33, 286–327. [Google Scholar] [CrossRef]
- Dasari, S.T.; Zhang, Y.; Yu, H. Antibacterial Activity and Cytotoxicity of Gold (I) and (III) Ions and Gold Nanoparticles. Biochem. Pharmacol. 2015, 4, 199. [Google Scholar] [CrossRef] [Green Version]
- Amin, R.M.; Mohamed, M.B.; Ramadan, M.A.; Verwanger, T.; Krammer, B. Rapid and Sensitive Microplate Assay for Screening the Effect of Silver and Gold Nanoparticles on Bacteria. Nanomedecine 2009, 4, 637–643. [Google Scholar] [CrossRef]
- Chatterjee, S.; Bandyopadhyay, A.; Sarkar, K. Effect of Iron Oxide and Gold Nanoparticles on Bacterial Growth Leading towards Biological Application. J. Nanobiotechnol. 2011, 9, 34. [Google Scholar] [CrossRef] [Green Version]
- Valsalam, S.; Agastian, P.; Esmai, G.A.; Ghilan, M.; Al-dhabi, N.A.; Valan, M. Biosynthesis of Silver and Gold Nanoparticles Using Musa acuminata colla Flower and Its Pharmaceutical Activity against Bacteria and Anticancer Efficacy. J. Photochem. Photobiol. B Biol. 2019, 201, 111670. [Google Scholar] [CrossRef] [PubMed]
- Velmurugan, P.; Lee, K.; Cho, M. Green Synthesis of Silver and Gold Nanoparticles Using Zingiber Officinale Root Extract and Antibacterial Activity of Silver Nanoparticles against Food Pathogens Green Synthesis of Silver and Gold Nanoparticles Using Zingiber Officinale Root Extract and A. Bioprocess Biosyst. Eng. 2014, 37, 1935–1943. [Google Scholar] [CrossRef] [PubMed]
- Rajan, A.; Vilas, V.; Philip, D. Studies on Catalytic, Antioxidant, Antibacterial and Anticancer Activities of Biogenic Gold Nanoparticles. J. Mol. Liq. 2015, 212, 331–339. [Google Scholar] [CrossRef]
- Lakshmanan, A.; Umamaheswari, C.; Nagarajan, N.S. A Facile Phyto-Mediated Synthesis of Gold Nanoparticles Using Aqueous Extract of Momordica cochinchinensis Rhizome and Their Biological Activities. J. Nanosci. Technol. 2016, 2, 76–80. [Google Scholar]
- Mata, R.; Bhaskaran, A.; Sadras, S.R. Green-Synthesized Gold Nanoparticles from Plumeria Alba Flower Extract to Augment Catalytic Degradation of Organic Dyes and Inhibit Bacterial Growth. Particuology 2015, 24, 78–86. [Google Scholar] [CrossRef]
- Dhayalan, M.; Immanuel, M.; Denison, J.; Ayyar, M.; Gandhi, N.N.; Krishnan, K. Biogenic Synthesis, Characterization of Gold and Silver Nanoparticles from Coleus forskohlii and Their Clinical Importance. J. Photochem. Photobiol. B Biol. 2018, 183, 251–257. [Google Scholar] [CrossRef]
- Ullah, R.; Bakht, J.; Shah, M.R.; Shafi, M. Bioinspired Synthesis and Characterization of Gold Nano-Particles from Medicinally Important Periploca Hydaspidis and Their in Vitro Antioxidant and Antimicrobial Activity. Pak. J. Pharm. Sci. 2019, 32, 1069–1080. [Google Scholar]
- Boomi, P.; Ganesan, R.M.; Poorani, G.; Gurumallesh Prabu, H.; Ravikumar, S.; Jeyakanthan, J. Biological Synergy of Greener Gold Nanoparticles by Using Coleus aromaticus Leaf Extract. Mater. Sci. Eng. C 2019, 99, 202–210. [Google Scholar] [CrossRef]
- Benedec, D.; Oniga, I.; Cuibus, F.; Sevastre, B.; Stiufiuc, G.; Duma, M.; Hanganu, D.; Iacovita, C.; Stiufiuc, R.; Lucaciu, C.M. Origanum vulgare Mediated Green Synthesis of Biocompatible Gold Nanoparticles Simultaneously Possessing Plasmonic, Antioxidant and Antimicrobial Properties. Int. J. Nanomed. 2018, 13, 1041–1058. [Google Scholar] [CrossRef] [Green Version]
- Basavegowda, N.; Lee, Y.R. Synthesis of Gold and Silver Nanoparticles Using Leaf Extract of Perilla Frutescens—A Biogenic Approach. J. Nanosci. Nanotechnol. 2014, 14, 4377–4382. [Google Scholar] [CrossRef]
- Paul, B.; Bhuyan, B.; Purkayastha, D.D.; Dhar, S.S. Photocatalytic and Antibacterial Activities of Gold and Silver Nanoparticles Synthesized Using Biomass of Parkia roxburghii Leaf. J. Photochem. Photobiol. B Biol. 2016, 154, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Liu, S.; Wang, J.; Lin, R.; Kawasaki, M.; Rus, E.; Silberstein, K.E.; Lowe, M.A.; Lin, F.; Nordlund, D.; et al. Spontaneous Incorporation of Gold in Palladium-Based Ternary Nanoparticles Makes Durable Electrocatalysts for Oxygen Reduction Reaction. Nat. Commun. 2016, 7, 11941. [Google Scholar] [CrossRef] [PubMed]
- Vimalraj, S.; Ashokkumar, T.; Saravanan, S. Biogenic Gold Nanoparticles Synthesis Mediated by Mangifera indica Seed Aqueous Extracts Exhibits Antibacterial, Anticancer and Anti-Angiogenic Properties. Biomed. Pharmacother. 2018, 105, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Soshnikova, V.; Kim, Y.J.; Singh, P.; Huo, Y.; Markus, J.; Ahn, S.; Castro-Aceituno, V.; Kang, J.; Chokkalingam, M.; Mathiyalagan, R.; et al. Cardamom Fruits as a Green Resource for Facile Synthesis of Gold and Silver Nanoparticles and Their Biological Applications. Artif. Cells Nanomed. Biotechnol. 2018, 46, 108–117. [Google Scholar] [CrossRef] [Green Version]
- Kadiyala, N.K.; Mandal, B.K.; Ranjan, S.; Dasgupta, N. Bioinspired Gold Nanoparticles Decorated Reduced Graphene Oxide Nanocomposite Using Syzygium cumini Seed Extract: Evaluation of Its Biological Applications. Mater. Sci. Eng. C 2018, 93, 191–205. [Google Scholar] [CrossRef]
- Basavegowda, N.; Idhayadhulla, A.; Lee, Y.R. Phyto-Synthesis of Gold Nanoparticles Using Fruit Extract of Hovenia dulcis and Their Biological Activities. Ind. Crop. Prod. 2014, 52, 745–751. [Google Scholar] [CrossRef]
- Lee, K.D.; Nagajyothi, P.C.; Sreekanth, T.V.M.; Park, S. Eco-Friendly Synthesis of Gold Nanoparticles (AuNPs) Using Inonotus obliquus and Their Antibacterial, Antioxidant and Cytotoxic Activities. J. Ind. Eng. Chem. 2015, 26, 67–72. [Google Scholar] [CrossRef]
- Gopinath, K.; Kumaraguru, S.; Bhakyaraj, K.; Mohan, S.; Venkatesh, K.S.; Esakkirajan, M.; Kaleeswarran, P.R.; Naiyf, S.A.; Kadaikunnan, S.; Govindarajan, M.; et al. Green Synthesis of Silver, Gold and Silver/Gold Bimetallic Nanoparticles Using the Gloriosa superba Leaf Extract and Their Antibacterial and Antibiofilm Activities. Microb. Pathog. 2016, 101, 1–11. [Google Scholar] [CrossRef]
- Pasquet, J.; Chevalier, Y.; Pelletier, J.; Couval, E.; Bouvier, D.; Bolzinger, M.A. The Contribution of Zinc Ions to the Antimicrobial Activity of Zinc Oxide. Colloids Surf. A Physicochem. Eng. Asp. 2014, 457, 263–274. [Google Scholar] [CrossRef]
- Abendrot, M.; Kalinowska-Lis, U. Zinc-Containing Compounds for Personal Care Applications. Int. J. Cosmet. Sci. 2018, 40, 319–327. [Google Scholar] [CrossRef] [Green Version]
- Happy, A.; Soumya, M.; Kumar, S.V.; Rajeshkumar, S.; Sheba, R.D.; Lakshmi, T.; Nallaswamy, V.D. Phyto-Assisted Synthesis of Zinc Oxide Nanoparticles Using Cassia Alata and Its Antibacterial Activity against Escherichia coli. Biochem. Biophys. Rep. 2019, 17, 208–211. [Google Scholar] [CrossRef] [PubMed]
- Dobrucka, R.; Długaszewska, J. Biosynthesis and Antibacterial Activity of Zno Nanoparticles Using Trifolium pratense Flower Extract. Saudi J. Biol. Sci. 2016, 23, 517–523. [Google Scholar] [CrossRef] [Green Version]
- Malaikozhundan, B.; Vaseeharan, B.; Vijayakumar, S.; Pandiselvi, K.; Kalanjiam, R.; Murugan, K.; Benelli, G. Biological Therapeutics of Pongamia pinnata Coated Zinc Oxide Nanoparticles against Clinically Important Pathogenic Bacteria, Fungi and MCF-7 Breast Cancer Cells. Microb. Pathog. 2017, 104, 268–277. [Google Scholar] [CrossRef]
- Vijayakumar, S.; Vinoj, G.; Malaikozhundan, B.; Shanthi, S.; Vaseeharan, B. Plectranthus amboinicus Leaf Extract Mediated Synthesis of Zinc Oxide Nanoparticles and Its Control of Methicillin Resistant Staphylococcus aureus Biofilm and Blood Sucking Mosquito Larvae. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 137, 886–891. [Google Scholar] [CrossRef] [PubMed]
- Khatami, M.; Alijani, H.Q.; Heli, H.; Sharifi, I. Rectangular Shaped Zinc Oxide Nanoparticles: Green Synthesis by Stevia and Its Biomedical Effiency. Ceram. Int. 2018, 44, 15596–15602. [Google Scholar] [CrossRef]
- Nazir, S.; Zaka, M.; Adil, M.; Abbasi, B.H.; Hano, C. Synthesis, Characterisation and Bactericidal Effect of ZnO Nanoparticles via Chemical and Bio-Assisted (Silybum marianum in Vitro Plantlets and Callus Extract) Methods: A Comparative Study. IET Nanobiotechnol. 2018, 12, 604–608. [Google Scholar] [CrossRef]
- Abbasi, B.H.; Zahir, A.; Ahmad, W.; Nadeem, M.; Giglioli-guivarc, N.; Hano, C. Biogenic Zinc Oxide Nanoparticles-Enhanced Biosynthesis of Lignans and Neolignans in Cell Suspension Cultures of Linum usitatissimum L Biogenic Zinc Oxide Nanoparticles-Enhanced Biosynthesis of Lignans And. Artif. Cells Nanomed. Biotechnol. 2019, 47, 1367–1373. [Google Scholar] [CrossRef] [Green Version]
- Azizi, S.; Mohamad, R.; Bahadoran, A.; Bayat, S.; Rahim, R.A.; Ariff, A.; Saad, W.Z. Effect of Annealing Temperature on Antimicrobial and Structural Properties of Bio-Synthesized Zinc Oxide Nanoparticles Using Flower Extract of Anchusa italica. Photochem. Photobiol. B Biol. 2016, 161, 441–449. [Google Scholar] [CrossRef]
- Ali, J.; Irshad, R.; Li, B.; Tahir, K.; Ahmad, A.; Shakeel, M.; Khan, N.U.; Khan, Z.U.H. Synthesis and Characterization of Phytochemical Fabricated Zinc Oxide Nanoparticles with Enhanced Antibacterial and Catalytic Applications. J. Photochem. Photobiol. B Biol. 2018, 183, 349–356. [Google Scholar] [CrossRef]
- Sharretts Plating Company. Platinum vs. Palladium Plating in Medicine. 2017. Available online: https://www.sharrettsplating.com/blog/platinum-vs-palladium-plating-medicine/ (accessed on 20 December 2021).
- Food and Drug Administration. FDA Backgrounder on Platinum in Silicone Breast Implants. 2018. Available online: https://www.fda.gov/medical-devices/breast-implants/fda-backgrounder-platinum-silicone-breast-implants (accessed on 20 December 2021).
- Sinitsyna, O.; Paralikar, P.; Pandit, R.; Rai, M. Platinum in Biomedical Applications. In Biomedical Applications of Metals; Springer: Berlin/Heidelberg, Germany, 2018; pp. 151–165. ISBN 9783319748146. [Google Scholar]
- Nishanthi, R.; Malathi, S.; Paul, S.J.; Palani, P. Green Synthesis and Characterization of Bioinspired Silver, Gold and Platinum Nanoparticles and Evaluation of Their Synergistic Antibacterial Activity after Combining with Different Classes of Antibiotics. Mater. Sci. Eng. C 2019, 96, 693–707. [Google Scholar] [CrossRef]
- Castro, L.; Blázquez, M.L.; González, F.; Muñoz, J.Á.; Ballester, A. Biosynthesis of Silver and Platinum Nanoparticles Using Orange Peel Extract: Characterisation and Applications. IET Nanobiotechnol. 2015, 9, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Chelli, V.R.; Golder, A.K. One Pot Green Synthesis of Pt, Co and Pt @ Co Core-Shell Nanoparticles Using Sechium edule. J. Chem. Technol. Biotechnol. 2019, 94, 911–918. [Google Scholar] [CrossRef]
- Subramanian, S.B.; Ramani, A.; Ganapathy, V.; Anbazhagan, V. Preparation of Self-Assembled Platinum Nanoclusters to Combat Salmonella Typhi Infection and Inhibit Biofilm Formation. Colloids Surf. B Biointerfaces 2018, 92, 479–490. [Google Scholar] [CrossRef] [PubMed]
- Tahir, K.; Nazir, S.; Ahmad, A.; Li, B.; Ullah, A.; Ul, Z.; Khan, H.; Ullah, F.; Ullah, Q.; Khan, A.; et al. Facile and Green Synthesis of Phytochemicals Capped Platinum Nanoparticles and in Vitro Their Superior Antibacterial Activity. J. Photochem. Photobiol. B Biol. 2017, 166, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Arockiya, F.; Rajathi, A. Phytofabrication of Nano-Crystalline Platinum Particles by Leaves of Cerbera manghas and Its Antibacterial Efficacy. Inter. J. Pharm. Bio. Sci. 2015, 5, 619–628. [Google Scholar]
- Manikandan, V.; Velmurugan, P.; Park, H.; Lovanh, N.; Seo, S.; Jayanthi, P.; Park, Y.; Cho, M. Synthesis and Antimicrobial Activity of Palladium Nanoparticles from Prunus × Yedoensis Leaf Extract. Mater. Lett. 2016, 185, 335–338. [Google Scholar] [CrossRef]
- Yu, X.; Yuan, L.; Zhu, N.; Wang, K.; Xia, Y. Fabrication of Antimicrobial Curcumin Stabilized Platinum Nanoparticles and Their Anti-Liver Fibrosis Activity for Potential Use in Nursing Care. J. Photochem. Photobiol. B Biol. 2019, 195, 27–32. [Google Scholar] [CrossRef]
- Surendra, T.V.; Mohana, S.; Valan, M.; Al-dhabi, N.A.; Rayalu, G.M. RSM Optimized Moringa oleifera Peel Extract for Green Synthesis of M. oleifera Capped Palladium Nanoparticles with Antibacterial and Hemolytic Property. J. Photochem. Photobiol. B Biol. 2016, 162, 550–557. [Google Scholar] [CrossRef]
- Anjana, P.M.; Bindhu, M.R.; Umadevi, M.; Rakhi, R.B. Antibacterial and Electrochemical Activities of Silver, Gold, and Palladium Nanoparticles Dispersed Amorphous Carbon Composites. Appl. Surf. Sci. 2019, 479, 96–104. [Google Scholar] [CrossRef]
- Azizi, S.; Mahdavi, M.; Sulaiman, H.; Rahim, R.A.; Rasedee, A. Green Synthesis Palladium Nanoparticles Mediated by White Tea (Camellia sinensis) Extract with Antioxidant, Antibacterial, and Antiproliferative Activities toward the Human Leukemia (MOLT-4) Cell Line. Int. J. Nanomed. 2017, 12, 8841–8853. [Google Scholar] [CrossRef] [Green Version]
- Hazarika, M.; Borah, D.; Bora, P.; Silva, A.R.; Das, P. Biogenic Synthesis of Palladium Nanoparticles and Their Applications as Catalyst and Antimicrobial Agent. PLoS ONE 2017, 12, e0184936. [Google Scholar] [CrossRef] [PubMed]
- Tahir, K.; Nazir, S.; Ahmad, A.; Li, B.; Shah, S.A.A.; Khan, A.U.; Khan, Z.U.H.; Khan, Q.; Khan, F.U. Biodirected Synthesis of Palladium Nanoparticles Using Phoenix Dactylifera Leaves Extract and Their Size Dependent Biomedical and Catalytic Applications. RSC Adv. 2016, 6, 1–39. [Google Scholar] [CrossRef]
- Parker, H.L.; Rylott, E.L.; Hunt, A.J.; Dodson, J.R.; Taylor, A.F.; Bruce, N.C.; Clark, J.H. Supported Palladium Nanoparticles Synthesized by Living Plants as a Catalyst for Suzuki-Miyaura Reactions. PLoS ONE 2014, 9, e087192. [Google Scholar] [CrossRef] [PubMed]
- Devi, D.K.; Pratap, S.V.; Haritha, R.; Sivudu, K.S.; Radhika, P.; Sreedhar, B. Gum Acacia as a Facile Reducing, Stabilizing, and Templating Agent for Palladium Nanoparticles. J. Appl. Polym. Sci. 2011, 121, 1765–1773. [Google Scholar] [CrossRef]
- Vaghela, H.; Shah, R.; Pathan, A. Palladium Nanoparticles Mediated Through Bauhinia Variegata: Potent In Vitro Anticancer Activity Against MCF-7 Cell Lines and Antimicrobial Assay. Curr. Nanomater. 2018, 3, 168–177. [Google Scholar] [CrossRef]
- Liu, D.; Wu, F. Biosynthesis of Pd Nanoparticle Using Onion Extract for Electrochemical Determination of Carbendazim. Int. J. Electrochem. Sci. 2017, 12, 2125–2134. [Google Scholar] [CrossRef]
- Sharmila, G.; Fathima, M.F.; Haries, S.; Geetha, S.; Kumar, N.M.; Muthukumaran, C. Green Synthesis, Characterization and Antibacterial Efficacy of Palladium Nanoparticles Synthesized Using Filicium Decipiens Leaf Extract. J. Mol. Struct. 2017, 12, 2125–2134. [Google Scholar] [CrossRef]
- Dinesh, M.; Roopan, S.M.; Selvaraj, C.I.; Arunachalam, P. Phyllanthus Emblica Seed Extract Mediated Synthesis of PdNPs against Antibacterial, Heamolytic and Cytotoxic Studies. J. Photochem. Photobiol. B Biol. 2017, 167, 64–71. [Google Scholar] [CrossRef]
- Duan, L.; Li, M.; Liu, H. Biosynthesised Palladium Nanoparticles Using Eucommia Ulmoides Bark Aqueous Extract and Their Catalytic Activity. IET Nanobiotechnol. 2015, 9, 349–354. [Google Scholar] [CrossRef]
- Dauthal, P.; Mukhopadhyay, M. Biosynthesis of Palladium Nanoparticles Using Delonix Regia Leaf Extract and Its Catalytic Activity for Nitro-Aromatics Hydrogenation. Ind. Eng. Chem. Res. 2013, 52, 18131–18139. [Google Scholar] [CrossRef]
- Mohammed, N.G.; Prasad, S.; Khandare, R.V.; Prasad, N.R. Extracellular One Pot Green Synthesis of Palladium Nanoparticles. Int. J. Nanomater. Nanostruct. 2014, 1, 1–8. [Google Scholar]
- Mallikarjuna, K.; Sushma, N.J.; Reddy, B.V.S.; Narasimha, G.; Raju, B.D.P. Palladium Nanoparticles: Single-Step Plant-Mediated Green Chemical Procedure Using Piper Betle Leaves Broth and Their Anti-Fungal Studies. Int. J. Chem. Anal. Sci. 2013, 4, 14–18. [Google Scholar] [CrossRef]
- Rabiee, N.; Bagherzadeh, M.; Kiani, M.; Ghadiri, A.M. Rosmarinus Officinalis Directed Palladium Nanoparticle Synthesis: Investigation of Potential Anti-Bacterial, Anti-Fungal and Mizoroki-Heck Catalytic Activities. Adv. Powder Technol. 2020, 31, 1402–1411. [Google Scholar] [CrossRef]
- Kora, A.J.; Rastogi, L. Catalytic Degradation of Anthropogenic Dye Pollutants Using Palladium Nanoparticles Synthesized by Gum Olibanum, a Glucuronoarabinogalactan Biopolymer. Ind. Crop. Prod. 2016, 81, 1–10. [Google Scholar] [CrossRef]
- Nadagouda, M.N.; Varma, R.S. Green Synthesis of Silver and Palladium Nanoparticles at Room Temperature Using Coffee and Tea Extract Green Synthesis of Silver and Palladium Nanoparticles at Room Temperature Using Coffee and Tea Extract. Green Chem. 2008, 10, 859–862. [Google Scholar] [CrossRef]
- Hoseinpour, V.; Ghaemi, N. Green Synthesis of Manganese Nanoparticles: Applications and Future Perspective–A Review. J. Photochem. Photobiol. B Biol. 2018, 189, 234–243. [Google Scholar] [CrossRef]
- Cherian, T.; Ali, K.; Saquib, Q.; Faisal, M.; Wahab, R. Cymbopogon Citratus Functionalized Green Synthesis of CuO-Nanoparticles: Novel Prospects as Antibacterial and Antibiofilm Agents. Biomolecules 2019, 10, 169. [Google Scholar] [CrossRef] [Green Version]
- Khani, R.; Roostaei, B.; Bagherzade, G.; Moudi, M. Green Synthesis of Copper Nanoparticles by Fruit Extract of Ziziphus spina-Christi (L.) Willd: Application for Adsorption of Triphenylmethane Dye and Antibacterial Assay. J. Mol. Liq. 2018, 255, 541–549. [Google Scholar] [CrossRef]
- Mali, S.C.; Raj, S.; Trivedi, R. Biosynthesis of Copper Oxide Nanoparticles Using Enicostemma Axillare (Lam.) Leaf Extract. Biochem. Biophys. Rep. 2019, 20, 100699. [Google Scholar] [CrossRef]
- Caroling, G.; Vinodhini, E.; Ranjitham, A.M.; Shanthi, P. Biosynthesis of Copper Nanoparticles Using Aqueous Phyllanthus Embilica (Gooseberry) Extract—Characterisation and Study of Antimicrobial Effects. Int. J. Nano. Chem. 2015, 63, 53–63. [Google Scholar]
- Sankar, R.; Manikandan, P.; Malarvizhi, V.; Fathima, T. Green Synthesis of Colloidal Copper Oxide Nanoparticles Using Carica Papaya and Its Application in Photocatalytic Dye Degradation. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 121, 746–750. [Google Scholar] [CrossRef] [PubMed]
- Sathiyavimal, S.; Vasantharaj, S.; Bharathi, D.; Saravanan, M. Biogenesis of Copper Oxide Nanoparticles (CuONPs) Using Sida acuta and Their Incorporation over Cotton Fabrics to Prevent the Pathogenicity of Gram Negative and Gram Positive Bacteria. J. Photochem. Photobiol. B Biol. 2018, 188, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Jinu, U.; Gomathi, M.; Saiqa, I.; Geetha, N.; Benelli, G.; Venkatachalam, P. Green Engineered Biomolecule-Capped Silver and Copper Nanohybrids Using Prosopis Cineraria Leaf Extract: Enhanced Antibacterial Activity against Microbial Pathogens of Public Health Relevance and Cytotoxicity on Human Breast Cancer Cells (MCF-7). Microb. Pathog. 2017, 105, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Prasad, P.R.; Kanchi, S.; Naidoo, E.B. In-Vitro Evaluation of Copper Nanoparticles Cytotoxicity on Prostate Cancer Cell Lines and Their Antioxidant, Sensing and Catalytic Activity: One-Pot Green Approach. Photochem. Photobiol. 2016, 161, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Vasantharaj, S.; Sathiyavimal, S.; Saravanan, M.; Senthilkumar, P.; Gnanasekaran, K.; Shanmugavel, M. Synthesis of Ecofriendly Copper Oxide Nanoparticles for Fabrication over Textile Fabrics: Characterization of Antibacterial Activity and Dye Degradation Potential. J. Photochem. Photobiol. B Biol. 2019, 191, 143–149. [Google Scholar] [CrossRef]
- Kaur, P.; Energy, T.; Thakur, R.; Chaudhury, A. Biogenesis of Copper Nanoparticles Using Peel Extract of Punica Granatum and Their Antimicrobial Activity against Opportunistic Pathogens. Green Chem. Lett. Rev. 2016, 9, 33–38. [Google Scholar] [CrossRef] [Green Version]
- Thakur, S.; Sharma, S.; Thakur, S.; Rai, R. Green Synthesis of Copper Nano-Particles Using Asparagus Adscendens Roxb. Root and Leaf Extract and Their Antimicrobial Activities. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 683–694. [Google Scholar] [CrossRef]
- Naika, H.R.; Lingaraju, K.; Manjunath, K.; Kumar, D.; Nagaraju, G.; Suresh, D.; Nagabhushana, H. Green Synthesis of CuO Nanoparticles Using Gloriosa superba L. Extract and Their Antibacterial Activity. J Taibah Univ. Sci. 2015, 9, 7–12. [Google Scholar] [CrossRef] [Green Version]
- Valli, G.; Geetha, S. Green Synthesis of Copper Nanoparticles Using Cassia Auriculata Leaves Extract. Int. J. TechnoChem Res. 2016, 2, 5–10. [Google Scholar]
- Bekele, S. Synthesis of Copper Oxide Nanoparticles Using Leaf Extract of Bersama Abyssinica and Its Application for the Treatment of Drug Resistant Bacteria. Marster’s Thesis, Adama Science and Technology University, Adama, Ethiopia, 2018. [Google Scholar]
- Kala, A.; Soosairaj, S. Green Synthesis of Copper Bionanoparticles to Control the Bacterial Leaf Blight Disease of Rice. Curr. Sci. 2016, 110, 2011–2014. [Google Scholar] [CrossRef]
- Chitra, K.; Reena, K.; Manikandan, A.; Antony, S.A. Antibacterial Studies and Effect of Poloxamer on Gold Nanoparticles by Zingiber Officinale Extracted Green Synthesis. J. Nanosci. Nanotechnol. 2015, 15, 4984–4991. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi-aloucheh, R.; Habibi-yangjeh, A.; Bayrami, A.; Asadi, A. Enhanced Anti-Bacterial Activities of ZnO Nanoparticles and ZnO/CuO Nanocomposites Synthesized Using Vaccinium arctostaphylos L. Fruit Extract. Artif. Cells Nanomed. Biotechnol. 2018, 46 (Suppl. 1), 1200–1209. [Google Scholar] [CrossRef] [Green Version]
- Rajeshkumar, S.; Menon, S.; S, V.K.; Tambuwala, M.M.; Bakshi, H.A.; Mehta, M.; Satija, S.; Gupta, G.; Kumar, D. Antibacterial and Antioxidant Potential of Biosynthesized Copper Nanoparticles Mediated through Cissus arnotiana Plant Extract. J. Photochem. Photobiol. B Biol. 2019, 197, 111531. [Google Scholar] [CrossRef] [PubMed]
- Qasim, S.; Zafar, A.; Saif, M.S.; Nazar, M.; Waqas, M.; Haq, A.U.; Tariq, T. Green Synthesis of Iron Oxide Nanorods Using Withania coagulans Extract Improved Photocatalytic Degradation and Antimicrobial Activity. J. Photochem. Photobiol. B Biol. 2020, 204, 111784. [Google Scholar] [CrossRef] [PubMed]
- Da, E.; Taha, A.; Afkar, E. Applied Sciences Green Synthesis of Iron Nanoparticles by Acacia nilotica Pods Extract and Its Catalytic, Adsorption, and Antibacterial Activities. Appl. Sci. 2018, 8, 1922. [Google Scholar] [CrossRef] [Green Version]
- Saranya, S.; Vijayaranai, K.; Pavithra, S.; Raihana, N.; Kumanan, K. In Vitro Cytotoxicity of Zinc Oxide, Iron Oxide and Copper Nanopowders Prepared by Green Synthesis. Toxicol. Rep. 2017, 4, 427–430. [Google Scholar] [CrossRef]
- Alam, T.; Asad, R.; Khan, A.; Ali, A.; Sher, H.; Ullah, Z.; Ali, M. Biogenic Synthesis of Iron Oxide Nanoparticles via Skimmia laureola and Their Antibacterial Efficacy against Bacterial Wilt Pathogen Ralstonia solanacearum. Mater. Sci. Eng. C 2019, 98, 101–108. [Google Scholar] [CrossRef]
- Kanagasubbulakshmi, S.; Kadirvelu, K. Green Synthesis of Iron Oxide Nanoparticles Using Lagenaria siceraria and Evaluation of Its Antimicrobial Activity. Def. Life Sci. J. 2017, 2, 422–427. [Google Scholar] [CrossRef]
- Daniel, K.; Natesan, S.; Kasi, N. Biosynthesis of Cu, ZVI, and Ag Nanoparticles Using Dodonaea viscosa Extract for Antibacterial Activity against Human Pathogens. J. Nanopart. Res. 2013, 15, 1–10. [Google Scholar] [CrossRef]
- Sathishkumar, G.; Logeshwaran, V.; Sarathbabu, S.; Jha, P.K.; Jeyaraj, M.; Rajkuberan, C.; Senthilkumar, N.; Sivaramakrishnan, S. Green Synthesis of Magnetic Fe3O4 Nanoparticles Using Couroupita guianensis aubl. Fruit Extract for Their Antibacterial and Cytotoxicity Activities. Artif. Cells Nanomed. Biotechnol. 2018, 46, 589–598. [Google Scholar] [CrossRef] [Green Version]
- Jeyasundari, J.; Praba, P.S.; Jacob, Y.B.A.; Vasantha, V.S.; Shanmugaiah, V. Green Synthesis and Characterization of Zero Valent Iron Nanoparticles from the Leaf Extract of Psidium guajava Plant and Their Antibacterial Activity. Chem. Sci. Rev. Lett. 2017, 6, 1244–1252. [Google Scholar]
- Irshad, R.; Tahir, K.; Li, B.; Ahmad, A.; Siddiqui, A.R.; Nazir, S. Antibacterial Activity of Biochemically Capped Iron Oxide Nanoparticles: A View towards Green Chemistry. J. Photochem. Photobiol. B Biol. 2017, 170, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Arokiyaraj, S.; Saravanan, M.; Prakash, N.K.U.; Arasu, M.V.; Vijayakumar, B.; Vincent, S. Enhanced Antibacterial Activity of Iron Oxide Magnetic Nanoparticles Treated with Argemone mexicana L. Leaf Extract: An in Vitro Study. Mater. Res. Bull. 2013, 48, 3323–3327. [Google Scholar] [CrossRef]
- Vasantharaj, S.; Sathiyavimal, S.; Senthilkumar, P.; Lewisoscar, F. Biosynthesis of Iron Oxide Nanoparticles Using Leaf Extract of Ruellia tuberosa: Antimicrobial Properties and Their Applications in Photocatalytic Degradation. J. Photochem. Photobiol. B Biol. 2019, 192, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Veeramanikandan, V.; Madhu, G.C.; Pavithra, V.; Jaianand, K.; Balaji, P. Green Synthesis, Characterization of Iron Oxide Nanoparticles Using Leucas Aspera Leaf Extract and Evaluation of Antibacterial and Antioxidant Studies. Int. J. Agric. Innov. Res. 2018, 6, 2319-1473. [Google Scholar]
- Jagathesan, G.; Rajiv, P. Biocatalysis and Agricultural Biotechnology Biosynthesis and Characterization of Iron Oxide Nanoparticles Using Eichhornia crassipes Leaf Extract and Assessing Their Antibacterial Activity. Biocatal. Agric. Biotechnol. 2018, 13, 90–94. [Google Scholar] [CrossRef]
- Pallela, P.N.V.K.; Ummey, S.; Ruddaraju, L.K.; Gadi, S.; Cherukuri, C.S.L.; Barla, S.; Pammi, S.V.N. Antibacterial Efficacy of Green Synthesized α-Fe2O3 Nanoparticles Using Sida cordifolia Plant Extract. Heliyon 2019, 5, e02765. [Google Scholar] [CrossRef]
- Radini, I.A.; Hasan, N.; Malik, M.A. Biosynthesis of Iron Nanoparticles Using Trigonella foenum-graecum Seed Extract for Photocatalytic Methyl Orange Dye Degradation and Antibacterial Applications. J. Photochem. Photobiol. B Biol. 2018, 183, 154–163. [Google Scholar] [CrossRef]
- Igwe, O.U.; Nwamezie, F. Green Synthesis of Iron Nanoparticles Using Flower Extracts of Piliostigma thonningii and Their Antibacterial Activity Evaluation Green Synthesis of Iron Nanoparticles Using Flower Extract of Piliostigma thonningii and Their Antibacterial Activity Evaluat. Chem. Int. 2018, 4, 60–66. [Google Scholar]
- Wang, F.; Niu, X.; Wang, W.; Jing, W.; Huang, Y.; Zhang, J. Green Synthesis of Pd Nanoparticles via Extracted Polysaccharide Applied to Glucose Detection. J. Taiwan Inst. Chem. Eng. 2018, 93, 87–93. [Google Scholar] [CrossRef]
- Tang, Q.; Xia, H.; Liang, W.; Huo, X.; Wei, X. Synthesis and Characterization of Zinc Oxide Nanoparticles from Morus nigra and Its Anticancer Activity of AGS Gastric Cancer Cells. J. Photochem. Photobiol. B Biol. 2020, 202, 111698. [Google Scholar] [CrossRef] [PubMed]
- Karthiga, P.; Shankar, T.; Karthick, K.; Swarnalatha, K. Phytomediated Synthesis of Silver Nanoparticles Against Microbial Pathogens and Cytotoxicity on Human Breast Cancer Cells (MCF-7). Resour. Effic. Technol. 2020, 2, 16–24. [Google Scholar] [CrossRef]
- Kuppusamy, P.; Yusoff, M.M.; Maniam, G.P. Biosynthesis of Metallic Nanoparticles Using Plant Derivatives and Their New Avenues in Pharmacological Applications—An Updated Report. Saudi Pharm. J. 2016, 24, 473–484. [Google Scholar] [CrossRef] [PubMed]
- Surendra, T.V.; Roopan, S.M.; Al-dhabi, N.A.; Arasu, M.V.; Sarkar, G. Vegetable Peel Waste for the Production of ZnO Nanoparticles and Its Toxicological Efficiency, Antifungal, Hemolytic, and Antibacterial Activities. Nanoscale Res. Lett. 2016, 11, 546. [Google Scholar] [CrossRef] [Green Version]
- Edayadulla, N.; Basavegowda, N.; Lee, Y.R. Green Synthesis and Characterization of Palladium Nanoparticles and Their Catalytic Performance for the Efficient Synthesis of Biologically Interesting Di (Indolyl) Indolin-2-Ones. J. Ind. Eng. Chem. 2014, 21, 1365–1372. [Google Scholar] [CrossRef]
- Islam, N.U.; Amin, R.; Shahid, M.; Amin, M.; Zaib, S.; Iqbal, J. A Multi-Target Therapeutic Potential of Prunus Domestica Gum Stabilized Nanoparticles Exhibited Prospective Anti-Inflammatory and Analgesic Properties. BMC Complement. Altern. Med. 2017, 17, 276. [Google Scholar] [CrossRef]
- Mahlaule-Glory, L.M.; Mbita, Z.; Ntsendwana, B.; Mathipa, M.M.; Mketo, N.; Hintsho-Mbita, N.C. ZnO Nanoparticles via Sutherlandia frutescens Plant Extract: Physical and Biological Properties. Mater. Res. Express 2019, 6, 085006. [Google Scholar] [CrossRef]
- Idelevich, E.A.; Becker, K. How to Accelerate Antimicrobial Susceptibility Testing. Clin. Microbiol. Infect. 2019, 25, 1347–1355. [Google Scholar] [CrossRef]
- Van Belkum, A.; Dunne, W.M. Next-Generation Antimicrobial Susceptibility Testing. J. Clin. Microbiol. 2013, 51, 2018–2024. [Google Scholar] [CrossRef] [Green Version]
- Suresh, A.K. Co-Relating Metallic Nanoparticle Characteristics and Bacterial Toxicity; Springer Nature: Cham, Switzerland, 2015. [Google Scholar]
- Ferreyra Maillard, A.P.V.; Gonçalves, S.; Santos, N.C.; López de Mishima, B.A.; Dalmasso, P.R.; Hollmann, A. Studies on Interaction of Green Silver Nanoparticles with Whole Bacteria by Surface Characterization Techniques. Biochim. Biophys. Acta Biomembr. 2019, 1861, 1086–1092. [Google Scholar] [CrossRef]
- Cui, L.; Chen, P.; Chen, S.; Yuan, Z.; Yu, C.; Ren, B.; Zhang, K. In Situ Study of the Antibacterial Activity and Mechanism of Action of Silver Nanoparticles by Surface-Enhanced Raman Spectroscopy. Anal. Chem. 2013, 85, 5436–5443. [Google Scholar] [CrossRef] [PubMed]
- Canciu, A.; Tertis, M.; Hosu, O.; Cernat, A.; Cristea, C.; Graur, F. Modern Analytical Techniques for Detection of Bacteria in Surface and Wastewaters. Sustainability 2021, 13, 7229. [Google Scholar] [CrossRef]
- Muhammad, H.; Akram, B.; Kazmi, S.A.; Ahmad, I. Populus Ciliata Leaves Extract Mediated Synthesis of Zinc Oxide Nanoparticles and Investigation of Their Anti-Bacterial Activities. Mater. Res. Express 2019, 6, 075064. [Google Scholar] [CrossRef]
- Pethakamsetty, L.; Kothapenta, K.; Nammi, H.R.; Ruddaraju, L.K.; Kollu, P.; Yoon, S.G.; Pammi, S.V.N. Green Synthesis, Characterization and Antimicrobial Activity of Silver Nanoparticles Using Methanolic Root Extracts of Diospyros sylvatica. J. Environ. Sci. 2017, 55, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Samira, S.; Mohamadi, A.; Mohseni, S. Biosynthesis, Characterization and Antimicrobial Activities of Zinc Oxide Nanoparticles from Leaf Extract of Mentha pulegium (L.). Microb. Pthogenes. 2019, 131, 239–245. [Google Scholar] [CrossRef]
- Raja, A.; Ashokkumar, S.; Marthandam, R.; Jayachandiran, J.; Khatiwada, C.P.; Kaviyarasy, K.; Raman, R.G.; Swaminathan, M. Eco-Friendly Preparation of Zinc Oxide Nanoparticles Using Tabernaemontana Divaricata and Its Photocatalytic and Antimicrobial Activity. J. Photochem. Photobiol. B Biol. 2018, 181, 53–58. [Google Scholar] [CrossRef]
- Balouiri, M.; Sadiki, M.S.; Ibnsouda, S.K. Methods for in Vitro Evaluating Antimicrobial Activity: A Review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef] [Green Version]
- Alonso, C.A.; Dominguez, C.; Heras, J.; Mata, E.; Pascual, V.; Torres, C.; Zarazaga, M. AntibiogramJ: A Tool for Analysing Images from Disk Diffusion Tests. Comput. Methods Programs Biomed. 2017, 143, 159–169. [Google Scholar] [CrossRef]
- Jorgensen, J.H.; Turnidge, J.D. Susceptibility Test Methods: Dilution and Disk Diffusion Methods. In Manual of Clinical Microbiology, 11th ed.; Wiley: Hoboken, NJ, USA, 2015; pp. 1253–1273. [Google Scholar] [CrossRef]
- Brook, I.; Wexler, H.M.; Goldstein, J.C. Antianaerobic Antimicrobials: Spectrum and Susceptibility Testing. Clin. Microbiol. Rev. 2013, 26, 526–546. [Google Scholar] [CrossRef] [Green Version]
- Kolya, H.; Maiti, P.; Pandey, A.; Tripathy, T. Green Synthesis of Silver Nanoparticles with Antimicrobial and Azo Dye (Congo Red) Degradation Properties Using Amaranthus Gangeticus Linn Leaf Extract. J. Anal. Sci. Technol. 2015, 6, 4–10. [Google Scholar] [CrossRef] [Green Version]
- Gomathi, M.; Rajkumar, P.V.; Prakasam, A.; Ravichandran, K. Green Synthesis of Silver Nanoparticles Using Datura stramonium Leaf Extract and Assessment of Their Antibacterial Activity. Resour. Technol. 2017, 3, 280–284. [Google Scholar] [CrossRef]
- Shameli, K.; Ahmad, M.B.; Jazayeri, S.D.; Shabanzadeh, P.; Sangpour, P.; Jahangirian, H.; Gharayebi, Y. Investigation of Antibacterial Properties Silver Nanoparticles Prepared via Green Method. Chem. Cent. J. 2012, 6, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lakshmeesha, T.R.; Sateesh, M.K.; Prasad, B.D.; Sharma, S.C.; Kavyashree, D.; Chandrasekhar, M.; Nagabhushana, H. Reactivity of Crystalline ZnO Superstructures against Fungi and Bacterial Pathogens: Synthesized Using Nerium Oleander Leaf Extract. Cryst. Growth Des. 2014, 14, 4068–4079. [Google Scholar] [CrossRef]
- Hamelian, M.; Varmira, K.; Veisi, H. Green Synthesis and Characterizations of Gold Nanoparticles Using Thyme and Survey Cytotoxic Effect, Antibacterial and Antioxidant Potential. J. Photochem. Photobiol. B Biol. 2018, 184, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Dorosti, N.; Jamshidi, F. Plant-mediated Gold Nanoparticles by Dracocephalum Kotschyi as Anticholinesterase Agent: Synthesis, Characterization, and Evaluation of Anticancer and Antibacterial Activity. J. Econ. Financ. Adm. Sci. 2016, 14, 235–245. [Google Scholar] [CrossRef]
- Escarcega-Gonzalez, C.E.; Garza-Cervantes, J.A.; Vazquez-Rodriguez, A.; Montelongo-Peralta, L.Z.; Trevino-Gonzalez, M.J.; Diaz Barriga Castro, E.; Saucedo-Salazar, E.M.; Chavez Morales, R.M.; Regalado Soto, D.I.; Trevino Gonzalez, F.M.; et al. In Vivo Antimicrobial Activity of Silver Nanoparticles Produced via a Green Chemistry Synthesis Using Acacia Rigidula as a Reducing and Capping Agent. Int. J. Nanomed. 2018, 13, 2349–2363. [Google Scholar] [CrossRef] [Green Version]
- Mogana, R.; Adhikari, A.; Tzar, M.N.; Ramliza, R.; Wiart, C. Antibacterial Activities of the Extracts, Fractions and Isolated Compounds from Canarium patentinervium Miq. against Bacterial Clinical Isolates. BMC Complement. Med. Ther. 2020, 20, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Wiegand, I.; Burak, S. Effect of Inoculum Density on Susceptibility of Plesiomonas shigelloides to Cephalosporins. J. Antimicrob. Chemother. 2004, 54, 418–423. [Google Scholar] [CrossRef] [Green Version]
- Schumacher, A.; Vranken, T.; Malhotra, A.; Arts, J.J.C.; Habibovic, P. In Vitro Antimicrobial Susceptibility Testing Methods: Agar Dilution to 3D Tissue-Engineered Models. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 187–208. [Google Scholar] [CrossRef] [Green Version]
- European Committee on Antibiotic Susceptibility Testing. Methods for the Determination of Susceptibility of Bacteria to Antimicrobial Agents, Terminology. Eucast Defin. Doc. 1998, 4, 291–296. [Google Scholar] [CrossRef] [Green Version]
- Clinical Laboratory Standards Institute. Methods for Determining Bactericidal Activity of Antimicrobial Agents; Approved Standard; CLSI Guideline M26-A; Clinical Laboratory Standards Institute: Wayne, PA, USA, 1999. [Google Scholar]
- Wiegand, I.; Hilpert, K.; Hancock, R.E.W. Agar and Broth Dilution Methods to Determine the Minimal Inhibitory Concentration (MIC) of Antimicrobial Substances. Nat. Protoc. 2015, 3, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Dafale, N.A.; Semwal, U.P.; Rajput, R.K.; Singh, G.N. Selection of Appropriate Analytical Tools to Determine the Potency and Bioactivity of Antibiotics and Antibiotic Resistance. J. Pharm. Anal. 2016, 6, 207–213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jorgensen, J.H.; Ferraro, M.J. Antimicrobial Susceptibility Testing: A Review of General Principles and Contemporary Practices. Clin. Infect. Dis. 2009, 49, 1749–1755. [Google Scholar] [CrossRef] [PubMed]
- Eloff, J.N. Avoiding Pitfalls in Determining Antimicrobial Activity of Plant Extracts and Publishing the Results. BMC Complement Altern. Med. 2019, 19, 106. [Google Scholar] [CrossRef] [Green Version]
- Shanmugakani, R.K.; Srinivasan, B.; Glesby, M.J.; Westblade, L.F.; Cárdenas, W.B.; Raj, T.; Erickson, D.; Mehta, S. Current State of the Art in Rapid Diagnostics for Antimicrobial Resistance. Lab Chip 2020, 20, 2607–2625. [Google Scholar] [CrossRef]
- Kumar, A.; Chordia, N. Role of Microbes in Human. Appl. Microbiol. 2017, 3, 2–4. [Google Scholar] [CrossRef] [Green Version]
- Gilbert, J.A.; Blaser, M.J.; Caporaso, J.G.; Jansson, J.K.; Lynch, S.V.; Knight, R. Current Understanding of the Human Microbiome. Nat. Med. 2018, 24, 392–400. [Google Scholar] [CrossRef]
- Chen, T.; Cheng, Z.; Yi, C.; Xu, Z. Synthesis of Platinum Nanoparticles Templated by Dendrimers Terminated with Alkyl Chains. Chem. Commun. 2018, 54, 9143–9146. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Liu, Y.; Du, Z.; Zhang, L.; Chen, J.; Shen, Z.; Liu, Q.; Qin, J.; Lv, H.; Wang, H.; et al. Skin Microbiota Analysis-Inspired Development of Novel Anti-Infectives. Microbiome 2020, 8, 85. [Google Scholar] [CrossRef]
- Byrd, A.L.; Belkaid, Y.; Segre, J.A. The Human Skin Microbiome. Nat. Publ. Gr. 2018, 16, 143–155. [Google Scholar] [CrossRef]
- Huffnagle, G.B.; Noverr, M.C. The Emerging World of the Fungal Microbiome. Trends Microbiol. 2013, 21, 334–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaturvedi, M.; Punj, A. Human Oral Microflora. Int. J. Curr. Adv. Res. 2018, 7, 14065–14070. [Google Scholar] [CrossRef]
- Thursby, E.; Juge, N. Introduction to the Human Gut Microbiota. Biochem. J. 2017, 474, 1823–1836. [Google Scholar] [CrossRef] [PubMed]
- Nagajyothi, P.C.; Sreekanth, T.V.M.; Tettey, C.O.; Jun, Y.I.; Mook, S.H. Characterization, Antibacterial, Antioxidant, and Cytotoxic Activities of ZnO Nanoparticles Using Coptidis rhizoma. Bioorg. Med. Chem. Lett. 2014, 24, 4298–4303. [Google Scholar] [CrossRef] [PubMed]
- Khalil, A.T.; Ovais, M.; Ullah, I.; Ali, M.; Shinwari, Z.K.; Khamlich, S.; Maaza, M. Sageretia thea (Osbeck.) Mediated Synthesis of Zinc Oxide Nanoparticles and Its Biological Applications. Nanomedicine 2017, 12, 1767–1789. [Google Scholar] [CrossRef]
- Pavithra, N.S.; Lingaraju, K.; Raghu, G.K.; Nagaraju, G. Citrus maxima (Pomelo) Juice Mediated Eco-Friendly Synthesis of ZnO Nanoparticles: Applications to Photocatalytic, Electrochemical Sensor and Antibacterial Activities. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 185, 11–19. [Google Scholar] [CrossRef]
- Al-Dhabi, N.A.; Arasu, M.V. Environmentally-Friendly Green Approach for the Production of Zinc Oxide Nanoparticles and Their. Nanomaterials 2018, 8, 500. [Google Scholar] [CrossRef] [Green Version]
- Pandiyan, N.; Murugesan, B.; Arumugam, M.; Sonamuthu, J. Ionic Liquid—A Greener Templating Agent with Justicia Adhatoda Plant Extract Assisted Green Synthesis of Morphologically Improved Ag-Au/ZnO Nanostructure and It’s Antibacterial and Anticancer Activities. J. Photochem. Photobiol. B Biol. 2019, 198, 111559. [Google Scholar] [CrossRef]
- El-Moslamy, S.H.; Shokry, H.H.; Rezk, A.H.; Fattah, Y.R.A. Bioprocess Strategies and Characterization of Anti-Multidrug Resistant Human Pathogens Copper/Copper Oxide Nanoparticles from Citrus Peel Waste Extracts. J. Nanomater. Mol. Nanotechnol. 2017, 6, 1000217. [Google Scholar] [CrossRef]
- Slavin, Y.N.; Asnis, J.; Häfeli, U.O.; Bach, H. Metal Nanoparticles: Understanding the Mechanisms behind Antibacterial Activity. J. Nanobiotechnol. 2017, 15, 65. [Google Scholar] [CrossRef]
- Kim, S.W.; Jung, J.H.; Lamsal, K.; Kim, Y.S.; Min, J.S.; Lee, Y.S. Antifungal Effects of Silver Nanoparticles (AgNPs) against Various Plant Pathogenic Fungi. Mycobiology 2012, 40, 53–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cavassin, E.D.; de Figueiredo, L.F.P.; Otoch, J.P.; Seckler, M.M.; de Oliveira, R.A.; Franco, F.F.; Marangoni, V.S.; Zucolotto, V.; Levin, A.S.S.; Costa, S.F. Comparison of Methods to Detect the in Vitro Activity of Silver Nanoparticles (AgNP) against Multidrug Resistant Bacteria. J. Nanobiotechnol. 2015, 13, 64. [Google Scholar] [CrossRef] [PubMed]
- Dorobantu, L.S.; Fallone, C.; Noble, A.J.; Veinot, J.; Ma, G.; Goss, G.G.; Burrell, R.E. Toxicity of Silver Nanoparticles against Bacteria, Yeast, and Algae. J. Nanopart. Res. 2015, 17, 172. [Google Scholar] [CrossRef]
- Mukha, I.P.; Eremenko, A.M.; Smirnova, N.P.; Mikhienkova, A.I.; Korchak, G.I.; Gorchev, V.F.; Chunikhin, A.Y. Antimicrobial Activity of Stable Silver Nanoparticles of a Certain Size. Appl. Biochem. Microbiol. 2013, 49, 199–206. [Google Scholar] [CrossRef]
- Taranath, T.C.; Patil, B.N. Limonia acidissima L. Leaf Mediated Synthesis of Zinc Oxide Nanoparticles: A Potent Tool against Mycobacterium Tuberculosis. Int. J. Mycobacteriol. 2016, 5, 197–204. [Google Scholar] [CrossRef] [Green Version]
- Aljabali, A.A.A.; Akkam, Y.; Salim, M.; Zoubi, A.; Al-batayneh, K.M.; Id, B.A.; Alrob, O.A.; Alkilany, A.M.; Benamara, M.; Id, D.J.E. Synthesis of Gold Nanoparticles Using Leaf Extract of Ziziphus zizyphus and Their Antimicrobial Activity. Nanomaterials 2018, 8, 174. [Google Scholar] [CrossRef] [Green Version]
- Alavi, M.; Karimi, N. Biosynthesis of Ag and Cu NPs by Secondary Metabolites of Usnic Acid and Thymol with Biological Macromolecules Aggregation and Antibacterial Activities against Multi Drug Resistant (MDR) Bacteria. Int. J. Biol. Macromol. 2019, 128, 893–901. [Google Scholar] [CrossRef]
- Seetharaman, P.; Chandrasekaran, R.; Gnanasekar, S.; Mani, I.; Sivarperumal, S. Biogenic Gold Nanoparticles Synthesized Using Crescentia cujete L. and Evaluation of Their Different Biological Activities. Biocatal. Agric. Biotechnol. 2017, 11, 75–82. [Google Scholar] [CrossRef]
- Behlol, K.; Ahmed, A.; Subramanian, S.; Sivasubramanian, A.; Veerappan, G.; Veerappan, A. Preparation of Gold Nanoparticles Using Salicornia brachiata Plant Extract and Evaluation of Catalytic and Antibacterial Activity. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 130, 54–58. [Google Scholar] [CrossRef]
- Baskar, S.; Selvan, G.; Anbarasu, R.; Raja, V. Green Synthesis of Gold Nanoparticles (Au-NPs) Using Barleria cristata and Study Their Pharmacological Applications. World J. Pharm. Res. 2016, 5, 1072–1085. [Google Scholar] [CrossRef]
- Sela, U.; Euler, C.W.; Correa da Rosa, J.; Fischetti, V.A. Strains of Bacterial Species Induce a Greatly Varied Acute Adaptive Immune Response: The Contribution of the Accessory Genome. PLoS Pathog. 2018, 14, e1006726. [Google Scholar] [CrossRef] [PubMed]
- Bundy, J.G.; Willey, T.L.; Castell, R.S.; Ellar, D.J.; Brindle, K.M. Discrimination of Pathogenic Clinical Isolates and Laboratory Strains of Bacillus Cereus by NMR-Based Metabolomic Profiling. FEMS Microbiol. Lett. 2005, 242, 127–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Medeiros, A.W.; Pereira, R.I.; Oliveira, D.V.; Martins, P.D.; d’Azevedo, P.A.; Van der Sand, S.; Frazzon, J.; Frazzon, A.P.G. Molecular Detection of Virulence Factors among Food and Clinical Enterococcus Faecalis Strains in South Brazil. Braz. J. Microbiol. 2014, 45, 327–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tayabali, A.F.; Nguyen, K.C.; Shwed, P.S.; Crosthwait, J.; Coleman, G.; Seligy, V.L. Comparison of the Virulence Potential of Acinetobacter Strains from Clinical and Environmental Sources. PLoS ONE 2012, 7, e37024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alnuaimi, A.D.; O’Brien-Simpson, N.M.; Reynolds, E.C.; Mccullough, M.J. Clinical Isolates and Laboratory Reference Candida Species and Strains Have Varying Abilities to Form Biofilms. FEMS Yeast Res. 2013, 13, 689–699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clinical Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 11th ed.; Approved Standard; CLSI Guideline M07; Clinical Laboratory Standards Institute: Wayne, PA, USA, 2018. [Google Scholar]
- European Committee on Antimicrobial Susceptibility Testing. Determination of Minimum Inhibitory Concentrations (MICs) of Antibacterial Agents by Broth Dilution. Clin. Microbiol. Infect. 2003, 9, 1–7. [Google Scholar]
- Åhman, J.; Matuschek, E.; Kahlmeter, G. EUCAST Evaluation of 21 Brands of Mueller–Hinton Dehydrated Media for Disc Diffusion Testing. Clin. Microbiol. Infect. 2020, 26, 1412.e1–1412.e5. [Google Scholar] [CrossRef] [Green Version]
- Udekwu, K.I.; Parrish, N.; Ankomah, P.; Baquero, F.; Levin, B.R. Functional Relationship between Bacterial Cell Density and the Efficacy of Antibiotics. J. Antimicrob. Chemother. 2009, 63, 745–757. [Google Scholar] [CrossRef]
- Matuschek, E.; Brown, D.F.J.; Kahlmeter, G. Development of the EUCAST Disk Diffusion Antimicrobial Susceptibility Testing Method and Its Implementation in Routine Microbiology Laboratories. Clin. Microbiol. Infect. 2014, 20, O255–O266. [Google Scholar] [CrossRef] [Green Version]
- Hudzicki, J. Kirby-Bauer Disk Diffusion Susceptibility Test Protocol. Am. Soc. Microbiol. 2016, 15, 55–63. [Google Scholar]
- Vandepitte, J.; Engbaek, K.; Rohner, P.; Piot, P.; Heuck, C.C. Basic Laboratory Procedures in Clinical Bacteriology, 2nd ed.; World Health Organization: Geneva, Switzerland, 2003. [Google Scholar]
- United States Pharmacopoeia. Antimicrobial Effectiveness Testing; USP 51; United States Pharmacopoeia: Rockville, MD, USA, 2010. [Google Scholar]
- European Pharmacopoeia. Efficacy of Antimicrobial Preservation; EP 6.4; Council of Europe: Strasbourg, France, 2010.
- Japanese Pharmacopoeia. Preservative Effectiveness Test, 15th ed.; Pharmaceuticals and Medical Devices Agency: Tokyo, Japan, 2016.
- Smith, K.P.; Kirby, J.E. The Inoculum Effect in the Era of Multidrug Resistance: Minor Differences in Inoculum Have Dramatic Effect on MIC Determination. Antimicrob. Agents Chemother. 2018, 62, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clinical Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 9th ed.; Approved Standard; CLSI Guideline M07; Clinical Laboratory Standards Institute: Wayne, PA, USA, 2015. [Google Scholar]
- Chahardoli, A.; Karimi, N.; Sadeghi, F.; Fattahi, A. Green Approach for Synthesis of Gold Nanoparticles from Nigella Arvensis Leaf Extract and Evaluation of Their Antibacterial, Antioxidant, Cytotoxicity and Catalytic Activities. Artif. Cells Nanomed. Biotechnol. 2018, 46, 579–588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishwarya, R.; Vaseeharan, B.; Kalyani, S.; Banumathi, B.; Govindarajan, M.; Alharbi, N.S.; Kadaikunnan, S.; Al-anbr, M.N.; Khaled, J.M.; Benelli, G. Facile Green Synthesis of Zinc Oxide Nanoparticles Using Ulva lactuca Seaweed Extract and Evaluation of Their Photocatalytic, Antibiofilm and Insecticidal Activity. J. Photochem. Photobiol. B Biol. 2018, 178, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Clinical Laboratory Standards Institute. Performance Standards for Antimicrobial Disk Susceptibility Tests, 13th ed.; Approved Standard; CLSI Guideline M02; Clinical Laboratory Standards Institute: Wayne, PA, USA, 2018. [Google Scholar]
- Clinical Laboratory Standards Institute. Method for Antifungal Disk Diffusion Susceptibility Testing of Yeasts, 3rd ed.; Approved Standard; CLSI Guideline M44; Clinical Laboratory Standards Institute: Wayne, PA, USA, 2018. [Google Scholar]
- Clinical Laboratory Standards Institute. Method for Antifungal Disk Diffusion Susceptibility Testing of Nondermatophyte Filamentous Fungi; Approved Standard; CLSI Guideline M51-A; Clinical Laboratory Standards Institute: Wayne, PA, USA, 2010. [Google Scholar]
- Clinical Laboratory Standards Institute. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts, 4th ed.; Approved Standard; CLSI Guideline M27; Clinical Laboratory Standards Institute: Wayne, PA, USA, 2017. [Google Scholar]
- Clinical and Laboratory Standards Institute. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi; Approved Guideline, 3rd ed.; CLSI Guideline M38; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2017. [Google Scholar]
- Flanagan, J.N.; Steck, T.R. The Relationship Between Agar Thickness and Antimicrobial Susceptibility Testing. Indian J. Microbiol. 2017, 57, 503–506. [Google Scholar] [CrossRef] [PubMed]
- Aisida, S.O.; Madubuonu, N.; Alnasir, M.H.; Ahmad, I.; Botha, S.; Maaza, M.; Ezema, F.I. Biogenic Synthesis of Iron Oxide Nanorods Using Moringa Oleifera Leaf Extract for Antibacterial Applications. Appl. Nanosci. 2019, 101, 305–315. [Google Scholar] [CrossRef]
- Ambika, S.; Sundrarajan, M. Antibacterial Behaviour of Vitex negundo Extract Assisted ZnO Nanoparticles against Pathogenic Bacteria. J. Photochem. Photobiol. B Biol. 2015, 146, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Devi, H.S.; Boda, M.A.; Shah, M.A.; Parveen, S.; Wani, A.H. Green Synthesis of Iron Oxide Nanoparticles Using Platanus Orientalis Leaf Extract for Antifungal Activity. Green Process. Synth. 2019, 8, 38–45. [Google Scholar] [CrossRef]
- Chamsa-ard, W.; Fawcett, D.; Fung, C.C.; Poinern, G.E.J. Biogenic Synthesis of Gold Nanoparticles from Waste Watermelon and Their Antibacterial Activity against Escherichia coli and Staphylococcus epidermidis. Int. J. Res. Med. Sci. 2019, 7, 2499–2505. [Google Scholar] [CrossRef]
- Supraja, N.; Prasad, T.N.V.K.V.; Dhanesh, A.; Anbumani, D. Synthesis, Characterization and Evaluation of Antimicrobial Efficacy and Brine Shrimp Lethality Assay of Alstonia scholaris Stem Bark Extract Mediated ZnONPs. Biochem. Biophys. Rep. 2018, 14, 69–77. [Google Scholar] [CrossRef]
- Clinical Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing, 32nd ed.; Approved Standard; CLSI Guideline M100; Clinical Laboratory Standards Institute: Wayne, PA, USA, 2022. [Google Scholar]
- European Committee on Antimicrobial Susceptibility Testing. Routine and Extended Internal Quality Control for MIC Determination and Disk Diffusion as Recommended by EUCAST; Version 12.0.; European Committee on Antimicrobial Susceptibility Testing: Växjö, Sweden, 2022. [Google Scholar]
- Mohamed, D.S.; El-Baky, R.M.A.; Sandle, T.; Mandour, S.A.; Ahmed, E.F. Antimicrobial Activity of Silver-Treated Bacteria against Other Multi-Drug Resistant Pathogens in Their Environment. Antibiotics 2020, 9, 181. [Google Scholar] [CrossRef] [Green Version]
- Konaté, K.; Mavoungou, J.F.; Lepengué, A.N.; Aworet-samseny, R.R.R.; Hilou, A.; Souza, A.; Dicko, M.H.; Batchi, B.M. Antibacterial Activity against β-Lactamase Producing Methicillin and Ampicillin-Resistants Staphylococcus aureus: Fractional Inhibitory Concentration Index (FICI) Determination. Ann. Clin. Microbiol. Antimicrob. 2012, 11, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Appiah, T.; Boakye, Y.D.; Agyare, C. Antimicrobial Activities and Time-Kill Kinetics of Extracts of Selected Ghanaian Mushrooms. Evid.-Based Complement. Altern. Med. 2017, 2017, 4534350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wani, I.A.; Ahmad, T. Size and Shape Dependant Antifungal Activity of Gold Nanoparticles: A Case Study of Candida. Colloids Surf. B Biointerfaces 2013, 101, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Hoseinzadeh, E.; Makhdoumi, P.; Taha, P.; Hossini, H.; Stelling, J.; Amjad Kamal, M.; Md Ashraf, G. A Review on Nano-Antimicrobials: Metal Nanoparticles, Methods and Mechanisms. Curr. Drug Metab. 2016, 18, 120–128. [Google Scholar] [CrossRef]
- Lu, Z.; Rong, K.; Li, J.; Yang, H.; Chen, R. Size-Dependent Antibacterial Activities of Silver Nanoparticles against Oral Anaerobic Pathogenic Bacteria. J. Mater. Sci. Mater. Med. 2013, 24, 1465–1471. [Google Scholar] [CrossRef]
- Osonga, F.J.; Kalra, S.; Miller, R.M.; Isika, D.; Sadik, O.A. Synthesis, Characterization and Antifungal Activities of Eco-Friendly Palladium Nanoparticles. RSC Adv. 2020, 10, 5894–5904. [Google Scholar] [CrossRef]
- Pajerski, W.; Ochonska, D.; Brzychczy-Wloch, M.; Indyka, P.; Jarosz, M.; Golda-Cepa, M.; Sojka, Z.; Kotarba, A. Attachment Efficiency of Gold Nanoparticles by Gram-Positive and Gram-Negative Bacterial Strains Governed by Surface Charges. J. Nanoparticle Res. 2019, 21, 186. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.H.; Park, J.C.; Jeon, G.E.; Kim, C.S.; Seo, J.H. Effect of the Size and Shape of Silver Nanoparticles on Bacterial Growth and Metabolism by Monitoring Optical Density and Fluorescence Intensity. Biotechnol. Bioprocess. Eng. 2017, 22, 210–217. [Google Scholar] [CrossRef]
- Pal, S.; Tak, Y.K.; Song, J.M. Does the Antibacterial Activity of Silver Nanoparticles Depend on the Shape of the Nanoparticle? A Study of the Gram-Negative Bacterium Escherichia coli. Appl. Environ. Microbiol. 2007, 73, 1712–1720. [Google Scholar] [CrossRef] [Green Version]
- Van Dong, P.; Ha, C.H.; Binh, L.T.; Kasbohm, J. Chemical Synthesis and Antibacterial Activity of Novel-Shaped Silver Nanoparticles. Int. Nano Lett. 2012, 2, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Javed, R.; Zia, M.; Naz, S.; Aisida, S.O.; ul Ain, N.; Ao, Q. Role of Capping Agents in the Application of Nanoparticles in Biomedicine and Environmental Remediation: Recent Trends and Future Prospects. J. Nanobiotechnol. 2020, 18, 172. [Google Scholar] [CrossRef] [PubMed]
- Restrepo, C.V.; Villa, C.C. Synthesis of Silver Nanoparticles, Influence of Capping Agents, and Dependence on Size and Shape: A Review. Environ. Nanotechnol. Monit. Manag. 2021, 15, 100428. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, M.; Portney, N.G.; Cui, D.; Budak, G.; Ozbay, E.; Ozkan, M.; Ozkan, C.S. Zeta Potential: A Surface Electrical Characteristic to Probe the Interaction of Nanoparticles with Normal and Cancer Human Breast Epithelial Cells. Biomed. Microdevices 2008, 10, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Wilson, W.W.; Wade, M.M.; Holman, S.C.; Champlin, F.R. Status of Methods for Assessing Bacterial Cell Surface Charge Properties Based on Zeta Potential Measurements. J. Microbiol. Methods 2001, 43, 153–164. [Google Scholar] [CrossRef]
- Wang, L.; Hu, C.; Shao, L. The Antimicrobial Activity of Nanoparticles: Present Situation and Prospects for the Future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef] [Green Version]
- Arakha, M.; Pal, S.; Samantarrai, D.; Panigrahi, T.K. Antimicrobial Activity of Iron Oxide Nanoparticle upon Modulation of Nanoparticle-Bacteria Interface. Sci. Rep. 2015, 5, 14813. [Google Scholar] [CrossRef] [Green Version]
- Fang, B.; Jiang, Y.; Nüsslein, K.; Rotello, V.M.; Santore, M.M. Antimicrobial Surfaces Containing Cationic Nanoparticles: How Immobilized, Clustered, and Protruding Cationic Charge Presentation Affects Killing Activity and Kinetics. Colloids Surf. B Biointerfaces 2015, 125, 255–263. [Google Scholar] [CrossRef]
- Halder, S.; Yadav, K.K.; Sarkar, R.; Mukherjee, S.; Saha, P.; Haldar, S.; Karmakar, S.; Sen, T. Alteration of Zeta Potential and Membrane Permeability in Bacteria: A Study with Cationic Agents. Springerplus 2015, 4, 672. [Google Scholar] [CrossRef] [Green Version]
- Abo-Shama, U.H.; El-Gendy, H.; Mousa, W.S.; Hamouda, R.A.; Yousuf, W.E.; Hetta, H.F.; Abdeen, E.E. Synergistic and Antagonistic Effects of Metal Nanoparticles in Combination with Antibiotics against Some Reference Strains of Pathogenic Microorganisms. Infect. Drug Resist. 2020, 13, 351–362. [Google Scholar] [CrossRef] [Green Version]
- Malawong, S.; Thammawithan, S.; Sirithongsuk, P.; Daduang, S.; Klaynongsruang, S.; Wong, P.T.; Patramanon, R. Silver Nanoparticles Enhance Antimicrobial Efficacy of Antibiotics and Restore That Efficacy against the Melioidosis Pathogen. Antibiotics 2021, 10, 839. [Google Scholar] [CrossRef]
- Heydari, R.; Rashidipour, M. Green Synthesis of Silver Nanoparticles Using Extract of Oak Fruit Hull (Jaft): Synthesis and In Vitro Cytotoxic Effect on MCF-7 Cells. Int. J. Breast Cancer 2015, 2015, 846743. [Google Scholar] [CrossRef] [PubMed]
- Balciunaitiene, A.; Viskelis, P.; Viskelis, J.; Streimikyte, P.; Liaudanskas, M.; Bartkiene, E.; Zavistanaviciute, P.; Zokaityte, E.; Starkute, V.; Ruzauskas, M.; et al. Green Synthesis of Silver Nanoparticles Using Extract of Artemisia absinthium L., Humulus lupulus L. and Thymus vulgaris L., Physico-Chemical Characterization, Antimicrobial and Antioxidant Activity. Processes 2021, 9, 1304. [Google Scholar] [CrossRef]
- Banerjee, P.; Satapathy, M.; Mukhopahayay, A.; Das, P. Leaf Extract Mediated Green Synthesis of Silver Nanoparticles from Widely Available Indian Plants: Synthesis, Characterization, Antimicrobial Property and Toxicity Analysis. Bioresour. Bioprocess. 2014, 1, 3. [Google Scholar] [CrossRef] [Green Version]
- Sibiya, P.N.; Moloto, M.J. Green Synthesis of Ag2S Nanoparticles: Effect of pH and Capping Agent on Size and Shape of NPs and Their Antibacterial Activity. Dig. J. Nanomater. Biostruct. 2018, 13, 411–418. [Google Scholar]
- Guckeisen, T.; Hosseinpour, S.; Peukert, W. Effect of PH and Urea on the Proteins Secondary Structure at the Water/Air Interface and in Solution. J. Colloid Interface Sci. 2021, 590, 38–49. [Google Scholar] [CrossRef] [PubMed]
- Vadlapudi, V.; Kaladhar, D. Review: Green Synthesis of Silver and Gold Nanoparticles. Middle-East J. Sci. Res. 2014, 19, 834–842. [Google Scholar]
- Friedman, M.; Jurgens, H.S. Effect of PH on the Stability of Plant Phenolic Compounds Mendel. J. Agric. Food Chem. 2000, 48, 2101–2110. [Google Scholar] [CrossRef]
- Chitra, K.; Annadurai, G. Antibacterial Activity of PH-Dependent Biosynthesized Silver Nanoparticles against Clinical Pathogen. Biomed. Res. Int. 2014, 2014, 725165. [Google Scholar] [CrossRef] [Green Version]
- Qu, F.; Xu, H.; Wei, H.; Lai, W. Effects of pH and Temperature on Antibacterial Activity of Silver Nanoparticles. In Proceedings of the 2010 3rd International Conference on Biomedical Engineering and Informatics, Yantai, China, 16–18 October 2010; pp. 2033–2037. [Google Scholar] [CrossRef]
- Kredy, H.M. The Effect of pH, Temperature on the Green Synthesis and Biochemical Activities of Silver Nanoparticles from Lawsonia Inermis Extract. J. Pharm. Sci. Res. 2018, 10, 2022–2026. [Google Scholar]
- Saliani, M.; Jalal, R. Effects of pH and Temperature on Antibacterial Activity of Zinc Oxide Nanofluid Against Escherichia coli O157:H7 and Staphylococcus Aureus. J. Microbiol. 2015, 8, e17115. [Google Scholar] [CrossRef] [Green Version]
- Arakha, M.; Saleem, M.; Mallick, B.C.; Jha, S. The Effects of Interfacial Potential on Antimicrobial Propensity of ZnO Nanoparticle. Sci. Rep. 2015, 5, 9578. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, H.; Menon, S.; Kumar, S.V.; Rajeshkumar, S. Mechanistic Study on Antibacterial Action of Zinc Oxide Nanoparticles Synthesized Using Green Route. Chem. Biol. Interact. 2018, 286, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, S.; Kuppusamy, K.A. Extracellular Synthesis of Zinc Oxide Nanoparticle Using Seaweeds of Gulf of Mannar, India. J. Nanobiotechnol. 2013, 11, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parashar, S.; Sharma, M.K.; Garg, C.; Garg, M. Green Synthesized Silver Nanoparticles as Silver Lining in Antimicrobial Resistance: A Review. Curr. Drug. Deliv. 2022, 19, 170–181. [Google Scholar] [CrossRef] [PubMed]
- LIOFILCHEM. Antibiotic Disc Interpretative Criteria and Quality Control. 2017. Available online: http://www.liofilchem.net/pdf/disc/disc_interpretative_table.pdf (accessed on 20 December 2021).
- European Committee on Antimicrobial Susceptibility Testing. Breakpoints Tables for Interpretation of MICs and Zone Diameters. 2019. Available online: https://www.eucast.org/clinical_breakpoints/ (accessed on 20 December 2021).
- Maťátkova, O.; Michailidu, J.; Miškovská, A.; Kolouchová, I.; Masák, J.; Čejková, A. Antimicrobial Properties and Applications of Metal Nanoparticles Biosynthesized by Green Methods. Biotechnol. Adv. 2022, 11, 107905. [Google Scholar] [CrossRef] [PubMed]
- Baron, S.; Ceccarelli, D.; Dalsgaard, I.; Granier, S.A.; Haenen, O.; Jansson, E.; Madsen, L.; Jouy, E.; Kempf, I.; Larvor, E.; et al. Influence of Incubation Time on Antimicrobial Susceptibility Testing of Pathogenic Vibrio anguillarum and Vibrio vulnificus Isolated from Fish. Aquaculture 2020, 524, 735258. [Google Scholar] [CrossRef]
- Ipe, D.S.; Kumar, P.T.S.; Love, R.M.; Hamlet, S.M. Silver Nanoparticles at Biocompatible Dosage Synergistically Increases Bacterial Susceptibility to Antibiotics. Front. Microbiol. 2020, 11, 74. [Google Scholar] [CrossRef]
- Ali, K.; Dwivedi, S.; Azam, A.; Saquib, Q.; Al-said, M.S.; Alkhedhairy, A.A.; Musarrat, J. Aloe Vera Extract Functionalized Zinc Oxide Nanoparticles as Nanoantibiotics against Multi-Drug Resistant Clinical Bacterial Isolates. J. Colloid Interface Sci. 2016, 472, 145–156. [Google Scholar] [CrossRef]
- Fayaz, A.M.; Balaji, K.; Girilal, M.; Yadav, R.; Kalaichelvan, P.T.; Venketesan, R. Biogenic Synthesis of Silver Nanoparticles and Their Synergistic Effect with Antibiotics: A Study against Gram-Positive and Gram-Negative Bacteria. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 103–109. [Google Scholar] [CrossRef]
- Baker, S.; Pasha, A.; Satish, S. Biogenic Nanoparticles Bearing Antibacterial Activity and Their Synergistic Effect with Broad Spectrum Antibiotics: Emerging Strategy to Combat Drug Resistant Pathogens. Saudi Pharm. J. 2017, 25, 44–51. [Google Scholar] [CrossRef] [Green Version]
- Wilson, C.; Lukowicz, R.; Merchant, S.; Valquier-Flynn, H.; Caballero, J.; Sandoval, J.; Okuom, M.; Huber, C.; Brooks, T.D.; Wilson, E.; et al. Quantitative and Qualitative Assessment Methods for Biofilm Growth: A Mini-Review. Res. Rev. J. Eng. Technol. 2017, 6, 6133255. [Google Scholar]
- Allkja, J.; Bjarnsholt, T.; Coenye, T.; Cos, P.; Fallarero, A.; Harrison, J.J.; Lopes, S.P.; Oliver, A.; Pereira, M.O.; Ramage, G.; et al. Minimum Information Guideline for Spectrophotometric and Fluorometric Methods to Assess Biofilm Formation in Microplates. Biofilm 2020, 2, 100010. [Google Scholar] [CrossRef] [PubMed]
- Azeredo, J.; Azevedo, N.F.; Briandet, R.; Cerca, N.; Coenye, T.; Costa, A.R.; Desvaux, M.; Di Bonaventura, G.; Hébraud, M.; Jaglic, Z.; et al. Critical Review on Biofilm Methods. Crit. Rev. Microbiol. 2017, 43, 313–351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manikandan, M.; Wu, H.; Hasan, N. Biosensors and Bioelectronics Cell Population Based Mass Spectrometry Using Platinum Nanodots for Algal and Fungal Studies. Biosens. Bioelectron. 2012, 35, 493–497. [Google Scholar] [CrossRef] [PubMed]
- Stepanović, S.; Vuković, D.; Hola, V.; Di Bonaventura, G.; Djukić, S.; Ćirković, I.; Ruzicka, F. Quantification of Biofilm in Microtiter Plates: Overview of Testing Conditions and Practical Recommendations for Assessment of Biofilm Production by Staphylococci. Apmis 2007, 115, 891–899. [Google Scholar] [CrossRef]
- Peeters, E.; Nelis, H.J.; Coenye, T. Comparison of Multiple Methods for Quantification of Microbial Biofilms Grown in Microtiter Plates. J. Microbiol. Methods 2008, 72, 157–165. [Google Scholar] [CrossRef] [Green Version]
- Sandberg, M.; Määttänen, A.; Peltonen, J.; Vuorela, P.M.; Fallarero, A. Automating a 96-Well Microtitre Plate Model for Staphylococcus Aureus Biofilms: An Approach to Screening of Natural Antimicrobial Compounds. Int. J. Antimicrob. Agents 2008, 32, 233–240. [Google Scholar] [CrossRef]
- Xu, Z.; Liang, Y.; Lin, S.; Chen, D.; Li, B.; Li, L.; Deng, Y. Crystal Violet and XTT Assays on Staphylococcus Aureus Biofilm Quantification. Curr. Microbiol. 2016, 73, 474–482. [Google Scholar] [CrossRef]
- Palanisamy, N.K.; Ferina, N.; Amirulhusni, A.N.; Mohd-Zain, Z.; Hussaini, J.; Ping, L.J.; Durairaj, R. Antibiofilm Properties of Chemically Synthesized Silver Nanoparticles Found against Pseudomonas aeruginosa. J. Nanobiotechnol. 2014, 12, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Sabaeifard, P.; Abdi-Ali, A.; Soudi, M.R.; Dinarvand, R. Optimization of Tetrazolium Salt Assay for Pseudomonas Aeruginosa Biofilm Using Microtiter Plate Method. J. Microbiol. Methods 2014, 105, 134–140. [Google Scholar] [CrossRef]
- Jagani, S.; Chelikani, R.; Kim, D.S. Effects of Phenol and Natural Phenolic Compounds on Biofilm Formation by Pseudomonas aeruginosa. Biofouling 2009, 25, 321–324. [Google Scholar] [CrossRef] [PubMed]
- Marcos-Zambrano, L.J.; Escribano, P.; Bouza, E.; Guinea, J. Production of Biofilm by Candida and Non-Candida Spp. Isolates Causing Fungemia: Comparison of Biomass Production and Metabolic Activity and Development of Cut-off Points. Int. J. Med. Microbiol. 2014, 304, 1192–1198. [Google Scholar] [CrossRef] [PubMed]
- Stepanović, S.; Vuković, D.; Ježek, P.; Pavlović, M.; Švabic-Vlahović, M. Influence of Dynamic Conditions on Biofilm Formation by Staphylococci. Eur. J. Clin. Microbiol. Infect. Dis. 2001, 20, 502–504. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yan, Z.; Xu, J. Quantitative Variation of Biofilms among Strains in Natural Populations of Candida albicans. Microbiology 2003, 149, 353–362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cruz, C.D.; Shah, S.; Tammela, P. Defining Conditions for Biofilm Inhibition and Eradication Assays for Gram-Positive Clinical Reference Strains. BMC Microbiol. 2018, 18, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.; Usman, J.; Kaleem, F.; Omair, M.; Khalid, A.; Iqbal, M. Evaluation of Different Detection Methods of Biofilm Formation in the Clinical Isolates. Braz. J. Infect. Dis. 2011, 15, 305–311. [Google Scholar] [CrossRef] [Green Version]
- Kragh, K.N.; Alhede, M.; Rybtke, M.; Stavnsberg, C.; Jensen, P.; Tolker-Nielsen, T.; Whiteley, M.; Bjarnsholt, T. The Inoculation Method Could Impact the Outcome of Microbiological Experiments. Appl. Environ. Microbiol. 2018, 84, e02264-17. [Google Scholar] [CrossRef] [Green Version]
- Azevedo, N.F.; Pinto, A.R.; Reis, N.M.; Vieira, M.J.; Keevil, C.W. Shear Stress, Temperature, and Inoculation Concentration Influence the Adhesion of Water-Stressed Helicobacter Pylori to Stainless Steel 304 and Polypropylene. Appl. Environ. Microbiol. 2006, 72, 2936–2941. [Google Scholar] [CrossRef] [Green Version]
- Deighton, M.A.; Capstick, J.; Domalewski, E.; Van Nguyen, T. Methods for Studying Biofilms Produced by Staphylococcus epidermidis. Methods Enzymol. 2001, 336, 177–195. [Google Scholar] [CrossRef]
- Kiers, P.J.M.; Bos, R.; van der Mei, H.C.; Busscher, H.J. The Electrophoretic Softness of the Surface of Staphylococcus epidermidis Cells Grown in a Liquid Medium and on a Solid Agar. Microbiology 2001, 147, 757–762. [Google Scholar] [CrossRef] [Green Version]
- Cheung, A.L.; Fischetti, V.A. Variation in the Expression of Cell Wall Proteins of Staphylococcus Aureus Grown on Solid and Liquid Media. Infect. Immun. 1988, 56, 1061–1065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohanty, S.; Mishra, S.; Jena, P.; Jacob, B.; Sarkar, B.; Sonawane, A. An Investigation on the Antibacterial, Cytotoxic, and Antibiofilm Efficacy of Starch-Stabilized Silver Nanoparticles. Nanomedicine 2012, 8, 916–924. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, J.B.; Izano, E.A.; Gopal, P.; Karwacki, M.T.; Kim, S.; Bose, J.L.; Bayles, K.W.; Horswill, A.R. Low Levels of β-Lactam Antibiotics Induce Extracellular DNA Release and Biofilm Formation in Staphylococcus aureus. mBio 2012, 3, e00198-12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanchez, C.J.; Mende, K.; Beckius, M.L.; Akers, K.S.; Romano, D.R.; Wenke, J.C.; Murray, C.K. Biofilm Formation by Clinical Isolates and the Implications in Chronic Infections. BMC Infect. Dis. 2013, 13, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Izano, E.A.; Amarante, M.A.; Kher, W.B.; Kaplan, J.B. Differential Roles of Poly-N-Acetylglucosamine Surface Polysaccharide and Extracellular DNA in Staphylococcus aureus and Staphylococcus epidermidis Biofilms. Appl. Environ. Microbiol. 2008, 74, 470–476. [Google Scholar] [CrossRef] [Green Version]
- Zmantar, T.; Kouidhi, B.; Miladi, H.; Mahdouani, K.; Bakhrouf, A. A Microtiter Plate Assay for Staphylococcus aureus Biofilm Quantification at Various PH Levels and Hydrogen Peroxide Supplementation. New Microbiol. 2010, 33, 137–145. [Google Scholar]
- Negri, M.; Gonçalves, V.; Silva, S.; Henriques, M.; Azeredo, J.; Oliveira, R. Crystal Violet Staining to Quantity Candida Adhesion to Epithelial Cells. Br. J. Biomed. Sci. 2010, 67, 120–125. [Google Scholar] [CrossRef]
- Monteiro, D.R.; Gorup, L.F.; Silva, S.; Negri, M.; de Camargo, E.R.; Oliveira, R.; Barbosa, D.B.; Henriques, M. Silver Colloidal Nanoparticles: Antifungal Effect against Adhered Cells and Biofilms of Candida Albicans and Candida Glabrata. Biofouling 2011, 27, 711–719. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Z. Biosynthesis of Gold Nanoparticles Using Green Alga Pithophora oedogonia with Their Electrochemical Performance for Determining Carbendazim in Soil. Int. J. Electrochem. Sci. 2016, 11, 4550–4559. [Google Scholar] [CrossRef]
- Khan, S.; Alam, F.; Azam, A.; Khan, A.U. Gold Nanoparticles Enhance Methylene Blue-Induced Photodynamic Therapy: A Novel Therapeutic Approach to Inhibit Candida Albicans Biofilm. Int. J. Nanomed. 2012, 7, 3245–3257. [Google Scholar] [CrossRef] [Green Version]
- Dovigo, L.N.; Pavarina, A.C.; Carmello, J.C.; MacHado, A.L.; Brunetti, I.L.; Bagnato, V.S. Susceptibility of Clinical Isolates of Candida to Photodynamic Effects of Curcumin. Lasers Surg. Med. 2011, 43, 927–934. [Google Scholar] [CrossRef] [PubMed]
- Deveau, A.; Hogan, D.A. Linking Quorum Sensing Regulation and Biofilm Formation by Candida albicans. Methods Mol. Biol. 2011, 692, 219–233. [Google Scholar] [PubMed]
- Christensen, G.D.; Simpson, W.A.; Younger, J.J.; Baddour, L.M.; Barrett, F.F.; Melton, D.M.; Beachey, E.H. Adherence of Coagulase-Negative Staphylococci to Plastic Tissue Culture Plates: A Quantitative Model for the Adherence of Staphylococci to Medical Devices. J. Clin. Microbiol. 1985, 22, 996–1006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kennedy, C.A.; O’Gara, J.P. Contribution of Culture Media and Chemical Properties of Polystyrene Tissue Culture Plates to Biofilm Development by Staphylococcus aureus. J. Med. Microbiol. 2004, 53, 1171–1173. [Google Scholar] [CrossRef]
- Stepanović, S.; Vuković, D.; Dakić, I.; Savić, B.; Švabić-Vlahović, M. A Modified Microtiter-Plate Test for Quantification of Staphylococcal Biofilm Formation. J. Microbiol. Methods 2000, 40, 175–179. [Google Scholar] [CrossRef]
- Mathur, T.; Singhal, S.; Khan, S.; Upadhyay, D.; Fatma, T.; Rattan, A. Detection of Biofilm Formation Among the Clinical Isolates of Staphylococci: An Evaluation of Three Different Screening Methods. Indian J. Med. Microbiol. 2006, 24, 25–29. [Google Scholar] [CrossRef]
- Cramton, S.E.; Gerke, C.; Gotz, F. In Vitro Methods to Study Staphylococcal Biofilm Formation. Methods Enzymol. 2001, 336, 239–255. [Google Scholar]
- Tormo, M.Á.; Martí, M.; Valle, J.; Manna, A.C.; Cheung, A.L.; Lasa, I.; Penadés, J.R. SarA Is an Essential Positive Regulator of Staphylococcus epidermidis Biofilm Development. J. Bacteriol. 2005, 187, 2348–2356. [Google Scholar] [CrossRef] [Green Version]
- Mack, D.; Bartscht, K.; Fischer, C.; Rohde, H.; De Grahl, C.; Dobinsky, S.; Horstkotte, M.A.; Kiel, K.; Knobloch, J.K.M. Genetic and Biochemical Analysis of Staphylococcus epidermidis Biofilm Accumulation. Methods Enzymol. 2001, 336, 215–239. [Google Scholar] [CrossRef]
- Petrelli, D.; Zampaloni, C.; D’Ercole, S.; Prenna, M.; Ballarini, P.; Ripa, S.; Vitali, L.A. Analysis of Different Genetic Traits and Their Association with Biofilm Formation in Staphylococcus epidermidis Isolates from Central Venous Catheter Infections. Eur. J. Clin. Microbiol. Infect. Dis. 2006, 25, 773–781. [Google Scholar] [CrossRef]
- Huang, H.; Shan, K.; Liu, J.; Tao, X.; Periyasamy, S. Synthesis, Optimization and Characterization of Silver Nanoparticles Using the Catkin Extract of Piper Longum for Bactericidal Effect against Food-Borne Pathogens via Conventional and Mathematical Approaches. Bioorg. Chem. 2020, 20, 104230. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Kumar, V.; Yadav, E.; Falls, N.; Singh, M.; Komal, U.; Verma, A. One-Pot Green Synthesis and Structural Characterisation of Silver Nanoparticles Using Aqueous Leaves Extract of Carissa Carandas: Antioxidant, Anticancer and Antibacterial Activities. IET Nanobiotechnol. 2018, 12, 748–756. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, S.; Gopinath, S.C.B.; Anbu, P.; Lakshmipriya, T.; Kasim, F.H.; Lee, C. Eco-Friendly Synthesis of Solanum Trilobatum Extract-Capped Silver Nanoparticles Is Compatible with Good Antimicrobial Activities. J. Mol. Struct. 2018, 1160, 80–91. [Google Scholar] [CrossRef]
- Lakshmanan, G.; Sathiyaseelan, A.; Kalaichelvan, P.T.; Murugesan, K. Plant-Mediated Synthesis of Silver Nanoparticles Using Fruit Extract of Cleome Viscosa L.: Assessment of Their Antibacterial and Anticancer Activity. Karbala Int. J. Mod. Sci. 2018, 4, 61–68. [Google Scholar] [CrossRef]
- Ibrahim, H.M.M. Green Synthesis and Characterization of Silver Nanoparticles Using Banana Peel Extract and Their Antimicrobial Activity against Representative Microorganisms. J. Radiat. Res. Appl. Sci. 2015, 8, 265–275. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, S.; Saifullah; Ahmad, M. Green Synthesis of Silver Nanoparticles Using Azadirachta Indica Aqueous Leaf Extract. J. Radiat. Res. Appl. Sci. 2016, 9, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Logeswari, P.; Silambarasan, S.; Abraham, J. Ecofriendly Synthesis of Silver Nanoparticles from Commercially Available Plant Powders and Their Antibacterial Properties. Sci. Iran. 2013, 20, 1049–1054. [Google Scholar] [CrossRef]
- Aygün, A.; Gülbağca, F.; Nas, M.S.; Alma, M.H.; Çalimili, M.H.; Ustaogulu, B.; Altunoglu, Y.C.; Baloglu, M.C.; Cellat, K.; Sen, F. Biological synthesis of silver nanoparticles using Rheum ribes and evaluation of their anticarcinogenic and antimicrobal potential a novel approach in phytonanotechnology. J. Pharm. Biomed. Anal. 2020, 179, 113012. [Google Scholar] [CrossRef]
- Mickymaray, S. One-Step Synthesis of Silver Nanoparticles Using Saudi Arabian Desert Seasonal Plant Sisymbrium Irio and Antibacterial Activity Against Multidrug- Resistant Bacterial Strains. Molecules 2019, 9, 662. [Google Scholar] [CrossRef] [Green Version]
- Ali, M.A.; Mosa, K.A.; El-Keblawy, A.; Alawadhi, H. Exogenous production of silver nanoparticles by Tephrosia apollinea living plants under drought stress and their antimicrobial activities. Nanomaterials 2019, 9, 1716. [Google Scholar] [CrossRef] [Green Version]
- Tanase, C.; Berta, L.; Coman, N.A.; Roșca, I.; Man, A.; Toma, F.; Mocan, A.; Nicolescu, A.; Jakab-Farkas, L.; Biró, D.; et al. Antibacterial and antioxidant potential of silver nanoparticles biosynthesized using the spruce bark extract. Nanomaterials. 2019, 9, 1541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Küp, F.Ö.; Çoşkunçay, S.; Duman, F. Biosynthesis of Silver Nanoparticles Using Leaf Extract of Aesculus Hippocastanum (Horse Chestnut): Evaluation of Their Antibacterial, Antioxidant and Drug Release System Activities. Mater. Sci. Eng. C 2020, 107, 110207. [Google Scholar] [CrossRef] [PubMed]
- Manosalva, N.; Tortella, G.; Cristina Diez, M.; Schalchli, H.; Seabra, A.B.; Durán, N.; Rubilar, O. Green Synthesis of Silver Nanoparticles: Effect of Synthesis Reaction Parameters on Antimicrobial Activity. World J. Microbiol. Biotechnol. 2019, 35, 88. [Google Scholar] [CrossRef] [PubMed]
- Baruah, D.; Yadav, R.N.S.; Yadav, A.; Das, A.M. Alpinia Nigra Fruits Mediated Synthesis of Silver Nanoparticles and Their Antimicrobial and Photocatalytic Activities. J. Photochem. Photobiol. B Biol. 2019, 201, 111649. [Google Scholar] [CrossRef] [PubMed]
- Aritonang, H.F.; Koleangan, H.; Wuntu, A.D. Synthesis of Silver Nanoparticles Using Aqueous Extract of Medicinal Plants (Impatiens Balsamina and Lantana Camara) Fresh Leaves and Analysis of Antimicrobial Activity. Int. J. Microbiol. 2019, 2019, 8642303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibrahim, E.H.; Kilany, M.; Ghramh, H.A.; Khan, K.A.; ul Islam, S. Cellular Proliferation/Cytotoxicity and Antimicrobial Potentials of Green Synthesized Silver Nanoparticles (AgNPs) Using Juniperus Procera. Saudi J. Biol. Sci. 2019, 26, 1689–1694. [Google Scholar] [CrossRef]
- Dada, A.O.; Adekola, F.A.; Dada, F.E.; Adelani-Akenda, A.T.; Bello, M.O.; Okonkwo, C.R.; Inyinbor, A.A.; Oluyori, A.P.; Olayanju, A.; Ajanaku, K.O.; et al. Silver Nanoparticle Synthesis by Acalypha wilkesiana Extract Phytochemical Screening, Characterization, Influence of Operational Parameters, and Preliminary Antibacterial Testing. Heliyon 2019, 5, e02517. [Google Scholar] [CrossRef] [Green Version]
- Dakshayani, S.S.; Marulasiddeshwara, M.B.; Kumar, M.N.S.; Ramesh, G.; Kumar, P.R.; Devaraja, S.; Hosamani, R. Antimicrobial, Anticoagulant and Antiplatelet Activities of Green Synthesized Silver Nanoparticles Using Selaginella (Sanjeevini) Plant Extract. Int. J. Biol. Macromol 2019, 131, 787–797. [Google Scholar] [CrossRef]
- Kumar, V.; Wadhwa, R.; Kumar, N.; Maurya, P.K. A Comparative Study of Chemically Synthesized and Camellia Sinensis Leaf Extract-Mediated Silver Nanoparticles. 3 Biotech 2019, 9, 7. [Google Scholar] [CrossRef]
- Anwar, A.; Masri, A.; Rao, K.; Rajendran, K.; Khan, N.A. Antimicrobial Activities of Green Synthesized Gums-Stabilized Nanoparticles Loaded with Flavonoids. Sci. Rep. 2019, 9, 3122. [Google Scholar] [CrossRef]
- Salehi, S.; Shandiz, S.; Ghanbar, F.; Darvish, M.; Ardestani, M. Phytosynthesis of Silver Nanoparticles Using Artemisia Marschalliana Sprengel Aerial Part Extract and Assessment of Their Antioxidant, Anticancer, and Antibacterial Properties. Int. J. Nanomed. 2016, 11, 1835–1846. [Google Scholar]
- Femi-Adepoju, A.G.; Dada, A.O.; Otun, K.O.; Adepoju, A.O.; Fatoba, O.P. Green Synthesis of Silver Nanoparticles Using Terrestrial Fern (Gleichenia pectinata (Willd.) C. Presl.): Characterization and Antimicrobial Studies. Heliyon 2019, 5, e01543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basera, P.; Lavania, M.; Agnihotri, A.; Lal, B. Analytical Investigation of Cymbopogon Citratus and Exploiting the Potential of Developed Silver Nanoparticle Against the Dominating Species of Pathogenic Bacteria. Front. Microbiol. 2019, 10, 282. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, F.Y.; El-khateeb, A.Y.; Mohamed, A.H. Rhus and Safflower Extracts as Potential Novel Food Antioxidant, Anticancer, and Antimicrobial Agents Using Nanotechnology. Foods 2019, 8, 139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanchooli, N.; Saeidi, S.; Barani, H.K.; Sanchooli, E. In Vitro Antibacterial Effects of Silver Nanoparticles Synthesized Using Verbena officinalis Leaf Extract on Yersinia ruckeri, Vibrio cholera and Listeria monocytogenes. Iran. J. Microbiol. 2018, 10, 400–408. [Google Scholar]
- Ashour, A.; Raafat, D.; El-gowelli, H.M.; El-Kamel, A.H. Green Synthesis of Silver Nanoparticles Using Cranberry Powder Aqueous Extract: Characterization and Antimicrobial Properties. Int. J. Nanomed. 2015, 10, 7207–7221. [Google Scholar]
- Fernando, H.N.; Kumarasinghe, K.G.U.R.; Gunasekara, T.D.C.P.; Wijekoon, H.P.S.K.; Ekanayaka, E.M.A.K.; Rajapaksha, S.P.; Fernando, S.S.N.; Jayaweera, P.M. Synthesis, Characterization and Antimicrobial Activity of Garcinol Capped Silver Nanoparticles. J. Microbiol. Biotechnol. 2019, 29, 1841–1851. [Google Scholar] [CrossRef]
- Rahman, A.U.; Khan, U.A.; Yuan, Q.; Wei, Y.; Ahmad, A. Tuber Extract of Arisaema Flavum Eco-Benignly and Effectively Synthesize Silver Nanoparticles: Photocatalytic and Antibacterial Response against Multidrug Resistant Engineered E. Coli QH4. J. Photochem. Photobiol. B Biol. 2019, 193, 31–38. [Google Scholar] [CrossRef]
- Abudalo, M.A.; Al-Mheidat, I.R.; Al-Shurafat, A.W.; Grinham, C. Synthesis of Silver Nanoparticles Using a Modi Fied Tollens’ Method in Conjunction with Phytochemicals and Assessment of Their Antimicrobial Activity. PeerJ 2019, 7, e6413. [Google Scholar] [CrossRef] [Green Version]
- Rashid, S.; Azeem, M.; Ali, S.; Maroof, M. Characterization and Synergistic Antibacterial Potential of Green Synthesized Silver Nanoparticles Using Aqueous Root Extracts of Important Medicinal Plants of Pakistan. Colloids Surf. B Biointerfaces 2019, 179, 317–325. [Google Scholar] [CrossRef]
- Bharathi, D.; Kalaichelvan, P.T.; Atmaram, V.; Anbu, S. Biogenic Synthesis of Silver Nanoparticles from Aqueous Flower Extract of Bougainvillea Spectabilis and Their Antibacterial Activity. J. Med. Plants 2016, 4, 248–252. [Google Scholar]
- Fleming, D.S.A.T. Synthesis of Silver Nanoparticles from Bauhinia Acuminata Aqueous Leaf Extract and Molecular Docking Analysis of Various Cancer Receptors. Int. J. Sci. Res. 2017, 6, 50–55. [Google Scholar]
- Ashraf, A.; Zafar, S.; Zahid, K.; Salahuddin, M.; Al-Ghanim, K.A.; Al-Misned, F.; Mahboob, S. Synthesis, Characterization, and Antibacterial Potential of Silver Nanoparticles Synthesized from Coriandrum sativum L. J. Infect. Public Health 2018, 12, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Kaur, J.; Gupta, N.; Kaur, M.; Chatli, A.S. Antibacterial Effects of Green Synthesized AgNPs from Datura Metel Leaf Extracts. Int. J. Pure Appl. Biosci. 2019, 7, 247–252. [Google Scholar] [CrossRef]
- Pande, N.; Jaspal, D.K.; Ambekar, J.; Jayachandran, V.P. Ecofriendly Synthesis and Applications of Silver Nanoparticles. J. Chem. Pharm. Res. 2014, 6, 403–410. [Google Scholar]
- Dada, A.O.; Inyinbor, A.A.; Idu, E.I.; Bello, O.M.; Oluyori, A.P.; Adelani-akande, T.A.; Okunola, A.A. Effect of Operational Parameters, Characterization and Antibacterial Studies of Green Synthesis of Silver Nanoparticles Using Tithonia Diversifolia. PeerJ 2018, 6, e5865. [Google Scholar] [CrossRef] [Green Version]
- Tamileswari, R.; Haniff Nisha, M.; Jesurani, S.; Kanagesan, S.; Hashim, M.; Catherine, S.; Alexander, P. Synthesis of Silver Nanoparticles Using the Vegetable Extract of Raphanus sativus (Radish) and Assessment of Their Antibacterial Activity. Int. J. Adv. Technol. Eng. Sci. 2015, 3, 207–212. [Google Scholar]
- Ehsan, M.; Yazdi, T.; Khara, J.; Housaindokht, M.R.; Sadeghnia, H.R.; Bahabadi, S.E.; Amiri, M.S.; Mosawee, H.; Taherzadeh, D. Role of Ribes Khorasanicum in the Biosynthesis of Silver Nanoparticles and Their Antibacterial Properties. IET Nanobiotechnol. 2018, 13, 8–13. [Google Scholar] [CrossRef]
- Tripathi, D.; Modi, A.; Narayan, G.; Pandey, S. Green and Cost Effective Synthesis of Silver Nanoparticles from Endangered Medicinal Plant Withania Coagulans and Their Potential Biomedical Properties. Mater. Sci. Eng. C 2019, 100, 152–164. [Google Scholar] [CrossRef]
- Wintachai, P.; Paosen, S.; Yupanqui, C.T.; Voravuthikunchai, S.P. Silver nanoparticles synthesized with Eucalyptus critriodora ethanol leaf extract stimulate antibacterial activity against clinically multidrug-resistant Acinetobacter baumannii isolated from pneumonia patients. Microb. Pathog. 2019, 126, 245–257. [Google Scholar] [CrossRef]
- Arya, G.; Kumar, N.; Gupta, N.; Kumar, A.; Nimesh, S. Antibacterial Potential of Silver Nanoparticles Biosynthesised Using Canarium Ovatum Leaves Extract. IET Nanobiotechnol. 2017, 11, 506–511. [Google Scholar] [CrossRef] [PubMed]
- Sinha, A.; Manjhi, J.; Kumar, V.; Rai, D.V. Evaluation of Antibacterial Properties of Silver Nanoparticles Prepared via Green Route Using Elaeocarpus Ganitrus (Rudraksha) Beads Extract. J. Bionanosci. 2018, 12, 553–561. [Google Scholar] [CrossRef]
- Suwan, T.; Khongkhunthian, S.; Sirithunyalug, J. Effect of Rice Variety and Reaction Parameters on Synthesis and Antibacterial Activity of Silver Nanoparticles. Drug Discov. Ther. 2018, 12, 267–274. [Google Scholar] [CrossRef] [Green Version]
- Allafchian, A.R.; Jalali, S.A.H.; Aghaei, F.; Farhang, H.R. Green Synthesis of Silver Nanoparticles Using Glaucium Corniculatum (L.) Curtis Extract and Evaluation of Its Antibacterial Activity. IET Nanobiotechnol. 2018, 12, 574–578. [Google Scholar] [CrossRef] [PubMed]
- Dhiman, J.; Kundu, V.; Kumar, S.; Kumar, R.; Chakarvarti, S.K. Eco-Friendly Synthesis and Characterization of Silver Nanoparticles and Evaluation of Their Antibacterial Activity. Am. J. Mat. Sci. Tech. 2014, 3, 13–21. [Google Scholar] [CrossRef]
- Goyal, S.; Gupta, N.; Kumar, A.; Chatterjee, S.; Nimesh, S. Antibacterial, Anticancer and Antioxidant Potential of Silver Nanoparticles Engineered Using Trigonella Foenum-Graecum Seed Extract. IET Nanobiotechnol. 2018, 12, 526–533. [Google Scholar] [CrossRef]
- Vijayan, R.; Joseph, S.; Mathew, B. Eco-Friendly Synthesis of Silver and Gold Nanoparticles with Enhanced Antimicrobial, Antioxidant, and Catalytic Activities. IET Nanobiotechnol. 2018, 12, 850–856. [Google Scholar] [CrossRef]
- Balashanmugan, P.; Kalaichelvan, P.T. Biosynthesis Characterization of Silver Nanoparticles Using Cassia Roxburghii DC. Aqueous Extract, and Coated on Cotton Cloth for Effective Antibacterial Activity. Int. J. Nanomed. 2015, 10, 87–97. [Google Scholar] [CrossRef] [Green Version]
- Indiradevi, M.P.; Nallamuthu, N.; Rajini, N.; Rajulu, A.V.; Hariram, N.; Siengchin, S. Cellulose Hybrid Nanocomposites Using Napier Grass Fibers with in Situ Generated Silver Nanoparticles as Fillers for Antibacterial Applications. Int. J. Biol. Macromol. 2018, 118, 99–106. [Google Scholar] [CrossRef]
- Jadou, A.; Al-Shahwany, A. Biogenic Synthesis and Characterization of Silver Nanoparticles Using Some Medical Plants and Evaluation of Their Antibacterial and Toxicity Potential. J AOAC Int. 2018, 101, 1905–1912. [Google Scholar] [CrossRef]
- Esfanddarani, H.M.; Kajani, A.A.; Bordbar, A. Green Synthesis of Silver Nanoparticles Using Flower Extract of Malva sylvestris and Investigation of Their Antibacterial Activity. IET Nanobiotechnol. 2018, 12, 412–416. [Google Scholar] [CrossRef] [PubMed]
- Shaik, M.R.; Albalawi, G.H.; Khan, S.T.; Khan, M.; Adil, S.F.; Kuniyil, M.; Al-warthan, A. “Miswak” Based Green Synthesis of Silver Nanoparticles: Evaluation and Comparison of Their Microbicidal Activities with the Chemical Synthesis. Molecules 2016, 21, 1478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elemike, E.E.; Fayemi, O.E.; Ekennia, A.C.; Onwudiwe, D.C.; Ebenso, E.E. Silver Nanoparticles Mediated by Costus Afer Leaf Electrochemical Properties. Molecules 2017, 22, 701. [Google Scholar] [CrossRef] [Green Version]
- Mohammed, A.E.; Al-Qahtani, A.; Al-Shamri, B.; Aabed, K. Antibacterial and cytotoxic potential of biosynthesized silver nanoparticles by some plant extracts. Nanomaterials 2018, 8, 382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patil, M.P.; Singh, R.D.; Koli, P.B.; Patil, K.T.; Jagdale, B.S.; Tipare, A.R.; Kim, G. Antibacterial Potential of Silver Nanoparticles Synthesized Using Madhuca Longifolia Flower Extract as a Green Resource. Microb. Pathog. 2018, 121, 184–189. [Google Scholar] [CrossRef]
- Riaz, M.; Altaf, M.; Faisal, A.; Shekheli, M.A.; Miana, G.A.; Khan, M.Q.; Shah, M.A.; Ilyas, S.Z.; Khan, A.A. Biogenic Synthesis of AgNPs with Saussurea Lappa C. B. Clarke and Studies on Their Biochemical Properties. J. Nanosc. Nanotech. 2018, 12, 8392–8398. [Google Scholar] [CrossRef]
- Riaz, M.; Altaf, M.; Khan, M.Q.; Manzoor, S.; Shekheli, M.A.; Shah, M.A.; Ilyas, S.Z.; Hussain, Z. Green Synthesis of Silver Nanoparticles Using Jurinea Dolomiaea and Biological Activities. J. Nanosc. Nanotech. 2018, 12, 8386–8391. [Google Scholar] [CrossRef]
- Saravanakumar, K.; Chelliah, R.; Shanmugam, S.; Varukattu, N.B.; Oh, D.; Kathiresan, K.; Wang, M. Green Synthesis and Characterization of Biologically Active Nanosilver from Seed Extract of Gardenia Jasminoides Ellis. J. Photochem. Photobiol. B Biol. 2018, 185, 126–135. [Google Scholar] [CrossRef]
- Shahriari, M.; Veisi, H.; Hekmati, M.; Nemmati, S. In Situ Green Synthesis of Ag Nanoparticles on Herbal Tea Extract (Stachys lavandulifolia)-Modified Magnetic Iron Oxide Nanoparticles as Antibacterial Agent and Their 4-Nitrophenol Catalytic Reduction Activity. Mater. Sci. Eng. C 2018, 90, 57–66. [Google Scholar] [CrossRef]
- Sinsinwar, S.; Sarkar, M.K.; Suriya, K.R.; Nithyanand, P.; Vadivel, V. Use of Agricultural Waste (Coconut Shell) for the Synthesis of Silver Nanoparticles and Evaluation of Their Antibacterial Activity against Selected Human Pathogens. Microb. Pathog. 2018, 124, 30–37. [Google Scholar] [CrossRef]
- Mohan, C.; Purwar, R.; Pal, A. Enhanced Potential of Biomimetic, Silver Nanoparticles Functionalized Antheraea mylitta (Tasar) Silk Fibroin Nanofibrous Mats for Skin Tissue Engineering. Int. J. Biol. Macromol. 2019, 130, 437–453. [Google Scholar] [CrossRef]
- Upadhyay, P.; Mishra, S.K.; Purohit, S.; Dubey, G.P.; Chauhan, S.; Srikrishna, S.; Upadhyay, P.; Mishra, S.K.; Purohit, S.; Dubey, G.P. Antioxidant, Antimicrobial and Cytotoxic Potential of Silver Nanoparticles Synthesized Using Flavonoid Rich Alcoholic Leaves Extract of Reinwardtia Indica. Drug Chem. Toxicol. 2018, 42, 65–75. [Google Scholar] [CrossRef]
- Zia, G.; Haleema, S.; Nazir, S.; Ejaz, K.; Ali, S. In Vitro Studies on Cytotoxic, DNA Protecting, Antibiofilm and Antibacterial Effects of Biogenic Silver Nanoparticles Prepared with Bergenia Ciliata Rhizome Extract. Curr. Pharm. Biotechnol. 2018, 19, 68–78. [Google Scholar] [CrossRef] [PubMed]
- De Barros, C.H.N.; Cruz, G.C.F.; Mayrink, W.; Tasic, L. Bio-Based Synthesis of Silver Nanoparticles from Orange Waste: Effects of Distinct Biomolecule Coatings on Size, Morphology, and Antimicrobial Activity. Nanotechnol. Sci. Appl. 2018, 11, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Francis, S.; Koshy, E.P.; Mathew, B. Green synthesis of Stereospermum suaveolens capped silver and gold nanoparticles and assessment of their innate antioxidant, antimicrobial and antiproliferative activities. Bioprocess Biosyst. Eng. 2018, 41, 939–951. [Google Scholar] [CrossRef]
- Das, M.P.; Livingstone, J.R.; Veluswamy, P.; Das, J. Exploration of Wedelia Chinensis Leaf-Assisted Silver Nanoparticles for Antioxidant, Antibacterial and in Vitro Cytotoxic Applications. J. Food Drug Anal. 2017, 26, 917–925. [Google Scholar] [CrossRef] [Green Version]
- Otunola, G.A.; Afolayan, A.J.; Ajayi, E.O.; Odeyemi, S.W. Characterization, antibacterial and antioxidant properties of silver nanoparticles synthesized from aqueous extracts of Allium sativum, Zingiber officinale, and Capsicum frutescens. Pharmacogn. Mag. 2017, 13, 201–208. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.; Sharma, B.; Deswal, R. Green Silver Nanoparticles from Novel Brassicaceae Cultivars with Enhanced Antimicrobial Potential than Earlier Reported Brassicaceae Members. J. Trace Elem. Med. Biol. 2018, 47, 1–11. [Google Scholar] [CrossRef]
- Singh, H.; Du, J.; Yi, T.H. Green and Rapid Synthesis of Silver Nanoparticles Using Borago Officinalis Leaf Extract: Anticancer and Antibacterial Activities. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1310–1316. [Google Scholar] [CrossRef] [Green Version]
- Ansari, M.A.; Alzohairy, M.A. One-Pot Facile Green Synthesis of Silver Nanoparticles Using Seed Extract of Phoenix Dactylifera and Their Bactericidal Potential against MRSA. Evid.-Based Complement. Altern. Med. 2018, 2018, 1860280. [Google Scholar] [CrossRef] [Green Version]
- Eren, A.; Baran, M. Green Synthesis, Characterization and Antimicrobial Activity of Silver Nanoparticles (AgNPs) from Maize (Zea mays L.). Appl. Ecol. Environ. Res. 2019, 17, 4097–4105. [Google Scholar] [CrossRef]
- Owaid, M.N.; Muslim, R.F.; Hamad, H.A. Mycosynthesis of Silver Nanoparticles Using Terminia Sp. Desert Truffle, Pezizaceae, and Their Antibacterial Activity. Jordan J. Biol. Sci. 2018, 11, 401–405. [Google Scholar]
- Mehmood, A.; Shabir, S.; Hussain, S.; Ahmad, K.S.; Hamid, A. Antibacterial and Antioxidant Activity of Biosynthesized Silver Nanoparticles from Ulmus Wallichiana Planch. Leaf Extract. Farmacia 2019, 67, 662–669. [Google Scholar] [CrossRef]
- Sangaonkar, G.M.; Pawar, K.D. Garcinia Indica Mediated Biogenic Synthesis of Silver Nanoparticles with Antibacterial and Antioxidant Activities. Colloids Surf. B Biointerfaces 2018, 164, 210–217. [Google Scholar] [CrossRef]
- Ahluwalia, V.; Elumalai, S.; Kumar, V.; Kumar, S.; Sangwan, R.S. Nano Silver Particle Synthesis Using Swertia Paniculata Herbal Extract and Its Antimicrobial Activity Vivek. Microb. Pathog. 2017, 114, 402–408. [Google Scholar] [CrossRef]
- Jaffri, S.B.; Ahmad, K.S. Augmented Photocatalytic, Antibacterial and Antifungal Activity of Prunosynthetic Silver Nanoparticles. Artif. Cells Nanomed. Biotechnol. 2018, 46, S127–S137. [Google Scholar] [CrossRef] [Green Version]
- Erci, F.; Cakir-Koc, R.; Isildak, I. Green synthesis of silver nanoparticles using Thymbra spicata (L.) var. spicata (zahter) aqueous leaf extract and evaluation of their morphology-dependent antibacterial and cytotoxic activity. Artif Cells Nanomed. Biotechnol. 2018, 46, 150–158. [Google Scholar] [CrossRef] [Green Version]
- Idrees, M.; Batool, S.; Kalsoom, T.; Raina, S.; Sharif, M.A.; Yasmeen, S. Biosynthesis of Silver Nanoparticles Using Sida Acuta Extract for Antimicrobial Actions and Corrosion Inhibition Potential. Environ. Technol. 2018, 112, 4073–4085. [Google Scholar] [CrossRef]
- Jain, S.; Mehata, M.S. Medicinal Plant Leaf Extract and Pure Flavonoid Mediated Green Synthesis of Silver Nanoparticles and Their Enhanced Antibacterial Property. Sci. Rep. 2017, 7, 15867. [Google Scholar] [CrossRef]
- Lakkakula, J.R.; Ndinteh, D.T.; Van Vuuren, S.F.; Olivier, D.K.; Krause, R.W.M. Synthesis of Silver Nanoparticles from a Desmodium Adscendens Extract and Its Antibacterial Evaluation on Wound Dressing Material. IET Nanobiotechnol. 2017, 11, 1017–1026. [Google Scholar] [CrossRef]
- Syafiuddin, A.; Salmiati; Hadibarata, T.; Hong, K.A.B.; Salim, R.M. Novel Weed-Extracted Silver Nanoparticles and Their Antibacterial Appraisal against a Rare Bacterium from River and Sewage Treatment Plan. Nanomaterials 2018, 8, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Devanesan, S.; Alsalhi, M.S.; Balaji, R.V.; Ranjitsingh, A.J.A.; Ahamed, A.; Alfuraydi, A.A.; Alqahtani, F.Y.; Aleanizy, F.S.; Othman, A.H. Antimicrobial and Cytotoxicity Effects of Synthesized Silver Nanoparticles from Punica Granatum Peel Extract. Nanoscale Res. Lett. 2018, 13, 315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohanta, Y.K.; Biswas, K.; Panda, S.K.; Bandyopadhyay, J.; De, D.; Jayabalan, R.; Bastia, A.K.; Mohanta, T.K. Phyto-Assisted Synthesis of Bio-Functionalised Silver Nanoparticles and Their Potential Anti-Oxidant, Anti-Microbial and Wound Healing Activities. IET Nanobiotechnol. 2017, 11, 1027–1034. [Google Scholar] [CrossRef] [PubMed]
- Patil, M.P.; Seo, Y.B.; Kim, G. Morphological Changes of Bacterial Cells upon Exposure of Silver-Silver Chloride Nanoparticles Synthesized Using Agrimonia Pilosa. Microb. Pathog. 2018, 116, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Patil, B.N.; Taranath, T.C. Limonia acidissima L. Leaf Mediated Synthesis of Silver and Zinc Oxide Nanoparticles and Their Antibacterial Activities. Microb. Pathog. 2018, 115, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Shriniwas, P.P. Antioxidant, Antibacterial and Cytotoxic Potential of Silver Nanoparticles Synthesized Using Terpenes Rich Extract of Lantana Camara L. Leaves. Biochem. Biophys. Rep. 2017, 10, 76–81. [Google Scholar] [CrossRef]
- Siritapetawee, J.; Limphirat, W.; Nantapong, N.; Songthamwat, D. Fabrication of Silver Chloride Nanoparticles Using a Plant Serine Protease in Combination with Photo-Activation and Investigation of Their Biological Activities. Biotechnol. Appl. Biochem. 2018, 64, 572–579. [Google Scholar] [CrossRef]
- Premkumar, J.; Sudhakar, T.; Dhakal, A.; Shrestha, J.B.; Krishnakumar, S.; Balashanmugam, P. Synthesis of Silver Nanoparticles (AgNPs) from Cinnamon against Bacterial Pathogens. Biocatal. Agric. Biotechnol. 2018, 15, 311–316. [Google Scholar] [CrossRef]
- Yuan, C.; Huo, C.; Gui, B.; Liu, J.; Chen, Y. Facile Phyto-Mediated Synthesis of Silver Nanoparticles Using Chinese Winter Jujube (Ziziphus jujuba Mill. Cv. Dongzao ) Extract and Their Antibacterial/Catalytic Properties. IET Nanobiotechnol. 2017, 11, 973–980. [Google Scholar] [CrossRef]
- Rodríguez-León, E.; Íñiguez-Palomares, R.A.; Navarro, R.E.; Rodríguez-Beas, C.; Larios-Rodríguez, E.; Alvarez-Cirerol, F.J.; Íñiguez-Palomares, C.; Ramírez-Saldaña, M.; Hernández, J.; Martínez-higuera, A.; et al. Silver Nanoparticles Synthesized with Rumex Hymenosepalus Extracts: Effective Broad-Spectrum Microbicidal Agents and Cytotoxicity Study. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1194–1206. [Google Scholar] [CrossRef] [Green Version]
- Hernández-Gómora, A.E.; Lara-Carrillo, E.; Robles-Navarro, J.B.; Scougall-Vilchis, R.J.; Hernández-López, S.; Medina-Solís, C.E.; Morales-Luckie, R.A. Biosynthesis of Silver Nanoparticles on Orthodontic Elastomeric Modules: Evaluation of Mechanical and Antibacterial Properties. Molecules 2017, 22, 1407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jha, D.; Thiruveedula, P.K.; Pathak, R.; Kumar, B.; Gautam, H.K.; Agnihotri, S.; Sharma, A.K.; Kumar, P. Multifunctional Biosynthesized Silver Nanoparticles Exhibiting Excellent Antimicrobial Potential against Multi-Drug Resistant Microbes along with Remarkable Anticancerous Properties. Mater. Sci. Eng. C 2017, 80, 659–669. [Google Scholar] [CrossRef] [PubMed]
- Fierascu, I.; Georgiev, M.I.; Ortan, A.; Fierascu, R.C.; Marius, S.; Ionescu, D.; Sutan, A.; Brinzan, A.; Ditu, L.M. Phyto-Mediated Metallic Nano- Architectures via Melissa Officinalis L.: Synthesis, Characterization and Biological Properties. Sci. Rep. 2017, 7, 12428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Francis, S.; Joseph, S.; Koshy, E.P.; Mathew, B. Microwave Assisted Green Synthesis of Silver Nanoparticles Using Leaf Extract of Elephantopus Scaber and Its Environmental and Biological Applications. Artif. Cells, Nanomed. Biotechnol. 2018, 46, 795–804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghanbar, F.; Mirzaie, A.; Ashrafi, F.; Noorbazargan, H.; Jalali, M.D.; Salehi, S.; Shandiz, S.A.S. Antioxidant, Antibacterial and Anticancer Properties of Phyto-Synthesised Artemisia Quttensis Podlech Extract Mediated AgNPs. IET Nanobiotechnol. 2017, 11, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Iyer, I.; Panda, R.T. Biosynthesis of Gold and Silver Nanoparticles Using Extracts of Callus Cultures of Pumpkin (Cucurbita maxima). J. Nanosci. Nanotechnol. 2018, 18, 5341–5353. [Google Scholar] [CrossRef]
- Kasithevar, M.; Periakaruppan, P.; Muthupandian, S.; Mohan, M. Antibacterial Efficacy of Silver Nanoparticles against Multi-Drug Resistant Clinical Isolates from Post-Surgical Wound Infections. Microb. Pathog. 2017, 107, 327–334. [Google Scholar] [CrossRef]
- Kelkawi, A.H.A.; Kajani, A.A.; Bordbar, A. Green Synthesis of Silver Nanoparticles Using Mentha Pulegium and Investigation of Their Antibacterial, Antifungal and Anticancer Activity. IET Nanobiotechnol. 2017, 11, 370–376. [Google Scholar] [CrossRef]
- Muniyan, A.; Ravi, K.; Mohan, U.; Panchamoorthy, R. Characterization and in Vitro Antibacterial Activity of Saponin-Conjugated Silver Nanoparticles against Bacteria That Cause Burn Wound Infection. World J. Microbiol. Biotechnol. 2017, 33, 147. [Google Scholar] [CrossRef]
- Osibe, D.A.; Chiejina, N.V.; Ogawa, K.; Aoyagi, H. Stable Antibacterial Silver Nanoparticles Produced with Seed-Derived Callus Extract of Catharanthus Roseus. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1266–1273. [Google Scholar] [CrossRef] [Green Version]
- Rasheed, T.; Bilal, M.; Iqbal, H.M.N.; Li, C. Green Biosynthesis of Silver Nanoparticles Using Leaves Extract of Artemisia Vulgaris and Their Potential Biomedical Applications. Colloids Surf. B Biointerfaces 2017, 158, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Rashid, M.O.; Akhter, K.N.; Chowdhury, J.A.; Hossen, F.; Hussain, S. Characterization of Phytoconstituents and Evaluation of Antimicrobial Activity of Silver- Extract Nanoparticles Synthesized from Momordica Charantia Fruit Extract. BMC Complement Altern. Med. 2017, 17, 336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Senthil, B.; Devasena, T.; Prakash, B.; Rajasekar, A. Non-Cytotoxic Effect of Green Synthesized Silver Nanoparticles and Its Antibacterial Activity. J. Photochem. Photobiol. B Biol. 2017, 177, 1–7. [Google Scholar] [CrossRef]
- Singla, R.; Soni, S.; Patial, V.; Kulurkar, P.M. Cytocompatible Anti-Microbial Dressings of S Yzygium Cumini Cellulose Nanocrystals Decorated with Silver Nanoparticles Accelerate Acute and Diabetic Wound Healing. Sci. Rep. 2017, 7, 10457. [Google Scholar] [CrossRef] [PubMed]
- Skandalis, N.; Dimopoulou, A.; Georgopoulou, A.; Gallios, N.; Papadopoulos, D.; Tsipas, D.; Theologidis, I.; Michailidis, N. The Effect of Silver Nanoparticles Size, Produced Using Plant Extract from Arbutus Unedo, on Their Antibacterial Efficacy. Nanomaterials 2017, 7, 178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Syafiuddin, A.; Salmiati; Hadibarata, T.; Salim, M.R.; Kueh, A.B.H.; Sari, A.A. A Purely Green Synthesis of Silver Nanoparticles Using Carica Papaya, Manihot Esculenta, and Morinda Citrifolia: Synthesis and Antibacterial Evaluations. Bioprocess Biosyst. Eng. 2017, 40, 1349–1361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, R.; Mathew, S.; Nayana, A.; Mathews, J.; Radhakrishnan, E. Microbially and Phytofabricated AgNPs with Different Mode of Bactericidal Action Were Identified to Have Comparable Potential for Surface Fabrication of Central Venous Catheters to Combat Staphylococcus Aureus Biofil. J. Photochem. Photobiol. B Biol. 2017, 171, 96–103. [Google Scholar] [CrossRef]
- Rashmi, V.; Sanjay, K. Green Synthesis, Characterization and Bioactivity of Plant-Mediated Silver Nanoparticles Using Decalepis Hamiltonii Root Extract. IET Nanobiotechnol. 2017, 11, 247–254. [Google Scholar] [CrossRef]
- Sundararajan, B.; Mahendran, G.; Thamaraiselvi, R.; Kumari, B.D.R. Biological Activities of Synthesized Silver Nanoparticles from Cardiospermum halicacabum L. Bull. Mater. Sci. 2016, 39, 423–431. [Google Scholar] [CrossRef] [Green Version]
- Ali, Z.A.; Yahya, R.; Sekaran, S.D.; Puteh, R. Green Synthesis of Silver Nanoparticles Using Apple Extract and Its Antibacterial Properties. Adv. Mater. Sci. Eng. 2016, 2016, 4102196. [Google Scholar] [CrossRef] [Green Version]
- Khan, F.A.; Zahoor, M.; Jalal, A.; Rahman, A.U. Green Synthesis of Silver Nanoparticles by Using Ziziphus Nummularia Leaves Aqueous Extract and Their Biological Activities. J. Nanomater. 2016, 2016, 8026843. [Google Scholar] [CrossRef] [Green Version]
- Anandalakshmi, K.; Venugobal, J.; Ramasamy, V. Characterization of silver nanoparticles by green synthesis method using Pedalium murex leaf extract and their antibacterial activity. Appl. Nanosci. 2016, 6, 399–408. [Google Scholar] [CrossRef] [Green Version]
- Pak, Z.H.; Abbaspour, H.; Karimi, N.; Fattahi, A. Eco-Friendly Synthesis and Antimicrobial Activity of Silver Nanoparticles Using Dracocephalum Moldavica Seed Extract. Appl. Sci. 2016, 6, 69. [Google Scholar] [CrossRef]
- Augustine, R.; Augustine, A.; Kalarikkal, N.; Thomas, S. Fabrication and Characterization of Biosilver Nanoparticles Loaded Calcium Pectinate Nano-Micro Dual-Porous Antibacterial Wound Dressings. Prog. Biomater. 2016, 5, 223–235. [Google Scholar] [CrossRef] [Green Version]
- Muthukrishnan, S.B.S.; Muthukumar, M.S.M. Biogenic Synthesis of Silver Nanoparticles and Their Antioxidant and Antibacterial Activity. Appl. Nanosci. 2016, 6, 755–766. [Google Scholar] [CrossRef] [Green Version]
- Bose, D.; Chatterjee, S. Biogenic Synthesis of Silver Nanoparticles Using Guava (Psidium Guajava) Leaf Extract and Its Antibacterial Activity against Pseudomonas Aeruginosa. Appl. Nanosci. 2016, 6, 895–901. [Google Scholar] [CrossRef] [Green Version]
- Choudhary, M.K.; Kataria, J.; Cameotra, S.S.; Singh, J. A Facile Biomimetic Preparation of Highly Stabilized Silver Nanoparticles Derived from Seed Extract of Vigna Radiata and Evaluation of Their Antibacterial Activity. Appl. Nanosci. 2015, 6, 105–111. [Google Scholar] [CrossRef] [Green Version]
- Dehghanizade, S.; Arasteh, J.; Mirzaie, A. Green Synthesis of Silver Nanoparticles Using Anthemis Atropatana Extract: Characterization and in Vitro Biological Activities. Artif. Cells Nanomed. Biotechnol. 2018, 46, 160–168. [Google Scholar] [CrossRef] [Green Version]
- Khatami, M.; Mehnipor, R.; Poor, M.H.S.; Jouzani, G.S. Facile Biosynthesis of Silver Nanoparticles Using Descurainia Sophia and Evaluation of Their Antibacterial and Antifungal Properties. J. Clust. Sci. 2016, 27, 1601–1612. [Google Scholar] [CrossRef]
- Lateef, A.; Azeez, M.A.; Asafa, T.B.; Yekeen, T.A. Cocoa Pod Husk Extract-Mediated Biosynthesis of Silver Nanoparticles: Its Antimicrobial, Antioxidant and Larvicidal Activities. J. Nanostructure Chem. 2016, 6, 159–169. [Google Scholar] [CrossRef] [Green Version]
- Logaranjan, K.; Raiza, A.J.; Gopinath, S.C.B.; Chen, Y. Shape- and Size-Controlled Synthesis of Silver Nanoparticles Using Aloe Vera Plant Extract and Their Antimicrobial Activity. Nanoscale Res. Lett. 2016, 11, 520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murali Krishna, I.; Bhagavanth Reddy, G.; Veerabhadram, G.; Madhusudhan, A. Eco-Friendly Green Synthesis of Silver Nanoparticles Using Salmalia Malabarica: Synthesis, Characterization, Antimicrobial, and Catalytic Activity Studies. Appl. Nanosci. 2016, 6, 681–689. [Google Scholar] [CrossRef] [Green Version]
- Xia, Q.H.; Ma, Y.J.; Wang, J.W. Biosynthesis of Silver Nanoparticles Using Taxus Yunnanensis Callus and Their Antibacterial Activity and Cytotoxicity in Human Cancer Cells. Nanomaterials 2016, 6, 160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oluwaniyi, O.O.; Adegoke, H.I.; Adesuji, E.T.; Alabi, A.B.; Bodeke, S.O.; Labulo, A.H.; Oseghale, C.O. Biosynthesis of Silver Nanoparticles Using Aqueous Leaf Extract of Thevetia Peruviana Juss and Its Antimicrobial Activities. Appl. Nanosci. 2015, 6, 903–912. [Google Scholar] [CrossRef]
- Sahu, N.; Soni, D.; Satpute, B.C.D.B. Synthesis of Silver Nanoparticles Using Flavonoids: Hesperidin, Naringin and Diosmin, and Their Antibacterial Effects and Cytotoxicity. Int. Nano Lett. 2016, 6, 173–181. [Google Scholar] [CrossRef] [Green Version]
- Vijayan, R.; Joseph, S.; Mathew, B. Indigofera Tinctoria Leaf Extract Mediated Green Synthesis of Silver and Gold Nanoparticles and Assessment of Their Anticancer, Antimicrobial, Antioxidant and Catalytic Properties. Artif. Cells Nanomed. Biotechnol. 2018, 46, 861–871. [Google Scholar] [CrossRef] [Green Version]
- Ponvel, K.; Narayanaraya, T.; Prabakaran, J. Biosynthesis of Silver Nanoparticles Using Root Extract of the Medicinal Plant Justicia Adhatoda: Characterization, Electrochemical Behavior and Applications. Int. J. Nano Dimens. 2015, 6, 339–349. [Google Scholar] [CrossRef]
- Jamil, M.; Murtaza, G.; Mehmood, A. Green Synthesis of Silver Nanoparticles Using Leaves Extract of Skimmia Laureola: Characterization and Antibacterial Activity. Mater. Lett. 2015, 153, 10–13. [Google Scholar] [CrossRef]
- Bose, D.; Chatterjee, S. Antibacterial Activity of Green Synthesized Silver Nanoparticles Using Vasaka (Justicia Adhatoda L.) Leaf Extract. Indian J. Microbial. 2015, 55, 163–167. [Google Scholar] [CrossRef] [Green Version]
- Soares, M.R.P.S.; Corrêa, R.O.; Stroppa, P.H.F.; Marques, F.C.; Andrade, G.F. Biosynthesis of Silver Nanoparticles Using Caesalpinia Ferrea (Tul.) Martius Extract: Physicochemical Characterization, Antifungal Activity and Cytotoxicity. PeerJ 2018, 6, e4361. [Google Scholar] [CrossRef] [Green Version]
- Doddapaneni, S.J.D.S.; Amgoth, C.; Kalle, A.M.; Suryadevara, S.N.; Alapati, K.S. Antimicrobial and Anticancer Activity of AgNPs Coated with Alphonsea Sclerocarpa Extract. 3 Biotech 2018, 8, 156. [Google Scholar] [CrossRef] [PubMed]
- Pham, D.C.; Nguyen, T.H.; Thi, U.; Ngoc, P.; Thuy, N.; Le, T.; Tran, T.V.; Nguyen, D.H. Of Chitosan-Silver Nanoparticles Synergize Fungicide Against Pyricularia Oryzae. J. Nanasci. Nanotechnol. 2018, 18, 5299–5305. [Google Scholar] [CrossRef] [PubMed]
- Velu, M.; Chang, J.L.W.; Lovanh, N. Fabrication, Optimization, and Characterization of Noble Silver Nanoparticles from Sugarcane Leaf (Saccharum officinarum) Extract for Antifungal Application. 3 Biotech 2017, 7, 147. [Google Scholar] [CrossRef] [PubMed]
- Netala, V.R.; Kotakadi, S.V.; Domdi, L.; Gaddam, S.A.; Bobbu, P. Biogenic Silver Nanoparticles: Efficient and Effective Antifungal Agents. Appl. Nanosci. 2015, 6, 475–484. [Google Scholar] [CrossRef] [Green Version]
- Dobrucka, R.; Długaszewska, J. Antimicrobial Activities of Silver Nanoparticles Synthesized by Using Water Extract of Arnicae Anthodium. Indian J. Microbiol. 2015, 55, 168–174. [Google Scholar] [CrossRef] [Green Version]
- Yugandhar, P.; Haribabu, R.; Savithramma, N. Synthesis, Characterization and Antimicrobial Properties of Green-Synthesised Silver Nanoparticles from Stem Bark Extract of Syzygium Alternifolium (Wt.) Walp. 3 Biotech 2015, 5, 1031–1039. [Google Scholar] [CrossRef] [Green Version]
- Joseph, S.; Mathew, B. Microwave Assisted Biosynthesis of Silver Nanoparticles Using the Rhizome Extract of Alpinia Galanga and Evaluation of Their Catalytic and Antimicrobial Activities. J. Nanoparticle 2014, 2014, 967802. [Google Scholar] [CrossRef] [Green Version]
- Kumaran, M.; Kalaichelvan, P. Exploitation of Endophytic Fungus, Guignardia Mangiferae for Extracellular Synthesis of Silver Nanoparticles and Their in Vitro Biological Activities. Microbiol. Res. 2015, 178, 9–17. [Google Scholar] [CrossRef]
- Khatami, M.; Pourseyedi, S. Phoenix Dactylifera (Date Palm) Pit Aqueous Extract Mediated Novel Route for Synthesis High Stable Silver Nanoparticles with High Antifungal and Antibacterial Activity. IET Nanobiotechnol. 2015, 9, 184–190. [Google Scholar] [CrossRef]
- Khatoon, N.; Mishra, A.; Alam, H. Biosynthesis, Characterization, and Antifungal Activity of the Silver Nanoparticles Against Pathogenic Candida Species. BioNanoScience 2015, 5, 65–74. [Google Scholar] [CrossRef]
- Mohanta, Y.K.; Panda, S.K.; Jayabalan, R.; Sharma, N. Antimicrobial, Antioxidant and Cytotoxic Activity of Silver Nanoparticles Synthesized by Leaf Extract of Erythrina Suberosa (Roxb.). Front. Mol. Biosci. 2017, 4, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Narayanan, K.B.; Park, H.H. Antifungal Activity of Silver Nanoparticles Synthesized Using Turnip Leaf Extract (Brassica rapa L.) against Wood Rotting Pathogens. Eur. J. Plant Pathol. 2014, 140, 185–192. [Google Scholar] [CrossRef]
- Nguyen, D.H.; Lee, J.S.; Park, K.D.; Ching, Y.C.; Nguyen, X.T.; Phan, V.H.G.; Thanh, T.; Thi, H. Green Silver Nanoparticles Formed by Phyllanthus Urinaria, Pouzolzia Zeylanica, and Scoparia Dulcis Leaf Extracts and the Antifungal Activity. Nanomaterials 2020, 10, 542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Padalia, H.; Moteriya, P.; Chanda, S. Green Synthesis of Silver Nanoparticles from Marigold Flower and Its Synergistic Antimicrobial Potential. Arab. J. Chem. 2014, 8, 732–741. [Google Scholar] [CrossRef] [Green Version]
- Sahni, G.; Panwar, A.; Kaur, B. Controlled Green Synthesis of Silver Nanoparticles by Allium Cepa and Musa Acuminata with Strong Antimicrobial Activity. Int. Nano Lett. 2015, 5, 93–100. [Google Scholar] [CrossRef] [Green Version]
- Shetty, P.; Supraja, N.; Garud, M.; Prasad, T.N.V.K.V. Synthesis, Characterization and Antimicrobial Activity of Alstonia Scholaris Bark-Extract-Mediated Silver Nanoparticles. J. Nanostruc. Chem. 2014, 4, 161–170. [Google Scholar] [CrossRef] [Green Version]
- Rao, M.L.; Bhumi, G.; Savithramma, N. Green Synthesis of Silver Nanoparticles by Allamanda Cathartica L. Leaf Extract and Evaluation for Antimicrobial Activity. Int. J. Pharm. Sci. Nanotechnol. 2013, 6, 2260–2268. [Google Scholar] [CrossRef]
- Pasupuleti, V.R.; Prasad, T.N.V.K.V.; Shiekh, R.A.; Balam, S.K. Biogenic silver nanoparticles using Rhinacanthus nasutus leaf extract: Synthesis, spectral analysis, and antimicrobial studies. Int. J. Nanomed. 2013, 8, 3355–3364. [Google Scholar] [CrossRef] [Green Version]
- Sesuvium, L.; Nabikhan, A.; Kandasamy, K.; Raj, A.; Alikunhi, N.M. Synthesis of Antimicrobial Silver Nanoparticles by Callus and Leaf Extracts from Saltmarsh Plant, Sesuvium portulacastrum L. Colloids Surf. B Biointerfaces 2010, 79, 488–493. [Google Scholar] [CrossRef]
- Ansar, S.; Tabassum, H.; Aladwan, N.S.M.; Ali, M.N. Eco Friendly Silver Nanoparticles Synthesis by Brassica Oleracea and Its Antibacterial, Anticancer and Antioxidant Properties. Sci. Rep. 2020, 10, 18564. [Google Scholar] [CrossRef]
- Niluxsshun, M.C.D.; Masilamani, K.; Mathiventhan, U. Green Synthesis of Silver Nanoparticles from the Extracts of Fruit Peel of Citrus Tangerina, Citrus Sinensis, and Citrus Limon for Antibacterial Activities. Bioinorg. Chem. Appl. 2021, 2021, 6695734. [Google Scholar] [CrossRef] [PubMed]
- Devanesan, S.; Alsalhi, M.S. Green Synthesis of Silver Nanoparticles Using the Flower Extract of Abelmoschus Esculentus for Cytotoxicity and Antimicrobial Studies. Int. J. Nanomed. 2021, 16, 3343–3356. [Google Scholar] [CrossRef] [PubMed]
- Nayem, S.M.A.; Sultana, N.; Haque, A.; Miah, B. Green Synthesis of Gold and Silver Nanoparticles by Using Amorphophallus Paeoniifolius Tuber Extract and Evaluation of Their Antibacterial Activity. Molecules 2020, 25, 4773. [Google Scholar] [CrossRef] [PubMed]
- Kota, S.; Dumpala, P.; Anantha, R.K.; Verma, M.K. Evaluation of Therapeutic Potential of the Silver/Silver Chloride Nanoparticles Synthesized with the Aqueous Leaf Extract of Rumex Acetosa. Sci. Rep. 2017, 1, 11566. [Google Scholar] [CrossRef] [Green Version]
- Khodadadi, S.; Mahdinezhad, N.; Fazeli-Nasab, B.; Heidari, M.J.; Fakheri, B.; Miri, A. Investigating the Possibility of Green Synthesis of Silver Nanoparticles Using Vaccinium Arctostaphlyos Extract and Evaluating Its Antibacterial Properties. Biomed Res. Int. 2021, 2021, 5572252. [Google Scholar] [CrossRef]
- Padilla-Camberos, E.; Sanchez-Hernandez, I.M.; Torres-Gonzalez, O.R.; Ramirez-Rodriguez, P.; Diaz, E.; Wille, H.; Flores-Fernandez, J.M. Biosynthesis of Silver Nanoparticles Using Stenocereus Queretaroensis Fruit Peel Extract: Study of Antimicrobial Activity. Materials 2021, 14, 4543. [Google Scholar] [CrossRef]
- Logeswari, P.; Silambarasan, S.; Abraham, J. Synthesis of Silver Nanoparticles Using Plants Extract and Analysis of Their Antimicrobial Property. J. Saudi Chem. Soc. 2015, 19, 311–317. [Google Scholar] [CrossRef] [Green Version]
- Li, R.; Chen, Z.; Ren, N.; Wang, Y.; Wang, Y.; Yu, F. Biosynthesis of Silver Oxide Nanoparticles and Their Photocatalytic and Antimicrobial Activity Evaluation for Wound Healing Applications in Nursing Care. J. Photochem. Photobiol. B Biol. 2019, 199, 111593. [Google Scholar] [CrossRef]
- Sharma, K.; Guleria, S.; Razdan, V.K. Green Synthesis of Silver Nanoparticles Using Ocimum Gratissimum Leaf Extract: Characterization, Antimicrobial Activity and Toxicity Analysis. J. Plant Biochem. Biotechnol. 2019, 29, 213–224. [Google Scholar] [CrossRef]
- Ebrahimzadeh, M.A.; Naghizadeh, A.; Mohammadi-aghdam, S.; Khojasteh, H. Enhanced Catalytic and Antibacterial Efficiency of Biosynthesized Convolvulus Fruticosus Extract Capped Gold Nanoparticles (CFE @ AuNPs ). J. Photochem. Photobiol. B Biol. 2020, 209, 111949. [Google Scholar] [CrossRef]
- Boomi, P.; Ganesan, R.; Poorani, G.; Gurumallesh, H. Phyto-Engineered Gold Nanoparticles (AuNPs) with Potential Antibacterial, Antioxidant, and Wound Healing Activities Under in Vitro and in Vivo Conditions. Int. J. Nanomed. 2020, 15, 7553–7568. [Google Scholar] [CrossRef] [PubMed]
- Ullah, R.; Shah, S.; Muhammad, Z.; Shah, S.A.; Faisal, S. In Vitro and in Vivo Applications of Euphorbia Wallichii Shoot Extract - Mediated Gold Nanospheres. Green Process. Synth. 2021, 10, 101–111. [Google Scholar] [CrossRef]
- Karthika, V.; Arumugam, A.; Gopinath, K.; Kaleeswarran, P.; Govindarajan, M.; Alharbi, N.S.; Kadaikunnan, S.; Khaled, J.M.; Benelli, G. Guazuma Ulmifolia Bark-Synthesized Ag, Au and Ag/Au Alloy Nanoparticles: Photocatalytic Potential, DNA/Protein Interactions, Anticancer Activity and Toxicity against 14 Species of Microbial Pathogens. J. Photochem. Photobiol. B Biol. 2017, 167, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Huo, Y.; Singh, P.; Kim, Y.J.; Soshnikova, V.; Markus, J.; Ahn, S.; Castro-aceituno, V.; Chokkalingam, M.; Bae, K.; Yang, D.C.; et al. Biological Synthesis of Gold and Silver Chloride Nanoparticles by Glycyrrhiza Uralensis and in Vitro Applications. Artif. Cells Nanomed. Biotechnol. 2017, 46, 303–312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basavegowda, N.; Kumar, G.D.; Tyliszczak, B.; Wzorek, Z.; Sobczak-Kupiec, A. One-Step Synthesis of Highly-Biocompatible Spherical Gold Nanoparticles Using Artocarpus Heterophyllus Lam. (Jackfruit) Fruit Extract and Its Effect on Pathogens. Ann. Agric. Environ. Med. 2015, 22, 84–89. [Google Scholar] [CrossRef] [Green Version]
- Shahriari, M.; Hemmati, S. Biosynthesis of Gold Nanoparticles Using Allium Noeanum Reut. Ex Regel Leaves Aqueous Extract; Characterization and Analysis of Their Cytotoxicity, Antioxidant, and Antibacterial Properties. Appl. Ornanometallic Chem. 2019, 33, e5189. [Google Scholar] [CrossRef]
- Dobrucka, R.; Dlugaszewska, J.; Kaczmarek, M. Antimicrobial and Cytostatic Activity of Biosynthesized Nanogold Prepared Using Fruit Extract of Ribes Nigrum. Arab. J. Chem. 2019, 12, 3902–3910. [Google Scholar] [CrossRef] [Green Version]
- Press, D. One-Step Green Synthesis and Characterization of Leaf Extract-Mediated Biocompatible Silver and Gold Nanoparticles from Memecylon Umbellatum. Int. J. Nanomed. 2013, 8, 1307–1315. [Google Scholar]
- Patra, J.K.; Baek, K.-H. Novel Green Synthesis of Gold Nanoparticles Using Citrullus Lanatus Rind and Investigation of Proteasome Inhibitory Activity, Antibacterial, and Antioxidant Potential. Int. J. Nanomed. 2015, 10, 7253–7264. [Google Scholar]
- Basavegowda, N.; Idhayadhulla, A.; Lee, Y.R. Preparation of Au and Ag Nanoparticles Using Artemisia Annua and Their in Vitro Antibacterial and Tyrosinase Inhibitory Activities. Mater. Sci. Eng. C 2014, 43, 58–64. [Google Scholar] [CrossRef]
- Yuan, C.; Huo, C.; Gui, B.; Cao, W. Green Synthesis of Gold Nanoparticles Using Citrus Maxima Peel Extract and Their Catalytic/Antibacterial Activities. IET Nanobiotechnol. 2017, 11, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Vijayan, R.; Joseph, S.; Mathew, B. Anticancer, Antimicrobial, Antioxidant, and Catalytic Activities of Green-Synthesized Silver and Gold Nanoparticles Using Bauhinia Purpurea Leaf Extract. Bioprocess Biosyst. Eng. 2019, 42, 305–319. [Google Scholar] [CrossRef] [PubMed]
- Tettey, C.O.; Nagajyothi, P.C.; Lee, S.E.; Ocloo, A.; Minh An, T.N.; Sreekanth, T.V.M.; Lee, K.D. Anti-Melanoma, Tyrosinase Inhibitory and Anti-Microbial Activities of Gold Nanoparticles Synthesized from Aqueous Leaf Extracts of Teraxacum Officinale. Int. J. Cosmet. Sci. 2012, 34, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Shankar, S.; Leejae, S.; Jaiswal, L.; Voravuthikunchai, S.P. Metallic Nanoparticles Augmented the Antibacterial Potency of Rhodomyrtus Tomentosa Acetone Extract against Escherichia Coli. Microb. Pathog. 2017, 107, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Du, J.; Singh, P.; Yi, T.H. Ecofriendly Synthesis of Silver and Gold Nanoparticles by Euphrasia Officinalis Leaf Extract and Its Biomedical Applications. Artif. Cells, Nanomed. Biotechnol. 2018, 46, 1163–1170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emmanuel, R.; Saravanan, M.; Ovais, M. Antimicrobial Efficacy of Drug Blended Biosynthesized Colloidal Gold Nanoparticles from Justicia Glauca against Oral Pathogens: A Nanoantibiotic Approach. Microb. Pathog. 2017, 113, 295–302. [Google Scholar] [CrossRef]
- Nagajyothi, P.C.; Lee, S.E.; An, M.; Lee, K.D. Green Synthesis of Silver and Gold Nanoparticles Using Lonicera Japonica Flower Extract. Bull. Korean Chem. Soc. 2012, 33, 2609–2612. [Google Scholar] [CrossRef] [Green Version]
- Ramamurthy, C.H.; Padma, M.; Daisy, I.; Mareeswaran, R.; Suyavaran, A.; Kumar, M.S.; Premkumar, K.; Thirunavukkarasu, C. The Extra Cellular Synthesis of Gold and Silver Nanoparticles and Their Free Radical Scavenging and Antibacterial Properties. Colloids Surf. B Biointerfaces 2013, 102, 808–815. [Google Scholar] [CrossRef]
- Mohan Kumar, K.; Mandal, B.K.; Sinha, M.; Krishnakumar, V. Terminalia Chebula Mediated Green and Rapid Synthesis of Gold Nanoparticles. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 86, 490–494. [Google Scholar] [CrossRef]
- Islam, N.U.; Jalil, K.; Shahid, M.; Rauf, A.; Muhammad, N.; Khan, A.; Shah, M.R.; Khan, M.A. Green Synthesis and Biological Activities of Gold Nanoparticles Functionalized with Salix Alba. Arab. J. Chem. 2019, 12, 2914–2925. [Google Scholar] [CrossRef] [Green Version]
- Bindhu, M.R.; Vijaya Rekha, P.; Umamaheswari, T.; Umadevi, M. Antibacterial Activities of Hibiscus Cannabinus Stem-Assisted Silver and Gold Nanoparticles. Mater. Lett. 2014, 131, 194–197. [Google Scholar] [CrossRef]
- Kuppusamy, P.; Ichwan, S.J.A.; Parine, N.R.; Yusoff, M.M.; Maniam, G.P.; Govindan, N. Intracellular Biosynthesis of Au and Ag Nanoparticles Using Ethanolic Extract of Brassica Oleracea L. and Studies on Their Physicochemical and Biological Properties. J. Environ. Sci. 2015, 29, 151–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muthuvel, A.; Adavallan, K.; Balamurugan, K.; Krishnakumar, N. Biosynthesis of Gold Nanoparticles Using Solanum Nigrum Leaf Extract and Screening Their Free Radical Scavenging and Antibacterial Properties. Biomed. Prev. Nutr. 2014, 4, 325–332. [Google Scholar] [CrossRef]
- Tamuly, C.; Hazarika, M.; Debnath, R.; Saikia, R. Effect of CTAB in Biosynthesis of Au-Nanoparticles Using Gymnocladus Assamicus and Its Biological Evaluation. Mater. Lett. 2013, 113, 103–106. [Google Scholar] [CrossRef]
- Nagaraj, B.; Malakar, B.; Divya, T.K.; Krishnamurthy, N.B.; Liny, P.; Dinesh, R.; Iconaru, S.L.; Ciobanu, C.S. Synthesis of plant mediated gold nanoparticles using flower extracts of Carthamus tinctorius L. (Safflower) and evaluation of their biological activities. Dig. J. Nanomater. Biostruct. 2012, 7, 1289–1295. [Google Scholar]
- Dhayalan, M.; Denison, M.I.J.; Anitha Jegadeeshwari, L.; Krishnan, K.; Nagendra Gandhi, N. In Vitro Antioxidant, Antimicrobial, Cytotoxic Potential of Gold and Silver Nanoparticles Prepared Using Embelia Ribes. Nat. Prod. Res. 2017, 31, 465–468. [Google Scholar] [CrossRef]
- Geethalakshmi, R.; Sarada, D.V.L. Gold and Silver Nanoparticles from Trianthema Decandra: Synthesis, Characterization, and Antimicrobial Properties. Int. J. Nanomed. 2012, 7, 5375–5384. [Google Scholar] [CrossRef] [Green Version]
- Godipurge, S.S.; Yallappa, S.; Biradar, N.J.; Biradar, J.S.; Dhananjaya, B.L.; Hegde, G.; Jagadish, K. Green Strategy for the Facile Synthesis of Biocompatible Au, Ag and Au-Ag Alloy Nanoparticles Using Aerial Parts of R. Hypocrateriformis Extract and Their Biological Evaluation. Enzyme Microb. Technol. 2016, 95, 174–184. [Google Scholar] [CrossRef]
- Yallappa, S.; Manjanna, J.; Dhananjaya, B.L. Phytosynthesis of Stable Au, Ag and Au-Ag Alloy Nanoparticles Using J. Sambac Leaves Extract, and Their Enhanced Antimicrobial Activity in Presence of Organic Antimicrobials. Spectrochim. Acta-Part A Mol. Biomol. Spectrosc. 2015, 137, 236–243. [Google Scholar] [CrossRef]
- Balasubramani, G.; Ramkumar, R.; Raja, R.K.; Aiswarya, D.; Rajthilak, C.; Perumal, P. Albizia Amara Roxb. Mediated Gold Nanoparticles and Evaluation of Their Antioxidant, Antibacterial and Cytotoxic Properties. J. Clust. Sci. 2017, 28, 259–275. [Google Scholar] [CrossRef]
- Basavegowda, N.; Sobczak-Kupiec, A.; Malina, D.; Yathirajan, H.S.; Keerthi, V.R.; Chandrashekar, N.; Dinkar, S.; Liny, P. Plant Mediated Synthesis of Gold Nanoparticles Using Fruit Extracts of Ananas Comosus (L.) (Pineapple) and Evaluation of Biological Activities. Adv. Mater. Lett. 2013, 4, 332–337. [Google Scholar] [CrossRef] [Green Version]
- Asariha, M.; Chahardoli, A.; Karimi, N.; Gholamhosseinpour, M.; Khoshroo, A.; Nemati, H.; Shokoohinia, Y.; Fattahi, A. Green Synthesis and Structural Characterization of Gold Nanoparticles from Achillea Wilhelmsii Leaf Infusion and in Vitro. Bull. Mater. Sci. 2020, 43, 57. [Google Scholar] [CrossRef]
- Karthik, R.; Chen, S.; Elangovan, A.; Muthukrishnan, P.; Shanmugam, R.; Lou, B.; Karthik, R.; Chen, S.; Elangovan, A.; Muthukrishnan, P.; et al. Phytomediated Biogenic Synthesis of Gold Nanoparticles Using Cerasus Serrulata and Its Utility in Detecting Hydrazine, Microbial Activity and DFT Studies. J. Colloid Interface Sci. 2016, 468, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Ullah, U.; Rauf, A.; El, E.; Farhan, S.; Khan, A.; Khan, A.; Majid, S. Green Synthesis, in Vivo and in Vitro Pharmacological Studies of Tamarindus Indica Based Gold Nanoparticles. Bioprocess Biosyst. Eng. 2021, 44, 1185–1192. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Becerril, M.; Fernando, R.; Sanchez, F.; López, M.; Angulo, C. Green Synthesis of Gold Nanoparticles Using Turnera Diffusa Willd Enhanced Antimicrobial Properties and Immune Response in Longfin Yellowtail Leukocytes. Aquac. Res. 2021, 52, 3391–3402. [Google Scholar] [CrossRef]
- Khan, S.; Runguo, W.; Tahir, K.; Jichuan, Z.; Zhang, L. Catalytic Reduction of 4-Nitrophenol and Photo Inhibition of Pseudomonas Aeruginosa Using Gold Nanoparticles as Photocatalyst. J. Photochem. Photobiol. B Biol. 2017, 170, 181–187. [Google Scholar] [CrossRef]
- Fanoro, O.T.; Parani, S.; Maluleke, R.; Lebepe, T.C.; Varghese, J.R.; Mavumengwana, V.; Oluwafemi, O.S. Facile Green, Room-Temperature Synthesis of Gold Nanoparticles Using Combretum Erythrophyllum Leaf Extract: Antibacterial and Cell Viability Studies against Normal and Cancerous Cells. Antibiotics 2021, 10, 893. [Google Scholar] [CrossRef]
- Layeghi-Ghalehsoukhteh, S.; Jalaei, J.; Fazeli, M.; Memarian, P.; Shekarforoush, S.S. Evaluation of ‘Green’ Synthesis and Biological Activity of Gold Nanoparticles Using Tragopogon Dubius Leaf Extract as an Antibacterial Agent. IET Nanobiotechnol. 2018, 12, 1118–1124. [Google Scholar] [CrossRef]
- El-borady, O.M.; Ayat, M.S.; Shabrawy, M.A.; Millet, P. Green Synthesis of Gold Nanoparticles Using Parsley Leaves Extract and Their Applications as an Alternative Catalytic, Antioxidant, Anticancer, and Antibacterial Agents. Adv. Powder Technol. 2020, 31, 4390–4400. [Google Scholar] [CrossRef]
- Fadaka, A.; Aluko, O.; Awawu, S.; Theledi, K. Green Synthesis of Gold Nanoparticles Using Pimenta Dioica Leaves Aqueous Extract and Their Application as Photocatalyst, Antioxidant, and Antibacterial Agents. J. Multidiscip. Appl. Nat. Sci. 2021, 1, 78–88. [Google Scholar] [CrossRef]
- Acay, H. Utilization of Morchella Esculenta-Mediated Green Synthesis Golden Nanoparticles in Biomedicine Applications. Prep. Biochem. Biotechnol. 2020, 51, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Vinayagam, R.; Santhoshkumar, M.; Eun, K.; David, E.; Gu, S. Bioengineered Gold Nanoparticles Using Cynodon Dactylon Extract and Its Cytotoxicity and Antibacterial Activities. Bioprocess Biosyst. Eng. 2021, 44, 1253–1262. [Google Scholar] [CrossRef] [PubMed]
- Moustafa, N.E.; Alomari, A.A. Green Synthesis and Bactericidal Activities of Isotropic and Anisotropic Spherical Gold Nanoparticles Produced Using Peganum Harmala L Leaf and Seed Extracts. Biotechnol. Appl. Biochem. 2019, 66, 664–672. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Wei, Y.; Ullah, S.; Shah, S.I.; Nasir, F.; Shah, A.; Iqbal, Z.; Tahir, K.; Khan, U.A.; Yuan, Q. Synthesis of Phytochemicals-Stabilized Gold Nanoparticles and Their Biological Activities against Bacteria and Leishmania. Microb. Pathog. 2017, 110, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Naraginti, S.; Li, Y. Preliminary Investigation of Catalytic, Antioxidant, Anticancer and Bactericidal Activity of Green Synthesized Silver and Gold Nanoparticles Using Actinidia Deliciosa. J. Photochem. Photobiol. B Biol. 2017, 170, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Markus, J.; Wang, D.; Kim, Y.J.; Ahn, S.; Mathiyalagan, R.; Wang, C.; Yang, D.C. Biosynthesis, Characterization, and Bioactivities Evaluation of Silver and Gold Nanoparticles Mediated by the Roots of Chinese Herbal Angelica Pubescens Maxim. Nanoscale Res. Lett. 2017, 12, 46. [Google Scholar] [CrossRef] [Green Version]
- Islam, N.U.; Amin, R.; Shahid, M.; Amin, M. Gummy Gold and Silver Nanoparticles of Apricot (Prunus Armeniaca) Confer High Stability and Biological Activity. Arab. J. Chem. 2019, 12, 3977–3992. [Google Scholar] [CrossRef] [Green Version]
- Reddy, G.R.; Morais, A.B.; Gandhi, N.N. Green Synthesis, Characterization and in Vitro Antibacterial Studies of Gold Nanoparticles by Using Senna Siamea Plant Seed Aqueous Extract at Ambient Conditions. Asian J. Chem. 2013, 25, 8541–8544. [Google Scholar] [CrossRef]
- Zha, J.; Dong, C.; Wang, X.; Zhang, X.; Xiao, X.; Yang, X. Green Synthesis and Characterization Monodisperse Gold Nanoparticles Using Ginkgo Biloba Leaf Extract. Opt. Int. J. Light Electron Opt. 2017, 380, 3773–3777. [Google Scholar] [CrossRef]
- Francis, G.; Thombre, R.; Leksminarayan, P. Bioinspired Synthesis of Gold Nanoparticles Using Ficus Benghalensis (Indian Banyan) Leaf Extract. Chem. Sci. Trans. 2014, 3, 470–474. [Google Scholar] [CrossRef]
- Anbarasu, R.; Selvan, G.; Baskar, S.; Raja, V. Synthesis of Evolvulus Alsinoides Derived Gold Nanoparticles for Medical Applications. Int. J. Adv. Sci. Res. 2016, 2, 38–44. [Google Scholar] [CrossRef] [Green Version]
- Jayaseelan, C.; Ramkumar, R.; Abdul, A.; Perumal, P. Green Synthesis of Gold Nanoparticles Using Seed Aqueous Extract of Abelmoschus Esculentus and Its Antifungal Activity. Ind. Crop. Prod. 2013, 45, 423–429. [Google Scholar] [CrossRef]
- Villalobos-Noriega, J.M.A.; Rodríguez-León, E.; Rodríguez-beas, C.; Larios-Rodríguez, E.; Plascencia-Jatomea, M.; Martínez-Higuera, A.; Acuña-campa, H.; García-galaz, A.; Mora-monroy, R.; Alvarez-cirerol, F.J.; et al. Au @ Ag Core @ Shell Nanoparticles Synthesized with Rumex Hymenosepalus as Antimicrobial Agent. Nanoscale Res. Lett. 2021, 16, 118. [Google Scholar] [CrossRef] [PubMed]
- Qais, F.A.; Ahmad, I.; Altaf, M.; Alotaibi, S.H. Biofabrication of Gold Nanoparticles Using Capsicum Annuum Extract and Its Antiquorum Sensing and Antibiofilm Activity against Bacterial Pathogens. ACS Omega 2021, 25, 16670–16682. [Google Scholar] [CrossRef] [PubMed]
- Amina, M.; Al Musayeib, N.M.; Alarfaj, N.A.; El-Tohamy, M.F.; Al-Hamoud, G.A. Antibacterial and Immunomodulatory Potentials of Biosynthesized Ag, Au, Ag-Au Bimetallic Alloy Nanoparticles Using the Asparagus Racemosus Root Extract. Nanomaterials 2020, 10, 2453. [Google Scholar] [CrossRef]
- Annamalai, A.; Christina, V.L.P.; Sudha, D.; Kalpana, M.; Lakshmi, P.T.V. Green Synthesis, Characterization and Antimicrobial Activity of Au NPs Using Euphorbia Hirta L. Leaf Extract. Colloids Surf. B Biointerfaces 2013, 108, 60–65. [Google Scholar] [CrossRef]
- Ganesan, R.M.; Gurumallesh Prabu, H. Synthesis of Gold Nanoparticles Using Herbal Acorus Calamus Rhizome Extract and Coating on Cotton Fabric for Antibacterial and UV Blocking Applications. Arab. J. Chem. 2019, 12, 2166–2174. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.; Chan, C.; Huang, S.; Lin, Y. Green Biosynthesis of Gold Nanoparticles Using Chenopodium Formosanum Shell Extract and Analysis of the Particles ’ Antibacterial Properties. J. Sci. Food Agric. 2019, 99, 3693–3702. [Google Scholar] [CrossRef]
- Naidu, K.S.B.; Murugan, N. Sershen Physico-Chemical and Antibacterial Properties of Gold Nanoparticles Synthesized Using Avicennia Marina Seeds Extract. Trans. R. Soc. S. Afr. 2019, 75, 33–39. [Google Scholar] [CrossRef]
- Pagno, C.H.; Costa, T.M.H.; de Menezes, E.W.; Benvenutti, E.V.; Hertz, P.F.; Matte, C.R.; Tosati, J.V.; Monteiro, A.R.; Rios, A.O.; Flôres, S.H. Development of Active Biofilms of Quinoa (Chenopodium Quinoa W.) Starch Containing Gold Nanoparticles and Evaluation of Antimicrobial Activity. Food Chem. 2015, 173, 755–762. [Google Scholar] [CrossRef]
- Khan, S.; Bakht, J.; Syed, F. green synthesis of gold nanoparticles using Acer pentapomicum leaves extract its characterization, antibacterial, antifungal and antioxidant bioassay. Dig. J. Nanomater. Biostruct. 2018, 13, 579–589. [Google Scholar]
- Jiménez, Z.E.; Markus, J.; Kim, Y.; Wang, D.; Soshnikova, V.; Yang, D.C. Ginseng-Berry-Mediated Gold and Silver Nanoparticle Synthesis and Evaluation of Their in Vitro Antioxidant, Antimicrobial, and Cytotoxicity Effects on Human Dermal Fibroblast and Murine Melanoma Skin Cell Lines. Int. J. Nanomed. 2017, 12, 709–723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanaraj, S.; Jabastin, J.; Sathiskumar, S.; Preethi, K. Production and Characterization of Bio-AuNPs to Induce Synergistic Effect Against Multidrug Resistant Bacterial Biofilm Production and Characterization of Bio-AuNPs to Induced Synergistic Effect Against Multidrug Resistant Bacterial Biofilm. J. Clust. Sci. 2016, 28, 227–244. [Google Scholar] [CrossRef]
- Bawadekji, A.; Oueslati, M.; Ali, A.; Basha, J. Biosynthesis of Gold Nanoparticles Using Pleurotus Ostreatus (Jacq. Ex. Fr.) Kummer Extract and Their Antibacterial and Antifungal Activities. J. Appl. Environ. Biol. Sci. 2018, 8, 142–147. [Google Scholar]
- Vidhya, A.T.; Ahmed, J. Cajanus Cajan Mediated Gold Nanoparticle Synthesis, Characterization, Antimicrobial Efficacy and Its Antifouling Application on Metal Coupons. Asian J. Pharm. Technol. Innov. 2017, 5, 41–54. [Google Scholar]
- Annavaram, V.; Posa, V.R.; Lakshmi, D.V.; Sumalatha, J.; Somala, A.R. Terminalia Bellirica Fruit Extract Mediated Synthesis of Gold Nanoparticles (AuNP) and Studies on Antimicrobial and Antioxidant Activity. Synth. React. Inorg. Met. Nano-Metal Chem. 2016, 47, 681–687. [Google Scholar] [CrossRef]
- Gude, V.; Upadhyaya, K.; Prasad, M.N.; Rao, N. Green Synthesis of Gold and Silver Nanoparticles Using Achyranthes Aspera L. Leaf Extract. Adv. Sci. Eng. Med. 2012, 4, 1–6. [Google Scholar] [CrossRef]
- Velammal, S.P.; Devi, T.A.; Amaladhas, T.P. Antioxidant, Antimicrobial and Cytotoxic Activities of Silver and Gold Nanoparticles Synthesized Using Plumbago Zeylanica Bark. J. Nanostruct. Chem. 2016, 6, 247–260. [Google Scholar] [CrossRef] [Green Version]
- Sunkari, S.; Gangapuram, B.R.; Dadigala, R.; Bandi, R.; Alle, M.; Guttena, V. Microwave-Irradiated Green Synthesis of Gold Nanoparticles for Catalytic and Anti-Bacterial Activity. J. Anal. Sci. Technol. 2017, 8, 13. [Google Scholar] [CrossRef] [Green Version]
- Adewale, A.S.; Yao, B.; Folorunso, A. Green Synthesis, Characterization, and Antibacterial Investigation of Synthesized Gold Nanoparticles (AuNPs) from Garcinia Kola Pulp Extract. Plasmonics 2021, 16, 157–165. [Google Scholar] [CrossRef]
- Dudhane, A.A.; Waghmode, S.R.; Dama, L.B.; Vaibhav, P. Synthesis and Characterization of Gold Nanoparticles Using Plant Extract of Terminalia Arjuna with Antibacterial Activity. Int. J. Nanosci. Nanotechnol. 2019, 15, 75–82. [Google Scholar]
- Guliani, A.; Kumari, A.; Acharya, A. Green Synthesis of Gold Nanoparticles Using Aqueous Leaf Extract of Populus Alba: Characterization, Antibacterial and Dye Degradation Activity. Int. J. Environ. Sci. Technol. 2021, 18, 4007–4018. [Google Scholar] [CrossRef]
- Muniyappan, N.; Pandeeswaran, M.; Amalraj, A. Green Synthesis of Gold Nanoparticles Using Curcuma Pseudomontana Isolated Curcumin: Its Characterization, Antimicrobial, Antioxidant and Anti-Inflammatory Activities. Environ. Chem. Ecotoxicol. 2021, 3, 117–124. [Google Scholar] [CrossRef]
- Hatipoğlu, A. Rapid Green Synthesis of Gold Nanoparticles: Synthesis, Characterization, and Antimicrobial Activities. Prog. Nutr. 2021, 23, e2021242. [Google Scholar] [CrossRef]
- Al-radadi, N.S. Facile One-Step Green Synthesis of Gold Nanoparticles (AuNp) Using Licorice Root Extract: Antimicrobial and Anticancer Study against HepG2 Cell Line. Arab. J. Chem. 2021, 14, 102956. [Google Scholar] [CrossRef]
- Wang, M.; Hu, Y. Green Synthesized Gold Nanoparticles Using Viola Betonicifolia Leaves Extract: Characterization, Antimicrobial, Antioxidant, and Cytobiocompatible Activities. Int. J. Nanomed. 2021, 16, 7319–7337. [Google Scholar] [CrossRef] [PubMed]
- Dogan, S.S.; Kocabas, A. Green Synthesis of ZnO Nanoparticles with Veronica Multifida and Their Antibiofilm Activity. Hum. Exp. Toxicol. 2019, 39, 19–327. [Google Scholar]
- Abbasi, B.A.; Iqbal, J.; Ahmad, R.; Zia, L.; Kanwal, S.; Mahmood, T.; Wang, C.; Chen, J.-T. Bioactivities of Geranium Wallichianum Leaf Extracts Conjugated with Zinc Oxide Nanoparticles. Biomolecules 2020, 10, 38. [Google Scholar] [CrossRef] [Green Version]
- Al-jumaili, A.; Mulvey, P.; Kumar, A.; Prasad, K.; Bazaka, K.; Warner, J.; Jacob, M. V Eco-Friendly Nanocomposites Derived from Geranium Oil and Zinc Oxide in One Step Approach. Sci. Rep. 2019, 9, 5973. [Google Scholar] [CrossRef]
- Lalithamba, H.S.; Raghavendra, M.; Uma, K.; Yatish, K.V.; Mousumi, D.; Nagendra, G. Capsicum Annuum Fruit Extract: A Novel Reducing Agent for the Green Synthesis of ZnO Nanoparticles and Their Multifunctional Applications. Acta Cchimica Slov. 2018, 65, 354–364. [Google Scholar] [CrossRef]
- Abbas, F.; Maqbool, Q.; Nazar, M.; Jabeen, N.; Hussain, S.Z.; Mehmood, N.; Sheikh, M.S.; Hussain, T.; Iftikhar, S. Green Synthesised Zinc Oxide Nanostructures through Periploca Aphylla Extract Shows Tremendous Antibacterial Potential against Multidrug Resistant Pathogens. IET Nanobiotechnol. 2017, 11, 935–941. [Google Scholar] [CrossRef] [PubMed]
- Azizi, S.; Mohamad, R.; Shahri, M.M. Green Microwave-Assisted Combustion Synthesis of Zinc Oxide Nanoparticles with Citrullus Colocynthis (L.) Schrad: Characterization and Biomedical Applications. Molecules 2017, 22, 301. [Google Scholar] [CrossRef] [Green Version]
- Gupta, M.; Tomar, R.S.; Kaushik, S.; Mishra, R.K.; Sharma, D. Effective Antimicrobial Activity of Green ZnO Nano Particles of Catharanthus Roseus. Front. Microbiol. 2018, 9, 2030. [Google Scholar] [CrossRef] [PubMed]
- Bala, N.; Saha, S.; Chakraborty, M.; Maiti, M.; Das, S.; Basu, R.; Nandy, P. Green Synthesis of Zinc Oxide Nanoparticles Using Hibiscus Subdariffa Leaf Extract: Effect of Temperature on Synthesis, Anti-Bacterial Activity and Anti-Diabetic Activity. RSC Adv. 2014, 5, 4993–5003. [Google Scholar] [CrossRef]
- Elumalai, K.; Velmurugan, S.; Ravi, S.; Kathiravan, V.; Ashokkumar, S. Green Synthesis of Zinc Oxide Nanoparticles Using Moringa Oleifera Leaf Extract and Evaluation of Its Antimicrobial Activity. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 143, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, M.; Anbuvannan, M.; Viruthagiri, G. Green Synthesis of ZnO Nanoparticles Using Solanum Nigrum Leaf Extract and Their Antibacterial Activity. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 136, 864–870. [Google Scholar] [CrossRef]
- Hajiashrafi, S.; Motakef-Kazemi, N. Green Synthesis of Zinc Oxide Nanoparticles Using Parsley Extract. Nanomed. Res. J. 2018, 3, 44–50. [Google Scholar] [CrossRef]
- Kahraman, O.; Binzet, R.; Turunc, E.; Dogen, A.; Arslan, H. Synthesis, Characterization, Antimicrobial and Electrochemical Activities of Zinc Oxide Nanoparticles Obtained from Sarcopoterium spinosum (L.) Spach Leaf Extract. Mater. Res. Express 2018, 5, 115017. [Google Scholar] [CrossRef]
- Sathishkumar, G.; Rajkuberan, C.; Manikandan, K.; Prabukumar, S.; Danieljohn, J.; Sivaramakrishnan, S. Facile Biosynthesis of Antimicrobial Zinc Oxide (ZnO) Nanoflakes Using Leaf Extract of Couroupita Reference: To Appear in: Materials Letters. Mater. Lett. 2016, 188, 383–386. [Google Scholar] [CrossRef]
- Yuvakkumar, R.; Suresh, J.; Nathanael, A.J.; Sundrarajan, M.; Hong, S.I. Novel Green Synthetic Strategy to Prepare ZnO Nanocrystals Using Rambutan (Nephelium Lappaceum L.) Peel Extract and Its Antibacterial Applications. Mater. Sci. Eng. C 2014, 41, 17–27. [Google Scholar] [CrossRef]
- Jafarirad, S.; Mehrabi, M.; Divband, B.; Kosari-nasab, M. Biofabrication of Zinc Oxide Nanoparticles Using Fruit Extract of Rosa Canina and Their Toxic Potential against Bacteria: A Mechanistic Approach. Mater. Sci. Eng. C 2016, 59, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Saha, R.; Karthik, S.; Balu, K.S. Influence of the Various Synthesis Methods on the ZnO Nanoparticles Property Made Using the Bark Extract of Terminalia Arjuna In Fl Uence of the Various Synthesis Methods on the ZnO Nanoparticles Property Made Using the Bark Extract of Terminalia Arjuna. Mater. Chem. Phys. 2018, 209, 208–216. [Google Scholar] [CrossRef]
- Vijayakumar, S.; Krishnakumar, C.; Arulmozhi, P.; Mahadevan, S.; Parameswari, N. Microbial Pathogenesis Biosynthesis, Characterization and Antimicrobial Activities of Zinc Oxide Nanoparticles from Leaf Extract of Glycosmis Pentaphylla (Retz.) DC. Microb. Pthogenes. 2018, 116, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Sonia, S.; Kumari, H.L.J.; Ruchmani, K.; Sivakumar, M. Antimicrobial and Antioxidant Potentials of Biosynthesized Colloidal Zinc Oxide Nanoparticles for a Fortified Cold Cream Formulation: A Potent Nanocosmeceutical Application. Mater. Sci. Eng. C 2017, 79, 581–589. [Google Scholar] [CrossRef]
- Mohammadi, F.; Bayrami, A.; Habibi-yangjeh, A.; Rahim, S. A Comprehensive Study on Antidiabetic and Antibacterial Activities of ZnO Nanoparticles Biosynthesized Using Silybum Marianum L Seed Extract. Mater. Sci. Eng. C 2019, 97, 397–405. [Google Scholar] [CrossRef]
- Fuku, X.; Diallo, A.; Maaza, M. Nanoparticles through Green Process of Punica Granatum L. and Their Antibacterial Activities. Int. J. Electrochem. 2016, 2016, 4682967. [Google Scholar] [CrossRef]
- Akhter, S.H.; Mahmood, Z.; Ahmad, S.; Mohammad, F. Plant-Mediated Green Synthesis of Zinc Oxide Nanoparticles Using Swertia Chirayita Leaf Extract, Characterization and Its Antibacterial Efficacy Against Some Common Pathogenic Bacteria. Bionanoscience 2018, 8, 811–817. [Google Scholar] [CrossRef]
- Dobrucka, R.; Dlugaszewska, J.; Kaczmarek, M. Cytotoxic and Antimicrobial Effects of Biosynthesized ZnO Nanoparticles Using of Chelidonium Majus Extract. Biomed. Microdevices 2018, 20, 5. [Google Scholar] [CrossRef] [Green Version]
- Velmurugan, P.; Park, J.-H.; Lee, S.-M.; Yi, Y.-J.; Cho, M.; Jang, J.-S.; Myung, H.; Bang, K.; Oh, B. Eco-Friendly Approach towards Green Synthesis of Zinc Oxide Nanocrystals and Its Potential Applications. Artif. Cells Nanomed. Biotechnol. 2016, 44, 1537–1543. [Google Scholar] [CrossRef]
- Elavarasan, N.; Kokila, K.; Inbasekar, G.; Sujatha, V. Evaluation of Photocatalytic Activity, Antibacterial and Cytotoxic Effects of Green Synthesized ZnO Nanoparticles by Sechium Edule Leaf Extract and Cytotoxic Effects of Green Synthesized ZnO. Res. Chem. Intermed. 2017, 43, 3361–3376. [Google Scholar] [CrossRef]
- Datta, A.; Patra, C.; Bharadwaj, H.; Kaur, S.; Dimri, N.; Khajuria, R. Green Synthesis of Zinc Oxide Nanoparticles Using Parthenium Hysterophorus Leaf Extract and Evaluation of Their Antibacterial Properties. J. Biotechnol. Biomater. 2017, 7, 3–7. [Google Scholar] [CrossRef]
- Lingaraju, K.; Naika, H.R.; Manjunath, K.; Basavaraj, R.B.; Nagabhushana, H.; Nagaraju, G.; Suresh, D. Biogenic Synthesis of Zinc Oxide Nanoparticles Using Ruta Graveolens (L.) and Their Antibacterial and Antioxidant Activities. Appl. Nanosci. 2016, 6, 703–710. [Google Scholar] [CrossRef] [Green Version]
- Sharmila, G.; Muthukumaran, C.; Santhiya, K.S.S.; Pradeep, R.S.; Kumar, N.M.; Suriyanarayanan, N.; Hirumarimurugan, M. Biosynthesis, Characterization, and Antibacterial Activity of Zinc Oxide Nanoparticles Derived from Bauhinia Tomentosa Leaf Extract. J. Nanostruct. Chem. 2018, 8, 293–299. [Google Scholar] [CrossRef] [Green Version]
- Suresh, D.; Nethravathi, P.C.; Udayabhanu; Rajanaika, H.; Nagabhushana, H.; Sharma, S.C. Green Synthesis of Multifunctional Zinc Oxide (ZnO) Nanoparticles Using Cassia Fistula Plant Extract and Their Photodegradative, Antioxidant and Antibacterial Activities. Mater. Sci. Semicond. Process. 2015, 31, 446–454. [Google Scholar] [CrossRef]
- Chandra, H. Phyto-Mediated Synthesis of Zinc Oxide Nanoparticles of Berberis Aristata: Characterization, Antioxidant Activity and Antibacterial Activity with Special Reference to Urinary Tract Pathogens. Mater. Sci. Eng. C 2019, 102, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Santhoshkumar, J.; Kumar, S.V.; Rajeshkumar, S. Resource-Efficient Technologies Synthesis of Zinc Oxide Nanoparticles Using Plant Leaf Extract against Urinary Tract Infection Pathogen. Resour. Technol. 2017, 102, 212–220. [Google Scholar] [CrossRef]
- Elumalai, K.; Velmurugan, S.; Ravi, S.; Kathiravan, V.; Adaikala Raj, G. Bio-Approach: Plant Mediated Synthesis of ZnO Nanoparticles and Their Catalytic Reduction of Methylene Blue and Antimicrobial Activity. Adv. Powder Technol. 2015, 26, 1639–1651. [Google Scholar] [CrossRef]
- Ansari, M.A.; Murali, M.; Prasad, D.; Alzohairy, M.A.; Almatroudi, A.; Alomary, M.N.; Udayashankar, A.C.; Singh, S.B.; Mousa, S.; Asiri, M.; et al. Cinnamomum Verum Bark Extract Mediated Green Synthesis of ZnO Nanoparticles and Their Antibacterial Potentiality. Biomolecules 2020, 10, 336. [Google Scholar] [CrossRef] [Green Version]
- Vaishnav, J.; Subha, V.; Kirubanandan, S.; Arulmozhi, M.; Renganathan, S. Green synthesis of zinc oxide nanoparticles by Celosia. J. Optoelectron. Biomed. Mater. 2017, 9, 59–71. [Google Scholar]
- Iqbal, J.; Ahsan, B.; Mahmood, T.; Kanwal, S.; Ahmad, R.; Ashraf, M. Plant-Extract Mediated Green Approach for the Synthesis of ZnONPs: Characterization and Evaluation of Cytotoxic, Antimicrobial and Antioxidant Potentials. J. Mol. Struct. 2019, 1189, 315–327. [Google Scholar] [CrossRef]
- Blessy, B.J.; Gomez, L.A. Biosynthesis and Characterization of ZnO Nanoparticle Using Cassia Auriculata Flower Extract and Their Antibacterial, Photocatalytic Activity. Int. J. Innov. Res. Sci. Eng. Technol. 2016, 5, 6437–6445. [Google Scholar] [CrossRef]
- Anbukkarasi, V.; Srinivasan, R.; Elangovan, N. Antimicrobial Activity of Green Synthesized Zinc Oxide Nanoparticles from Emblica Officinalis. Int. J. Pharm. Sci. Rev. Res. 2015, 33, 110–115. [Google Scholar]
- Suriyaprabha, R.; Balu, K.S.; Karthik, S.; Prabhu, M.; Rajendran, V.; Aicher, W.K.; Maaza, M. A Sensitive Re Fi Ning of in Vitro and in Vivo Toxicological Behavior of Green Synthesized ZnO Nanoparticles from the Shells of Jatropha Curcas for Multifunctional Biomaterials Development. Ecotoxicol. Environ. Saf. 2019, 184, 109621. [Google Scholar] [CrossRef] [PubMed]
- Prachi, A.M.; Patel, R.; Singh, N.; Negi, D.S.; Rawat, S. Green synthesis of zinc oxide nanoparticles using Rubia cordifolia root extract against different bacterial pathogens. Indo Am. J. Pharm. Res. 2017, 7, 759–765. [Google Scholar]
- Mahendra, C.; Murali, M.; Manasa, G.; Pooja, P.; Abhilash, M.R.; Lakshmeesha, T.R.; Satish, A.; Amruthesh, K.N.; Sudarshana, M.S. Antibacterial and Antimitotic Potential of Bio-Fabricated Zinc Oxide Nanoparticles of Cochlospermum Religiosum (L.). Microb. Pathog. 2017, 110, 620–629. [Google Scholar] [CrossRef]
- Khajuria, A.K.; Bisht, N.S.; Kumar, G. Synthesis of Zinc Oxide Nanoparticles Using Leaf Extract of Viola Canescens Wall. Ex, Roxb. and Their Antimicrobial Activity. J. Pharmagnosy Phytochem. 2017, 6, 1301–1304. [Google Scholar]
- Ananthalakshmi, R.; Rajarathinam, S.R.X.; Lavanya, A.; Sadiq, A.M.; Gomathi, C. Synthesis and Characterization of Zinc Oxide Nanoparticles Using Aqueous Extract of Banana Peel (Musa Acuminata L.). Int. J. Basic Apllied Res. 2018, 7, 98–107. [Google Scholar]
- Steffy, K.; Shanthi, G.; Maroky, A.S.; Selvakumar, S. Enhanced Antibacterial Effects of Green Synthesized ZnO NPs Using Aristolochia Indica against Multi-Drug Resistant Bacterial Pathogens from Diabetic Foot Ulcer. J. Infect. Public Health 2017, 11, 463–471. [Google Scholar] [CrossRef]
- Asha, P.; Francis, J. One pot green synthesis of Zno nanoparticles using Azolla extract and assessing its biological activities. Int. J. Curr. Res. 2015, 7, 22520–22527. [Google Scholar]
- Prabhu, S.; Vaideki, K.; Anitha, S.; Rajendran, R. Synthesis of ZnO Nanoparticles Using Melia Dubia Leaf Extract and Its Characterization. IET Nanobiotechnol. 2017, 11, 62–65. [Google Scholar] [CrossRef]
- Shakibaie, M.; Alipour-Esmaeili-Anari, F.; Adeli-Sardou, M.; Ameri, A.; Doostmohammadi, M.; Forootanfar, H.; Ameri, A. Antibacterial and Anti-Biofilm Effects of Microwave-Assisted Biologically Synthesized Zinc Nanoparticles. Nanomed. J. 2019, 6, 223–231. [Google Scholar] [CrossRef]
- Vahidi, A.; Vaghari, H.; Najian, Y.; Najian, M.J.; Jafarizadeh-Malmiri, H. Evaluation of Three Different Green Fabrication Methods for the Synthesis of Crystalline ZnO Nanoparticles Using Pelargonium Zonale Leaf Extract. Green Process Synth. 2019, 8, 302–308. [Google Scholar] [CrossRef]
- Awwad, A.M.; Amer, M.W.; Salem, N.M.; Abdeen, A.O. Green Synthesis of Zinc Oxide Nanoparticles (ZnO-NPs) Using Ailanthus Altissima Fruit Extracts and Antibacterial Activity. Int. Sci. Organ. 2020, 6, 151–159. [Google Scholar]
- Nadeem, A.; Naz, S.; Sarfraz, J.; Mannan, A.; Zia, M. Synthesis, Characterization and Biological Activities of Monometallic and Bimetallic Nanoparticles Using Mirabilis Jalapa Leaf Extract. Biotechnol. Rep. 2019, 24, e00338. [Google Scholar] [CrossRef]
- Umar, H.; Kavaz, D.; Rizaner, N. Biosynthesis of Zinc Oxide Nanoparticles Using Albizia Lebbeck Stem Bark, and Evaluation of Its Antimicrobial, Antioxidant, and Cytotoxic Activities on Human Breast Cancer Cell Lines. Int. J. Nanomed. 2019, 14, 87–100. [Google Scholar] [CrossRef] [Green Version]
- Yasotha, P.; Kalaiselvi, V.; Vidhya, N.; Ramya, V. Green Synthesis and Characterization of Zinc Oxide Nanoparticles Using Ocimum Tenuiflorum. Int. J. Adv. Sci. Eng. 2020, 2020, 1584–1588. [Google Scholar] [CrossRef]
- Suresh, J.; Pradheesh, G.; Alexramani, V.; Sundrarajan, M.; Hong, S.I. Green Synthesis and Characterization of Zinc Oxide Nanoparticle Using Insulin Plant (Costus Pictus D. Don) and Investigation of Its Antimicrobial as Well as Anticancer Activities. Adv. Nat. Sci. Nanosci. Nanotechnol. 2018, 9, 015008. [Google Scholar] [CrossRef]
- Prakash, M.J.; Kalyanasundharam, S. Biosynthesis, Characterisation, Free Radical Scavenging Activity and Anti-Bacterial Effect of Plant- Mediated Zinc Oxide Nanoparticles Using Pithecellobium dulce and Lagenaria siceraria Leaf Extract. World Sci. News 2015, 18, 100–117. [Google Scholar]
- Shanavas, S.; Duraimurugan, J.; Kumar, G.S.; Ramesh, R.; Acevedo, R.; Anbarasan, P.M.; Maadeswaran, P. Ecofriendly Green Synthesis of ZnO Nanostructures Using Artabotrys Hexapetalu and Bambusa Vulgaris Plant Extract and Investigation on Their Photocatalytic and Antibacterial Activity. Mater. Res. Express 2019, 6, 105098. [Google Scholar] [CrossRef]
- Jamdagni, P.; Khatri, P.; Rana, J.S. Green Synthesis of Zinc Oxide Nanoparticles Using Flower Extract of Nyctanthes Arbor-Tristis and Their Antifungal Activity. J. King Saud Univ. Sci. 2018, 30, 168–175. [Google Scholar] [CrossRef] [Green Version]
- Anupama, C.; Kaphle, A.; Udayabhanu; Nagaraju, G. Aegle Marmelos Assisted Facile Combustion Synthesis of Multifunctional ZnO Nanoparticles: Study of Their Photoluminescence, Photo Catalytic and Antimicrobial Activities. J. Mater. Sci. Mater. Electron. 2018, 29, 4238–4249. [Google Scholar] [CrossRef]
- Padalia, H.; Chanda, S. Characterization, Antifungal and Cytotoxic Evaluation of Green Synthesized Zinc Oxide Nanoparticles Using Ziziphus Nummularia Leaf Extract. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1751–1761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Narendhran, S.; Sivaraj, R. Biogenic ZnO Nanoparticles Synthesized Using L. Aculeata Leaf Extract And Their Antifungal Activity against Plant Fungal Pathogens. Bull. Mater. Sci. 2016, 39, 1–5. [Google Scholar] [CrossRef]
- Ramachandrappa, L.T.; Kalagatur, N.K.; Mudili, V.; Mohan, C.D.; Shobith, R.; Prasad, B.D.; Ashwini, B.S.; Hashem, A.; Alqarawi, A.A.; Malik, J.A.; et al. Biofabrication of Zinc Oxide Nanoparticles With Syzygium Aromaticum Flower Buds Extract and Finding Its Novel Application in Controlling the Growth and Mycotoxins of Fusarium Graminearum. Front. Microbiol. 2019, 10, 1244. [Google Scholar] [CrossRef] [Green Version]
- Shobha, N.; Nanda, N.; Giresha, A.S.; Manjappa, P.; Dharmappa, K.K.; Nagabhushana, B.M. Synthesis and Characterization of Zinc Oxide Nanoparticles Utilizing Seed Source of Ricinus Communis and Study of Its Antioxidant, Antifungal and Anticancer Activity. Mater. Sci. Eng. C 2018, 97, 842–850. [Google Scholar] [CrossRef] [PubMed]
- Sirumbayee, E.; Anusuya, S. Preparation of Zinc Oxide Nanoparticles Using Azima Tetracantha Lam. Leaf Extract and Its Potential for the Removal of Contaminants from Water. J. Nanosci. Technol. 2020, 6, 862–865. [Google Scholar]
- Madhumitha, G.; Fowsiya, J.; Gupta, N.; Kumar, A.; Singh, M. Green Synthesis, Characterization and Antifungal and Photocatalytic Activity of Pithecellobium Dulce Peel–Mediated ZnO Nanoparticles. J. Phys. Chem. Solids 2019, 127, 43–51. [Google Scholar] [CrossRef]
- Nandhini, M.; Rajini, S.B.; Udayashankar, A.C.; Niranjana, S.R.; Lund, O.S.; Shetty, H.S.; Prakash, H.S. Biofabricated Zinc Oxide Nanoparticles as an Eco-Friendly Alternative for Growth Promotion and Management of Downy Mildew of Pearl Millet. Crop Prot. 2019, 121, 103–112. [Google Scholar] [CrossRef]
- Sharma, D.; Sabela, I.M.; Kanchi, S.; Mdluli, S.P.; Singh, G.; Stenstr, T.A.; Bisetty, K. Biosynthesis of ZnO Nanoparticles Using Jacaranda Mimosifolia Flowers Extract: Synergistic Antibacterial Activity and Molecular Simulated Facet Specific Adsorption Studies. Photochem. Photobiol. B Biol. 2016, 162, 199–207. [Google Scholar] [CrossRef] [Green Version]
- Velmurugan, P.; Shim, J.; Oh, B. Prunus x Yedoensis Tree Gum Mediated Synthesis of Platinum Nanoparticles with Antifungal Activity against Phytopathogens. Mater. Lett. 2016, 174, 61–65. [Google Scholar] [CrossRef]
- Kumar, P.V.; Jelastin, S.M.; Prakash, K.S. Journal of Environmental Chemical Engineering Green Synthesis Derived Pt-Nanoparticles Using Xanthium Strumarium Leaf Extract and Their Biological Studies. J. Environ. Chem. Eng. 2019, 7, 103146. [Google Scholar] [CrossRef]
- Jeyapaul, U.; Kala, M.J.; Bosco, A.J.; Piruthiviraj, P.; Easuraja, M. An Eco-Friendly Approach for Synthesis of Platinum Nanoparticles Using Leaf Extracts of Jatropa Gossypifolia and Jatropa Glandulifera and Their Antibacterial Activity. Orient. J. Chem. 2018, 34, 779–790. [Google Scholar] [CrossRef] [Green Version]
- Ali, N.H.; Mohammed, A.M. Biosynthesis and Characterization of Platinum Nanoparticles Using Iraqi zahidi Dates and Evaluation of Their Biological Applications. Biotechnol. Rep. 2021, 30, e00635. [Google Scholar] [CrossRef] [PubMed]
- Fanoro, O.T.; Parani, S.; Maluleke, R.; Lebepe, T.C.; Varghese, R.J.; Mgedle, N.; Mavumengwana, V.; Oluwafemi, O.S. Biosynthesis of Smaller-Sized Platinum Nanoparticles Using the Leaf Extract of Combretum Erythrophyllum and Its Antibacterial Activities. Antibiotics 2021, 10, 1275. [Google Scholar] [CrossRef] [PubMed]
- Selvi, A.M.; Palanisamy, S.; Jeyanthi, S.; Vinosha, M.; Mohandoss, S.; Tabarsa, M.; You, S.; Kannapiran, E.; Prabhu, N.M. Synthesis of Tragia Involucrata Mediated Platinum Nanoparticles for Comprehensive Therapeutic Applications: Antioxidant, Antibacterial and Mitochondria-Associated Apoptosis in HeLa Cells. Process Biochem. 2020, 20, 21–33. [Google Scholar] [CrossRef]
- Eltaweil, A.S.; Fawzy, M.; Hosny, M.; El-monaem, E.M.A.; Tamer, T.M.; Omer, A.M. Potential Antimicrobial, Antioxidant, and Catalytic Applications Green Synthesis of Platinum Nanoparticles Using Atriplex Halimus Leaves for Potential Antimicrobial, Antioxidant, and Catalytic Applications. Arab. J. Chem. 2021, 15, 103517. [Google Scholar] [CrossRef]
- Ghadiri, A.M.; Fatahi, Y.; Dinarvand, R.; Webster, T.J. High-Gravity-Assisted Green Synthesis of Palladium Nanoparticles: The Flowering of Nanomedicine. Nanomed. Nanotechnol. Biol. Med. 2020, 30, 102297. [Google Scholar] [CrossRef]
- Attar, A.; Yapaoz, M.A. Biosynthesis of Palladium Nanoparticles Using Diospyros Kaki Leaf Extract and Determination of Antibacterial Efficacy. Prep. Biochem. Biotechnol. 2018, 48, 629–634. [Google Scholar] [CrossRef]
- Sharmila, G.; Haries, S.; Farzana Fathima, M.; Geetha, S.; Manoj Kumar, N.; Muthukumaran, C. Enhanced Catalytic and Antibacterial Activities of Phytosynthesized Palladium Nanoparticles Using Santalum Album Leaf Extract. Powder Technol. 2017, 320, 22–26. [Google Scholar] [CrossRef]
- Vijilani, C.; Bindhu, M.R.; Fincy, F.C.; Mohamed, S.; Alsahi, S.; Sabitha, S.; Saravanakumar, K.; Sandhanasamy, D.; Umadevi, M.; Aljaafreh, M.J.; et al. Antimicrobial and Catalytic Activities of Biosynthesized Gold, Silver and Palladium Nanoparticles from Solanum Nigurum Leaves. J. Photochem. Photobiol. B Biol. 2019, 202, 111713. [Google Scholar] [CrossRef]
- Tahir, K.; Nazir, S.; Li, B.; Ahmad, A.; Nasir, T.; Khan, A.U.; Asim, S.; Shah, A.; Ul, Z.; Khan, H.; et al. Sapium sebiferum leaf extract mediated synthesis of palladium nanoparticles and in vitro investigation of their bacterial and photocatalytic activities. J. Photochem. Photobiol. B Biol. 2016, 164, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Prakashkumar, N.; Vignesh, M.; Brindhadevi, K.; Phuong, N.; Pugazhendhi, A.; Suganthy, N. Progress in Organic Coatings Enhanced Antimicrobial, Antibiofilm and Anticancer Activities of Biocompatible Neem Gum Coated Palladium Nanoparticles. Prog. Org. Coat. 2021, 151, 106098. [Google Scholar] [CrossRef]
- Kora, A.J.; Rastogi, L. Green Synthesis of Palladium Nanoparticles Using Gum Ghatti (Anogeissus latifolia) and Its Application as an Antioxidant and Catalyst. Arab. J. Chem. 2018, 11, 1097–1106. [Google Scholar] [CrossRef] [Green Version]
- Gnanasekar, S.; Murugaraj, J.; Dhivyabharathi, B.; Vilwanathan, R.; Sivaperumal, S. Antibacterial and Cytotoxicity Effects of Biogenic Palladium Nanoparticles Synthesized Using Fruit Extract of Couroupita Guianensis. J. Econ. Financ. Adm. Sci. 2017, 16, 59–65. [Google Scholar] [CrossRef]
- Bhakyaraj, K.; Kumaraguru, S.; Gopinath, K.; Sabitha, V.; Kaleeswarran, P.R.; Karthika, V.; Sudha, A.; Muthukumaran, U.; Jayakumar, K.; Mohan, S.; et al. Eco-friendly synthesis of palladium nanoparticles using Melia azedarach leaf extract and their evaluation for antimicrobial and larvicidal activities. J. Clust. Sci. 2017, 28, 463–476. [Google Scholar] [CrossRef]
- Sonbol, H.; Ameen, F.; Alyahya, S.; Almansob, A.; Alwakeel, S. Padina Boryana Mediated Green Synthesis of Crystalline Palladium Nanoparticles as Potential Nanodrug against Multidrug Resistant Bacteria and Cancer Cells. Sci. Rep. 2021, 11, 5444. [Google Scholar] [CrossRef]
- Kalaiselvi, A.; Mohana, S.; Madhumitha, G.; Ramalingam, C. Synthesis and Characterization of Palladium Nanoparticles Using Catharanthus Roseus Leaf Extract and Its Application in the Photo-Catalytic Degradation. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 135, 116–119. [Google Scholar] [CrossRef]
- Babu, R.; Chidambaram, K. Watermelon Rind-Mediated Green Synthesis of Noble Palladium Nanoparticles: Catalytic Application Watermelon Rind-Mediated Green Synthesis of Noble Palladium Nanoparticles: Catalytic Application. Appl. Nanosci. 2015, 5, 223–228. [Google Scholar] [CrossRef] [Green Version]
- Veisi, H.; Ghorbani-vaghei, R.; Hemmati, S.; Haji, M. Green and Effective Route for the Synthesis of Monodispersed Palladium Nanoparticles Using Herbal Tea Extract (Stachys Lavandulifolia) as Reductant, Stabilizer and Capping Agent, and Their Application as Homogeneous and Reusable Catalyst in Suzuki Cou. Appl. Organomet. Chem. 2014, 29, 26–32. [Google Scholar] [CrossRef]
- Manoj, L.; Vishwakarma, V. Phytochemical Synthesis of Palladium-Gold Nanoparticles Using in-Vitro Grown Hypericin Rich Shoot Culture of Hypericum Hookerianum. Int. J. ChemTech Res. 2016, 9, 34–38. [Google Scholar]
- Zeleke, T.D. Copper Nanoparticles Synthesized Using Echinops Sp. Root Extract for Antimicrobial Applications. Int. J. Nano Dimens 2021, 12, 145–155. [Google Scholar]
- Amin, F.; Khattak, B.; Alotaibi, A.; Qasim, M.; Ahmad, I.; Ullah, R.; Bourhia, M.; Gul, A.; Zahoor, S.; Ahmad, R. Green Synthesis of Copper Oxide Nanoparticles Using Aerva Javanica Leaf Extract and Their Characterization and Investigation of In Vitro Antimicrobial Potential and Cytotoxic Activities. BMC Complement. Altern. Med. 2021, 2021, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Murthy, H.C.A.; Desalegn, T.; Kassa, M.; Abebe, B.; Assefa, T. Synthesis of Green Copper Nanoparticles Using Medicinal Plant Hagenia Abyssinica (Brace) JF. Gmel. Leaf Extract: Antimicrobial Properties. J. Nanomater. 2020, 2020, 3924081. [Google Scholar] [CrossRef]
- Rajesh, K.M.; Ajitha, B.; Reddy, Y.A.K.; Suneetha, Y.; Reddy, P.S. Assisted Green Synthesis of Copper Nanoparticles Using Syzygium Aromaticum Bud Extract: Physical, Optical and Antimicrobial Properties. Opt. Int. J. Light Electron Opt. 2018, 154, 593–600. [Google Scholar] [CrossRef]
- Kothai, S.; Umamaheswari, R. Green Synthesis, Characterization of Copper Nanoparticles Derived from Ocimum Sanctum Leaf Extract and Their Antimicrobial Activities. J. Chem. Chem. Sci. 2018, 8, 984–992. [Google Scholar] [CrossRef]
- Rai, A.; Lall, R. Antimicrobial, Antioxidant and Cytotoxic Activity of Green Synthesized Copper Nanoparticle of Parthenium hysterophorus L. Int. J. Multidiscip. Res. Anal. 2021, 4, 101–116. [Google Scholar] [CrossRef]
- Abboud, Y.; Saffaj, T.; Chagraoui, A.; El Bouari, A.; Brouzi, K.; Tanane, O.; Ihssane, B. Biosynthesis, Characterization and Antimicrobial Activity of Copper Oxide Biosynthesis, Characterization and Antimicrobial Activity of Copper Oxide Nanoparticles (CONPs) Produced Using Brown Alga Extract (Bifurcaria Bifurcata). Appl. Nanosci. 2014, 4, 571–576. [Google Scholar] [CrossRef] [Green Version]
- Subhankari, I.; Nayak, P. Antimicrobial Activity of Copper Nanoparticles Synthesised by Ginger (Zingiber Officinale) Extract. World J. Nano Sci. Technol. 2013, 2, 10–13. [Google Scholar] [CrossRef]
- Gopinath, M.; Subbaiya, R.; Selvam, M.M.; Suresh, D. Synthesis of Copper Nanoparticles from Nerium Oleander Leaf Aqueous Extract and Its Antibacterial Activity. Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 814–818. [Google Scholar]
- Hassanien, R.; Husein, D.Z.; Al-Hakkani, M.F. Biosynthesis of Copper Nanoparticles Using Aqueous Tilia Extract: Antimicrobial and Anticancer Activities. Heliyon 2018, 4, e01077. [Google Scholar] [CrossRef] [Green Version]
- Joseph, A.T.; Prakash, P.; Narvi, S.S. Phytofabrication and Characterization of Copper Nanoparticles Using Allium Sativum and Its Antibacterial Activity. Int. J. Sci. Eng. Technol. 2016, 4, 463–472. [Google Scholar]
- Vinod Vellora, T.; Miroslav Černík, P. Green Synthesis of Copper Oxide Nanoparticles Using Gum Karaya as a Biotemplate and Their Antibacterial Application. Int. J. Nanomed. 2013, 8, 889–898. [Google Scholar]
- Ali, K.; Ahmed, B.; Ansari, S.M.; Saquib, Q.; Al-khedhairy, A.A. Comparative in Situ ROS Mediated Killing of Bacteria with Bulk Analogue, Eucalyptus Leaf Extract (ELE)-Capped and Bare Surface Copper Oxide Nanoparticles. Mater. Sci. Eng. C 2019, 100, 747–758. [Google Scholar] [CrossRef] [PubMed]
- Sutradhar, P.; Saha, M.; Maiti, D. Microwave Synthesis of Copper Oxide Nanoparticles Using Tea Leaf and Coffee Powder Extracts and Its Antibacterial Activity. J. Nanostruct. Chem. 2014, 86, 4–9. [Google Scholar] [CrossRef] [Green Version]
- Sivaraj, R.; Rahman, P.K.S.M.; Rajiv, P.; Narendhran, S.; Venckatesh, R. Biosynthesis and Characterization of Acalypha Indica Mediated Copper Oxide Nanoparticles and Evaluation of Its Antimicrobial and Anticancer Activity. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 129, 255–258. [Google Scholar] [CrossRef]
- Das, P.E.; Yousef, I.A.A.; Majdalawieh, A.F.; Narasimhan, S. Green Synthesis of Encapsulated Copper Nanoparticles Using a Hydroalcoholic Extract of Moringa Oleifera Leaves and Assessment of Their Antioxidant and Antimicrobial Activities. Molecules 2020, 25, 555. [Google Scholar] [CrossRef] [Green Version]
- Angrasan, J.K.V.M.; Subbaiya, R. Original Research Article Biosynthesis of Copper Nanoparticles by Vitis Vinifera Leaf Aqueous Extract and Its Antibacterial Activity. Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 768–774. [Google Scholar]
- Arya, A.; Gupta, K.; Chundawat, T.S.; Vaya, D. Biogenic Synthesis of Copper and Silver Nanoparticles Using Green Alga Botryococcus Braunii and Its Antimicrobial Activity. Bioinorg. Chem. Appl. 2018, 2018, 7879403. [Google Scholar] [CrossRef] [Green Version]
- Kumar Vijay, P.P.; Ummey, S.; Pratap, K.; Kalyani, R.L.; Pammi, S.V. Green Synthesis of Copper Oxide Nanoparticles Using Aloe Vera Leaf Extract and Its Antibacterial Activity Against Fish Bacterial Pathogens. BioNanoScience 2015, 5, 63–122. [Google Scholar] [CrossRef]
- Sharma, G.; Kr, D.G.; Jasuja, N.D.; C, S.J. Pterocarpus Marsupium Derived Phyto-Synthesis of Copper Oxide Nanoparticles and Their Antimicrobial Activities. J. Microb. Biochem. Technol. 2016, 7, 140. [Google Scholar] [CrossRef] [Green Version]
- Das, P.; Ghosh, S.; Ghosh, R.; Dam, S.; Baskey, M. Madhuca Longifolia Plant Mediated Green Synthesis of Cupric Oxide Nanoparticles: A Promising Environmentally Sustainable Material for Waste Water Treatment and Efficient Antibacterial Agent. J. Photochem. Photobiol. B Biol. 2018, 189, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Jayandran, M.; Haneefa, M.M.; Balasubramanian, V. Green Synthesis of Copper Nanoparticles Using Natural Reducer and Stabilizer and an Evaluation of Antimicrobial Activity. J. Chem. Pharm. Res. 2015, 7, 251–259. [Google Scholar]
- Gültekİn, D.D.; Güngör, A.A.; Önem, H.; Babagİl, A. Synthesis of Copper Nanoparticles Using a Different Method: Determination of Their Antioxidant and Antimicrobial Activity. J. Turkish. Chem. Soc. 2016, 3, 623–636. [Google Scholar] [CrossRef] [Green Version]
- Saranyaadevi, K.; Subha, V.; Ravindran, R.S.E.; Renganathan, S. Synthesis and Characterization of Copper Nanoparticle Using Capparis Zeylanica Leaf Extract. Int. J. ChemTech Res. 2014, 6, 4533–4541. [Google Scholar]
- Subbaiya, R.; Selvam, M.M. Green Synthesis of Copper Nanoparticles from Hibicus Rosasinensis and Their Antimicrobial, Antioxidant Activities. Res. J. Pharm. Biol. Chem. Sci. 2015, 6, 1183–1190. [Google Scholar]
- Mane, V.A.; Patil, N.P.; Gaikwad, S.S. extracellular synthesis of copper nanoparticles using different plant extract. Int. J. Appl. Nat. Sci. 2019, 5, 33–38. [Google Scholar]
- Acharyulu, N.P.S.; Dubey, R.S.; Swaminadham, V.; Kalyani, R.L.; Kollu, P.; Pammi, S.V.N. Green Synthesis of CuO Nanoparticles Using Phyllanthus Amarus Leaf Extract and Their Antibacterial Activity Against Multidrug Resistance Bacteria. Int. J. Eng. Res. Technol. 2014, 3, 639–641. [Google Scholar]
- Gondwal, M.; Pant, G.J. Synthesis and Catalytic and Biological Activities of Silver and Copper Nanoparticles Using Cassia Occidentalis. Int. J. Biomater. 2018, 2018, 6735426. [Google Scholar] [CrossRef] [Green Version]
- Hassan, D.; Fouda, A.; Radwan, A.A.; Salem, S.S.; Barghoth, M.G.; Awad, M.A.; Abdo, A.M.; El, M.S. Endophytic actinomycetes Streptomyces spp mediated biosynthesis of copper oxide nanoparticles as a promising tool for biotechnological applications. J. Biol. Inorg. Chem. 2019, 24, 377–393. [Google Scholar] [CrossRef]
- Khan, A.S.; Noreen, F.; Kanwal, S.; Ibqal, A.; Hussain, G. Green Synthesis of ZnO and Cu-Doped ZnO Nanoparticles from Leaf Extracts of Abutilon Indicum, Clerodendrum Infortunatum, Clerodendrum Inerme and Investigation of Their Biological and Photocatalytic Activities. Mater. Sci. Eng. C 2018, 82, 46–59. [Google Scholar] [CrossRef]
- Maqbool, Q.; Iftikhar, S.; Nazar, M.; Abbas, F.; Saleem, A. Green Fabricated CuO Nanobullets via Olea Europaea Leaf Extract Shows Auspicious Antimicrobial Potential. IET Nanobiotechnol. 2017, 11, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Sivaraj, R.; Rahman, P.K.S.M.; Rajiv, P.; Abdul, H.; Venckatesh, R. Biogenic Copper Oxide Nanoparticles Synthesis Using Tabernaemontana Divaricate Leaf Extract and Its Antibacterial Activity against Urinary Tract Pathogen. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 133, 178–181. [Google Scholar] [CrossRef] [PubMed]
- Rajeshkumar, S.; Rinitha, G. Nanostructural Characterization of Antimicrobial and Antioxidant Copper Nanoparticles Synthesized Using Novel Persea Americana Seeds. OpenNano 2018, 3, 18–27. [Google Scholar] [CrossRef]
- Saif, S.; Tahir, A.; Asim, T.; Chen, Y. Plant Mediated Green Synthesis of CuO Nanoparticles: Comparison of Toxicity of Engineered and Plant Mediated CuO Nanoparticles towards Daphnia Magna. Nanomaterials 2016, 6, 205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ullah, H.; Wilfred, C.D.; Shaharun, M.S. Green Synthesis of Copper Nanoparticle Using Ionic Liquid-Based Extraction from Polygonum Minus and Their Applications. Environ. Technol. 2019, 40, 3705–3712. [Google Scholar] [CrossRef] [PubMed]
- Zangeneh, M.; Ghaneialvar, H.; Akbaribazm, M.; Ghanimatdan, M. Novel Synthesis of Falcaria Vulgaris Leaf Extract Conjugated Copper Nanoparticles with Potent Cytotoxicity, Antioxidant, Antifungal, Antibacterial, and Cutaneous Wound Healing Activities under In Vitro and In Vivo Condition. J. Photochem. Photobiol. B Biol. 2019, 197, 111556. [Google Scholar] [CrossRef]
- Sharma, P.; Pant, S.; Dave, V.; Tak, K.; Sadhu, V. Green Synthesis and Characterization of Copper Nanoparticles by Tinospora Cardifolia to Produce Nature-Friendly Copper Nano-Coated Fabric and Their Antimicrobial Evaluation. J. Microbiol. Methods 2019, 160, 107–116. [Google Scholar] [CrossRef]
- Awwad, A.; Albiss, B.; Salem, N. Antibacterial Activity of Synthesized Copper Oxide Nanoparticles Using Malva sylvestris Leaf Extract. SMU Med. J. 2015, 2, 91–101. [Google Scholar]
- Sravanthi, M.; Kular, D.M.; Usha, B.; Ravichandra, M. Biological synthesis and characterization of copper oxide nanoparticles using Antigonon leptopus leaf extract and their antibacterial proprerties. Int. J. Adv. Res. 2018, 4, 589–602. [Google Scholar] [CrossRef] [Green Version]
- Khatamifar, M.; Fatemi, S.J. Green Synthesis of Pure Copper Oxide Nanoparticles Using Quercus Infectoria Galls Extract, Thermal Behavior and Their Antimicrobial Effects. Part. Sci. Technol. 2021, 1, 18–26. [Google Scholar] [CrossRef]
- Shende, S.; Ingle, A.P.; Gade, A.; Rai, M.; Copper, Á. Green Synthesis of Copper Nanoparticles by Citrus Medica Linn. (Idilimbu) Juice and Its Antimicrobial Activity. World J. Microbiol. Biotechnol. 2015, 31, 865–873. [Google Scholar] [CrossRef] [PubMed]
- Khatami, M.; Heli, H.; Jahani, P.M.; Azizi, H.; Nobre, A.L. Copper/Copper Oxide Nanoparticles Synthesis Using Stachys Lavandulifolia and Its Antibacterial Activity. IET Nanobiotechnol. 2017, 11, 709–713. [Google Scholar] [CrossRef]
- Lee, H.; Song, J.Y.; Kim, B.S. Biological Synthesis of Copper Nanoparticles Using Magnolia Kobus Leaf Extract and Their Antibacterial Activity. J. Chem. Technol. Biotechnol. 2013, 88, 1971–1977. [Google Scholar] [CrossRef]
- Thiruvengadam, M.; Min, I.; Thandapani, C.; Mohammad, G.; Ansari, A. Synthesis, Characterization and Pharmacological Potential of Green Synthesized Copper Nanoparticles. Bioprocess Biosyst. Eng. 2019, 42, 1769–1777. [Google Scholar] [CrossRef] [PubMed]
- Mali, S.C.; Dhaka, A.; Githala, C.K.; Trivedi, R. Green Synthesis of Copper Nanoparticles Using Celastrus Paniculatus Willd. Leaf Extract and Their Photocatalytic and Antifungal Properties. Biotechnol. Rep. 2020, 27, e00518. [Google Scholar] [CrossRef]
- Naradala, J. Antibacterial Activity of Copper Nanoparticles Synthesized by Bambusa Arundinacea Leaves Extract. Biointerface Resaerch Appl. Chem. 2021, 12, 1230–1236. [Google Scholar]
- Senthil, M.; Ramesh, C. Biogenic synthesis of Fe3O4 nanoparticles using Tridax procumbens leaf extract and its antibacterial activity on Pseudomonas aeruginosa. Dig. J. Nanomater. Biostruct. 2012, 7, 1655–1660. [Google Scholar]
- Henam, S.D.; Ahmad, F.; Shah, M.A.; Parveen, S.; Wani, A.H. Microwave Synthesis of Nanoparticles and Their Antifungal Activities. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 213, 337–341. [Google Scholar] [CrossRef]
- Amutha, S.; Sridhar, S. Green Synthesis of Magnetic Iron Oxide Nanoparticle Using Leaves of Glycosmis Mauritiana and Their Antibacterial Activity against Human Pathogens. J. Innov. Pharm. Biol. Sci. 2018, 5, 22–26. [Google Scholar]
- Naseem, T.; Farrukh, M.A. Antibacterial Activity of Green Synthesis of Iron Nanoparticles Using Lawsonia Inermis and Gardenia Jasminoides Leaves Extract. J. Chem. 2015, 2015, 912342. [Google Scholar] [CrossRef] [Green Version]
- Majid, A.; Naz, F.; Jamro, H.A.; Ansari, B.; Abbasi, S.; Lal, S.; Ujjan, S.A. Facile Green Synthesis of Iron Oxide Nanoparticles Using Phoenix Dactylifera L. Seed Extract and Their Antibacterial Applications. J. Pharm. Res. Int. 2021, 33, 21–29. [Google Scholar] [CrossRef]
- Ahmad, W.; Jaiswal, K.K.; Amjad, M.; Jaiswal, K.K.; Amjad, M. Euphorbia Herita Leaf Extract as a Reducing Agent in a Facile Green Synthesis of Iron Oxide Nanoparticles and Antimicrobial Activity Evaluation Iron Oxide Nanoparticles and Antimicrobial Activity Evaluation. Inorg. Nano-Metal Chem. 2020, 51, 1147–1154. [Google Scholar] [CrossRef]
- Hosseen, S.; Yusuf, M.; Chandra, S.; Das, T.; Saha, O.; Rahaman, M.; Islam, J. Green Synthesis of Iron Oxide Nanoparticle Using Carica Papaya Leaf Extract: Application for Photocatalytic Degradation of Remazol Yellow RR Dye and Antibacterial Activity. Heliyon 2020, 6, e04603. [Google Scholar] [CrossRef]
- Jamzad, M.; Kamari, M. Green Synthesis of Iron Oxide Nanoparticles by the Aqueous Extract of Laurus Nobilis L. Leaves and Evaluation of the Antimicrobial Activity. J. Nanostruct. Chem. 2020, 10, 193–201. [Google Scholar] [CrossRef]
- Chauhan, S.; Sheo, L.; Upadhyay, B. Biosynthesis of Iron Oxide Nanoparticles Using Plant Derivatives of Lawsonia Inermis (Henna) and Its Surface Modification for Biomedical Application. Nanotechnol. Environ. Eng. 2019, 4, 8. [Google Scholar] [CrossRef]
- Tyagi, P.K.; Gupta, S.; Tyagi, S.; Kumar, M.; Pandiselvam, R.; Durna, S.; Sharifi-rad, J.; Gola, D.; Arya, A. Green Synthesis of Iron Nanoparticles from Spinach Leaf and Banana Peel Aqueous Extracts and Evaluation of Antibacterial Potential. J. Nanomater. 2021, 11, 4871453. [Google Scholar] [CrossRef]
- Vitta, Y.; Figueroa, M.; Ciangherotti, C. Synthesis of Iron Nanoparticles from Aqueous Extract of Eucalyptus Robusta Sm and Evaluation of Antioxidant and Antimicrobial Activity. Mater. Sci. Energy Technol. 2019, 3, 97–103. [Google Scholar] [CrossRef]
- Shaker, L.; Alimardani, V.; Mohammad, A. Green Synthesis of Iron-Based Nanoparticles Using Chlorophytum Comosum Leaf Extract: Methyl Orange Dye Degradation and Antimicrobial Properties. Heliyon 2021, 7, e06159. [Google Scholar] [CrossRef]
- Arasu, M.V.; Arokiyaraj, S.; Viayaraghavan, P.; Kumar, J.; Duraipandiyan, V.; Al-dhabi, N.A.; Kaviyarasu, K. One Step Green Synthesis of Larvicidal, and Azo Dye Degrading Antibacterial Nanoparticles by Response Surface Methodology. J. Photochem. Photobiol. B Biol. 2019, 190, 154–162. [Google Scholar] [CrossRef]
- Groiss, S.; Selvaraj, R.; Thivaharan, V.; Ramesh, V. Structural Characterization, Antibacterial and Catalytic Effect of Iron Oxide Nanoparticles Synthesised Using the Leaf Extract of Cynometra Ramiflora. J. Mol. Struct. 2016, 1128, 572–578. [Google Scholar] [CrossRef]
- Yadav, J.; Kumar, S.; Budhwar, L.; Yadav, A.; Yadav, M. Characterization and Antibacterial Activity of Synthesized Silver and Iron Nanoparticles Using Aloe Vera. J. Nanomed. Nanotechnol. 2016, 7, 1000384. [Google Scholar] [CrossRef]
- Mirza, A.U.; Kareem, A.; Nami, S.A.A.; Khan, S.M.; Rehman, S.; Bhat, S.A. Biogenic Synthesis of Iron Oxide Nanoparticles Using Agrewia Optiva and Prunus Persica Phyto Species: Characterization, Antibacterial and Antioxidant Activity. J. Photochem. Photobiol. B Biol. 2018, 185, 262–274. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, W.; Aslam, M.; Nazir, M.; Siddiquah, A. Mediated Novel Bioinspired Lead Oxide (PbO) and Iron Oxide (Fe2O3) Nanoparticles: In-Vitro Biological Applications, Biocompatibility and Their Potential towards HepG2 Cell Line. Mater. Sci. Eng. C 2019, 103, 109740. [Google Scholar] [CrossRef] [PubMed]
- Urabe, A.A.; Aziz, W.J. Assessment of antimicrobial activity phytofabricated iron oxide nanoparticles. Plant Arch. 2019, 19, 600–604. [Google Scholar]
- Johnson, A.; Uwa, P. Eco-Friendly Synthesis of Iron Nanoparticles Using Uvaria Chamae: Characterization and Biological Activity. Inorg. Nano-Metal Chem. 2019, 49, 431–442. [Google Scholar] [CrossRef]
- Zangeneh, M.M. Falcaria Vulgaris Leaf Aqueous Extract Mediated Synthesis of Iron Nanoparticles and Their Therapeutic Potentials under in Vitro and in Vivo Condition. Appl. Organomet. Chem. 2019, 33, e5246. [Google Scholar] [CrossRef]
- Farouk, F.; Abdelmageed, M.; Azam, M.; Azzazy, H.M.E. Synthesis of Magnetic Iron Oxide Nanoparticles Using Pulp and Seed Aqueous Extract of Citrullus Colocynth and Evaluation of Their Antimicrobial Activity. Biotechnol. Lett. 2019, 7, 231–240. [Google Scholar] [CrossRef]
- Ahmad, H.; Rajagopal, K.; Shah, A.H.; Bhat, A.H. Study of Bio-Fabrication of Iron Nanoparticles and Their Fungicidal Property against Phytopathogens of Apple Orchards. IET Nanobiotechnol. 2016, 11, 230–235. [Google Scholar] [CrossRef]
- Khalil, A.T.; Ovais, M.; Ullah, I.; Ali, M.; Khan, Z.; Maaza, M.; Talha, A.; Ovais, M.; Ullah, I.; Ali, M.; et al. Green Chemistry Letters and Reviews Biosynthesis of Iron Oxide (Fe2O3) Nanoparticles via Aqueous Extracts of Sageretia Thea (Osbeck.) and Their Pharmacognostic Properties. Green Chem. Lett. Rev. 2017, 10, 186–201. [Google Scholar] [CrossRef] [Green Version]
- Suganya, D.; Rajan, M.R.; Ramesh, R. Green synthesis of iron oxide nanoparticles from leaf of Passiflora foetida and its antibacterial activity. Int. J. Curr. Res. 2016, 8, 225–229. [Google Scholar]
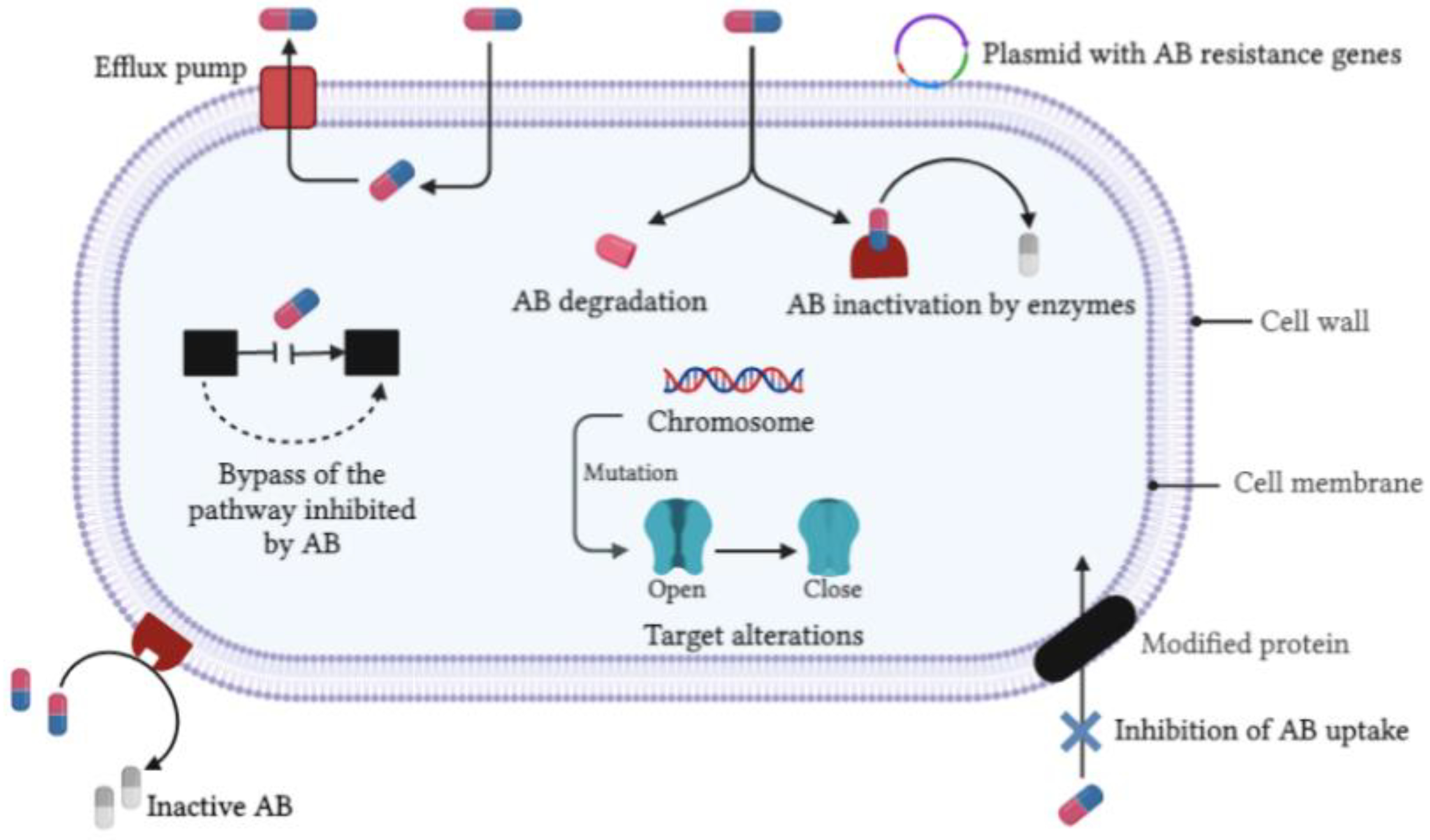
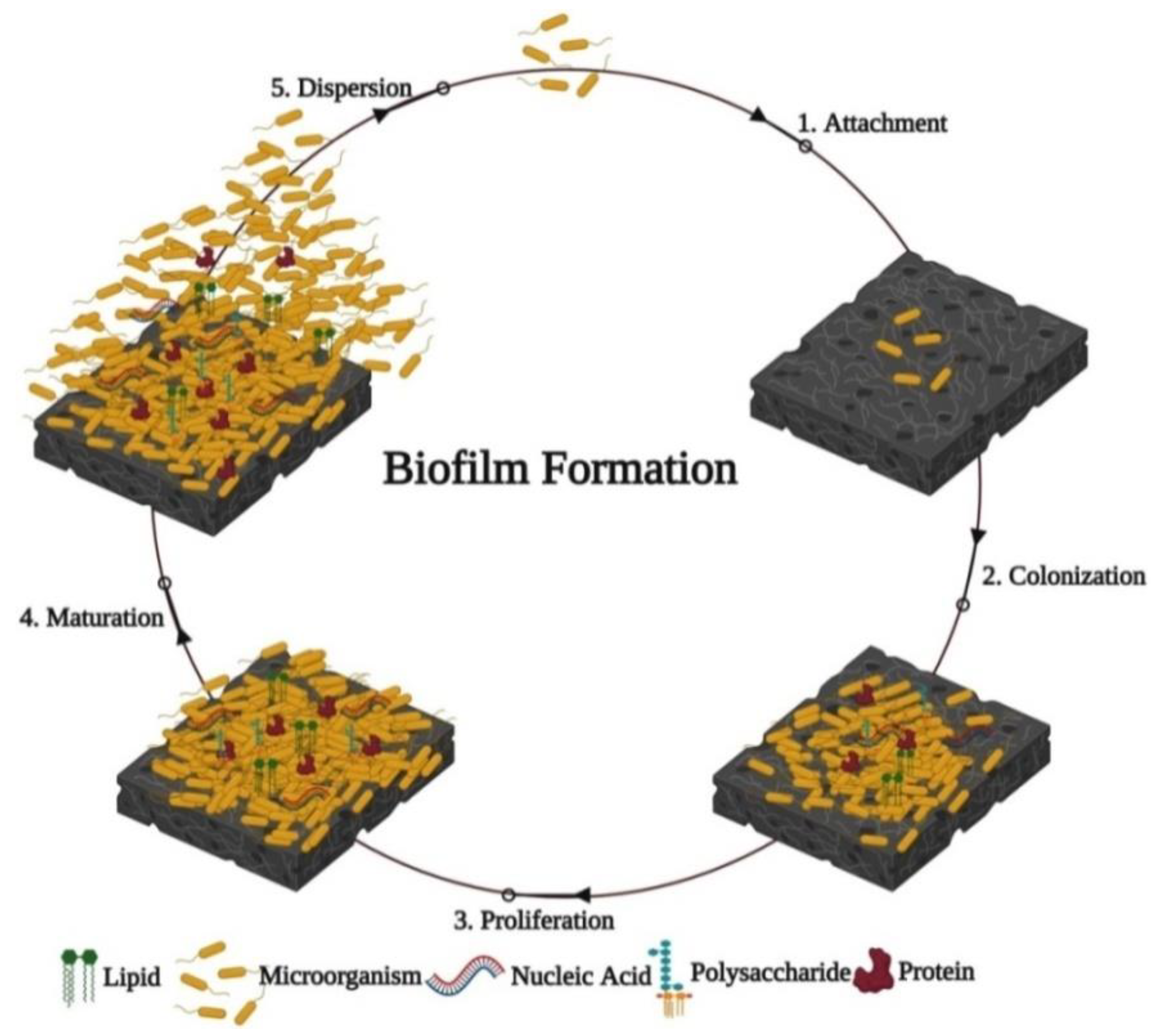






| Plant Type | Part Used | Operative Conditions for Synthesis | NP Characteristics (Shape and Size) | Microbiological Analyzes (Operative Conditions) | Refs. | ||
|---|---|---|---|---|---|---|---|
| Methods, Incubation Temperature, Incubation Time, pH, Inoculum Density, Positive Control | Tested Bacteria and Fungi | MIC, ZOI or PI * | |||||
| Cassia alata | Leaves | Zinc acetate 10 mM/plant extract 10% (1:1 v/v) 80 °C 2 h pH 12 | Spherical 60–80 nm | Dilution 37 °C 24 h pH NM 1 × 105 CFU/mL No control | E. coli | 20 μg/mL | [212] |
| Trifolium pratense | Flowers | Zinc oxide 500 mM/plant extract 2.25% (1:1 v/v) 90 °C 24 h pH 6 | Hexagonal 60–70 nm | Diffusion 35 ± 1 °C 18 h pH NM 5 × 105 CFU/mL No control | S. aureus ATCC 4163 E. coli ATCC 25922 P. aeruginosa ATCC 6749 S. aureus (clinical strain) P. aeruginosa (clinical strain) | 31 31 28 31 29 mm | [213] |
| Pongamia pinnata | Seed | Zinc acetate 20 mM/plant extract 20% (4:50 v/v) 60 °C 2 h pH 12 | Spherical 30–40 nm | Diffusion 37 °C 24 h pH NM Inoculum size NM Ciprofloxacin 5 μg | P. aeruginosa HQ 693274.1 Bacillus licheniformis M235407.1 Vibrio parahaemolyticus HQ 693275.1 | 14 17 12 mm | [214] |
| Plectranthus amboinicus | Leaves | Zinc nitrate 0.05 mM/plant extract 12% (1:5 v/v) 150 °C 6 h pH NM | Hexagonal 20–50 nm | Diffusion 37 °C 24 h pH 7.4 1 × 105 CFU/mL No control | S. aureus ATCC 33591 | 13 mm | [215] |
| Stevia rebaudiana | Leaves | Zinc acetate 100 mM/plant extract 14% (1:1 v/v) 70–80 °C 2 h pH NM | Rectangular 10–90 nm | Dilution 37 °C 24 h pH NM 1.5 × 108 CFU/mL No control | S. aureus E. coli | 2 2 µg/mL | [216] |
| Silybum marianum | Seed | Zinc sulfate 1 mM/plant extract 6% (1:50 v/v) 37 °C 24 h pH 12 | Flowers 60 nm | Diffusion 37 °C 24 h pH NM 5 × 106 CFU/mL Cefixime ** Roxithromycin ** | S. aureus ATCC 6538 K. pneumoniae ATCC 1705 B. subtilis ATCC 6633 E. coli ATCC 25922 P. aeruginosa ATCC 15442 | 20 17 9 10 17 mm | [217] |
| Linum usitatissimum | Root | Zinc nitrate 0.1 mM/plant extract 10% (1:10 w/v) 60 °C 3 h pH NM | Hexagonal 35 nm | Diffusion 37 °C 24 h pH NM Inoculum size NM Amoxicillin 10 µg/mL | S. aureus ATCC 6538 E. coli ATCC 15224 K. pneumoniae ATCC 4619 | 14 14 12 mm | [218] |
| Anchusa italic | Flowers | Zinc acetate 100 mM/plant extract 25% (1:10 v/v) 70 °C 6 h pH NM | Hexagonal 7.6 ± 2.0 nm | Diffusion 37 °C 24 h pH NM 1 × 108 CFU/mL No control | Bacillus megaterium S. aureus E. coli Salmonella typhimurium | 13.6 14.6 13 14.4 mm | [219] |
| Conyza canadensis | Leaves | Zinc nitrate 150 mM/plant extract 6% (1:2 v/v) 80 °C 20 min pH NM | Spherical 10–50 nm | Diffusion 37 °C 24 h pH NM Inoculum size NM Ciprofloxacin 0.5 mg | E. coli S. aureus | 16 14 mm | [220] |
| Plant Type | Part Used | Operative Conditions for Synthesis | NP Characteristics (Shape and Size) | Microbiological Analyzes (Operative Conditions) | Refs. | ||
|---|---|---|---|---|---|---|---|
| Methods, Incubation Temperature, Incubation Time, pH, Inoculum Density, Positive Control | Tested Bacteria and Fungi | MIC, DOI or PI * | |||||
| Garcinia mangostana | Fruit | Hexachloroplatinic acid 1 mM/plant extract 3% (1:1 v/v) 50–70 °C 15 min pH NM | Spherical 20–25 nm | Diffusion 35 °C 24–48 h pH NM 1 × 105 CFU/mL Penicillin G 2 μg Methicillin 5 μg Vancomycin 30 μg Gentamicin 50 μg Streptomycin 10 μg Ciprofloxacin 5 μg Azithromycin 30 μg Clotrimoxazol 25 μg | Staphylococcus spp. Bacillus spp. Pseudomonas spp. Klebsiella spp. | 10 0 12 11 mm | [224] |
| Citrus sinensis | Peel | Hexachloroplatinic acid 10 mM/plant extract 10% (9:1 v/v) 80 °C 24 h pH NM | Spherical 50 nm | Diffusion 30 ± 1 °C 24 h pH 4 Inoculum size NM No control | Aeromonas hydrophila | 4 mm | [225] |
| Sechium edule | Fruit | Platinum (II) chloride 1 mM/plant extract 12.5% (1:1 v/v) 100 ± 5 °C 12 h pH 9 | Spherical 28 nm | Diffusion 37 °C 24 h pH NM 5 × 105 CFU/mL Ciprofloxacin 30 μg Cefprozil 30 μg | B. subtilis E. coli | 25 24 mm | [226] |
| Spinacia oleracea | Leaves | Hexachloroplatinic acid 20 mM/plant extract 75% (2:1 v/v) 100 °C 24 h pH NM | Rod 154 nm | Diffusion 37 °C24 h pH NM 1 × 105 CFU/mL No control | S. typhi MTCC 098 | 13 mm | [227] |
| Taraxacum laevigatum | Powder | Hexachloroplatinic acid 10 mM/plant extract 5% (5:1 v/v) 90 °C 10 min pH NM | Spherical 2–7 nm | Diffusion 37 °C 24 h pH NM 5 × 105 CFU/mL Streptomycin ** | B. subtilis P. aeruginosa | 18 15 mm | [228] |
| Cerbera manghas | Leaves | Hexachloroplatinic acid 1 mM/plant extract 2% (19:1 v/v) 25 °C 2 h pH NM | Spherical 9–12 nm | Diffusion 37 °C 24 h pH NM 1 × 108 CFU/mL Streptomycin 0.25 mg/mL | Vibrio cholerae S. aureus Streptococcus pyogenes S. typhi E. coli | 20 19 13 12 11 mm | [229] |
| Prunus yedoensis | Gum | Hexachloroplatinic acid 100 mM/plant extract 25% (5:1 v/v) 80 °C 5 h pH NM | Spherical and oval 10–50 nm | Diffusion 37 °C 48 h pH NM Inoculum size NM Nystatin ** | Phytophthora capsici Phytophthora drechsleri Didymella bryoniae Colletotrichum acutatum Cladosporium fulvum | 0 0 0 15 18 mm | [230] |
| Curcuma longa | Seed | Hexachloroplatinic acid 1 mM/plant extract 1% (1:1 v/v) 80 °C 2 h pH 10 | Spherical 9 nm | Dilution 37 °C 3 h pH NM 1 × 108 CFU/mL No control | E. coli CD-496 E. coli CD-2 E. coli CD-3 E. coli CD-19 E. coli CD-549 S. aureus CD-1578 MRSA CD 489 | 16 16 16 16 16 64 32 nM | [231] |
| Plant Type | Part Used | Operative Conditions for Synthesis | Np Characteristics (Shape and Size) | Microbiological Analyzes (Operative Conditions) | Refs. | ||
|---|---|---|---|---|---|---|---|
| Methods, Incubation Temperature, Incubation Time, pH, Inoculum Density, Positive Control | Tested Bacteria and Fungi | MIC, DOI or PI * | |||||
| Moringa oleifera | Peel | Palladium acetate 10 mM/plant extract 10% (4:1 v/v) 80 °C 5 min pH NM | Spherical 27 nm | Diffusion 37 °C 24 h pH NM Inoculum size NM Amoxicillin ** | S. aureus E. coli | 1 1 mm | [232] |
| Prunus yedoensis | Leaves | Palladium chloride 1 mM/plant extract 25% (9:1 v/v) 80 °C 30 min pH NM | Spherical 50–150 nm | Diffusion 37 °C 24 h pH NM Inoculum size NM Amoxicillin ** | B. subtilis P. aeruginosa | 6 5 mm | [230] |
| Cissus quadrangularis | Stem | Palladium chloride 0.05 mM/plant extract 10% (1:5 v/v) 37 °C 10 min pH NM | Spherical 12–26 nm | Diffusion 37 °C 24 h pH NM Inoculum size NM No control | E. coli | 17 mm | [233] |
| Camellia sinensis | Leaves | Palladium chloride 1 mM/plant extract 1% (1:1 v/v) 40 °C 30 min pH NM | Spherical 6–18 nm | Diffusion 37 °C 24 h pH NM 1 × 108 CFU/mL Streptomycin ** | S. epidermidis S273 E. coli E266 | 17 14 mm | [234] |
| Garcinia pedunculata | Leaves | Palladium acetate 1 mM/plant extract 20% (2:1 v/v) 121 °C 15 min pH NM | Spherical 2–4 nm | Diffusion 37 °C 24 h pH NM Inoculum size NM No control | Cronobacter sakazakii AMD04 | 0.3 mm | [235] |
| Phoenix dactylifera | Leaves | Palladium chloride 3 mM/plant extract 10% (5:1 v/v) 37 °C 10 min pH NM | Spherical 2–5 nm | Diffusion 37 °C 24 h pH NM Inoculum size NM No control | P. aeruginosa | 26 mm | [236] |
| Arabidopsis thaliana | Leaves | Palladium chloride 5 mM/plant extract 1% (10:1 v/v) 80 °C 24 h pH NM | Spherical 20–40 nm | Diffusion 37 °C 24 h pH NM Inoculum size NM No control | S. aureus | 29 mm | [237] |
| Acacia senegalensis | Gum | Tetrachloropalladic acid 1 mM/plant extract 0.2% (1:1 v/v) 100 °C 6 h pH NM | Spherical 10 nm | Diffusion 37 °C 24 h pH NM 0.5 × 105 CFU/mL No control | Bacillus cereus S. aureus Streptococcus agalatiae | 18 16 17 mm | [238] |
| Bauhinia variegate | Bark | Palladium chloride 1 mM/plant extract 10% (4:1 v/v) 60 °C 30 min pH NM | Irregular 2–9 nm | Diffusion 37 °C 24 h pH NM Inoculum size NM No control | B. subtilis MTCC 441 S. aureus MTCC 737 E. coli MTCC 1687 C. albicans MTCC 183 | 16 6 1 7 mm | [239] |
| Allium cepa | Bulb | Palladium chloride 10 mM/plant extract 10% (1:5 v/v) 100 °C 2 h pH NM | Spherical 19 nm | Diffusion 37 °C 24 h pH NM 1 × 106 CFU/mL No control | Bacillus cereus S. aureus Micrococcus spp. E. coli Klebsiella spp. Proteus spp. | 36 27 40 22 18 17 mm | [240] |
| Filicium decipiens | Leaves | Palladium chloride 1 mM/plant extract 10% (9:1 v/v) 37 °C 96 h pH NM | Spherical 2–22 nm | Diffusion 37 °C 24 h pH NM 1 × 105 CFU/mL Levofloxacin ** | B. subtilis S. aureus E. coli P. aeruginosa | 12 12 27 24 mm | [241] |
| Phyllanthus emblica | Seed | Palladium acetate 870 mM/plant extract 10% (4:1 v/v) 60 °C 3 h pH NM | Spherical 28 nm | Diffusion 37 °C 24 h pH NM Inoculum size NM Streptomycin 50 µg/mL | B. subtilis S. aureus P. aeruginosa Proteus mirabilis | 8.9 8.2 7.6 4.3 mm | [242] |
| Eucommia ulmoides | Bark | Palladium chloride 10 mM/plant extract 20% (5:1 v/v) 80 °C 30 min pH 6 | Spherical 2 nm | Diffusion 37 °C 24 h pH NM Inoculum size NM No control | S. aureus E. coli | 2529 mm | [243] |
| Delonix regia | Leaves | Palladium chloride 0.5 mM/plant extract 25% (9:1 v/v) 28 °C 3 h pH NM | Spherical 2–4 nm | Diffusion 37 °C 24 h pH NM Inoculum size NM No control | Streptococcus mitis | 12 mm | [244] |
| Coriandrum sativum | Seed | Palladium chloride 10 mM/plant extract 5% (1:5 v/v) 60 °C 3 h pH NM | Spherical 113 nm | Diffusion 37 °C 18 h pH NM 5 × 108 CFU/mL Streptomycin 50 µg/mL | S. aureus E. coli Salmonella enterica | 13 8.5 10 mm | [245] |
| Piper betle | Leaves | Palladium chloride 1 mM/plant extract 20% (1:10 v/v) 30 °C 1 h pH NM | Spherical 4 nm | Diffusion 30 °C 48 h pH NM Inoculum size NM Clotrimazol 1 mg/mL | A. niger | 34 mm | [246] |
| Rosmarinus officinalis | Leaves | Palladium acetate 100 mM/plant extract 10% (2:1 v/v) 37 °C 24 h pH NM | Spherical 15 nm | Diffusion 37 °C 20 h pH NM 1 × 106 CFU/mL Ciprofloxacin ** | S. aureus E. coli S. epidermidis Micrococcus luteus | 24 25 21 20 mm | [247] |
| Difusion 32 °C 1 week pH NM 1 × 106 CFU/mL Nystatin ** | C. albicans Candida parapsilolis Candida glabrata Candida krusei | 0 0 0 0 mm | |||||
| Boswellia serrata | Gum | Palladium chloride 1 mM/plant extract 0.5% (1:1 v/v) 121 °C 30 min pH NM | Spherical 2–9 nm | Diffusion 37 °C 24 h pH NM 1 × 106 CFU/mL Gentamicin 10 µg | S. aureus ATCC 25923 P. aeruginosa ATCC 27853 | 2628 mm | [248] |
| Coffea arabica | Powder | Palladium chloride 100 mM/plant extract 8% (1:5 v/v) 60 °C 3 h pH NM | Spherical 20–60 nm | Diffusion 37 °C 24 h pH NM 2 × 108 CFU/mL Ampicillin ** | Enterococcus faecalis S. typhi S. epidermidis | 12 12 12 mm | [249] |
| Morus alba | Fruit | Palladium chloride 2 mM/plant extract 20% (5:1 v/v) 80 °C 3 h pH NM | Spherical and non-regular 50–150 nm | Diffusion 37± °C 24 h pH NM 1.5 × 108 CFU/mL Amoxicillin ** | Listeria monocytogenes ATCC 19115 E. coli O157:H7 | 26 29 mm | [120] |
| Plant Type | Part Used | Operative Conditions for Synthesis | NP Characteristics (Shape and Size) | Microbiological Analyzes (Operative Conditions) | Refs. | ||
|---|---|---|---|---|---|---|---|
| Methods, Incubation Temperature, Incubation Time, pH, Inoculum Density, Positive Control | Tested Bacteria and Fungi | MIC, DOI or PI * | |||||
| Cymbopogon citratus | Leaves | Copper (II) sulfate 0.25 mM/plant extract 50% (2:1 v/v) 60 °C 3 h pH 12 | Spherical, hexagonal and oval 12–14 nm | Diffusion 37 °C 24 h pH NM 1 × 107 CFU/mL No control | E. coli ESβL-336 MSSA MRSA | 20 18 16 mm | [251] |
| Ziziphus spina-christi | Fruits | Copper (II) sulfate 20 mM/plant extract 6% (1:10 v/v) 80 °C 1 h pH NM | Spherical 9 nm | Diffusion 37 °C 24 h pH NM Inoculum size NM No control | E. coli S. aureus | 2 2 mm | [252] |
| Enicostemma axillare | Leaves | Copper (II) sulfate 5 mM/plant extract 10% (10:1 v/v) 50 °C 24 h pH 7 | Spherical 44 nm | Diffusion 37 °C 24 h pH NM Inoculum size NM No control | E. coli P. aeruginosa K. pneumoniae S. aureus Proteus vulgaris | 4 6 8 11 8 mm | [253] |
| Phyllanthus emblica | Fruits | Copper (II) sulfate 20 mM/plant extract 50% (3:1 v/v) 80 °C 15 min pH 10 | Flakes 15–30 nm | Diffusion 37 °C 24 h pH NM Inoculum size NM Ciprofloxacin 25 μg | E. coli S. aureus | 14 14 mm | [254] |
| Carica papaya | Leaves | Copper (II) sulfate 5 mM/plant extract 10% (9:1 v/v) 60 °C 10 min pH NM | Rod 40 nm | Diffusion 37 °C 24 h pH NM Inoculum size NM No control | E. coli S. aureus P. aeruginosa | 9 9 10 mm | [255] |
| Sida acuta | Leaves | Copper (II) sulphate 1000 mM/plant extract 4% (2:1 v/v) 100 °C 5–7 h pH NM | Spherical 50 nm | Diffusion 37 °C 24 h pH NM Inoculum size NM No control | E. coli Proteus vulgaris | 15 11 mm | [256] |
| Prosopis cineraria | Leaves | Copper (I) acetate 5 mM/plant extract 10% (1:1 v/v) Room temperature 24 h pH NM | Hexagonal 19–32 nm | Diffusion 37 °C 24 h pH NM Inoculum size NM Cefotaxim ** | Proteus vulgaris P. aeruginosa K. pneumoniae E. coli S. aureus S. epidermidis | 17 18 22 22 19 23 mm | [257] |
| Syzygium aromaticum | Buds | Copper (II) acetate 1 mM/plant extract 100% (5:1 v/v) 30 °C 15 min pH NM | Spherical 20 nm | Diffusion 37 °C 24 h pH NM Inoculum size NM No control | Staphylococcus spp. E. coli Pseudomonas spp. Bacillus spp. | 5 6 7 8 mm | [258] |
| Diffusion 37 °C 72 h pH NM Inoculum size NM No control | A. niger A. flavus Penicillium spp. | 5 5 6 mm | |||||
| Ruellia tuberosa | Leaves | Copper (II) sulfate 1 mM/plant extract 5% (1:1 v/v) 100 °C 7–8 h pH NM | Nanorod 20–100 nm | Diffusion 37 °C 24 h pH NM Inoculum size NM Streptomycin ** | S. aureus K. pneumoniae E. coli | 13 14 18 mm | [259] |
| Punica granatum | Peels | Copper (II) sulfate 50 mM/plant extract 10% (1:1 v/v) 80 °C 10 min pH NM | Spherical 15–20 nm | Diffusion 37 °C 24 h pH NM Inoculum size NM Streptomycin ** | P. aeruginosa MTCC 424 Salmonella enterica MTCC 1253 Micrococcus luteus MTCC 1809 Enterobactera erogenes MTCC 2823 | 19 20 20 19 mm | [260] |
| Asparagus adscendens | Leaves | Copper (II) sulfate 1 mM/plant extract 5% (10:1 v/v) 100 °C 1 h pH NM | Spherical 10–15 nm | Diffusion 37 °C 24 h pH NM 1 × 108 CFU/mL Ampicillin 25 µg/mL | E. coli B. subtilis S. typhi K. pneumoniae S. aureus | 20 18 21 18 17 mm | [261] |
| Gloriosa superba | Leaves | Copper (II) sulfate 1 mM/plant extract 5% (4:1 v/v) 60 °C 3–4 min pH NM | Spherical 5–10 nm | Diffusion 37 °C 24–36 h pH NM Inoculum size NM Ciprofloxacin 0.5 µg/µL | Klebsiella aerogenes NCIM 2098 E. coli NCIM 5051 S. aureus NCIM 5022 Pseudomonas desmolyticum NCIM 2028 | 15 13 6 5 mm | [262] |
| Cassia auriculata | Leaves | Copper (II) sulfate1 mM/plant extract 5% (4:1 v/v) Room temperature 5 h pH NM | Clusters 38 nm | Diffusion 37 °C 24 h pH NM 1 × 108 CFU/mL Amoxicillin ** | E. coli P. aeruginosa S. aureus Proteus mirabilis Bacillus cereus K. pneumoniae | 16 10 14 16 18 14 mm | [263] |
| Bersama abyssinica | Leaves | Copper (I) acetate 100 mM/plant extract 10% (1:1 v/v) 80 °C 2 h pH NM | Spherical 31 nm | Diffusion 37 °C 24 h pH NM Inoculum size NM Gentamicin ** | S. aureus B. subtilis E. coli P. aeruginosa | 12 6 14 6 mm | [264] |
| Datura innoxia | Leaves | Copper (II) sulfate 1 mM/plant extract 5% (1:1 v/v) 100 °C 1 h pH NM | Nanoclusters 90–200 nm | Diffusion 37 °C 24 h pH NM Inoculum size NM Plantomycin * | Xanthomos oryzae pv. oryzae | 24 mm | [265] |
| Zingiber officinale | Rhizome | Copper (II) sulfate 5 mM/plant extract 30% (5:3 v/v) 60 °C 4 h pH NM | Spherical 31 nm | Diffusion 37 °C 24 h pH NM Inoculum size NM Ciprofloxacin ** | E. coli | 22 mm | [266] |
| Vaccinium arctostaphylos | Fruit | Copper (II) acetate/plant extract 5% (1:20 w/v) 60 °C 24 h pH 10 | Spherical 14 nm | Diffusion 37 °C 24 h pH NM Inoculum size NM Nitrofurantoïn ** | E. coli | 22 mm | [267] |
| Cissus arnotiana | Leaves | Copper (II) sulphate 10 mM/plant extract 1% (9:1 v/v) 60 °C 4 h pH NM | Spherical 60–90 nm | Diffusion 37 °C 18 h pH NM Inoculum size NM Ampicillin ** | E. coli Streptococcus spp. Rhizobium spp. Klebsiella spp. | 22 20.2 16.3 18.3 mm | [268] |
| Plant Type | Part Used | Operative Conditions for Synthesis | NP Characteristics (Shape and Size) | Microbiological Analyzes (Operative Conditions) | Refs. | ||
|---|---|---|---|---|---|---|---|
| Methods, Incubation Temperature, Incubation Time, pH, Inoculum Density, Positive Control | Tested Bacteria and Fungi | MIC, DOI or PI * | |||||
| Withania coagulans | Berries | Iron (III) chloride 2000 mM/plant extract 12% (5:1 v/v) 90 °C 30 min pH NM | Rod 16 nm | Diffusion 37 °C 24 h pH NM Inoculum size NM No control | P. aeruginosa S. aureus | 24 23 mm | [269] |
| Acacia nilotica | Pods | Iron (II) sulfate 100 mM/plant extract 10% (3:2 v/v) Room temperature 24 h pH 6 | Irregular 39 nm | Diffusion 30 °C 24 h pH NM Inoculum size NM No control | E. coli MRSA S. typhi S. aureus C. albicans | 17 24 23 25 25 mm | [270] |
| Musa ornate | Flowers | Iron (III) chloride 1 mM/plant extract 10% (1:1 v/v) 70–80 °C 1 h pH NM | Spherical 20–40 nm | Diffusion 37 °C 24 h pH NM Inoculum size NM No control | Streptococcus agalactiae S. aureus Salmonella enterica E. coli | 28 32 0 0 mm | [271] |
| Skimmia laureola | Leaves | Iron (III) chloride 100 mM/plant extract 10% (1:1 v/v) Room temperature 30 min pH NM | Spherical 56–350 nm | Diffusion 37 °C 24 h pH NM Inoculum size NM Streptomycin 200 ppm | Ralstornia solanacearum | 18 mm | [272] |
| Lagenaria siceraria | Leaves | Iron (III) chloride 10 mM/plant extract 5% (1:1 v/v) 40 °C 60 min pH NM | Cubic 30–100 nm | Diffusion 37 °C 24 h pH NM Inoculum size NM Ampicillin 20 mg/mL | S. aureus E. coli | 14 17 mm | [273] |
| Trigonella foenum-graecum | Seed | Iron (II) chloride 1000 mM/plant extract 5% (1:2 v/v) Room temperature 2 h pH 10 | Spherical ∼20 nm | Diffusion 37 °C 24 h pH NM Inoculum size NM No control | E. coli ATCC 11775 S. aureus ATCC 6538 | 22 24 mm | [148] |
| Dodonaea vicosa | Leaves | Iron (III) chloride 10 mM/plant extract 20% (2:1 v/v) 50 °C 1 h pH NM | Spherical 50–60 nm | Diffusion 37 °C 24 h pH NM Inoculum size NM No control | E. coli MTCC 443 K. pneumoniae NCIM 2079 B. subtilis MTCC 441 S. aureus MTCC 4032 Pseudomonas fluorescens MTCC 121 | 8 10 12 14 24 mm | [274] |
| Couroupita guianensis | Peel | Iron (III) chloride 100 mM/plant extract 5% (1:1 v/v) 80 °C 30 min pH 10 | Spherical 7–80 nm | Diffusion 37 °C 24 h pH NM 1 × 105 CFU/mL Streptomycin 1 mg | S. aureus MTCC 96 E. coli MTCC 2939 S. typhi MTCC 3917 K. pneumoniae MTCC 530 | 8 15 15 12 mm | [275] |
| Psidium guajava | Fruit | Iron (III) chloride 500 mM/plant extract 4% (4:1 v/v) 100 °C 1 h pH NM | Hexagonal 27 nm | Diffusion 37 °C 18–24 h pH NM Inoculum size NM Gentamicin 10 μg | Bacillus cereus E. coli K. pneumoniae S. aureus | 14 17 10 14 mm | [276] |
| Punica granatum | Peel | Iron (III) chloride 150 mM/plant extract 4.6% (5:2 v/v) 20 °C 5 h pH NM | Spherical 20–90 nm | Diffusion 30 °C 24 h pH NM Inoculum size NM Streptomycin ** | P. aeruginosa | 22 mm | [277] |
| Argemone mexicana | Leaves | Iron (III) chloride 25 mM/plant extract 3% (1:1 v/v) 45 °C 12 h pH NM | Spherical 10–30 nm | Diffusion 37 °C 24 h pH NM 1 × 106 CFU/mL Streptomycin 30 µg | E. coli MTCC 443 B. subtilis MTCC 425 Proteus mirabilis MTCC 441 | 13 18 10 mm | [278] |
| Ruellia tuberosa | Leaves | Iron (II) sulphate 1000 mM/plant extract 5% (1:1 v/v) 80 °C 30 min pH NM | Hexagonal 53 nm | Diffusion 37 °C 24 h pH NM 1 × 106 CFU/mL Streptomycin ** | K.pneumoniae E. coli S.aureus | 13 16 11 mm | [279] |
| Leucas aspera | Leaves | Iron (III) chloride 5 mM/plant extract 20% (1:1 v/v) 80 °C 15 min pH NM | Irregular rhombic 117 nm | Diffusion 37 °C 24 h pH NM Inoculum size NM Ampicillin 10 µg | E. coli K. pneumoniae Proteus mirabilis Salmonella enterica Shigella flexneri Vibrio cholera P. aeruginosa Bacillus cereus S. aureus Listeria monocytogens | 10 10 11 19 22 10 20 00 11 12 mm | [280] |
| Eichhornia crassipes | Leaves | Ferrous sulphate 100 mM/plant extract 5% (1:1 v/v) 55 °C 2 h pH 10 | Rod 10–100 nm | Diffusion 37 °C 24 h pH NM Inoculum size NM No control | S. aureus Pseudomonas fluorescens E. coli | 23 23 20 mm | [281] |
| Sida cordifolia | Whole the plant | Iron nitrate 10 mM/plant extract 5% (2:1 v/v) 60 °C 5 min pH NM | Spherical 10–22 nm | Diffusion 37 °C 24 h pH NM Inoculum size NM No control | B. subtilis S. aureus E. coli K. pneumoniae | 17 15 15 19 mm | [282] |
| Trigonella foenum-graecum | Seed | Iron (III) chloride 10 mM/plant extract 0.04% (1:20 v/v) 30 °C 15 min pH NM | Spherical 11 nm | Diffusion 37 °C 24 h pH NM Inoculum size NM Streptomycin ** | E. coli S. aureus | 22 19 mm | [283] |
| Piliostigma thonningii | Flowers | Iron (II) chloride 1 mM/plant extract 20% (9:1 v/v) 80 °C 2 min pH NM | Spherical 20–100 nm | Diffusion 37 °C 24 h pH NM Inoculum size NM Gentamycin ** | E. coli S. aureus | 21 20 mm | [284] |
| Methods | Microorganism | Growth Medium | Final Inoculum Size | Incubation Temperature | Incubation Time 1 |
|---|---|---|---|---|---|
| Disk diffusion | Bacteria | MHA | 1–2 × 108 CFU/mL | 35 ± 2 °C | 16–18 h |
| Fungi | MHA + GMB (Yeast) | 1–5 × 106 CFU/mL (yeast) | 35 ± 2 °C | 20–24 h | |
| Non-supplemented MHA (molds) | 0.4–5 × 106 CFU/mL (molds) | – | – | ||
| Broth dilution | Bacteria | MHB | 5 × 105 CFU/mL | 35 ± 2 °C | 20 h 2 |
| Fungi | RPMI 1640 (yeast) | 0.5–2.5 × 103 CFU/mL (yeast) | 35 °C | 24–48 h (yeast) 3 | |
| RPMI 1640 (molds) | 0.4–5 × 104 CFU/mL (molds) | 35 °C | 48 h (molds) 4 | ||
| Agar dilution | Bacteria | MHA | 1 × 104 CFU/spot | 35 ± 2 °C | 16–20 h |
| Time-kill test | Bacteria | MHB | 5 × 105 CFU/mL | 35 ± 2 °C | 0, 4, 18 and 24 h |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luzala, M.M.; Muanga, C.K.; Kyana, J.; Safari, J.B.; Zola, E.N.; Mbusa, G.V.; Nuapia, Y.B.; Liesse, J.-M.I.; Nkanga, C.I.; Krause, R.W.M.; et al. A Critical Review of the Antimicrobial and Antibiofilm Activities of Green-Synthesized Plant-Based Metallic Nanoparticles. Nanomaterials 2022, 12, 1841. https://doi.org/10.3390/nano12111841
Luzala MM, Muanga CK, Kyana J, Safari JB, Zola EN, Mbusa GV, Nuapia YB, Liesse J-MI, Nkanga CI, Krause RWM, et al. A Critical Review of the Antimicrobial and Antibiofilm Activities of Green-Synthesized Plant-Based Metallic Nanoparticles. Nanomaterials. 2022; 12(11):1841. https://doi.org/10.3390/nano12111841
Chicago/Turabian StyleLuzala, Miryam M., Claude K. Muanga, Joseph Kyana, Justin B. Safari, Eunice N. Zola, Grégoire V. Mbusa, Yannick B. Nuapia, Jean-Marie I. Liesse, Christian I. Nkanga, Rui W. M. Krause, and et al. 2022. "A Critical Review of the Antimicrobial and Antibiofilm Activities of Green-Synthesized Plant-Based Metallic Nanoparticles" Nanomaterials 12, no. 11: 1841. https://doi.org/10.3390/nano12111841
APA StyleLuzala, M. M., Muanga, C. K., Kyana, J., Safari, J. B., Zola, E. N., Mbusa, G. V., Nuapia, Y. B., Liesse, J.-M. I., Nkanga, C. I., Krause, R. W. M., Balčiūnaitienė, A., & Memvanga, P. B. (2022). A Critical Review of the Antimicrobial and Antibiofilm Activities of Green-Synthesized Plant-Based Metallic Nanoparticles. Nanomaterials, 12(11), 1841. https://doi.org/10.3390/nano12111841