Gold Nanocluster-Based Fluorometric Banoxantrone Assay Enabled by Photoinduced Electron Transfer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
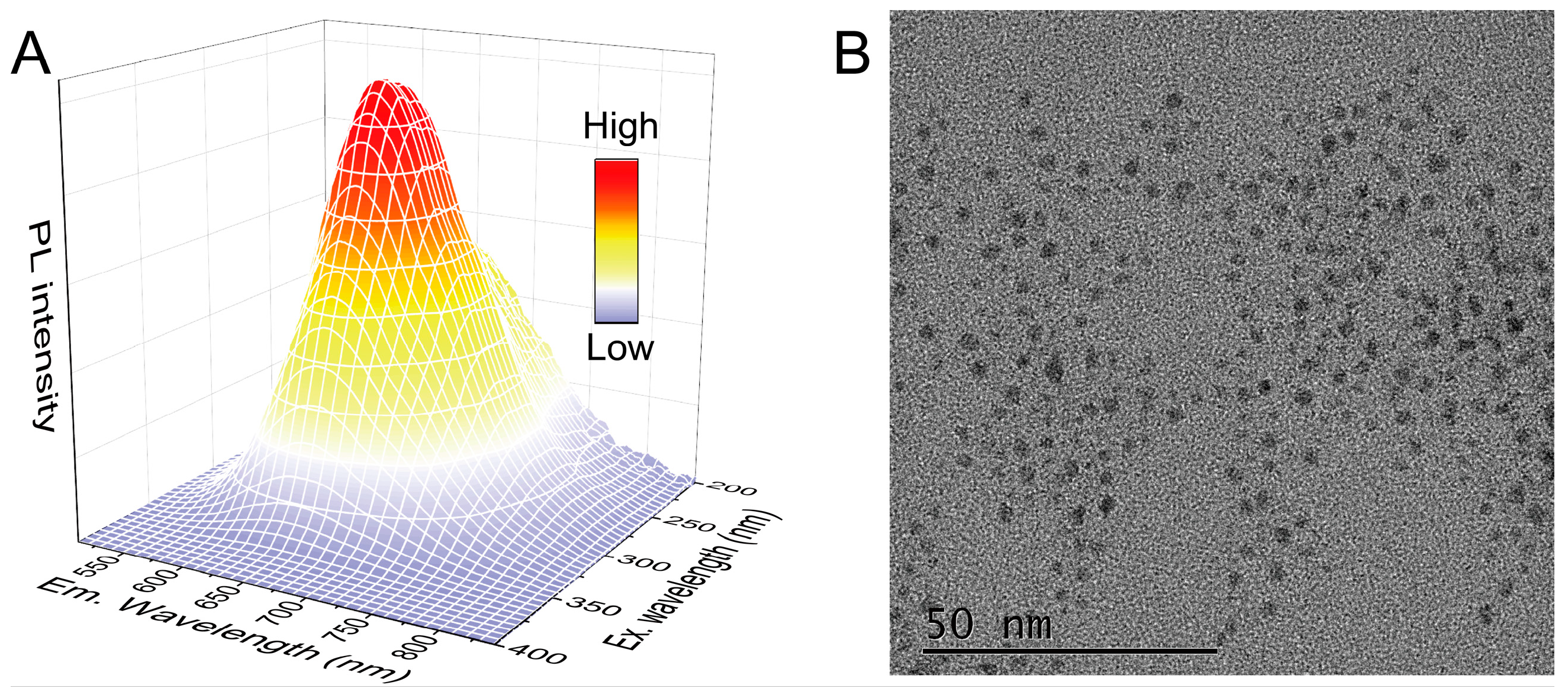
2.2. Preparation of CC/DTT-AuNCs
2.3. AQ4N Determination
2.4. AQ4N Extraction and Quantification
2.5. Computational Methodology
2.6. Instruments
3. Results and Discussion
3.1. Designing Strategy
3.2. Luminescence Quenching Mechanism Study
3.3. Optimizing the Test Conditions for AQ4N
3.4. Sensing Performance
3.5. Detection of AQ4N in Real Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Patterson, L.H.; McKeown, S.R. AQ4N: A New Approach to Hypoxia-Activated Cancer Chemotherapy. Br. J. Cancer 2000, 83, 1589–1593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albertella, M.R.; Loadman, P.M.; Jones, P.H.; Phillips, R.M.; Rampling, R.; Burnet, N.; Alcock, C.; Anthoney, A.; Vjaters, E.; Dunk, C.R.; et al. Hypoxia-Selective Targeting by the Bioreductive Prodrug AQ4N in Patients with Solid Tumors: Results of a Phase I Study. Clin. Cancer Res. 2008, 14, 1096–1104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shao, F.; Wu, Y.; Tian, Z.; Liu, S. Biomimetic Nanoreactor for Targeted Cancer Starvation Therapy and Cascade Amplificated Chemotherapy. Biomaterials 2021, 274, 120869. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Wu, Y.; Li, K.; Wang, Y.; Wu, J.; Zeng, Y.; Wu, D. Versatile Hyaluronic Acid Modified AQ4N-Cu(II)-Gossypol Infinite Coordination Polymer Nanoparticles: Multiple Tumor Targeting, Highly Efficient Synergistic Chemotherapy, and Real-Time Self-Monitoring. Biomaterials 2018, 154, 197–212. [Google Scholar] [CrossRef] [PubMed]
- Swaine, D.J.; Loadman, P.M.; Bibby, M.C.; Graham, M.A.; Patterson, L.H. High-Performance Liquid Chromatographic Analysis of AQ4N, an Alkylaminoanthraquinone N-Oxide. J. Chromatogr. B 2000, 742, 239–245. [Google Scholar] [CrossRef]
- Atkinson, S.J.; Loadman, P.M.; Sutton, C.; Patterson, L.H.; Clench, M.R. Examination of the Distribution of the Bioreductive Drug AQ4N and Its Active Metabolite AQ4 in Solid Tumours by Imaging Matrix-Assisted Laser Desorption/Ionisation Mass Spectrometry. Rapid Commun. Mass Spectrom. 2007, 21, 1271–1276. [Google Scholar] [CrossRef]
- Daly, B.; Ling, J.; de Silva, A.P. Current Developments in Fluorescent PET (Photoinduced Electron Transfer) Sensors and Switches. Chem. Soc. Rev. 2015, 44, 4203–4211. [Google Scholar] [CrossRef] [Green Version]
- Sun, W.; Li, M.; Fan, J.; Peng, X. Activity-Based Sensing and Theranostic Probes Based on Photoinduced Electron Transfer. Acc. Chem. Res. 2019, 52, 2818–2831. [Google Scholar] [CrossRef]
- Escudero, D. Revising Intramolecular Photoinduced Electron Transfer (PET) from First-Principles. Acc. Chem. Res. 2016, 49, 1816–1824. [Google Scholar] [CrossRef]
- Chi, W.; Chen, J.; Liu, W.; Wang, C.; Qi, Q.; Qiao, Q.; Tan, T.M.; Xiong, K.; Liu, X.; Kang, K.; et al. A General Descriptor ΔE Enables the Quantitative Development of Luminescent Materials Based on Photoinduced Electron Transfer. J. Am. Chem. Soc. 2020, 142, 6777–6785. [Google Scholar] [CrossRef]
- Marcus, R.A. Electron Transfer Reactions in Chemistry. Theory and Experiment. Rev. Mod. Phys. 1993, 65, 599–610. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Sun, J.; Zhao, J.; Lu, S.; Yang, X. Photo-Induced Electron Transfer-Based Versatile Platform with G-Quadruplex/Hemin Complex as Quencher for Construction of DNA Logic Circuits. Anal. Chem. 2018, 90, 3437–3442. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhu, J.; Guo, S.; Li, T.; Li, J.; Wang, E. Photoinduced Electron Transfer of DNA/Ag Nanoclusters Modulated by G-Quadruplex/Hemin Complex for the Construction of Versatile Biosensors. J. Am. Chem. Soc. 2013, 135, 2403–2406. [Google Scholar] [CrossRef] [PubMed]
- Pantazis, A.; Westerberg, K.; Althoff, T.; Abramson, J.; Olcese, R. Harnessing Photoinduced Electron Transfer to Optically Determine Protein Sub-Nanoscale Atomic Distances. Nat. Commun. 2018, 9, 4738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, G.; Zhu, Y.; Chen, L.; Zhang, X. Photoinduced Electron Transfer (PET) Based Label-Free Aptasensor for Platelet-Derived Growth Factor-Bb and Its Logic Gate Application. Biosens. Bioelectron. 2015, 63, 552–557. [Google Scholar] [CrossRef]
- Nawimanage, R.R.; Prasai, B.; Hettiarachchi, S.U.; McCarley, R.L. Rapid, Photoinduced Electron Transfer-Modulated, Turn-on Fluorescent Probe for Detection and Cellular Imaging of Biologically Significant Thiols. Anal. Chem. 2014, 86, 12266–12271. [Google Scholar] [CrossRef] [Green Version]
- Dong, B.; Lu, Y.; Zhang, N.; Song, W.; Lin, W. Ratiometric Imaging of Cysteine Level Changes in Endoplasmic Reticulum during H2O2-Induced Redox Imbalance. Anal. Chem. 2019, 91, 5513–5516. [Google Scholar] [CrossRef] [Green Version]
- Tang, Y.; Gu, C.; Wang, C.; Song, B.; Zhou, X.; Lou, X.; He, M. Evanescent Wave Aptasensor for Continuous and Online Aminoglycoside Antibiotics Detection Based on Target Binding Facilitated Fluorescence Quenching. Biosens. Bioelectron. 2018, 102, 646–651. [Google Scholar] [CrossRef]
- Tang, Y.; Gu, C.; Wang, C.; Song, B.; Zhou, X.; Lou, X.; He, M. Highly Water-Stable Dye@Ln-MOFs for Sensitive and Selective Detection toward Antibiotics in Water. ACS Appl. Mater. Interfaces 2019, 11, 21201–21210. [Google Scholar]
- Dwivedi, S.K.; Gupta, R.C.; Srivastava, P.; Singh, P.; Koch, B.; Maiti, B.; Misra, A. Dual Fluorophore Containing Efficient Photoinduced Electron Transfer Based Molecular Probe for Selective Detection of Cr3+ and PO43− Ions through Fluorescence “Turn–On–Off” Response in Partial Aqueous and Biological Medium: Live Cell Imaging and Logic Application. Anal. Chem. 2018, 90, 10974–10981. [Google Scholar]
- Uchiyama, S.; Fukatsu, E.; McClean, G.D.; de Silva, A.P. Measurement of Local Sodium Ion Levels near Micelle Surfaces with Fluorescent Photoinduced-Electron-Transfer Sensors. Angew. Chem. Int. Ed. 2016, 55, 768–771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, Y.; Malola, S.; Matus, M.F.; Chen, T.; Yao, Q.; Shi, R.; Häkkinen, H.; Xie, J. Reversible Isomerization of Metal Nanoclusters Induced by Intermolecular Interaction. Chem 2021, 7, 2227–2244. [Google Scholar] [CrossRef]
- Wu, Z.; Du, Y.; Liu, J.; Yao, Q.; Chen, T.; Cao, Y.; Zhang, H.; Xie, J. Aurophilic Interactions in the Self-Assembly of Gold Nanoclusters into Nanoribbons with Enhanced Luminescence. Angew. Chem. Int. Ed. 2019, 58, 8139–8144. [Google Scholar] [CrossRef] [PubMed]
- Qian, S.; Wang, Z.; Zuo, Z.; Wang, X.; Wang, Q.; Yuan, X. Engineering Luminescent Metal Nanoclusters for Sensing Applications. Coord. Chem. Rev. 2022, 451, 214268. [Google Scholar] [CrossRef]
- Li, Q.; Zhou, M.; So, W.Y.; Huang, J.; Li, M.; Kauffman, D.R.; Cotlet, M.; Higaki, T.; Peteanu, L.A.; Shao, Z.; et al. A Mono-Cuboctahedral Series of Gold Nanoclusters: Photoluminescence Origin, Large Enhancement, Wide Tunability, and Structure–Property Correlation. J. Am. Chem. Soc. 2019, 141, 5314–5325. [Google Scholar] [CrossRef]
- Shang, L.; Xu, J.; Nienhaus, G.U. Recent Advances in Synthesizing Metal Nanocluster-Based Nanocomposites for Application in Sensing, Imaging and Catalysis. Nano Today 2019, 28, 100767. [Google Scholar] [CrossRef]
- Kwak, K.; Lee, D. Electrochemistry of Atomically Precise Metal Nanoclusters. Acc. Chem. Res. 2019, 52, 12–22. [Google Scholar] [CrossRef]
- Zhou, M.; Du, X.; Wang, H.; Jin, R. The Critical Number of Gold Atoms for a Metallic State Nanocluster: Resolving a Decades-Long Question. ACS Nano 2021, 15, 13980–13992. [Google Scholar] [CrossRef]
- Zhou, G.; Lu, G.; Prezhdo, O.V. Modeling Auger Processes with Nonadiabatic Molecular Dynamics. Nano Lett. 2021, 21, 756–761. [Google Scholar] [CrossRef]
- Huang, K.; Fang, Q.; Sun, W.; He, S.; Yao, Q.; Xie, J.; Chen, W.; Deng, H. Cucurbit[n]uril Supramolecular Assemblies-Regulated Charge Transfer for Luminescence Switching of Gold Nanoclusters. J. Phys. Chem. Lett. 2022, 13, 419–426. [Google Scholar] [CrossRef]
- Deng, H.H.; Huang, K.Y.; Zhu, C.T.; Shen, J.F.; Zhang, X.P.; Peng, H.P.; Xia, X.H.; Chen, W. Bell-Shaped Electron Transfer Kinetics in Gold Nanoclusters. J. Phys. Chem. Lett. 2021, 12, 876–883. [Google Scholar] [CrossRef] [PubMed]
- Stamplecoskie, K.G.; Kamat, P.V. Size-Dependent Excited State Behavior of Glutathione-Capped Gold Clusters and Their Light-Harvesting Capacity. J. Am. Chem. Soc. 2014, 136, 11093–11099. [Google Scholar] [CrossRef] [PubMed]
- Stamplecoskie, K.G.; Chen, Y.S.; Kamat, P.V. Excited-State Behavior of Luminescent Glutathione-Protected Gold Clusters. J. Phys. Chem. C 2014, 118, 1370–1376. [Google Scholar] [CrossRef]
- Kato, D.; Sakai, H.; Saegusa, T.; Tkachenko, N.V.; Hasobe, T. Synthesis, Structural and Photophysical Properties of Pentacene Alkanethiolate Monolayer-Protected Gold Nanoclusters and Nanorods: Supramolecular Intercalation and Photoinduced Electron Transfer with C60. J. Phys. Chem. C 2017, 121, 9043–9052. [Google Scholar] [CrossRef]
- Huang, K.Y.; He, H.X.; He, S.B.; Zhang, X.P.; Peng, H.P.; Lin, Z.; Deng, H.H.; Xia, X.H.; Chen, W. Gold Nanocluster-Based Fluorescence Turn-off Probe for Sensing of Doxorubicin by Photoinduced Electron Transfer. Sens. Actuators B 2019, 296, 126656. [Google Scholar] [CrossRef]
- Chen, S.; Yu, Y.L.; Wang, J.H. Inner Filter Effect-Based Fluorescent Sensing Systems: A Review. Anal. Chim. Acta 2018, 999, 13–26. [Google Scholar] [CrossRef]
- Farid, S.; Dinnocenzo, J.P.; Merkel, P.B.; Young, R.H.; Shukla, D.; Guirado, G. Reexamination of the Rehm–Weller Data Set Reveals Electron Transfer Quenching That Follows a Sandros–Boltzmann Dependence on Free Energy. J. Am. Chem. Soc. 2011, 133, 11580–11587. [Google Scholar] [CrossRef]
- Fu, Y.; Cen, D.; Zhang, T.; Jiang, S.; Wang, Y.; Cai, X.; Li, X.; Han, G. Implantable Fibrous Scaffold with Hierarchical Microstructure for the ‘On-Site’ Synergistic Cancer Therapy. Chem. Eng. J. 2020, 402, 126204. [Google Scholar] [CrossRef]
- Rosenberg, M.; Junker, A.K.R.; Sørensen, T.J.; Laursen, B.W. Fluorescence pH Probes Based on Photoinduced Electron Transfer Quenching of Long Fluorescence Lifetime Triangulenium Dyes. ChemPhotoChem 2019, 3, 233–242. [Google Scholar] [CrossRef]
- Wehlin, S.A.M.; Troian Gautier, L.; Li, G.; Meyer, G.J. Chloride Oxidation by Ruthenium Excited-States in Solution. J. Am. Chem. Soc. 2017, 139, 12903–12906. [Google Scholar] [CrossRef]
- Kavarnos, G.J.; Turro, N.J. Photosensitization by Reversible Electron Transfer: Theories, Experimental Evidence, and Examples. Chem. Rev. 1986, 86, 401–449. [Google Scholar] [CrossRef]
- Kovacs, D.; Mathieu, E.; Kiraev, S.R.; Wells, J.A.L.; Demeyere, E.; Sipos, A.; Borbas, K.E. Coordination Environment-Controlled Photoinduced Electron Transfer Quenching in Luminescent Europium Complexes. J. Am. Chem. Soc. 2020, 142, 13190–13200. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.H.; Huang, K.Y.; He, S.B.; Xue, L.P.; Peng, H.P.; Zha, D.J.; Sun, W.M.; Xia, X.H.; Chen, W. Rational Design of High-Performance Donor–Linker–Acceptor Hybrids Using a Schiff Base for Enabling Photoinduced Electron Transfer. Anal. Chem. 2020, 92, 2019–2026. [Google Scholar] [CrossRef] [PubMed]
- Xiu, L.F.; Huang, K.Y.; Zhu, C.T.; Zhang, Q.; Peng, H.P.; Xia, X.H.; Chen, W.; Deng, H.H. Rare-Earth Eu3+/Gold Nanocluster Ensemble-Based Fluorescent Photoinduced Electron Transfer Sensor for Biomarker Dipicolinic Acid Detection. Langmuir 2021, 37, 949–956. [Google Scholar] [CrossRef]
- Deng, H.H.; Zhang, L.N.; He, S.B.; Liu, A.L.; Li, G.W.; Lin, X.H.; Xia, X.H.; Chen, W. Methionine-Directed Fabrication of Gold Nanoclusters with Yellow Fluorescent Emission for Cu2+ Sensing. Biosens. Bioelectron. 2015, 65, 397–403. [Google Scholar] [CrossRef]
- Peng, H.; Jian, M.; Deng, H.; Wang, W.; Huang, Z.; Huang, K.; Liu, A.; Chen, W. Valence States Effect on Electrogenerated Chemiluminescence of Gold Nanocluster. ACS Appl. Mater. Interfaces 2017, 9, 14929–14934. [Google Scholar] [CrossRef]
- Deng, H.H.; Wu, G.W.; Zou, Z.Q.; Peng, H.P.; Liu, A.L.; Lin, X.H.; Xia, X.H.; Chen, W. pH-Sensitive Gold Nanoclusters: Preparation and Analytical Applications for Urea, Urease, and Urease Inhibitor Detection. Chem. Commun. 2015, 51, 7847–7850. [Google Scholar] [CrossRef]
- Sun, J.; Yue, Y.; Wang, P.; He, H.; Jin, Y. Facile and Rapid Synthesis of Water-Soluble Fluorescent Gold Nanoclusters for Sensitive and Selective Detection of Ag+. J. Mater. Chem. C 2013, 1, 908–913. [Google Scholar] [CrossRef]
- Sun, J.; Wu, H.; Jin, Y. Synthesis of Thiolated Ag/Au Bimetallic Nanoclusters Exhibiting an Anti-Galvanic Reduction Mechanism and Composition-Dependent Fluorescence. Nanoscale 2014, 6, 5449–5457. [Google Scholar] [CrossRef]
- Tang, Y.; Xu, J.; Xiong, C.; Xiao, Y.; Zhang, X.; Wang, S. Enhanced Electrochemiluminescence of Gold Nanoclusters via Silver Doping and Their Application for Ultrasensitive Detection of Dopamine. Analyst 2019, 144, 2643–2648. [Google Scholar] [CrossRef]
- Luo, Z.; Yuan, X.; Yu, Y.; Zhang, Q.; Leong, D.T.; Lee, J.Y.; Xie, J. From Aggregation-Induced Emission of Au(I)–Thiolate Complexes to Ultrabright Au(0)@Au(I)–Thiolate Core–Shell Nanoclusters. J. Am. Chem. Soc. 2012, 134, 16662–16670. [Google Scholar] [CrossRef] [PubMed]





| Sample | Our method (μM, Mean ± SD, n = 3) | HPLC Method (μM, mean ± SD, n = 3) | F-Test a | t-Test a |
|---|---|---|---|---|
| 1 | 82.5 ± 1.2 | 78.2 ± 0.02 | 7.93 | 1.42 |
| 2 | 81.8 ± 0.6 | 78.3 ± 0.04 | 5.23 | 1.79 |
| 3 | 74.6 ± 1.9 | 78.4 ± 0.08 | 5.87 | 1.18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, K.-Y.; Weng, W.-H.; Huang, X.; Huang, H.-X.; Noreldeen, H.A.A.; Deng, H.-H.; Chen, W. Gold Nanocluster-Based Fluorometric Banoxantrone Assay Enabled by Photoinduced Electron Transfer. Nanomaterials 2022, 12, 1861. https://doi.org/10.3390/nano12111861
Huang K-Y, Weng W-H, Huang X, Huang H-X, Noreldeen HAA, Deng H-H, Chen W. Gold Nanocluster-Based Fluorometric Banoxantrone Assay Enabled by Photoinduced Electron Transfer. Nanomaterials. 2022; 12(11):1861. https://doi.org/10.3390/nano12111861
Chicago/Turabian StyleHuang, Kai-Yuan, Wen-Hui Weng, Xin Huang, Hong-Xiang Huang, Hamada A. A. Noreldeen, Hao-Hua Deng, and Wei Chen. 2022. "Gold Nanocluster-Based Fluorometric Banoxantrone Assay Enabled by Photoinduced Electron Transfer" Nanomaterials 12, no. 11: 1861. https://doi.org/10.3390/nano12111861







