Engineering Coinage Metal Nanoclusters for Electroluminescent Light-Emitting Diodes
Abstract
:1. Introduction
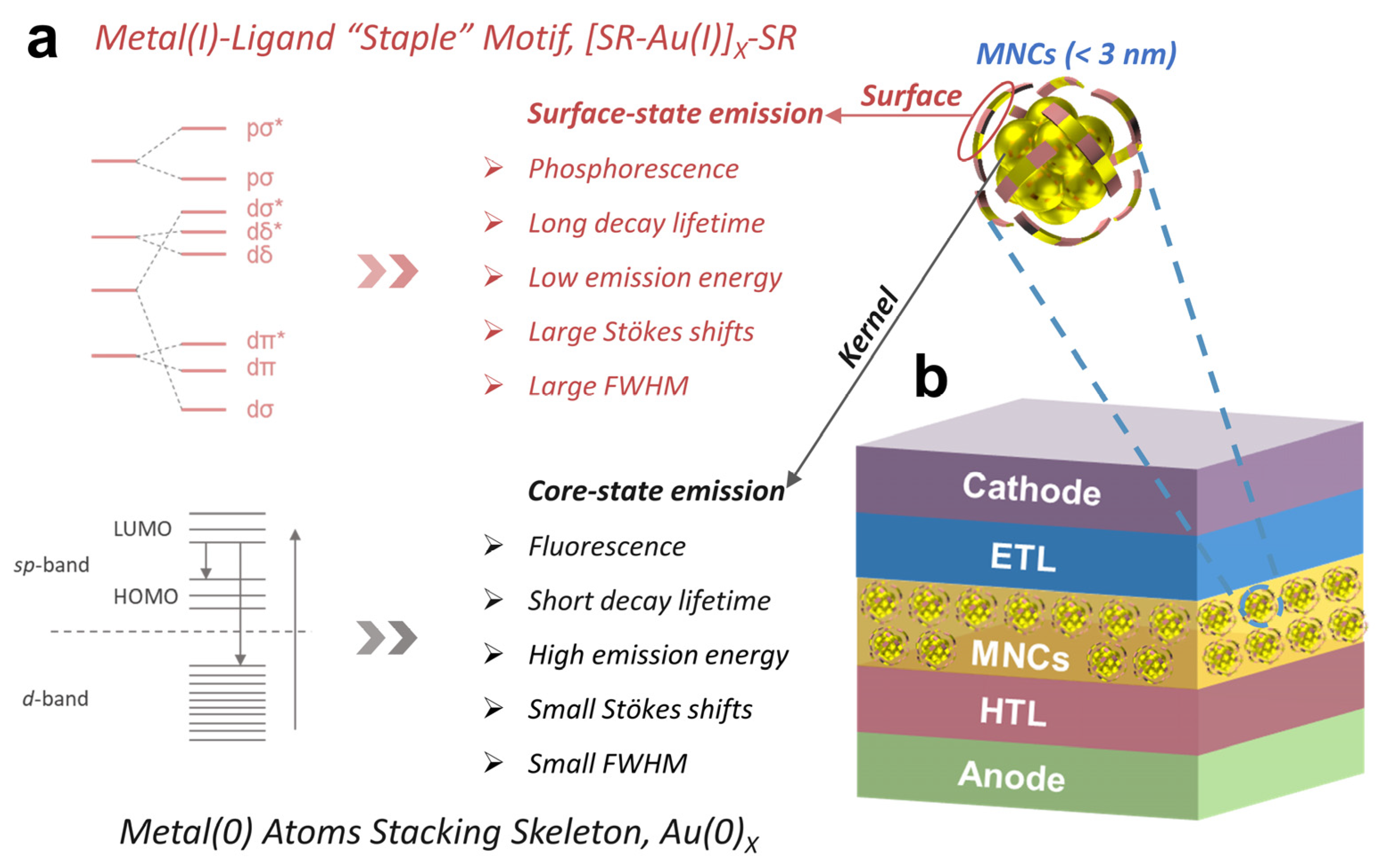
2. Correlating the Photoemission of MNCs to Their LED Applications
2.1. Metal Core-Related Photoemission
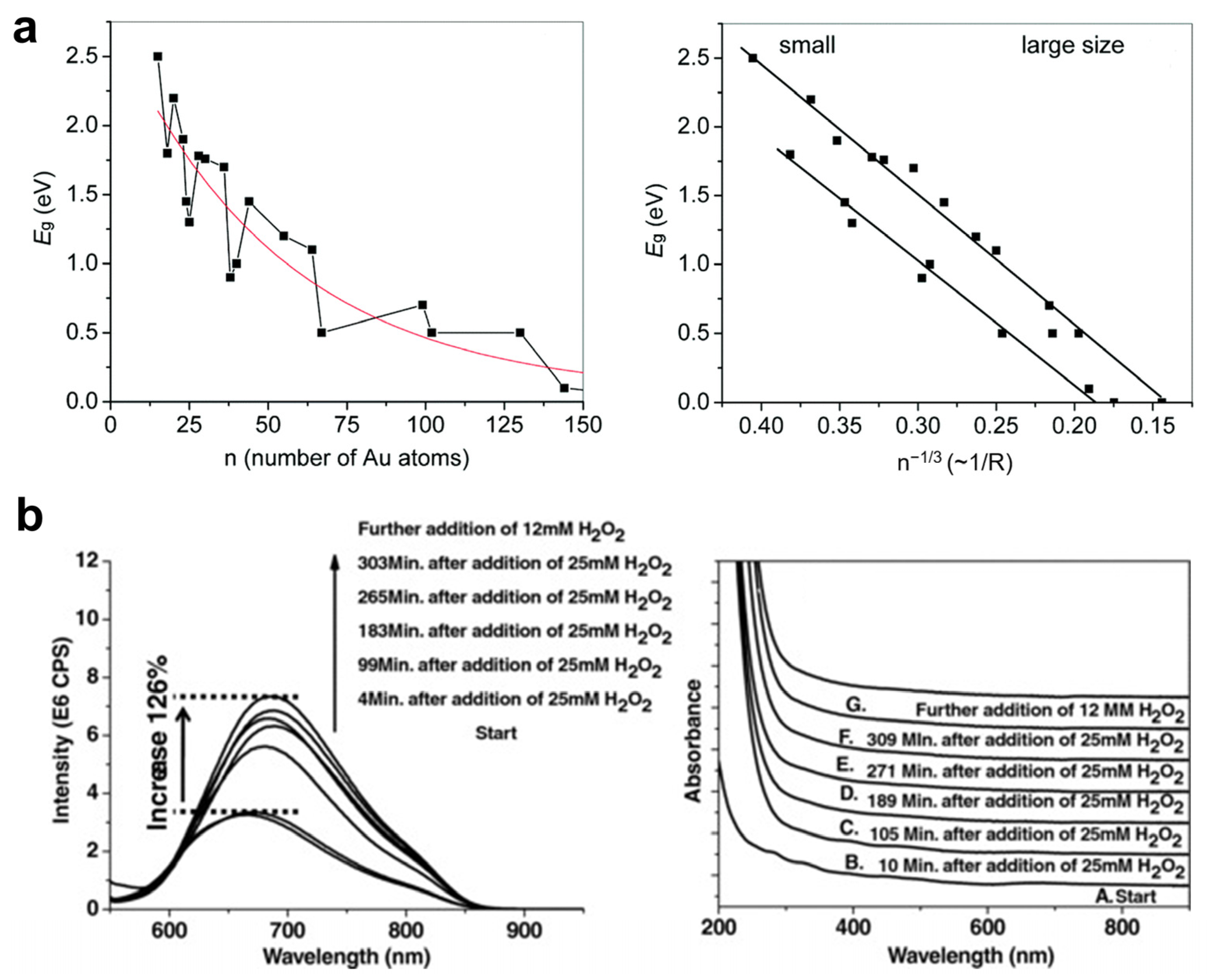
2.1.1. Effect of the Size of Metal Core
2.1.2. Effect of the Oxidation State of Metal Core
2.1.3. Effect of the Composition and Structure of Metal Core
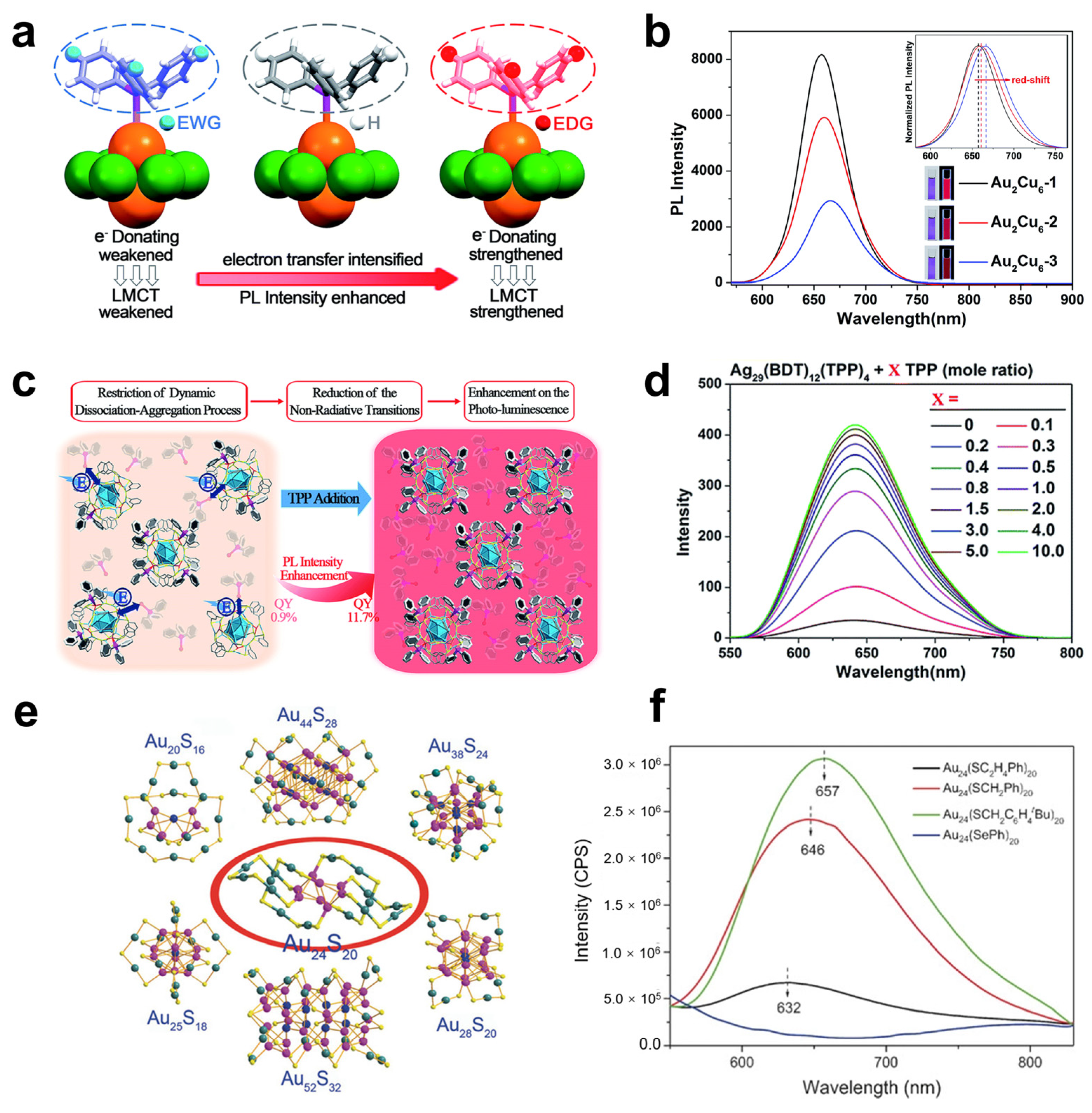
2.2. Ligand Shell-Related Photoemission
2.2.1. Effect of the Structure and Steric Hindrance of the Ligand Shell
2.2.2. Effect of the Tailorability of the Ligand Shell
2.3. Structure Modulation of MNCs
3. Electroluminescent Light-Emitting Diodes with Metal Nanoclusters
3.1. Monometallic Nanoclusters
3.2. Heterometallic Nanoclusters
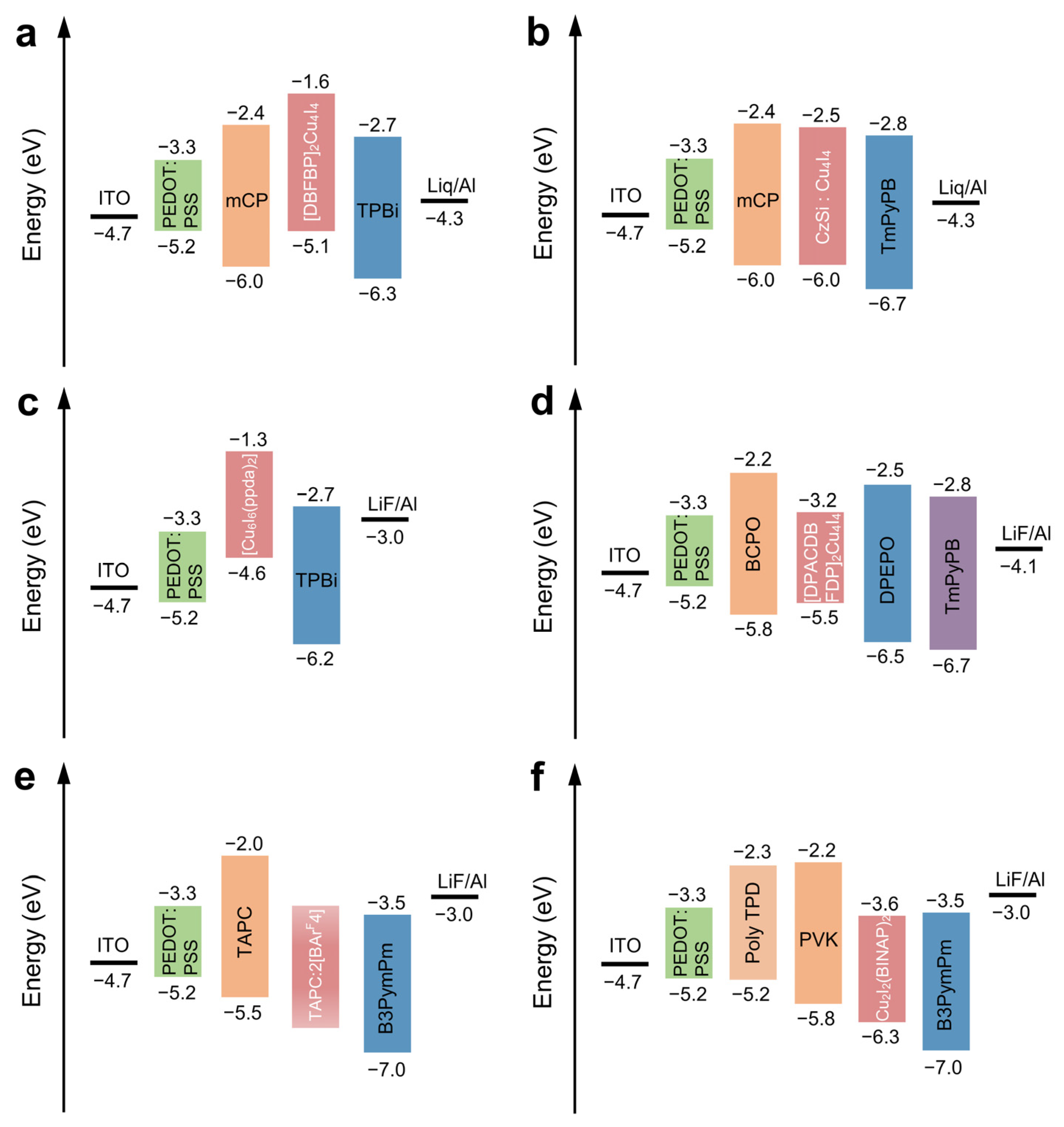
3.3. Copper Iodide Nanoclusters
4. Conclusions and Perspective
- Compared with traditional QDs materials, the PLQYs of MNCs is still at a low level. Co-optimization of the metal core and surface motifs needs to be employed to improve the PLQYs.
- The emission type of MNCs directly determines the performance of electroluminescent LEDs. At present, most of the electroluminescent LEDs are fluorescent MNCs, while phosphorescent MNCs have also been trialed and a high device efficiency has been obtained. Delayed fluorescence is an important feature of a new generation of luminescent materials. Triplet excitons can be effectively captured by the reverse intersystem crossing from the triplet to the singlet. It has a high radiation decay rate and fluorescence efficiency. In theory, the internal quantum efficiency can reach 100%, and it has been widely used in OLEDs and has made great progress. Therefore, exploring the development of delayed fluorescence MNCs’ materials and applying them as light-emitting layers to electroluminescent LEDs is bound to be of great significance for the improvement of device efficiency.
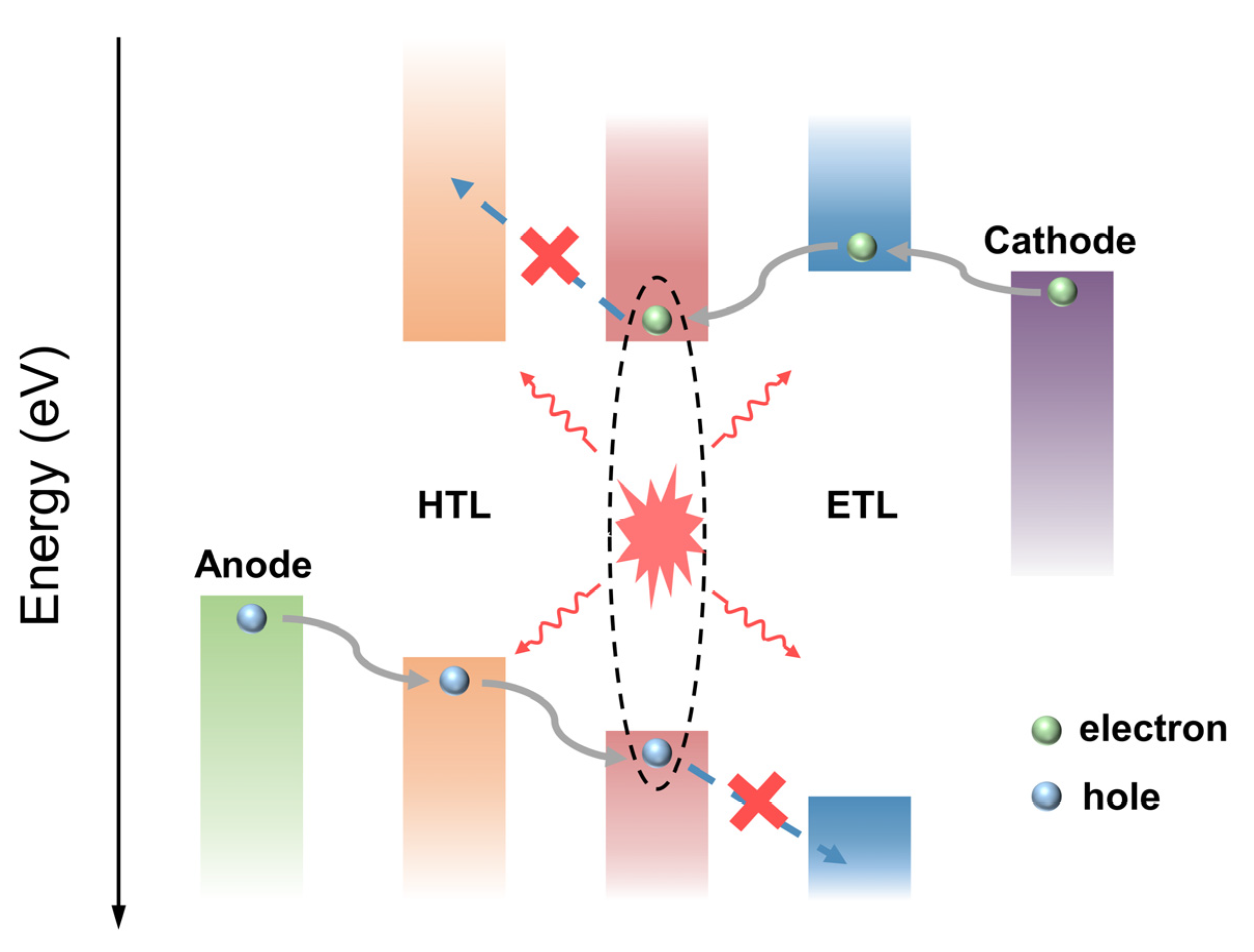
- Due to the imperfect structure, energy level, and emission mechanism of MNCs, the structure optimization and detailed working mechanism of MNC-based electroluminescent LEDs still need to be further investigated. Through the synergistic effect of the appropriate combination of functional layers with appropriate energy levels and the optimized light-emitting layer, the performance of the device (including the brightness and EQE) can be greatly improved. There is no doubt that with the development of high-performance LEDs, the importance of interface optimization will become more and more prominent. By adjusting the energy levels and thicknesses of the electron and hole transport layers, a relatively balanced electron and hole can be obtained in the light-emitting layer, which is beneficial to the radiative recombination of carriers in the light-emitting layer. At the same time, it is of great significance to confine the carriers in the light-emitting layer region to reduce the loss caused by non-radiative recombination. Drawing on the device design ideas, the analysis and optimization methods of OLEDs and perovskite LEDs will be conducive to developing the MNCs’ light-emitting layer LED structures and is expected to accelerate the development and device improvement of electroluminescent LEDs based on MNCs.
- In addition to applying the luminescence properties of MNCs to the luminescent layer of the electroluminescent LEDs, they can also be applied to the transport layer of the device to improve the carrier transmission performance or make the energy levels between the layers better matched by adjusting the energy levels of the transport layers, so as to jointly improve the performance of the device. At present, there are very few studies on improving the device transport layer based on MNCs, and researchers should further explore this field to enrich the application prospects of MNC-electroluminescent LEDs.
- At present, the research work based on MNCs is based on the processing and preparation of organic phase solvents, but organic solvents will cause great harm to the environment and operators. At the same time, a large number of fluorescent MNCs are water soluble, but there is no report on the application of aqueous MNCs as light-emitting layers, and there is still a large research space for MNCs in this field.
- Chiral metal nanoclusters have become a research hotspot in nanoscience and nanotechnology due to their potential applications in asymmetric catalysis, drug design, and chiral recognition and separation. At present, a series of optically pure monochiral NCs have been obtained through ligand engineering, and the application of these chiral MNCs in electroluminescent LED devices has promising application prospects. Some researchers have carried out related work in copper-iodine NCs and achieved excellent results, and the application of other MNCs, such as chiral Au NCs in electroluminescent LEDs, also needs further exploration.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, D.-W.; Li, M.; Chen, C.-F. Recent advances in circularly polarized electroluminescence based on organic light-emitting diodes. Chem. Soc. Rev. 2020, 49, 1331–1343. [Google Scholar] [CrossRef]
- Xiang, H.; Wang, R.; Chen, J.; Li, F.; Zeng, H. Research progress of full electroluminescent white light-emitting diodes based on a single emissive layer. Light Sci. Appl. 2021, 10, 206. [Google Scholar] [CrossRef]
- Vasilopoulou, M.; Yusoff, M.; Daboczi, M.; Conforto, J.; Gavim, A.E.X.; da Silva, W.J.; Macedo, A.G.; Soultati, A.; Pistolis, G.; Schneider, F.K. High efficiency blue organic light-emitting diodes with below-bandgap electroluminescence. Nat. Commun. 2021, 12, 4868. [Google Scholar] [CrossRef]
- Shen, Y.; Wu, H.Y.; Li, Y.Q.; Shen, K.C.; Gao, X.; Song, F.; Tang, J.X. Interfacial Nucleation Seeding for Electroluminescent Manipulation in Blue Perovskite Light-Emitting Diodes. Adv. Funct. Mater. 2021, 31, 2103870. [Google Scholar] [CrossRef]
- Liu, Q.; Smeets, S.; Mertens, S.; Xia, Y.; Valencia, A.; D’Haen, J.; Maes, W.; Vandewal, K. Narrow electroluminescence linewidths for reduced nonradiative recombination in organic solar cells and near-infrared light-emitting diodes. Joule 2021, 5, 2365–2379. [Google Scholar] [CrossRef]
- Ai, L.; Shi, R.; Yang, J.; Zhang, K.; Zhang, T.; Lu, S. Efficient Combination of G-C3N4 and CDs for Enhanced Photocatalytic Performance: A Review of Synthesis, Strategies, and Applications. Small 2021, 17, 2007523. [Google Scholar] [CrossRef]
- Sun, G.; Shi, J.-W.; Mao, S.; Ma, D.; He, C.; Wang, H.; Cheng, Y. Dodecylamine coordinated tri-arm CdS nanorod wrapped in intermittent ZnS shell for greatly improved photocatalytic H2 evolution. Chem. Eng. J. 2022, 429, 132382. [Google Scholar] [CrossRef]
- Gao, L.; Chen, H.; Wang, R.; Wei, S.; Kuklin, A.V.; Mei, S.; Zhang, F.; Zhang, Y.; Jiang, X.; Luo, Z. Ultra-Small 2D PbS Nanoplatelets: Liquid-Phase Exfoliation and Emerging Applications for Photo-Electrochemical Photodetectors. Small 2021, 17, 2005913. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, M.; Jin, R. Programmable metal nanoclusters with atomic precision. Adv. Mater. 2021, 33, 2006591. [Google Scholar] [CrossRef]
- Lin, Z.; Goswami, N.; Xue, T.; Chai, O.J.H.; Xu, H.; Liu, Y.; Su, Y.; Xie, J. Engineering metal nanoclusters for targeted therapeutics: From targeting strategies to therapeutic applications. Adv. Funct. Mater. 2021, 31, 2105662. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, M.; Song, Y.; Higaki, T.; Wang, H.; Jin, R. Double-helical assembly of heterodimeric nanoclusters into supercrystals. Nature 2021, 594, 380–384. [Google Scholar] [CrossRef]
- Luo, X.-M.; Gong, C.-H.; Pan, F.; Si, Y.; Yuan, J.-W.; Asad, M.; Dong, X.-Y.; Zang, S.-Q.; Mak, T.C. Small symmetry-breaking triggering large chiroptical responses of Ag70 nanoclusters. Nat. Commun. 2022, 13, 1177. [Google Scholar] [CrossRef]
- Liu, W.D.; Wang, J.Q.; Yuan, S.F.; Chen, X.; Wang, Q.M. Chiral superatomic nanoclusters Ag47 induced by the ligation of amino acids. Angew. Chem. Int. Ed. 2021, 60, 11430–11435. [Google Scholar] [CrossRef]
- Yao, Q.; Chen, T.; Yuan, X.; Xie, J. Toward total synthesis of thiolate-protected metal nanoclusters. Acc. Chem. Res. 2018, 51, 1338–1348. [Google Scholar] [CrossRef]
- Yuan, X.; Luo, Z.; Yu, Y.; Yao, Q.; Xie, J. Luminescent noble metal nanoclusters as an emerging optical probe for sensor development. Chem. Asian J. 2013, 8, 858–871. [Google Scholar] [CrossRef]
- Yao, Q.; Wu, Z.; Liu, Z.; Lin, Y.; Yuan, X.; Xie, J. Molecular reactivity of thiolate-protected noble metal nanoclusters: Synthesis, self-assembly, and applications. Chem. Sci. 2021, 12, 99–127. [Google Scholar] [CrossRef]
- Jin, R.; Li, G.; Sharma, S.; Li, Y.; Du, X. Toward active-site tailoring in heterogeneous catalysis by atomically precise metal nanoclusters with crystallographic structures. Chem. Rev. 2020, 121, 567–648. [Google Scholar] [CrossRef]
- Wu, Z.; Yao, Q.; Zang, S.; Xie, J. Directed self-assembly of ultrasmall metal nanoclusters. ACS Mater. Lett. 2019, 1, 237–248. [Google Scholar] [CrossRef]
- Yang, T.-Q.; Peng, B.; Shan, B.-Q.; Zong, Y.-X.; Jiang, J.-G.; Wu, P.; Zhang, K. Origin of the photoluminescence of metal nanoclusters: From metal-centered emission to ligand-centered emission. Nanomaterials 2020, 10, 261. [Google Scholar] [CrossRef] [Green Version]
- Kang, X.; Zhu, M. Tailoring the photoluminescence of atomically precise nanoclusters. Chem. Soc. Rev. 2019, 48, 2422–2457. [Google Scholar] [CrossRef]
- Maity, S.; Bain, D.; Patra, A. Engineering atomically precise copper nanoclusters with aggregation induced emission. J. Phys. Chem. C 2019, 123, 2506–2515. [Google Scholar] [CrossRef]
- Aikens, C.M. Electronic and geometric structure, optical properties, and excited state behavior in atomically precise thiolate-stabilized noble metal nanoclusters. Acc. Chem. Res. 2018, 51, 3065–3073. [Google Scholar] [CrossRef]
- Huang, Y.; Hsiang, E.-L.; Deng, M.-Y.; Wu, S.-T. Mini-LED, Micro-LED and OLED displays: Present status and future perspectives. Light Sci. Appl. 2020, 9, 105. [Google Scholar] [CrossRef]
- Salehi, A.; Fu, X.; Shin, D.H.; So, F. Recent advances in OLED optical design. Adv. Funct. Mater. 2019, 29, 1808803. [Google Scholar] [CrossRef]
- Luo, Y.; Li, S.; Zhao, Y.; Li, C.; Pang, Z.; Huang, Y.; Yang, M.; Zhou, L.; Zheng, X.; Pu, X. An ultraviolet thermally activated delayed fluorescence OLED with total external quantum efficiency over 9%. Adv. Mater. 2020, 32, 2001248. [Google Scholar] [CrossRef]
- Wang, Z.; Shen, X.; Tang, C.; Li, X.; Hu, J.; Zhu, J.; Yu, W.W.; Song, H.; Bai, X. Efficient and Stable CF3PEAI-Passivated CsPbI3 QDs toward Red LEDs. ACS Appl. Mater. Interfaces 2022, 14, 8235–8242. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, S.; Li, X.; Yuan, M.; Turyanska, L.; Yang, X. Core/shell perovskite nanocrystals: Synthesis of highly efficient and environmentally stable FAPbBr3/CsPbBr3 for LED applications. Adv. Funct. Mater. 2020, 30, 1910582. [Google Scholar] [CrossRef]
- Pimputkar, S.; Speck, J.S.; DenBaars, S.P.; Nakamura, S. Prospects for LED lighting. Nature photonics. 2009, 3, 180–182. [Google Scholar] [CrossRef]
- Pu, C.; Dai, X.; Shu, Y.; Zhu, M.; Deng, Y.; Jin, Y.; Peng, X. Electrochemically-stable ligands bridge the photoluminescence-electroluminescence gap of quantum dots. Nat. Commun. 2020, 11, 937. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Duan, X.; Lv, Y.-K.; Zhu, L.; Zhang, Z.; Yu, B.; Jin, Y.; Si, Y.; Wang, Z.; Li, B. Encapsulating metal nanoclusters inside porous organic cage towards enhanced radio-sensitivity and solubility. Chem. Eng. J. 2021, 426, 130872. [Google Scholar] [CrossRef]
- Bodiuzzaman, M.; Dar, W.A.; Pradeep, T. Cocrystals of atomically precise noble metal nanoclusters. Small 2021, 17, 2003981. [Google Scholar] [CrossRef]
- Rival, J.V.; Mymoona, P.; Lakshmi, K.M.; Pradeep, T.; Shibu, E.S. Self-Assembly of Precision Noble Metal Nanoclusters: Hierarchical Structural Complexity, Colloidal Superstructures, and Applications. Small 2021, 17, 2005718. [Google Scholar] [CrossRef]
- Kang, X.; Wei, X.; Liu, X.; Wang, S.; Yao, T.; Wang, S.; Zhu, M. A reasonable approach for the generation of hollow icosahedral kernels in metal nanoclusters. Nat. Commun. 2021, 12, 6186. [Google Scholar] [CrossRef]
- Brütting, W.; Berleb, S.; Mückl, A.G. Device physics of organic light-emitting diodes based on molecular materials. Org. Electron. 2001, 2, 1–36. [Google Scholar] [CrossRef]
- Qian, L.; Zheng, Y.; Xue, J.; Holloway, P.H. Stable and efficient quantum-dot light-emitting diodes based on solution-processed multilayer structures. Nat. Photonics 2011, 5, 543–548. [Google Scholar] [CrossRef]
- Pan, J.; Quan, L.N.; Zhao, Y.; Peng, W.; Murali, B.; Sarmah, S.P.; Yuan, M.; Sinatra, L.; Alyami, N.M.; Liu, J. Highly efficient perovskite-quantum-dot light-emitting diodes by surface engineering. Adv. Mater. 2016, 28, 8718–8725. [Google Scholar] [CrossRef]
- Lin, K.; Xing, J.; Quan, L.N.; De Arquer, F.; Gong, X.; Lu, J.; Xie, L.; Zhao, W.; Zhang, D.; Yan, C. Perovskite light-emitting diodes with external quantum efficiency exceeding 20 per cent. Nature 2018, 562, 245–248. [Google Scholar] [CrossRef]
- Fakharuddin, A.; Gangishetty, M.K.; Abdi-Jalebi, M.; Chin, S.-H.; bin Mohd Yusoff, A.; Congreve, D.N.; Tress, W.; Deschler, F.; Vasilopoulou, M.; Bolink, H.J. Perovskite light-emitting diodes. Nat. Electron. 2022, 5, 203–216. [Google Scholar] [CrossRef]
- Jia, Z.; Yuan, C.; Liu, Y.; Wang, X.-J.; Sun, P.; Wang, L.; Jiang, H.; Jiang, J. Strategies to approach high performance in Cr3+-doped phosphors for high-power NIR-LED light sources. Light Sci. Appl. 2020, 9, 86. [Google Scholar]
- Mattei, G.; Mazzoldi, P.; Bernas, H. Metal nanoclusters for optical properties. In Materials Science with Ion Beams; Springer: Berlin, Germany, 2009; Volume 23, pp. 287–316. [Google Scholar]
- Du, Y.; Sheng, H.; Astruc, D.; Zhu, M. Atomically precise noble metal nanoclusters as efficient catalysts: A bridge between structure and properties. Chem. Rev. 2019, 120, 526–622. [Google Scholar] [CrossRef]
- Tang, Q.; Hu, G.; Fung, V.; Jiang, D.-E. Insights into interfaces, stability, electronic properties, and catalytic activities of atomically precise metal nanoclusters from first principles. Acc. Chem. Res. 2018, 45, 273–276. [Google Scholar] [CrossRef] [PubMed]
- Guan, Z.-J.; Hu, F.; Li, J.-J.; Wen, Z.-R.; Lin, Y.-M.; Wang, Q.-M. Isomerization in alkynyl-protected gold nanoclusters. J. Am. Chem. Soc. 2020, 142, 2995–3001. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Lin, H.; Cao, Y.; Yao, Q.; Xie, J. Interactions of metal nanoclusters with light: Fundamentals and applications. Adv. Mater. 2022, 34, 2103918. [Google Scholar] [CrossRef]
- Zhang, B.; Chen, J.; Cao, Y.; Chai, O.J.H.; Xie, J. Ligand Design in Ligand-Protected Gold Nanoclusters. Small 2021, 17, 2004381. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.; Li, Y.; Zhu, M.; Jin, R. Atomically precise alloy nanoclusters: Syntheses, structures, and properties. Chem. Soc. Rev. 2020, 49, 6443–6514. [Google Scholar] [CrossRef]
- Kang, X.; Zhu, M. Transformation of atomically precise nanoclusters by ligand-exchange. Chem. Mater. 2019, 31, 9939–9969. [Google Scholar] [CrossRef]
- Lopez-Martinez, E.; Gianolio, D.; Garcia-Orrit, S.; Vega-Mayoral, V.; Cabanillas-Gonzalez, J.; Sanchez-Cano, C.; Cortajarena, A.L. Tuning the Optical Properties of Au Nanoclusters by Designed Proteins. Adv. Opt. Mater. 2022, 10, 2101332. [Google Scholar] [CrossRef]
- Zheng, J.; Zhang, C.; Dickson, R.M. Highly fluorescent, water-soluble, size-tunable gold quantum dots. Phys. Rev. Lett. 2004, 93, 077402. [Google Scholar] [CrossRef]
- Jin, R. Atomically precise metal nanoclusters: Stable sizes and optical properties. Nanoscale 2015, 7, 1549–1565. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, M.; Yang, J.; Zheng, X.; Cai, W.; Meng, G.; Qian, H.; Wang, H.; Jin, R. Well-Defined Nanoclusters as Fluorescent Nanosensors: A Case Study on Au25(SG)18. Small 2012, 8, 2028–2035. [Google Scholar] [CrossRef]
- Kang, X.; Xiong, L.; Wang, S.; Yu, H.; Jin, S.; Song, Y.; Chen, T.; Zheng, L.; Pan, C.; Pei, Y. Shape-Controlled Synthesis of Trimetallic Nanoclusters: Structure Elucidation and Properties Investigation. Chem. Eur. J. 2016, 22, 17145–17150. [Google Scholar] [CrossRef] [PubMed]
- Joshi, C.P.; Bootharaju, M.S.; Alhilaly, M.J.; Bakr, O.M. [Ag25(SR)18]−: The “golden” silver nanoparticle. J. Am. Chem. Soc. 2015, 137, 11578–11581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Yuan, J.; Yao, C.; Chen, J.; Li, L.; Bao, X.; Yang, J.; Wu, Z. Crystal and solution photoluminescence of MAg24(SR)18 (M=Ag/Pd/Pt/Au) nanoclusters and some implications for the photoluminescence mechanisms. J. Phys. Chem. C 2017, 121, 13848–13853. [Google Scholar] [CrossRef]
- Kang, X.; Zhou, M.; Wang, S.; Jin, S.; Sun, G.; Zhu, M.; Jin, R. The tetrahedral structure and luminescence properties of Bi-metallic Pt1Ag28(SR)18(PPh3)4 nanocluster. Chem. Sci. 2017, 8, 2581–2587. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Jin, S.; Xiong, L.; Chen, M.; Zhang, J.; Zou, X.; Pei, Y.; Wang, S.; Zhu, M. Ag50(Dppm)6(SR) 30 and Its Homologue AuxAg50–x(Dppm)6(SR)30 Alloy Nanocluster: Seeded Growth, Structure Determination, and Differences in Properties. J. Am. Chem. Soc. 2017, 139, 1618–1624. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Fung, V.; Yao, Q.; Chen, T.; Zang, S.; Jiang, D.-E.; Xie, J. Control of single-ligand chemistry on thiolated Au25 nanoclusters. Nat. Commun. 2020, 11, 5498. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Zhang, B.; Yao, Q.; Yang, Y.; Xie, J.; Yan, N. Recent advances in the synthesis and catalytic applications of ligand-protected, atomically precise metal nanoclusters. Coord. Chem. Rev. 2016, 322, 1–29. [Google Scholar] [CrossRef]
- Kang, X.; Li, X.; Yu, H.; Lv, Y.; Sun, G.; Li, Y.; Wang, S.; Zhu, M. Modulating photo-luminescence of Au2Cu6 nanoclusters via ligand-engineering. RSC Adv. 2017, 7, 28606–28609. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.; Jin, R. On the ligand’s role in the fluorescence of gold nanoclusters. Nano Lett. 2010, 10, 2568–2573. [Google Scholar] [CrossRef]
- Gan, Z.; Lin, Y.; Luo, L.; Han, G.; Liu, W.; Liu, Z.; Yao, C.; Weng, L.; Liao, L.; Chen, J. Fluorescent gold nanoclusters with interlocked staples and a fully thiolate-bound kernel. Angew. Chem. Int. Ed. 2016, 55, 11567–11571. [Google Scholar] [CrossRef]
- Kang, X.; Wang, S.; Zhu, M. Observation of a new type of aggregation-induced emission in nanoclusters. Chem. Sci. 2018, 9, 3062–3068. [Google Scholar] [CrossRef] [PubMed]
- Ding, D.; Li, K.; Liu, B.; Tang, B.Z. Bioprobes based on AIE fluorogens. Acc. Chem. Res. 2013, 46, 2441–2453. [Google Scholar] [CrossRef] [PubMed]
- Goswami, N.; Yao, Q.; Luo, Z.; Li, J.; Chen, T.; Xie, J. Luminescent metal nanoclusters with aggregation-induced emission. J. Phys. Chem. Lett. 2016, 7, 962–975. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Duchesne, P.N.; Yu, M.; Jiang, X.; Ning, X.; Vinluan, R.D., III; Zhang, P.; Zheng, J. Luminescent gold nanoparticles with size-independent emission. Angew. Chem. 2016, 128, 9040–9044. [Google Scholar] [CrossRef]
- Wu, Z.; Yao, Q.; Chai, O.J.H.; Ding, N.; Xu, W.; Zang, S.; Xie, J. Unraveling the impact of gold (I)–thiolate motifs on the aggregation-induced emission of gold nanoclusters. Angew. Chem. 2020, 132, 10020–10025. [Google Scholar] [CrossRef]
- Zhuang, S.; Liao, L.; Yuan, J.; Xia, N.; Zhao, Y.; Wang, C.; Gan, Z.; Yan, N.; He, L.; Li, J. Fcc versus Non-fcc Structural Isomerism of Gold Nanoparticles with Kernel Atom Packing Dependent Photoluminescence. Angew. Chem. Int. Ed. 2019, 58, 4510–4514. [Google Scholar] [CrossRef]
- Zhou, M.; Higaki, T.; Hu, G.; Sfeir, M.Y.; Chen, Y.; Jiang, D.-e.; Jin, R. Three-orders-of-magnitude variation of carrier lifetimes with crystal phase of gold nanoclusters. Science 2019, 364, 279–282. [Google Scholar] [CrossRef]
- Tang, M.-C.; Chan, M.-Y.; Yam, V.W.-W. Molecular Design of Luminescent Gold (III) Emitters as Thermally Evaporable and Solution-Processable Organic Light-Emitting Device (OLED) Materials: Focus Review. Chem. Rev. 2021, 121, 7249–7279. [Google Scholar] [CrossRef]
- Kashida, J.; Shoji, Y.; Ikabata, Y.; Taka, H.; Sakai, H.; Hasobe, T.; Nakai, H.; Fukushima, T. An Air-and Water-Stable B4N4-Heteropentalene Serving as a Host Material for a Phosphorescent OLED. Angew. Chem. Int. Ed. 2021, 60, 23812–23818. [Google Scholar] [CrossRef]
- Burmeister, D.; Tran, H.A.; Müller, J.; Guerrini, M.; Cocchi, C.; Plaickner, J.; Kochovski, Z.; List-Kratochvil, E.J.; Bojdys, M.J. Optimized Synthesis of Solution-Processable Crystalline Poly (Triazine Imide) with Minimized Defects for OLED Application. Angew. Chem. Int. Ed. 2022, 61, 202111749. [Google Scholar] [CrossRef]
- Ma, J.; Idris, M.; Li, T.Y.; Ravinson, D.S.; Fleetham, T.; Kim, J.; Djurovich, P.I.; Forrest, S.R.; Thompson, M.E. Symmetric “Double Spiro” Wide Energy Gap Hosts for Blue Phosphorescent OLED Devices. Adv. Opt. Mater. 2022, 10, 2101530. [Google Scholar] [CrossRef]
- Chang, M.-H.; Das, D.; Varde, P.V.; Pecht, M. Light emitting diodes reliability review. Microelectron. Reliab. 2012, 52, 762–782. [Google Scholar] [CrossRef]
- Bergh, A.A.; Dean, P.J. Light-emitting diodes. Oxford 1976, 54, 526–529. [Google Scholar] [CrossRef]
- Bourget, C.M. An introduction to light-emitting diodes. Hortscience 2008, 43, 1944–1946. [Google Scholar] [CrossRef] [Green Version]
- Niesen, B.; Rand, B.P. Thin Film Metal Nanocluster Light-Emitting Devices. Adv. Mater. 2014, 26, 1446–1449. [Google Scholar] [CrossRef]
- Koh, T.-W.; Hiszpanski, A.; Sezen, M.; Naim, A.; Galfsky, T.; Trivedi, A.; Loo, Y.-L.; Menon, V.; Rand, B. Metal nanocluster light-emitting devices with suppressed parasitic emission and improved efficiency: Exploring the impact of photophysical properties. Nanoscale 2015, 7, 9140–9146. [Google Scholar] [CrossRef]
- Chao, Y.-C.; Cheng, K.-P.; Lin, C.-Y.; Chang, Y.-L.; Ko, Y.-Y.; Hou, T.-Y.; Huang, C.-Y.; Chang, W.H.; Lin, C.-A.J. Non-toxic gold nanoclusters for solution-processed white light-emitting diodes. Sci. Rep. 2018, 8, 8860. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.J.; Wang, J.Y.; Zhu, X.F.; Zeng, X.C.; Chen, Z.N. Phosphorescent Cationic Au4Ag2 Alkynyl Cluster Complexes for Efficient Solution-Processed Organic Light-Emitting Diodes. Adv. Funct. Mater. 2015, 25, 3033–3042. [Google Scholar] [CrossRef]
- Xu, L.-J.; Zhang, X.; Wang, J.-Y.; Chen, Z.-N. High-efficiency solution-processed OLEDs based on cationic Ag 6 Cu heteroheptanuclear cluster complexes with aromatic acetylides. J. Mater. Chem. C 2016, 4, 1787–1794. [Google Scholar] [CrossRef]
- Natarajan, N.; Shi, L.-X.; Xiao, H.; Wang, J.-Y.; Zhang, L.-Y.; Zhang, X.; Chen, Z.-N. Using phosphorescent PtAu3 clusters for superior solution-processable organic light emitting diodes with very small efficiency roll-off. J. Mater. Chem. C 2018, 6, 8966–8976. [Google Scholar] [CrossRef]
- Natarajan, N.; Shi, L.-X.; Xiao, H.; Wang, J.-Y.; Zhang, L.-Y.; Zhang, X.; Chen, Z.-N. PtAu3 cluster complexes with narrow-band emissions for solution-processed organic light emitting diodes. J. Mater. Chem. C 2019, 7, 2604–2614. [Google Scholar] [CrossRef]
- Huang, Y.-Z.; Shi, L.-X.; Wang, J.-Y.; Su, H.-F.; Chen, Z.-N. Elaborate Design of Ag8Au10 Cluster [2] Catenane Phosphors for High-Efficiency Light-Emitting Devices. ACS Appl. Mater. Interfaces 2020, 12, 57264–57270. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Z.; Yang, M.; Wang, J.-Y.; Huang, Y.-Z.; Xie, P.; Chen, Z.-N. High-efficiency solution-processed light-emitting diode based on a phosphorescent Ag3Cu5 cluster complex. J. Mater. Chem. C 2021, 9, 5528–5534. [Google Scholar] [CrossRef]
- Xie, M.; Han, C.; Zhang, J.; Xie, G.; Xu, H. White electroluminescent phosphine-chelated copper iodide nanoclusters. Chem. Mater. 2017, 29, 6606–6610. [Google Scholar] [CrossRef]
- Xie, M.; Han, C.; Liang, Q.; Zhang, J.; Xie, G.; Xu, H. Highly efficient sky blue electroluminescence from ligand-activated copper iodide clusters: Overcoming the limitations of cluster light-emitting diodes. Sci. Adv. 2019, 5, 9853–9857. [Google Scholar] [CrossRef] [Green Version]
- Zhang, N.; Hu, H.; Qu, L.; Huo, R.; Zhang, J.; Duan, C.; Meng, Y.; Han, C.; Xu, H. Overcoming Efficiency Limitation of Cluster Light-Emitting Diodes with Asymmetrically Functionalized Biphosphine Cu4I4 Cubes. J. Am. Chem. Soc. 2022, 144, 6551–6557. [Google Scholar] [CrossRef]
- Xu, K.; Chen, B.-L.; Zhang, R.; Liu, L.; Zhong, X.-X.; Wang, L.; Li, F.-Y.; Li, G.-H.; Alamry, K.A.; Li, F.-B. From a blue to white to yellow emitter: A hexanuclear copper iodide nanocluster. Dalton Trans. 2020, 49, 5859–5868. [Google Scholar] [CrossRef]
- Roose, J.; Tang, B.Z.; Wong, K.S. Circularly-polarized luminescence (CPL) from chiral AIE molecules and macrostructures. Small 2016, 12, 6495–6512. [Google Scholar] [CrossRef]
- Wang, J.-J.; Zhou, H.-T.; Yang, J.-N.; Feng, L.-Z.; Yao, J.-S.; Song, K.-H.; Zhou, M.-M.; Jin, S.; Zhang, G.; Yao, H.-B. Chiral Phosphine–Copper Iodide Hybrid Cluster Assemblies for Circularly Polarized Luminescence. J. Am. Chem. Soc. 2021, 143, 10860–10864. [Google Scholar] [CrossRef]
- Zheng, J.; Wang, J.-N.; Wang, T.; Wu, K.; Wei, R.-J.; Lu, W.; Li, D. Phosphorescent Metal Rotaxane-like Bimetallic Ag/Au Clusters. J. Phys. Chem. C 2021, 125, 9400–9410. [Google Scholar] [CrossRef]
- Zheng, K.; Xie, J. Engineering ultrasmall metal nanoclusters as promising theranostic agents. Trends Chem. 2020, 2, 665–679. [Google Scholar] [CrossRef]
- Troyano, J.; Zamora, F.; Delgado, S. Copper (I)–iodide cluster structures as functional and processable platform materials. Chem. Soc. Rev. 2021, 50, 4606–4628. [Google Scholar] [CrossRef] [PubMed]
- Benito, Q.; Le Goff, X.F.; Nocton, G.; Fargues, A.; Garcia, A.; Berhault, A.; Kahlal, S.; Saillard, J.-Y.; Martineau, C.; Trébosc, J. Geometry flexibility of copper iodide clusters: Variability in luminescence thermochromism. Inorg. Chem. 2015, 54, 4483–4494. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xie, M.; Xin, Y.; Han, C.; Xie, L.; Yi, M.; Xu, H. Organophosphine-Sandwiched Copper Iodide Cluster Enables Charge Trapping. Angew. Chem. Int. Ed. 2021, 60, 24894–24900. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Djurovich, P.I.; Whited, M.T.; Thompson, M.E. Cu4I4 clusters supported by P∧N-type ligands: New structures with tunable emission colors. Inorg. Chem. 2012, 51, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Wang, F.; Zhang, J.; Bu, X. Luminescent MTN-type cluster–organic framework with 2.6 nm cages. J. Am. Chem. Soc. 2012, 134, 17881–17884. [Google Scholar] [CrossRef]
- Hu, M.; Feng, H.-T.; Yuan, Y.-X.; Zheng, Y.-S.; Tang, B.Z. Chiral AIEgens–Chiral recognition, CPL materials and other chiral applications. Coord. Chem. Rev. 2020, 416, 213329. [Google Scholar] [CrossRef]
- Huck, N.P.; Jager, W.F.; De Lange, B.; Feringa, B.L. Dynamic control and amplification of molecular chirality by circular polarized light. Science 1996, 273, 1686–1688. [Google Scholar] [CrossRef] [Green Version]
- Ishii, A.; Miyasaka, T. Direct detection of circular polarized light in helical 1D perovskite-based photodiode. Sci. Adv. 2020, 6, 3270–3274. [Google Scholar] [CrossRef]




| Definition | Abbreviations |
|---|---|
| metal nanoclusters | MNCs |
| nanoclusters | NCs |
| light emitting diodes | LEDs |
| quantum dots | QDs |
| metal nanoparticles | MNPs |
| highest occupied molecular orbital | HOMO |
| lowest unoccupied molecular orbital | LUMO |
| photoluminescence quantum yields | PLQYs |
| organic light emitting diodes | OLEDs |
| quantum dots light emitting diodes | QLEDs |
| electron transport layer | ETL |
| electron injection layer | EIL |
| hole transport layer | HTL |
| hole injection layer | HIL |
| energy gap | Eg |
| ligand to metal charge transfer | LMCT |
| aggregation induced emission | AIE |
| ligand to metal-metal charge transfer | LMMCT |
| 4-tert-butyl sulfide | TBBT |
| face center cubic | FCC |
| body centered cubic | BCC |
| indium tin oxide | ITO |
| lithium fuoride | LiF |
| poly(N-vinyl carbazole) | PVK |
| N,N′-Bis(3-methylphenyl)-N,N′-diphenylbenzidine | TPD |
| hexagonal close packed | HCP |
| cetyltrimethylammonium bromide | CTAB |
| external quantum efficiency | EQE |
| ethoxylated polyethyleneimine | PEIE |
| copper thiocyanate | CuSCN |
| N,N-dicarbazolyl-3,5-benzene | mCP |
| 2,6-(PPh2)2C6H3 | PCP |
| tris(diphenylphosphine)methane | CH(PPh2)3 |
| tris(4-(9H-carbazol-9yl)phenyl)amine | TCTA |
| 1,3-bis(5(4-(tert-butyl)phenyl)-1,3,4-oxadiazol-2-yl)benzene | OXD-7 |
| full width at half maximum | FWHM |
| current efficiency | CE |
| 3,6-di-tert-butyl-1,8-diformyl-9H-carbazole | H3decz |
| 2,9-bis(diphenylphosphine)-dibenzofuran | DBFDP |
| 9,9-dimethylacridine | DMAC |
| (dimethylamino)phenyl(phenyl)phosphino]-N,N-dimethylaniline | ppda |
| thermally activated delayed fluorescence | TADF |
| metal to ligand charge transfer | MLCT |
| halogen to ligand charge transfer | XLCT |
| International Commission on illumination | CIE |
| density functional theory | DFT |
| circularly polarized light | CPL |
| bis(diphenylphosphino)-binaphthalene | BINAP |
| Light−Emitting Layer | PLQY(%) | Lmax(cd m−2) | EQEmax(%) | Wavelength (nm) | Ref. | |
|---|---|---|---|---|---|---|
| Monometallic NCs | Au25 or Ag25 NCs | N | N | 0.013 | 750 | [76] |
| GSH: Au NCs | 15 | 40 | 0.12 | 625 | [77] | |
| Au NCs | 4.99 | 100 | 0.08 | W | [78] | |
| Heterometallic NCs | Au4Ag2 NCs | 10.3 | 8804 | 7.0 | 539 | [79] |
| Ag6Cu NCs | 53 | 184 | 13.9 | 573 | [80] | |
| PtAu3 NCs | 90 | 1000 | 18 | 588 | [81] | |
| PtAu3 NCs | 87.3 | 6539 | 16.6 | 556 | [82] | |
| Ag8Au10 NCs | 63 | 14,859 | 15.7 | 567 | [83] | |
| Ag3Cu5 NCs | 18 | 8554 | 14.7 | 585 | [84] | |
| Copper iodide NCs | [DBFDP]2Cu4I4 NCs | 5 | 1500 | 0.73 | W | [85] |
| [DtBCzDBFDP]2Cu4I4 NCs | 65 | 7000 | 7.9 | 491 | [86] | |
| [DPACDBFDP]2Cu4I4 NCs | 81 | 4000 | 19.5 | 500 | [87] | |
| Cu6I6(ppda)2 NCs | 36 | N | 0.31 | 564 | [88] | |
| Cu4(PCP)3(BF4) NCs | 93 | 8900 | 11.2 | 518 | [89] | |
| Cu2I2(BINAP)2 NCs | 4.7 | 1200 | 0.54 | 515 | [90] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, T.; Wang, Z.; Zhang, Y.; Wu, Z. Engineering Coinage Metal Nanoclusters for Electroluminescent Light-Emitting Diodes. Nanomaterials 2022, 12, 3837. https://doi.org/10.3390/nano12213837
Li T, Wang Z, Zhang Y, Wu Z. Engineering Coinage Metal Nanoclusters for Electroluminescent Light-Emitting Diodes. Nanomaterials. 2022; 12(21):3837. https://doi.org/10.3390/nano12213837
Chicago/Turabian StyleLi, Tingting, Zhenyu Wang, Ying Zhang, and Zhennan Wu. 2022. "Engineering Coinage Metal Nanoclusters for Electroluminescent Light-Emitting Diodes" Nanomaterials 12, no. 21: 3837. https://doi.org/10.3390/nano12213837
APA StyleLi, T., Wang, Z., Zhang, Y., & Wu, Z. (2022). Engineering Coinage Metal Nanoclusters for Electroluminescent Light-Emitting Diodes. Nanomaterials, 12(21), 3837. https://doi.org/10.3390/nano12213837






