Reactive Oxygen Species Formed by Metal and Metal Oxide Nanoparticles in Physiological Media—A Review of Reactions of Importance to Nanotoxicity and Proposal for Categorization
Abstract
:1. Introduction
2. ROS Formation on Metal and Metal Oxide NPs: General Mechanisms
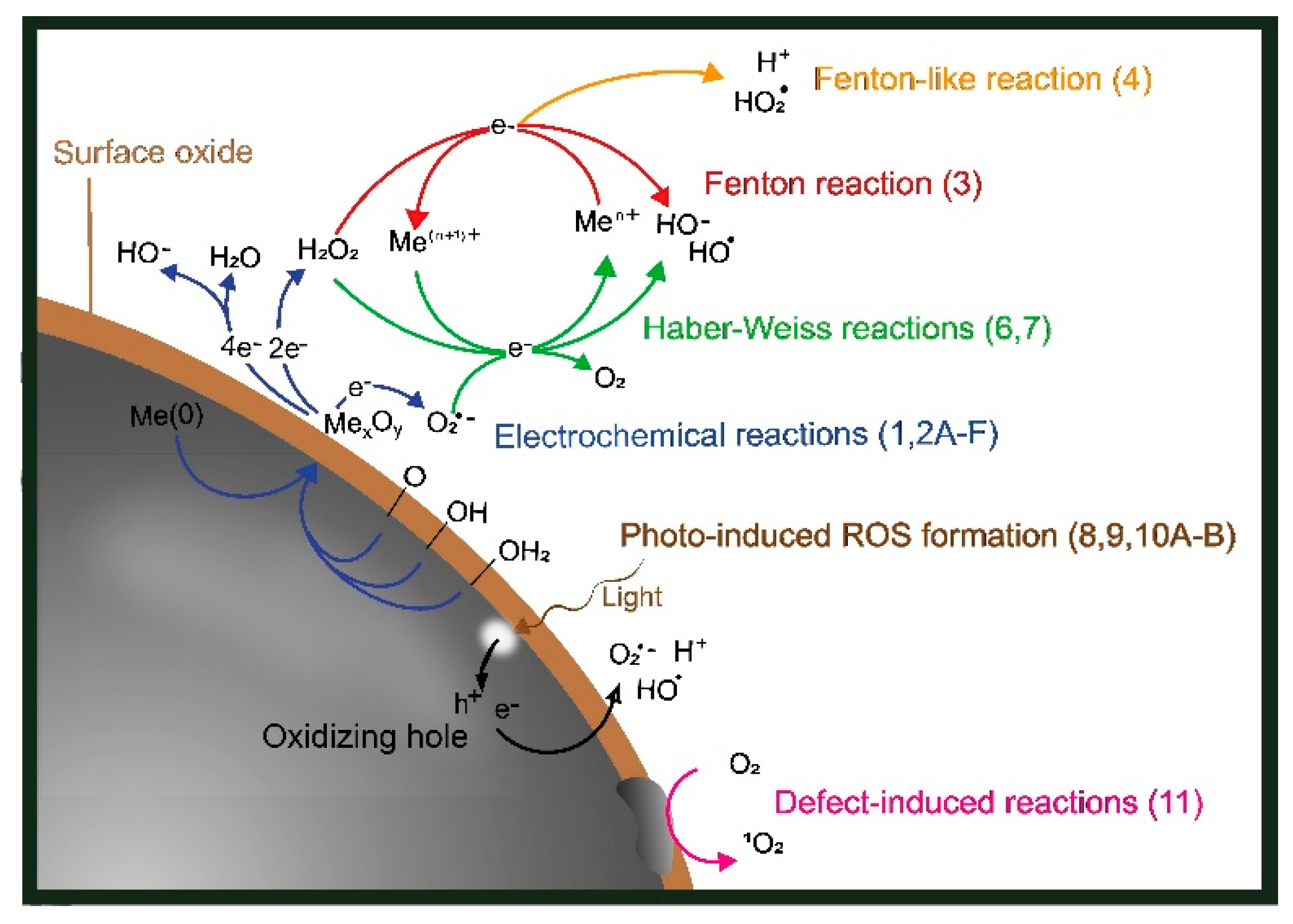
2.1. ROS Formation via Electrochemical Corrosion Reactions
2.2. Radical Transformation via Fenton and Haber–Weiss Reactions
2.3. Light-Induced ROS Formation
2.4. ROS Generation via Surface Catalytic Reactions
3. Importance of Metallic NPs and Their Characteristics for ROS Formation in Biological Settings
3.1. Effect of Biomolecule Adsorption onto Metallic NPs on the Formation of ROS
3.2. Importance of Metal Speciation and ROS Formation in Biological Systems
3.3. Effect of Interactions between Metallic NPs and Biological Redox Couples on ROS Formation
3.4. Correlation between ROS Formation and Properties of Metal and Metal Oxide NPs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Symbol | Denotation |
| ROS | Reactive oxygen species, including non-radicals |
| Radical | Species with an unpaired electron (e−) |
| O2 | Molecular (triplet) oxygen, two unpaired e−, stable |
| 1O2 | Singlet molecular oxygen, e− paired, unstable |
| O2•− | Superoxide radical |
| H2O2 | Hydrogen peroxide |
| HO− | Hydroxide ion |
| HO• | Hydroxyl radical |
| HOO• | Hydroperoxyl radical |
| e− | Electron |
| h+ | Positive hole |
| Me | Metal |
References
- Hochella, M.F.; Mogk, D.W.; Ranville, J.; Allen, I.C.; Luther, G.W.; Marr, L.C.; McGrail, B.P.; Murayama, M.; Qafoku, N.P.; Rosso, K.M. Natural, incidental, and engineered nanomaterials and their impacts on the Earth system. Science 2019, 363, eaau8299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soto, K.; Garza, K.M.; Murr, L.E. Cytotoxic effects of aggregated nanomaterials. Acta Biomater. 2007, 3, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Grassian, V.H. When size really matters: Size-dependent properties and surface chemistry of metal and metal oxide nanoparticles in gas and liquid phase environments. J. Phys. Chem. C 2008, 112, 18303–18313. [Google Scholar] [CrossRef]
- Kuech, T.R.; Hamers, R.J.; Pedersen, J.A. Chemical transformation of metal, metal oxide, and metal chalcogenide nanoparticles in the environment. In Engineered Nanoparticles and the Environment: Biophysicochemical Processes and Toxicity: Biophysicochemical Processes and Toxicity; Xing, B., Vecitis, C.D., Senesi, N., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2016; pp. 261–291. [Google Scholar]
- Nel, A.; Xia, T.; Mädler, L.; Li, N. Toxic potential of materials at the nanolevel. Science 2006, 311, 622–627. [Google Scholar] [CrossRef] [Green Version]
- Oberdörster, G.; Oberdörster, E.; Oberdörster, J. Nanotoxicology: An emerging discipline evolving from studies of ultrafine particles. Environ. Health Perspect. 2005, 113, 823–839. [Google Scholar] [CrossRef]
- Wardman, P. Reduction potentials of one-electron couples involving free radicals in aqueous solution. J. Phys. Chem. Ref. Data 1989, 18, 1637–1755. [Google Scholar] [CrossRef] [Green Version]
- Soenen, S.J.; Rivera-Gil, P.; Montenegro, J.-M.; Parak, W.J.; De Smedt, S.C.; Braeckmans, K. Cellular toxicity of inorganic nanoparticles: Common aspects and guidelines for improved nanotoxicity evaluation. Nano Today 2011, 6, 446–465. [Google Scholar] [CrossRef]
- Kumar, A.; Pandey, A.K.; Singh, S.S.; Shanker, R.; Dhawan, A. Engineered ZnO and TiO2 nanoparticles induce oxidative stress and DNA damage leading to reduced viability of Escherichia coli. Free Radic. Biol. Med. 2011, 51, 1872–1881. [Google Scholar] [CrossRef]
- Wang, Y.; Cai, R.; Chen, C. The Nano–bio interactions of nanomedicines: Understanding the biochemical driving forces and redox reactions. Acc. Chem. Res. 2019, 52, 1507–1518. [Google Scholar] [CrossRef]
- Nosaka, Y.; Nosaka, A.Y. Generation and detection of reactive oxygen species in photocatalysis. Chem. Rev. 2017, 117, 11302–11336. [Google Scholar] [CrossRef]
- von Moos, N.; Slaveykova, V.I. Oxidative stress induced by inorganic nanoparticles in bacteria and aquatic microalgae–state of the art and knowledge gaps. Nanotoxicology 2014, 8, 605–630. [Google Scholar] [CrossRef] [PubMed]
- Kaweeteerawat, C.; Ivask, A.; Liu, R.; Zhang, H.; Chang, C.H.; Low-Kam, C.; Fischer, H.; Ji, Z.; Pokhrel, S.; Cohen, Y. Toxicity of metal oxide nanoparticles in Escherichia coli correlates with conduction band and hydration energies. Environ. Sci. Technol. 2015, 49, 1105–1112. [Google Scholar] [CrossRef]
- McCarrick, S.; Cappellini, F.; Kessler, A.; Moelijker, N.; Derr, R.; Hedberg, J.; Wold, S.; Blomberg, E.; Odnevall Wallinder, I.; Hendriks, G. ToxTracker reporter cell lines as a tool for mechanism-based (geno) toxicity screening of nanoparticles—metals, oxides and quantum dots. Nanomaterials 2020, 10, 110. [Google Scholar] [CrossRef] [Green Version]
- Liu, G.; Gao, J.; Ai, H.; Chen, X. Applications and potential toxicity of magnetic iron oxide nanoparticles. Small 2013, 9, 1533–1545. [Google Scholar] [CrossRef]
- Wahajuddin; Arora, S. Superparamagnetic iron oxide nanoparticles: Magnetic nanoplatforms as drug carriers. Int. J. Nanomed. 2012, 7, 3445–3471. [Google Scholar] [CrossRef] [Green Version]
- Rosen, G.M.; Pou, S.; Ramos, C.L.; Cohen, M.S.; Britigan, B.E. Free radicals and phagocytic cells. FASEB J. 1995, 9, 200–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhakshinamoorthy, A.; Navalon, S.; Alvaro, M.; Garcia, H. Metal nanoparticles as heterogeneous Fenton catalysts. ChemSusChem 2012, 5, 46–64. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, W.; Niu, J.; Chen, Y. Mechanism of photogenerated reactive oxygen species and correlation with the antibacterial properties of engineered metal-oxide nanoparticles. ACS Nano 2012, 6, 5164–5173. [Google Scholar] [CrossRef]
- Saleh, N.B.; Milliron, D.J.; Aich, N.; Katz, L.E.; Liljestrand, H.M.; Kirisits, M.J. Importance of doping, dopant distribution, and defects on electronic band structure alteration of metal oxide nanoparticles: Implications for reactive oxygen species. Sci. Total Environ. 2016, 568, 926–932. [Google Scholar] [CrossRef] [Green Version]
- Lakshmi Prasanna, V.; Vijayaraghavan, R. Insight into the mechanism of antibacterial activity of ZnO: Surface defects mediated reactive oxygen species even in the dark. Langmuir 2015, 31, 9155–9162. [Google Scholar] [CrossRef]
- Gilbert, J.L. Corrosion in the human body: Metallic implants in the complex body environment. Corrosion 2017, 73, 1478–1495. [Google Scholar] [CrossRef]
- Burello, E.; Worth, A.P. A theoretical framework for predicting the oxidative stress potential of oxide nanoparticles. Nanotoxicology 2011, 5, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ji, Z.; Xia, T.; Meng, H.; Low-Kam, C.; Liu, R.; Pokhrel, S.; Lin, S.; Wang, X.; Liao, Y.-P. Use of metal oxide nanoparticle band gap to develop a predictive paradigm for oxidative stress and acute pulmonary inflammation. ACS Nano 2012, 6, 4349–4368. [Google Scholar] [CrossRef]
- Hedberg, J.; Karlsson, H.L.; Hedberg, Y.; Blomberg, E.; Odnevall Wallinder, I. The importance of extracellular speciation and corrosion of copper nanoparticles on lung cell membrane integrity. Colloids Surf. B Biointerfaces 2016, 141, 291–300. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, J.; Memon, A.G.; Shaikh, A.A.; Ismail, T.; Giwa, A.S.; Mahmood, A. Insight into single-element nobel metal anisotropic silver nanoparticle shape-dependent selective ROS generation and quantification. RSC Adv. 2021, 11, 8314–8322. [Google Scholar] [CrossRef] [PubMed]
- Forest, V.; Leclerc, L.; Hochepied, J.-F.; Trouvé, A.; Sarry, G.; Pourchez, J. Impact of cerium oxide nanoparticles shape on their in vitro cellular toxicity. Toxicol. Vitr. 2017, 38, 136–141. [Google Scholar] [CrossRef] [Green Version]
- Slavin, Y.N.; Asnis, J.; Häfeli, U.O.; Bach, H. Metal nanoparticles: Understanding the mechanisms behind antibacterial activity. J. Nanobiotechnology 2017, 15, 65. [Google Scholar] [CrossRef]
- Hayyan, M.; Hashim, M.A.; AlNashef, I.M. Superoxide ion: Generation and chemical implications. Chem. Rev. 2016, 116, 3029–3085. [Google Scholar] [CrossRef] [Green Version]
- Mach, W.J.; Thimmesch, A.R.; Pierce, J.T.; Pierce, J.D. Consequences of hyperoxia and the toxicity of oxygen in the lung. Nurs Res. Pr. 2011, 2011, 260482–260487. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Medlin, J.W.; Grönbeck, H. On the reaction mechanism of direct H2O2 formation over Pd catalysts. ACS Catal 2021, 11, 2735–2745. [Google Scholar] [CrossRef]
- Uhlig, H. The role of catalysis in corrosion processes. Adv. Catal. 1957, 9, 379–392. [Google Scholar] [CrossRef]
- Kleijn, S.E.F.; Lai, S.C.S.; Koper, M.T.M.; Unwin, P.R. Electrochemistry of nanoparticles. Angew. Chem. Int. Ed. 2014, 53, 3558–3586. [Google Scholar] [CrossRef]
- Grunberg, L. The formation of hydrogen peroxide on fresh metal surfaces. Proc. Phys. Society. Sect. B 1953, 66, 153–161. [Google Scholar] [CrossRef]
- Lawless, K.R. The oxidation of metals. Rep. Prog. Phys. 1974, 37, 231–316. [Google Scholar] [CrossRef]
- Grunberg, L.; Wright, K.H.R. Russell effect on evaporated metal films. Nature 1952, 170, 456–457. [Google Scholar] [CrossRef]
- Liu, J.; Hurt, R.H. Ion release kinetics and particle persistence in aqueous nano-silver colloids. Environ. Sci. Technol. 2010, 44, 2169–2175. [Google Scholar] [CrossRef] [PubMed]
- Olszowka, S.A.; Manning, M.A.; Barkatt, A. Copper dissolution and hydrogen peroxide formation in aqueous media. Corrosion 1992, 48, 411–418. [Google Scholar] [CrossRef]
- Shi, M.; Kwon, H.S.; Peng, Z.; Elder, A.; Yang, H. Effects of surface chemistry on the generation of reactive oxygen species by copper nanoparticles. ACS Nano 2012, 6, 2157–2164. [Google Scholar] [CrossRef] [Green Version]
- Colcleugh, D.W.; Graydon, W.F. The kinetics of hydrogen peroxide formation during the dissolution of polycrystalline copper. J. Phys. Chem. 1962, 66, 1370–1372. [Google Scholar] [CrossRef]
- Scarcello, E.; Herpain, A.; Tomatis, M.; Turci, F.; Jacques, P.; Lison, D. Hydroxyl radicals and oxidative stress: The dark side of Fe corrosion. Colloids Surf. B Biointerfaces 2020, 185, 110542. [Google Scholar] [CrossRef]
- Keenan, C.R.; Sedlak, D.L. Factors affecting the yield of oxidants from the reaction of nanoparticulate zero-valent iron and oxygen. Environ. Sci. Technol. 2008, 42, 1262–1267. [Google Scholar] [CrossRef]
- Plieth, W. Electrochemical properties of small clusters of metal atoms and their role in the surface enhanced Raman scattering. J. Phys. Chem. 1982, 86, 3166–3170. [Google Scholar] [CrossRef] [Green Version]
- Borm, P.; Klaessig, F.C.; Landry, T.D.; Moudgil, B.; Pauluhn, J.; Thomas, K.; Trottier, R.; Wood, S. Research strategies for safety evaluation of nanomaterials, part V: Role of dissolution in biological fate and effects of nanoscale particles. Toxicol. Sci. 2006, 90, 23–32. [Google Scholar] [CrossRef] [Green Version]
- Ivanova, O.S.; Zamborini, F.P. Size-dependent electrochemical oxidation of silver nanoparticles. J. Am. Chem. Soc. 2009, 132, 70–72. [Google Scholar] [CrossRef]
- Hedberg, J.; Blomberg, E.; Odnevall Wallinder, I. In the search for nanospecific effects of dissolution of metallic nanoparticles at freshwater-like conditions: A critical review. Environ. Sci. Technol. 2019, 53, 4030–4044. [Google Scholar] [CrossRef] [PubMed]
- Dale, A.L.; Lowry, G.V.; Casman, E.A. Accurate and fast numerical algorithms for tracking particle size distributions during nanoparticle aggregation and dissolution. Environ. Sci. Nano 2017, 4, 89–104. [Google Scholar] [CrossRef]
- Allen, S.L.; Sharma, J.N.; Zamborini, F.P. Aggregation-dependent oxidation of metal nanoparticles. J. Am. Chem. Soc. 2017, 139, 12895–12898. [Google Scholar] [CrossRef]
- Dabera, G.D.M.R.; Walker, M.; Sanchez, A.M.; Pereira, H.J.; Beanland, R.; Hatton, R.A. Retarding oxidation of copper nanoparticles without electrical isolation and the size dependence of work function. Nat. Commun. 2017, 8, 1894. [Google Scholar] [CrossRef] [Green Version]
- Daniel, M.-C.; Astruc, D. Gold nanoparticles: Assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem. Rev. 2004, 104, 293–346. [Google Scholar] [CrossRef]
- Wertheim, G. Electronic structure of metal clusters. Z. Für Phys. D At. Mol. Clust. 1989, 12, 319–326. [Google Scholar] [CrossRef]
- Jiang, K.; Back, S.; Akey, A.J.; Xia, C.; Hu, Y.; Liang, W.; Schaak, D.; Stavitski, E.; Nørskov, J.K.; Siahrostami, S. Highly selective oxygen reduction to hydrogen peroxide on transition metal single atom coordination. Nat. Commun. 2019, 10, 3997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.; Du, P.; Jeon, J.K.; Jang, G.H.; Hwang, M.P.; Han, H.S.; Park, K.; Lee, K.H.; Lee, J.W.; Jeon, H. Magnesium corrosion triggered spontaneous generation of H2O2 on oxidized titanium for promoting angiogenesis. Angew. Chem. Int. Ed. 2015, 54, 14753–14757. [Google Scholar] [CrossRef] [PubMed]
- Lison, D.; Carbonnelle, P.; Mollo, L.; Lauwerys, R.; Fubini, B. Physicochemical mechanism of the interaction between cobalt metal and carbide particles to generate toxic activated oxygen species. Chem. Res. Toxicol. 1995, 8, 600–606. [Google Scholar] [CrossRef] [PubMed]
- Weiss, J. The free radical mechanism in the reactions of hydrogen peroxide. Adv. Catal. 1952, 4, 343–365. [Google Scholar] [CrossRef]
- Yamaguchi, R.; Kurosu, S.; Suzuki, M.; Kawase, Y. Hydroxyl radical generation by zero-valent iron/Cu (ZVI/Cu) bimetallic catalyst in wastewater treatment: Heterogeneous Fenton/Fenton-like reactions by Fenton reagents formed in-situ under oxic conditions. Chem. Eng. J. 2018, 334, 1537–1549. [Google Scholar] [CrossRef]
- He, W.; Zhou, Y.-T.; Wamer, W.G.; Boudreau, M.D.; Yin, J.-J. Mechanisms of the pH dependent generation of hydroxyl radicals and oxygen induced by Ag nanoparticles. Biomaterials 2012, 33, 7547–7555. [Google Scholar] [CrossRef]
- Wang, B.; Yin, J.-J.; Zhou, X.; Kurash, I.; Chai, Z.; Zhao, Y.; Feng, W. Physicochemical origin for free radical generation of iron oxide nanoparticles in biomicroenvironment: Catalytic activities mediated by surface chemical states. J. Phys. Chem. C 2013, 117, 383–392. [Google Scholar] [CrossRef]
- Šulce, A.; Bulke, F.; Schowalter, M.; Rosenauer, A.; Dringen, R.; Kunz, S. Reactive oxygen species (ROS) formation ability and stability of small copper (Cu) nanoparticles (NPs). RSC Adv. 2016, 6, 76980–76988. [Google Scholar] [CrossRef]
- Lee, H.; Lee, H.-j.; Kim, H.-E.; Kweon, J.; Lee, B.-D.; Lee, C. Oxidant production from corrosion of nano-and microparticulate zero-valent iron in the presence of oxygen: A comparative study. J. Hazard. Mater. 2014, 265, 201–207. [Google Scholar] [CrossRef]
- Pinto, I.S.X.; Pacheco, P.H.V.V.; Coelho, J.V.; Lorençon, E.; Ardisson, J.D.; Fabris, J.D.; de Souza, P.P.; Krambrock, K.W.H.; Oliveira, L.C.A.; Pereira, M.C. Nanostructured δ-FeOOH: An efficient Fenton-like catalyst for the oxidation of organics in water. Appl. Catalysis. B Environ. 2012, 119–120, 175–182. [Google Scholar] [CrossRef]
- Wydra, R.J.; Oliver, C.E.; Anderson, K.W.; Dziubla, T.D.; Hilt, J.Z. Accelerated generation of free radicals by iron oxide nanoparticles in the presence of an alternating magnetic field. RSC Adv. 2015, 5, 18888–18893. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Qian, J.; Yu, A.; Pan, B. Singlet oxygen mediated iron-based Fenton-like catalysis under nanoconfinement. Proc. Natl. Acad. Sci. USA 2019, 116, 6659–6664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richardson, D.R.; Ponka, P. Identification of a mechanism of iron uptake by cells which is stimulated by hydroxyl radicals generated via the iron-catalysed Haber-Weiss reaction. Biochim. Et Biophys. Acta Mol. Cell Res. 1995, 1269, 105–114. [Google Scholar] [CrossRef] [Green Version]
- Kehrer, J.P. The Haber–Weiss reaction and mechanisms of toxicity. Toxicology 2000, 149, 43–50. [Google Scholar] [CrossRef]
- Leitao, E.F.V.; Ventura, E.; Desouza, M.A.F.; Riveros, J.M.; Domonte, S.A. Spin-forbidden branching in the mechanism of the intrinsic Haber-Weiss reaction. ChemistryOpen 2017, 6, 360–363. [Google Scholar] [CrossRef] [Green Version]
- Blanksby, S.J.; Bierbaum, V.M.; Ellison, G.B.; Kato, S. Superoxide Does React with Peroxides: Direct Observation of the Haber–Weiss Reaction in the Gas Phase. Angew. Chem. Int. Ed. 2007, 46, 4948–4950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, A.U.; Kasha, M. Singlet molecular oxygen in the Haber-Weiss reaction. Proc. Natl. Acad. Sci. USA 1994, 91, 12365–12367. [Google Scholar] [CrossRef] [Green Version]
- Shi, X.; Dalal, N.S. The role of superoxide radical in chromium(VI)-generated hydroxyl radical: The Cr(VI) Haber-Weiss cycle. Arch. Biochem. Biophys. 1992, 292, 323–327. [Google Scholar] [CrossRef]
- Liochev, S.I.; Fridovich, I. The Haber-Weiss cycle-70 years later: An alternative view. Redox Rep. 2002, 7, 55–57. [Google Scholar] [CrossRef]
- Sugden, K.D.; Burris, R.B.; Rogers, S.J. An oxygen dependence in chromium mutagenesis. Mutat. Res. Lett. 1990, 244, 239–244. [Google Scholar] [CrossRef]
- Egan, T.J.; Barthakur, S.R.; Aisen, P. Catalysis of the Haber-Weiss reaction by iron-diethylenetriaminepentaacetate. J. Inorg. Biochem. 1992, 48, 241–249. [Google Scholar] [CrossRef]
- Sutton, H.C. Efficiency of chelated iron compounds as catalysts for the Haber-Weiss reaction. J. Free Radic. Biol. Med. 1985, 1, 195–202. [Google Scholar] [CrossRef]
- Yang, M.; Soroka, I.; Jonsson, M. Hydroxyl radical production in aerobic aqueous solution containing metallic tungsten. Catal. Commun. 2015, 71, 93–96. [Google Scholar] [CrossRef]
- Fu, P.P.; Xia, Q.; Hwang, H.-M.; Ray, P.C.; Yu, H. Mechanisms of nanotoxicity: Generation of reactive oxygen species. J. Food Drug Anal. 2014, 22, 64–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoffmann, M.R.; Martin, S.T.; Choi, W.; Bahnemann, D.W. Environmental applications of semiconductor photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Dhanjai, D.; Sinha, A.; Zhao, H.; Chen, J.; Mugo, S.M. Water analysis | determination of chemical oxygen demand. In Encyclopedia of Analytical Science, 3rd ed.; Worsfold, P., Poole, C., Townshend, A., Miró, M., Eds.; Academic Press: Oxford, UK, 2019; pp. 258–270. [Google Scholar]
- Balamurugan, B.; Aruna, I.; Mehta, B.; Shivaprasad, S. Size-dependent conductivity-type inversion in Cu 2 O nanoparticles. Phys. Rev. B 2004, 69, 165419. [Google Scholar] [CrossRef]
- Savić, T.D.; Čomor, M.I.; Nedeljković, J.M.; Veljković, D.Ž.; Zarić, S.D.; Rakić, V.M.; Janković, I.A. The effect of substituents on the surface modification of anatase nanoparticles with catecholate-type ligands: A combined DFT and experimental study. Phys. Chem. Chem. Phys. 2014, 16, 20796–20805. [Google Scholar] [CrossRef]
- Neumann, C.C.; Batchelor-McAuley, C.; Tschulik, K.; Toh, H.S.; Shumbula, P.; Pillay, J.; Tshikhudo, R.; Compton, R.G. The surface energy of single nanoparticles probed via anodic stripping voltammetry. ChemElectroChem 2014, 1, 87–89. [Google Scholar] [CrossRef]
- Scanlon, M.D.; Peljo, P.; Méndez, M.A.; Smirnov, E.; Girault, H.H. Charging and discharging at the nanoscale: Fermi level equilibration of metallic nanoparticles. Chem. Sci. 2015, 6, 2705–2720. [Google Scholar] [CrossRef] [Green Version]
- Long, T.C.; Saleh, N.; Tilton, R.D.; Lowry, G.V.; Veronesi, B. Titanium dioxide (P25) produces reactive oxygen species in immortalized brain microglia (BV2): Implications for nanoparticle neurotoxicity. Environ. Sci. Technol. 2006, 40, 4346–4352. [Google Scholar] [CrossRef]
- Jiang, J.; Oberdörster, G.; Elder, A.; Gelein, R.; Mercer, P.; Biswas, P. Does nanoparticle activity depend upon size and crystal phase? Nanotoxicology 2008, 2, 33–42. [Google Scholar] [CrossRef] [Green Version]
- Varkey, A.; Fort, A. Some optical properties of silver peroxide (AgO) and silver oxide (Ag2O) films produced by chemical-bath deposition. Sol. Energy Mater. Sol. Cells 1993, 29, 253–259. [Google Scholar] [CrossRef]
- Zhang, W.; Li, Y.; Niu, J.; Chen, Y. Photogeneration of reactive oxygen species on uncoated silver, gold, nickel, and silicon nanoparticles and their antibacterial effects. Langmuir 2013, 29, 4647–4651. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Florez, V.; Mendez-Sanchez, S.C.; Patrón-Soberano, O.A.; Rodríguez-González, V.; Blach, D.; Martínez, F. Gold nanoparticle-mediated generation of reactive oxygen species during plasmonic photothermal therapy: A comparative study for different particle sizes, shapes, and surface conjugations. J. Mater. Chem. B 2020, 8, 2862–2875. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.-L.; Wang, H.-J.; Zhang, H.; Tian, Z.-Q.; Li, J.-F. Probing hot electron behaviors by surface-enhanced raman spectroscopy. Cell Rep. Phys. Sci. 2020, 1, 100184. [Google Scholar] [CrossRef]
- Labouret, T.; Audibert, J.F.; Pansu, R.B.; Palpant, B. Plasmon-assisted production of reactive oxygen species by single gold nanorods. Small 2015, 11, 4475–4479. [Google Scholar] [CrossRef] [PubMed]
- Anpo, M.; Che, M.; Fubini, B.; Garrone, E.; Giamello, E.; Paganini, M.C. Generation of superoxide ions at oxide surfaces. Top. Catal. 1999, 8, 189. [Google Scholar] [CrossRef]
- Applerot, G.; Lellouche, J.; Lipovsky, A.; Nitzan, Y.; Lubart, R.; Gedanken, A.; Banin, E. Understanding the antibacterial mechanism of CuO nanoparticles: Revealing the route of induced oxidative stress. Small 2012, 8, 3326–3337. [Google Scholar] [CrossRef]
- Lousada, C.M.; Johansson, A.J.; Brinck, T.; Jonsson, M. Mechanism of H2O2 decomposition on transition metal oxide surfaces. J. Phys. Chem. C 2012, 116, 9533–9543. [Google Scholar] [CrossRef]
- Watanabe, M.; Sei, H.; Stonehart, P. The influence of platinum crystallite size on the electroreduction of oxygen. J. Electroanal. Chem. Interfacial Electrochem. 1989, 261, 375–387. [Google Scholar] [CrossRef]
- Weiss, J. The catalytic decomposition of hydrogen peroxide on different metals. Trans. Faraday Soc. 1935, 31, 1547–1557. [Google Scholar] [CrossRef]
- Claesson, P.M.; Blomberg, E.; Fröberg, J.C.; Nylander, T.; Arnebrant, T. Protein interactions at solid surfaces. Adv. Colloid Interface Sci. 1995, 57, 161–227. [Google Scholar] [CrossRef]
- Hedberg, Y.; Wang, X.; Hedberg, J.; Lundin, M.; Blomberg, E.; Odnevall Wallinder, I. Surface-protein interactions on different stainless steel grades: Effects of protein adsorption, surface changes and metal release. J. Mater. Sci. Mater. Med. 2013, 24, 1015–1033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hedberg, Y.S. Role of proteins in the degradation of relatively inert alloys in the human body. npj Mater. Degrad. 2018, 2, 26. [Google Scholar] [CrossRef]
- Mudunkotuwa, I.A.; Grassian, V.H. Biological and environmental media control oxide nanoparticle surface composition: The roles of biological components (proteins and amino acids), inorganic oxyanions and humic acid. Environ. Sci. Nano 2015, 2, 429–439. [Google Scholar] [CrossRef]
- Lundqvist, M.; Stigler, J.; Elia, G.; Lynch, I.; Cedervall, T.; Dawson, K.A. Nanoparticle size and surface properties determine the protein corona with possible implications for biological impacts. Proc. Natl. Acad. Sci. USA 2008, 105, 14265–14270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cedervall, T.; Lynch, I.; Lindman, S.; Berggård, T.; Thulin, E.; Nilsson, H.; Dawson, K.A.; Linse, S. Understanding the nanoparticle–protein corona using methods to quantify exchange rates and affinities of proteins for nanoparticles. Proc. Natl. Acad. Sci. USA 2007, 104, 2050–2055. [Google Scholar] [CrossRef] [Green Version]
- Lynch, I.; Dawson, K.A.; Lead, J.R.; Valsami-Jones, E. Macromolecular coronas and their importance in nanotoxicology and nanoecotoxicology. In Nanoscience and the Environment; Lead, J.R., Valsami-Jones, E., Palmer, R.E., Eds.; Frontiers of Nanoscience; Elsevier: Amsterdam, The Netherlands, 2014; Volume 7, pp. 127–156. [Google Scholar]
- Fleischer, C.C.; Payne, C.K. Nanoparticle–cell interactions: Molecular structure of the protein corona and cellular outcomes. Acc. Chem. Res. 2014, 47, 2651–2659. [Google Scholar] [CrossRef]
- Wang, X.; Herting, G.; Odnevall Wallinder, I.; Blomberg, E. Adsorption of bovine serum albumin on silver surfaces enhances the release of silver at pH neutral conditions. Phys. Chem. Chem. Phys. 2015, 17, 18524–18534. [Google Scholar] [CrossRef] [Green Version]
- Karlsson, H.L.; Cronholm, P.; Hedberg, Y.; Tornberg, M.; De Battice, L.; Svedhem, S.; Odnevall Wallinder, I. Cell membrane damage and protein interaction induced by copper containing nanoparticles—Importance of the metal release process. Toxicology 2013, 313, 59–69. [Google Scholar] [CrossRef]
- Norde, W. Colloids and Interfaces in Life Sciences and Bionanotechnology, 2nd ed.; CRC Press, Taylor & Francis Group, LLC: Boca Raton, CA, USA, 2011. [Google Scholar]
- Imamura, K.; Oshita, M.; Iwai, M.; Kuroda, T.; Watanabe, I.; Sakiyama, T.; Nakanishi, K. Influences of properties of protein and adsorption surface on removal kinetics of protein adsorbed on metal surface by H2O2-electrolysis treatment. J. Colloid Interface Sci. 2010, 345, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Schwaminger, S.P.; Blank-Shim, S.A.; Scheifele, I.; Fraga-García, P.; Berensmeier, S. Peptide binding to metal oxide nanoparticles. Faraday Discuss 2017, 24, 233–250. [Google Scholar] [CrossRef] [PubMed]
- Limo, M.J.; Sola-Rabada, A.; Boix, E.; Thota, V.; Westcott, Z.C.; Puddu, V.; Perry, C.C. Interactions between metal oxides and biomolecules: From fundamental understanding to applications. Chem. Rev. 2018, 118, 11118–11193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Von Dem Bussche, A.; Kabadi, P.K.; Kane, A.B.; Hurt, R.H. Biological and environmental transformations of copper-based nanomaterials. ACS Nano 2013, 7, 8715–8727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Herting, G.; Wallinder, I.O.; Blomberg, E. Adsorption of lysozyme on silver and its influence on silver release. Langmuir 2014, 30, 13877–13889. [Google Scholar] [CrossRef]
- Williams, D.; Clark, G. The corrosion of pure cobalt in physiological media. J. Mater. Sci. 1982, 17, 1675–1682. [Google Scholar] [CrossRef]
- Lundin, M.; Hedberg, Y.; Jiang, T.; Herting, G.; Wang, X.; Thormann, E.; Blomberg, E.; Odnevall Wallinder, I. Adsorption and protein-induced metal release from chromium metal and stainless steel. J. Colloid Interface Sci. 2012, 366, 155–164. [Google Scholar] [CrossRef]
- Wagener, V.; Faltz, A.-S.; Killian, M.S.; Schmuki, P.; Virtanen, S. Protein interactions with corroding metal surfaces: Comparison of Mg and Fe. Faraday Discuss 2015, 18, 347–360. [Google Scholar] [CrossRef] [Green Version]
- Mei, N.; Hedberg, J.; Odnevall Wallinder, I.; Blomberg, E. Influence of biocorona formation on the transformation and dissolution of cobalt nanoparticles under physiological conditions. ACS Omega 2019, 4, 21778–21791. [Google Scholar] [CrossRef] [Green Version]
- Cronholm, P.; Karlsson, H.L.; Hedberg, J.; Lowe, T.A.; Winnberg, L.; Elihn, K.; Odnevall Wallinder, I.; Möller, L. Intracellular uptake and toxicity of Ag and CuO nanoparticles: A comparison between nanoparticles and their corresponding metal ions. Small 2013, 9, 970–982. [Google Scholar] [CrossRef]
- Hsiao, I.L.; Hsieh, Y.-K.; Wang, C.-F.; Chen, I.C.; Huang, Y.-J. Trojan-horse mechanism in the cellular uptake of silver nanoparticles verified by direct intra- and extracellular silver speciation analysis. Environ. Sci. Technol. 2015, 49, 3813–3821. [Google Scholar] [CrossRef] [PubMed]
- Vroman, L.; Adams, A.L. Identification of rapid changes at plasma–solid interfaces. J. Biomed. Mater. Res. 1969, 3, 43–67. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Xu, M.; Wang, R.; Yin, Y.; Lynch, I.; Liu, S. The crucial role of environmental coronas in determining the biological effects of engineered nanomaterials. Small 2020, 16, 2003691. [Google Scholar] [CrossRef] [PubMed]
- Messina, G.M.; Bocchinfuso, G.; Giamblanco, N.; Mazzuca, C.; Palleschi, A.; Marletta, G. Orienting proteins by nanostructured surfaces: Evidence of a curvature-driven geometrical resonance. Nanoscale 2018, 10, 7544–7555. [Google Scholar] [CrossRef] [Green Version]
- May, P.M. JESS at thirty: Strengths, weaknesses and future needs in the modelling of chemical speciation. Appl. Geochem. 2015, 55, 3–16. [Google Scholar] [CrossRef]
- May, P.M.; Linder, P.W.; Williams, D.R. Computer simulation of metal-ion equilibria in biofluids: Models for the low-molecular-weight complex distribution of calcium(II), magnesium(II), manganese(II), iron(III), copper(II), zinc(II), and lead(II) ions in human blood plasma. J. Chem. Soc. Dalton Trans. 1977, 588–595. [Google Scholar] [CrossRef]
- Tran-Ho, L.-C.; May, P.M.; Hefter, G.T. Complexation of copper (I) by thioamino acids. Implications for copper speciation in blood plasma. J. Inorg. Biochem. 1997, 68, 225–231. [Google Scholar] [CrossRef]
- Saran, M.; Michel, C.; Stettmaier, K.; Bors, W. Arguments against the significance of the Fenton reaction contributing to signal pathways under in vivo conditions. Free Radic. Res. 2000, 33, 567–579. [Google Scholar] [CrossRef]
- Hellack, B.; Nickel, C.; Albrecht, C.; Kuhlbusch, T.A.J.; Boland, S.; Baeza-Squiban, A.; Wohlleben, W.; Schins, R.P.F. Analytical methods to assess the oxidative potential of nanoparticles: A review. Environ. Sci. Nano 2017, 4, 1920–1934. [Google Scholar] [CrossRef]
- Sullivan, L.B.; Chandel, N.S. Mitochondrial reactive oxygen species and cancer. Cancer Metab. 2014, 2, 17. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Schoonen, M.A. The absolute energy positions of conduction and valence bands of selected semiconducting minerals. Am. Mineral. 2000, 85, 543–556. [Google Scholar] [CrossRef]
- Bahl, A.; Hellack, B.; Wiemann, M.; Giusti, A.; Werle, K.; Haase, A.; Wohlleben, W. Nanomaterial categorization by surface reactivity: A case study comparing 35 materials with four different test methods. NanoImpact 2020, 19, 100234. [Google Scholar] [CrossRef]
- Keskin, C.S.; Özdemir, A.; Keskin, S.Y. Cysteine interactions in glutathione mediated assembly of silver nanoparticles in the presence of metal ions. J. Nano Res. 2012, 18–19, 63–76. [Google Scholar] [CrossRef]
- Ramezani, F.; Ramezani, F.; Amanlou, M.; Amanlou, M.; Rafii-Tabar, H.; Rafii-Tabar, H. Comparison of amino acids interaction with gold nanoparticle. Amino Acids 2014, 46, 911–920. [Google Scholar] [CrossRef] [PubMed]
- Auffan, M.; Rose, J.; Wiesner, M.R.; Bottero, J.-Y. Chemical stability of metallic nanoparticles: A parameter controlling their potential cellular toxicity in vitro. Environ. Pollut. 2009, 157, 1127–1133. [Google Scholar] [CrossRef]
- Rushton, E.K.; Jiang, J.; Leonard, S.S.; Eberly, S.; Castranova, V.; Biswas, P.; Elder, A.; Han, X.; Gelein, R.; Finkelstein, J. Concept of assessing nanoparticle hazards considering nanoparticle dosemetric and chemical/biological response metrics. J. Toxicol. Environ. Health Part A 2010, 73, 445–461. [Google Scholar] [CrossRef]
- He, W.; Liu, Y.; Wamer, W.G.; Yin, J.-J. Electron spin resonance spectroscopy for the study of nanomaterial-mediated generation of reactive oxygen species. J. Food Drug Anal. 2014, 22, 49–63. [Google Scholar] [CrossRef]
- Gajewicz, A.; Puzyn, T.; Odziomek, K.; Urbaszek, P.; Haase, A.; Riebeling, C.; Luch, A.; Irfan, M.A.; Landsiedel, R.; van der Zande, M. Decision tree models to classify nanomaterials according to the DF4nanoGrouping scheme. Nanotoxicology 2018, 12, 1–17. [Google Scholar] [CrossRef] [Green Version]
- He, D.; Miller, C.J.; Waite, T.D. Fenton-like zero-valent silver nanoparticle-mediated hydroxyl radical production. J. Catal. 2014, 317, 198–205. [Google Scholar] [CrossRef]
- Chen, W.-H.; Xiong, J.-H.; Teng, X.; Mi, J.-X.; Hu, Z.-B.; Wang, H.; Chen, Z. A novel heterogeneous Co(II)-Fenton-like catalyst for efficient photodegradation by visible light over extended pH. Sci. China. Chem. 2020, 63, 1825–1836. [Google Scholar] [CrossRef]
- Mittal, S.; Pandey, A.K. Cerium oxide nanoparticles induced toxicity in human lung cells: Role of ROS mediated DNA damage and apoptosis. Biomed. Res. Int. 2014, 2014, 891934. [Google Scholar] [CrossRef] [PubMed]
- Heckert, E.G.; Seal, S.; Self, W.T. Fenton-like reaction catalyzed by the rare earth inner transition metal cerium. Environ. Sci. Technol. 2008, 42, 5014–5019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, J.; Cheng, Y.; Zhou, M.; Zhao, S.; Lin, S.; Wang, X.; Wu, J.; Li, S.; Wei, H. ROS scavenging Mn3O4 nanozymes for in vivo anti-inflammation. Chem. Sci. 2018, 9, 2927–2933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cappellini, F.; Hedberg, Y.; McCarrick, S.; Hedberg, J.; Derr, R.; Hendriks, G.; Odnevall Wallinder, I.; Karlsson, H.L. Mechanistic insight into reactivity and (geno)toxicity of well-characterized nanoparticles of cobalt metal and oxides. Nanotoxicology 2018, 12, 602–620. [Google Scholar] [CrossRef]
- Liu, Y.; Hong, H.; Lu, X.; Wang, W.; Liu, F.; Yang, H. l-ascorbic acid protected against extrinsic and intrinsic apoptosis induced by cobalt nanoparticles through ROS attenuation. Biol. Trace Elem. Res. 2016, 175, 428–439. [Google Scholar] [CrossRef]
- Cao, D.; Chao, J.; Sun, L.; Wang, G. Catalytic behavior of Co3O4 in electroreduction of H2O2. J. Power Sources 2008, 179, 87–91. [Google Scholar] [CrossRef]
- Uzunboy, S.; Demirci Çekiç, S.; Apak, R. Determination of cobalt(II)-hydrogen peroxide-induced DNA oxidative damage and preventive antioxidant activity by CUPRAC colorimetry. Anal. Lett. 2019, 52, 2663–2676. [Google Scholar] [CrossRef]
- Lan, A.P.; Chen, J.; Chai, Z.F.; Hu, Y. The neurotoxicity of iron, copper and cobalt in Parkinson’s disease through ROS-mediated mechanisms. Biometals 2016, 29, 665–678. [Google Scholar] [CrossRef]
- Angelé-Martínez, C.; Nguyen, K.V.T.; Ameer, F.S.; Anker, J.N.; Brumaghim, J.L. Reactive oxygen species generation by copper(II) oxide nanoparticles determined by DNA damage assays and EPR spectroscopy. Nanotoxicology 2017, 11, 278–288. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; He, J.; Shi, R.; Yang, P. Preparation and photo Fenton-like activities of high crystalline CuO fibers. Appl. Surf. Sci. 2017, 422, 1042–1051. [Google Scholar] [CrossRef]
- Hou, X.; Huang, X.; Ai, Z.; Zhao, J.; Zhang, L. Ascorbic acid/Fe@Fe2O3: A highly efficient combined Fenton reagent to remove organic contaminants. J. Hazard. Mater. 2016, 310, 170–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, Z.; Wang, B.; Yu, J.; Ma, C.; Zhou, C.; Chen, T.; Yan, Q.; Wang, K.; Sun, L. Density functional study on the heterogeneous oxidation of NO over α-Fe2O3 catalyst by H2O2: Effect of oxygen vacancy. Appl. Surf. Sci. 2017, 413, 292–301. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, B.; Wang, Q.; Xing, S. Facile synthesis of α-FeOOH/γ-Fe2O3 by a pH gradient method and the role of γ-Fe2O3 in H2O2 activation under visible light irradiation. Chem. Eng. J. 2018, 354, 75–84. [Google Scholar] [CrossRef]
- Lin, P.-J.; Yeh, C.-H.; Jiang, J.-C. Theoretical insight into hydroxyl production via H2O2 decomposition over the Fe3O4(311) surface. RSC Adv. 2021, 11, 36257–36264. [Google Scholar] [CrossRef]
- Wilcoxon, J.P. Nanoparticles—preparation, characterization and physical properties. In Metal Nanoparticles and Nanoalloys; Johnston, R.L., Wilcoxon, J.P., Palmer, R.E., Eds.; Frontiers of Nanoscience; Elsevier: Amsterdam, The Netherlands, 2012; Volume 3, pp. 43–127. [Google Scholar]
- Hedberg, Y.S.; Pradhan, S.; Capellini, F.; Karlsson, M.-E.; Blomberg, E.; Karlsson, H.L.; Odnevall Wallinder, I.; Hedberg, J.F. Electrochemical surface oxide characteristics of metal nanoparticles (Mn, Cu and Al) and the relation to toxicity. Electrochim. Acta 2016, 212, 360–371. [Google Scholar] [CrossRef] [Green Version]
- Noventa, S.; Hacker, C.; Rowe, D.; Elgy, C.; Galloway, T. Dissolution and bandgap paradigms for predicting the toxicity of metal oxide nanoparticles in the marine environment: An in vivo study with oyster embryos. Nanotoxicology 2018, 12, 63–78. [Google Scholar] [CrossRef]
- Latvala, S.; Hedberg, J.; Di Bucchianico, S.; Möller, L.; Odnevall Wallinder, I.; Elihn, K.; Karlsson, H.L. Nickel release, ROS generation and toxicity of Ni and NiO micro- and nanoparticles. PLoS ONE 2016, 11, e0159684. [Google Scholar] [CrossRef]
- Barakat, M.A.; Kumar, R. Photocatalytic Activity Enhancement of Titanium Dioxide Nanoparticles: Degradation of Pollutants in Wastewater. In Photocatalytic Activity Enhancement of Titanium Dioxide Nanoparticles; Sharma, S.K., Ed.; SpringerBriefs in Molecular Science; Springer: Cham, Switzerland, 2016; pp. 1–29. [Google Scholar]
- Madkour, L.H. Reactive Oxygen Species (ROS), Nanoparticles, and Endoplasmic Reticulum (ER) Stress-Induced Cell Death Mechanisms; Academic Press: London, UK, 2020. [Google Scholar]
- Szilágyi, I.M.; Fórizs, B.; Rosseler, O.; Szegedi, Á.; Németh, P.; Király, P.; Tárkányi, G.; Vajna, B.; Varga-Josepovits, K.; László, K.; et al. WO3 photocatalysts: Influence of structure and composition. J. Catal. 2012, 294, 119–127. [Google Scholar] [CrossRef] [Green Version]
- Lefatshe, K.; Mola, G.T.; Muiva, C.M. Reduction of hazardous reactive oxygen species (ROS) production of ZnO through Mn inclusion for possible UV-radiation shielding application. Heliyon 2020, 6, e04186. [Google Scholar] [CrossRef]
- Graedel, T.E. Corrosion mechanisms for silver exposed to the atmosphere. J. Electrochem. Soc. 1992, 139, 1963–1970. [Google Scholar] [CrossRef]
- Gliga, A.R.; Skoglund, S.; Odnevall Wallinder, I.; Fadeel, B.; Karlsson, H.L. Size-dependent cytotoxicity of silver nanoparticles in human lung cells: The role of cellular uptake, agglomeration and Ag release. Fibre Part. Toxicol. 2013, 11, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ivask, A.; Titma, T.; Visnapuu, M.; Vija, H.; Kakinen, A.; Sihtmae, M.; Pokhrel, S.; Madler, L.; Heinlaan, M.; Kisand, V. Toxicity of 11 metal oxide nanoparticles to three mammalian cell types in vitro. Curr. Top. Med. Chem. 2015, 15, 1914–1929. [Google Scholar] [CrossRef] [PubMed]
- Li, J.J.; Hartono, D.; Ong, C.-N.; Bay, B.-H.; Yung, L.-Y.L. Autophagy and oxidative stress associated with gold nanoparticles. Biomaterials 2010, 31, 5996–6003. [Google Scholar] [CrossRef] [PubMed]
- Carlander, U.; Midander, K.; Hedberg, Y.S.; Johanson, G.; Bottai, M.; Karlsson, H.L. Macrophage-assisted dissolution of gold nanoparticles. ACS Appl. Bio. Mater. 2019, 2, 1006–1016. [Google Scholar] [CrossRef] [PubMed]
- Rodhe, Y.; Skoglund, S.; Odnevall Wallinder, I.; Potácová, Z.; Möller, L. Copper-based nanoparticles induce high toxicity in leukemic HL60 cells. Toxicol. Vitr. 2015, 29, 1711–1719. [Google Scholar] [CrossRef]







| Corrosion | Band Gap Bio-Redox | Fenton | Fenton-Like | Haber–Weiss | SurfaceCatalytic | Photo- Catalytic | Tier | |
|---|---|---|---|---|---|---|---|---|
| Ag | [133,134] | [133] | [85] | 1 | ||||
| Au | [18,85] | 4 | ||||||
| CeO2 | [135,136] | [137] | 2 | |||||
| Co | [138,139] | [134] | 1 | |||||
| Co3O4 | [140] | 2 | ||||||
| CoO | [24] | [141,142] | 2 | |||||
| Cr | [134] | 1 | ||||||
| Cr2O3 | [24] | 3 | ||||||
| Cr3O4 | [24] | 3 | ||||||
| Cu | [25] | [134,142] | 1 | |||||
| CuO | [143] | [144] | 2 | |||||
| Fe | [41] | [18] | [64] | 1 | ||||
| Fe2O3 | [18,145,146] | [147] | 2 | |||||
| Fe3O4 | [18] | [148] | 2 | |||||
| FeS2 | [149] | 4 | ||||||
| Mn | [150] | [18,134] | 1 | |||||
| Mn2O3 | [24] | 2 | ||||||
| Mn3O4 | [18] | [137] | 2 | |||||
| MnO2 | [151] | [18] | 3 | |||||
| MoS2 | [149] | 4 | ||||||
| Ni | [152] | [85] | 1 | |||||
| Ni2O3 | [24] | 3 | ||||||
| Pd | [18] | 4 | ||||||
| Si | [85] | 4 | ||||||
| TiO2 | [24] | [153,154] | 2 | |||||
| WO3 | [155] | 4 | ||||||
| WS2 | [149] | 4 | ||||||
| ZnO | [151] | [21] | [21,154,156] | 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kessler, A.; Hedberg, J.; Blomberg, E.; Odnevall, I. Reactive Oxygen Species Formed by Metal and Metal Oxide Nanoparticles in Physiological Media—A Review of Reactions of Importance to Nanotoxicity and Proposal for Categorization. Nanomaterials 2022, 12, 1922. https://doi.org/10.3390/nano12111922
Kessler A, Hedberg J, Blomberg E, Odnevall I. Reactive Oxygen Species Formed by Metal and Metal Oxide Nanoparticles in Physiological Media—A Review of Reactions of Importance to Nanotoxicity and Proposal for Categorization. Nanomaterials. 2022; 12(11):1922. https://doi.org/10.3390/nano12111922
Chicago/Turabian StyleKessler, Amanda, Jonas Hedberg, Eva Blomberg, and Inger Odnevall. 2022. "Reactive Oxygen Species Formed by Metal and Metal Oxide Nanoparticles in Physiological Media—A Review of Reactions of Importance to Nanotoxicity and Proposal for Categorization" Nanomaterials 12, no. 11: 1922. https://doi.org/10.3390/nano12111922
APA StyleKessler, A., Hedberg, J., Blomberg, E., & Odnevall, I. (2022). Reactive Oxygen Species Formed by Metal and Metal Oxide Nanoparticles in Physiological Media—A Review of Reactions of Importance to Nanotoxicity and Proposal for Categorization. Nanomaterials, 12(11), 1922. https://doi.org/10.3390/nano12111922






