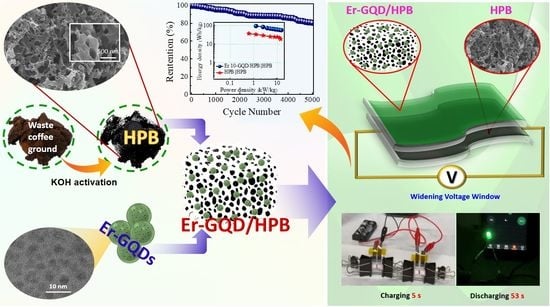
Erbium-Doped GQD-Embedded Coffee-Ground-Derived Porous Biochar for Highly Efficient Asymmetric Supercapacitor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Synthesis of Er-GQD/HPB Nanocomposites
2.3. Characterization
2.4. Electrochemical Analysis
3. Results and Discussion
3.1. Nanocomposite Characterization
3.2. Electrochemical Characterization of Er-GQD/HPB Nanocomposites
3.3. Electrochemical Performance of Asymmetric Supercapacitor
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chatterjee, D.P.; Nandi, A.K. A Review on the Recent Advances in Hybrid Supercapacitors. J. Mater. Chem. A 2021, 9, 15880–15918. [Google Scholar] [CrossRef]
- Bigdeloo, M.; Kowsari, E.; Ehsani, A.; Chinnappan, A.; Ramakrishna, S.; AliAkbari, R. Review on Innovative Sustainable Nanomaterials to Enhance the Performance of Supercapacitors. J. Energy Storage 2021, 37, 102474. [Google Scholar] [CrossRef]
- Raza, W.; Ali, F.; Raza, N.; Luo, Y.W.; Kim, K.H.; Yang, J.H.; Kumar, S.; Mehmood, A.; Kwon, E.E. Recent Advancements in Supercapacitor Technology. Nano Energy 2018, 52, 441–473. [Google Scholar] [CrossRef]
- Shao, Y.L.; El-Kady, M.F.; Sun, J.Y.; Li, Y.G.; Zhang, Q.H.; Zhu, M.F.; Wang, H.Z.; Dunn, B.; Kaner, R.B. Design and Mechanisms of Asymmetric Supercapacitors. Chem. Rev. 2018, 118, 9233–9280. [Google Scholar] [CrossRef]
- Dubey, P.; Shrivastav, V.; Maheshwari, P.H.; Sundriyal, S. Recent Advances in Biomass Derived Activated Carbon Electrodes for Hybrid Electrochemical Capacitor Applications: Challenges and Opportunities. Carbon 2020, 170, 1–29. [Google Scholar] [CrossRef]
- Luo, X.; Chen, Y.; Mo, Y. A Review of Charge Storage in Porous Carbon-Based Supercapacitors. Carbon 2021, 17, 427–428. [Google Scholar] [CrossRef]
- Gao, M.; Wang, W.K.; Zheng, Y.M.; Zhao, Q.B.; Yu, H.Q. Hierarchically Porous Biochar for Supercapacitor and Electrochemical H2O2 Production. Chem. Eng. J. 2020, 402, 126171. [Google Scholar] [CrossRef]
- Lei, W.D.; Yang, B.K.; Sun, Y.J.; Xiao, L.W.; Tang, D.Y.; Chen, K.; Sun, J.; Ke, J.; Zhuang, Y. Self-Sacrificial Template Synthesis of Heteroatom Doped Porous Biochar for Enhanced Electrochemical Energy Storage. J. Power Sources 2021, 488, 229455. [Google Scholar] [CrossRef]
- Yang, S.; Wang, S.; Liu, X.; Li, L. Biomass Derived Interconnected Hierarchical Micro-Meso-Macro- Porous Carbon with Ultrahigh Capacitance for Supercapacitors. Carbon 2021, 147, 540–549. [Google Scholar] [CrossRef]
- Nirmaladevi, S.; Boopathiraja, R.; Kandasamy, S.K.; Sathishkumar, S.; Parthibavarman, M. Wood Based Biochar Supported MnO2 Nanorods for High Energy Asymmetric Supercapacitor Applications. Surf. Interfaces 2021, 27, 101548. [Google Scholar] [CrossRef]
- Song, Y.; Qu, W.; He, Y.; Yang, H.; Du, M.; Wang, A.; Yang, Q.; Chen, Y. Synthesis and Processing Optimization of N-doped Hierarchical Porous Carbon Derived from Corncob for High Performance Supercapacitors. J. Energy Storage 2020, 32, 101877. [Google Scholar] [CrossRef]
- Zhao, H.Y.; Xia, J.; Yin, D.D.; Luo, M.; Yan, C.H.; Du, Y.P. Rare Earth Incorporated Electrode Materials for Advanced Energy Storage. Coord. Chem. Rev. 2019, 390, 32–49. [Google Scholar] [CrossRef]
- Nabi, G.; Raza, W.; Kamran, M.A.; Alharbi, T.; Rafique, M.; Tahir, M.B.; Hussain, S.; Khalid, N.R.; Aain, Q.; Malik, N.; et al. Role of Cerium-Doping in CoFe2O4 Electrodes for High Performance Supercapacitors. J. Energy Storage 2020, 29, 101452. [Google Scholar] [CrossRef]
- Mane, V.J.; Malavekar, D.B.; Ubale, S.B.; Bulakhe, R.N.; In, I.; Lokhande, C.D. Binder Fee Lanthanum Doped Manganese Oxide@Graphene Oxide Composite as High Energy Density Electrode Material for Flexible Symmetric Solid State Supercapacitor. Electrochim. Acta 2020, 335, 135613. [Google Scholar] [CrossRef]
- Dinari, M.; Allami, H.; Momeni, M.M. Construction of Ce-Doped NiCo-LDH@CNT Nanocomposite Electrodes for High-Performance Supercapacitor Application. Energy Fuels 2021, 35, 1831–1841. [Google Scholar] [CrossRef]
- Zahir, N.; Magri, P.; Luo, W.; Gaumet, J.J.; Pierrat, P. Recent Advances on Graphene Quantum Dots for Electrochemical Energy Storage Devices. Energy Environ. Mater. 2022, 5, 201–214. [Google Scholar] [CrossRef]
- Bui, T.A.N.; Nguyen, T.G.; Darmanto, W.; Doong, R.A. 3-Dimensional Ordered Reduced Graphene Oxide Embedded with N-Doped Graphene Quantum Dots for High Performance Supercapacitors. Electrochim. Acta 2020, 361, 137018. [Google Scholar] [CrossRef]
- Xing, R.; Gong, X.; Zhuang, X.; Li, Y.; Bulin, C.; Ge, X.; Zhang, B. Synthesis and Improved Electrochemical Properties of Nitrogen-doped Graphene Quantum Dot–Modified Polyaniline. J. Nanopart. Res. 2022, 24, 32. [Google Scholar] [CrossRef]
- Kuzhandaivel, H.; Manickam, S.; Balasingam, S.K.; Franklin, M.C.; Kim, H.J.; Nallathambi, K.S. Sulfur and Nitrogen-doped Graphene Quantum Dots/PANI Nanocomposites for Supercapacitors. New J. Chem. 2021, 45, 4101–4110. [Google Scholar] [CrossRef]
- Rahimpour, K.; Mofrad, R.T. Novel Hybrid Supercapacitor Based on Ferrocenyl Modified Graphene Quantum Dot and Polypyrrole Nanocomposite. Electrochim. Acta 2020, 345, 136207. [Google Scholar] [CrossRef]
- Ganganboina, A.B.; Park, E.Y.; Doong, R.A. Boosting the Energy Storage Performance of V2O5 Nanosheets by Intercalating Conductive Graphene Quantum Dots. Nanoscale 2020, 12, 16944–16955. [Google Scholar] [CrossRef]
- Sim, Y.; Kim, S.J.; Janani, G.; Chae, Y.; Surendran, S.; Kim, H.; Yoo, S.; Seok, D.C.; Jung, Y.H.; Jeon, C.; et al. The Synergistic Effect of Nitrogen and Fluorine co-Doping in Graphene Quantum Dot Catalysts for Full Water Splitting and Supercapacitor. Appl. Surf. Sci. 2020, 507, 145157. [Google Scholar] [CrossRef]
- Sekar, K.; Raji, G.; Tong, L.; Zhu, Y.; Liu, S.; Xing, R. Boosting the Electrochemical Performance of MoS2 Nanospheres-N-Doped-GQDs-rGO Three-Dimensional Nanostructure for Energy Storage and Conversion Applications. Appl. Surf. Sci. 2020, 504, 144441. [Google Scholar] [CrossRef]
- Zhang, S.; Gu, H.; Tang, T.; Du, W.; Gao, M.; Liu, Y.F.; Jian, D.; Pan, H. In Situ Encapsulation of the Nanoscale Er2O3 Phase to Drastically Suppress Voltage Fading and Capacity Degradation of a Li-and Mn Rich Layered Oxide Cathode for Lithium Ion Batteries. ACS Appl. Mater. Interfaces 2017, 9, 33863–33875. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.J.; Zhang, Y.; Huang, Y.D.; Wang, X.C.; Ding, J.; Guo, Y.; Tang, X.C. Enhancing Lithium Ion Diffusion Kinetic in Hierarchical Lithium Titanate@Erbium Oxide from Coating to Doping via Facile One-Step co-Precipitation. J. Colloid Interface Sci. 2021, 584, 900–906. [Google Scholar] [CrossRef]
- Chen, K.; Xue, D.F. Formation of Electroactive Colloids via in Situ Coprecipitation Under Electric Field: Erbium Chloride Alkaline Aqueous Pseudocapacitor. J. Colloid Interface Sci. 2014, 430, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Huynh, T.V.; Nguyen, T.N.A.; Darmanto, W.; Doong, R.A. Erbium-Doped Graphene Quantum Dots with Up-and Down-Conversion Luminescence for Effective Detection of Ferric Ions in Water and Human Serum. Sens. Actuators B Chem. 2021, 328, 129056. [Google Scholar] [CrossRef]
- Andrade, T.S.; Vakros, J.; Mantzavinos, D.; Lianos, P. Biochar Obtained by Carbonization of Spent Coffee Grounds and Its Application in the Construction of an Energy Storage Device. Chem. Eng. J. Adv. 2020, 4, 100061. [Google Scholar] [CrossRef]
- Tran, H.L.; Darmanto, W.; Doong, R.A. Ultrasensitive Detection of Tetracycline Using Boron and Nitrogen Co-Doped Graphene Quantum Dots from Natural Carbon Source as the Paper-Based Nanosensing Probe in Difference Matrices. Nanomaterials 2020, 10, 1883. [Google Scholar] [CrossRef]
- Chen, S.S.; Yu, I.K.M.D.; Cho, W.; Song, H.; Tsang, D.C.W.; Tessonnier, J.P.; Ok, Y.S.; Poon, C.S. Selective Glucose Isomerization to Fructose via a Nitrogen-Doped Solid Base Catalyst Derived from Spent Coffee Grounds. ACS Sustain. Chem. Eng. 2018, 6, 16113–16120. [Google Scholar] [CrossRef] [Green Version]
- Khedulkar, A.P.; Dang, V.D.; Pandit, B.; Bui, T.A.N.; Tran, H.L.; Doong, R.A. Flower-like Nickel Hydroxide@tea Leaf-Derived Biochar Composite for High-Performance Supercapacitor Application. J. Colloid Interface Sci. 2022, 623, 845–855. [Google Scholar] [CrossRef] [PubMed]
- Hung, Y.H.; Liu, T.Y.; Chen, H.Y. Renewable Coffee Waste-Derived Porous Carbons as Anode Materials for High-Performance Sustainable Microbial Fuel Cells. ACS Sustain. Chem. Eng. 2019, 7, 16991–16999. [Google Scholar] [CrossRef]
- Carneiro, J.S.D.S.; Filho, J.F.L.; Nardis, B.O.; Ribeiro-Soares, J.; Zinn, Y.L.; Melo, L.C.A. Carbon Stability of Engineered Biochar-Based Phosphate Fertilizers. ACS Sustain. Chem. Eng. 2018, 6, 14203–14212. [Google Scholar] [CrossRef]
- Kharangarh, P.R.; Umapathy, S.; Singh, G. Investigation of Sulfur Related Defects in Graphene Quantum Dots for Tuning Photoluminescence and High Quantum Yield. Appl. Surf. Sci. 2018, 449, 363–370. [Google Scholar] [CrossRef]
- Tajik, S.; Dourandish, Z.; Zhang, K.Q.; Beitollahi, H.; Le, Q.V.; Jang, H.W.; Shokouhimehr, M. Carbon and Graphene Quantum Dots: A Review on Syntheses, Characterization, Biological and Sensing Applications for Neurotransmitter Determination. RSC Adv. 2020, 10, 15406–15429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dang, V.D.; Adorna, J.; Annadurai, T.; Bui, T.A.N.; Tran, H.L.; Lin, L.Y.; Doong, R.A. Indirect Z-Scheme Nitrogen-Doped Carbon Dot Decorated Bi2MoO6/g-C3N4 Photocatalyst for Enhanced Visible-Light-Driven Degradation of Ciprofloxacin. Chem. Eng. J. 2021, 442, 130103. [Google Scholar] [CrossRef]
- Liu, S.H.; Huang, S.H. Valorization of Coffee Grounds to Biochar-Derived Adsorbents for CO2 Adsorption. J. Clean. Prod. 2018, 175, 354–360. [Google Scholar] [CrossRef]
- Li, Z.; Bu, F.; Wei, J.J.; Yao, W.W.; Wang, L.; Chen, Z.W.; Pan, D.Y.; Wu, M.H. Boosting the Energy Storage Densities of Supercapacitors by Incorporating N-Doped Graphene Quantum Dots into Cubic Porous Carbon. Nanoscale 2018, 10, 22871–22883. [Google Scholar] [CrossRef]
- Marik, S.; Labrugere, C.; Toulemonde, O.; Moran, E.; Franco, M.A.A. Core-Level Photoemission Spectra of Mo0.3Cu0.7Sr2ErCu2Oy, a Superconducting Perovskite Derivative. Unconventional Structure-Property Relationships. Dalton Trans. 2015, 44, 10795–10805. [Google Scholar] [CrossRef]
- Samir, M.; Ahmed, N.; Ramadan, M.; Allam, N.K. Electrospun Mesoporous Mn−V−O@C Nanofibers for High Performance Asymmetric Supercapacitor Devices with High Stability. ACS Sustain. Chem. Eng. 2019, 7, 13471–13480. [Google Scholar] [CrossRef]
- Samuel, E.; Kim, T.G.; Park, C.W.; Joshi, B.; Swihart, M.T.; Yoon, S.S. Supersonically Sprayed Zn2SnO4/SnO2/CNT Nanocomposites for High-Performance Supercapacitor Electrodes. ACS Sustain. Chem. Eng. 2019, 7, 14031–14040. [Google Scholar] [CrossRef]
- Pandit, B.; Karade, S.S.; Sankapal, B.R. Hexagonal VS2 Anchored MWCNTs: First Approach to Design Flexible Solid-State Symmetric Supercapacitor Device. ACS Appl. Mater. Interfaces 2017, 9, 44880–44891. [Google Scholar] [CrossRef]
- Hu, B.; Xu, C.; Aslam, M.K.; Cen, Y.; Hu, J.; Li, Y.; Liu, Y.; Guo, C.Z.; Yu, D.; Chen, C.G. La-Doped V2O5·nH2O@OAB and Flexible Fe2O3@rGO as Binder-Free Thin Film Electrodes for Asymmetric Supercapacitors. Chem. Eng. J. 2020, 389, 123534. [Google Scholar] [CrossRef]
- Noori, A.; Kady, M.F.E.; Rahmanifar, M.S.; Kaner, R.B.; Mousavi, M.F. Towards Establishing Standard Performance Metrics for Batteries, Supercapacitors and Beyond. Chem. Soc. Rev. 2019, 48, 1272–1341. [Google Scholar] [CrossRef] [PubMed]
- Sangabathula, O.; Sharma, C.S. One-Pot Hydrothermal Synthesis of Molybdenum Nickel Sulfide with Graphene Quantum Dots as a Novel Conductive Additive for Enhanced Supercapacitive Performance. Mater. Adv. 2020, 1, 2763–2772. [Google Scholar] [CrossRef]
- Liang, J.; Peng, H.; Wang, Z.; Zhao, R.; Zhang, W.; Ma, G.; Lei, Z. Rare Earth Metal Lanthanum-Organic Frameworks Derived Three-Dimensional Mesoporous Interconnected Carbon Nanosheets for Advanced Energy Storage. Electrochim. Acta 2020, 353, 136597. [Google Scholar] [CrossRef]
- Qiu, H.R.; Sun, X.; An, S.L.; Lan, D.; Cui, J.L.; Zhang, Y.Q.; He, W.X. Microwave Synthesis of Histidine-Functionalized Graphene Quantum Dots/Ni-Co LDH with Flower Ball Structure for Supercapacitor. J. Colloid Interf. Sci. 2020, 567, 264–273. [Google Scholar] [CrossRef]







| Electrode Materials | Electrolyte | Potential (V) | Specific Capacitance (F g−1) | Current Density (A g−1) | Refs. |
|---|---|---|---|---|---|
| GQD-Fc/PPy | 1 M Na2SO4 | −0.2 to 1 | 284 | 2.5 | [20] |
| NGQDs/PANI | 1 M H2SO4 | −0.2 to 0.8 | 506 | 0.5 | [18] |
| 3DMoS2-N-GQDs-rGO | 1 M Na2SO4 | −0.8 to 0.2 | 416.5 | 1 | [23] |
| S,N-GQDP2 | 1 M H2SO4 | −0.4 to 0.6 | 645 | 0.5 | [19] |
| N-doped AC | 6 M KOH | −1 to 0 | 382.6 | 0.5 | [11] |
| HMC-24 | 1 M H2SO4 | −0.4 to 0.6 | 447 | 0.2 | [8] |
| BC@MnO2 | 1 M Na2SO4 | −0.2 to 1 | 512 | 0.5 | [10] |
| Er 10-GQD/HPB | 2 M KOH | −1.2 to 0 | 699 | 1 | This work |
| Element | Value |
|---|---|
| Rs | 1.5 |
| Rct | 1.2 |
| Wo1 | 0.2 |
| CPE1 | 0.5 |
| CPE2 | 0.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bui, T.A.N.; Huynh, T.V.; Tran, H.L.; Doong, R.-a. Erbium-Doped GQD-Embedded Coffee-Ground-Derived Porous Biochar for Highly Efficient Asymmetric Supercapacitor. Nanomaterials 2022, 12, 1939. https://doi.org/10.3390/nano12111939
Bui TAN, Huynh TV, Tran HL, Doong R-a. Erbium-Doped GQD-Embedded Coffee-Ground-Derived Porous Biochar for Highly Efficient Asymmetric Supercapacitor. Nanomaterials. 2022; 12(11):1939. https://doi.org/10.3390/nano12111939
Chicago/Turabian StyleBui, Thi Ai Ngoc, Trung Viet Huynh, Hai Linh Tran, and Ruey-an Doong. 2022. "Erbium-Doped GQD-Embedded Coffee-Ground-Derived Porous Biochar for Highly Efficient Asymmetric Supercapacitor" Nanomaterials 12, no. 11: 1939. https://doi.org/10.3390/nano12111939
APA StyleBui, T. A. N., Huynh, T. V., Tran, H. L., & Doong, R.-a. (2022). Erbium-Doped GQD-Embedded Coffee-Ground-Derived Porous Biochar for Highly Efficient Asymmetric Supercapacitor. Nanomaterials, 12(11), 1939. https://doi.org/10.3390/nano12111939