TiO2 as an Anode of High-Performance Lithium-Ion Batteries: A Comprehensive Review towards Practical Application
Abstract
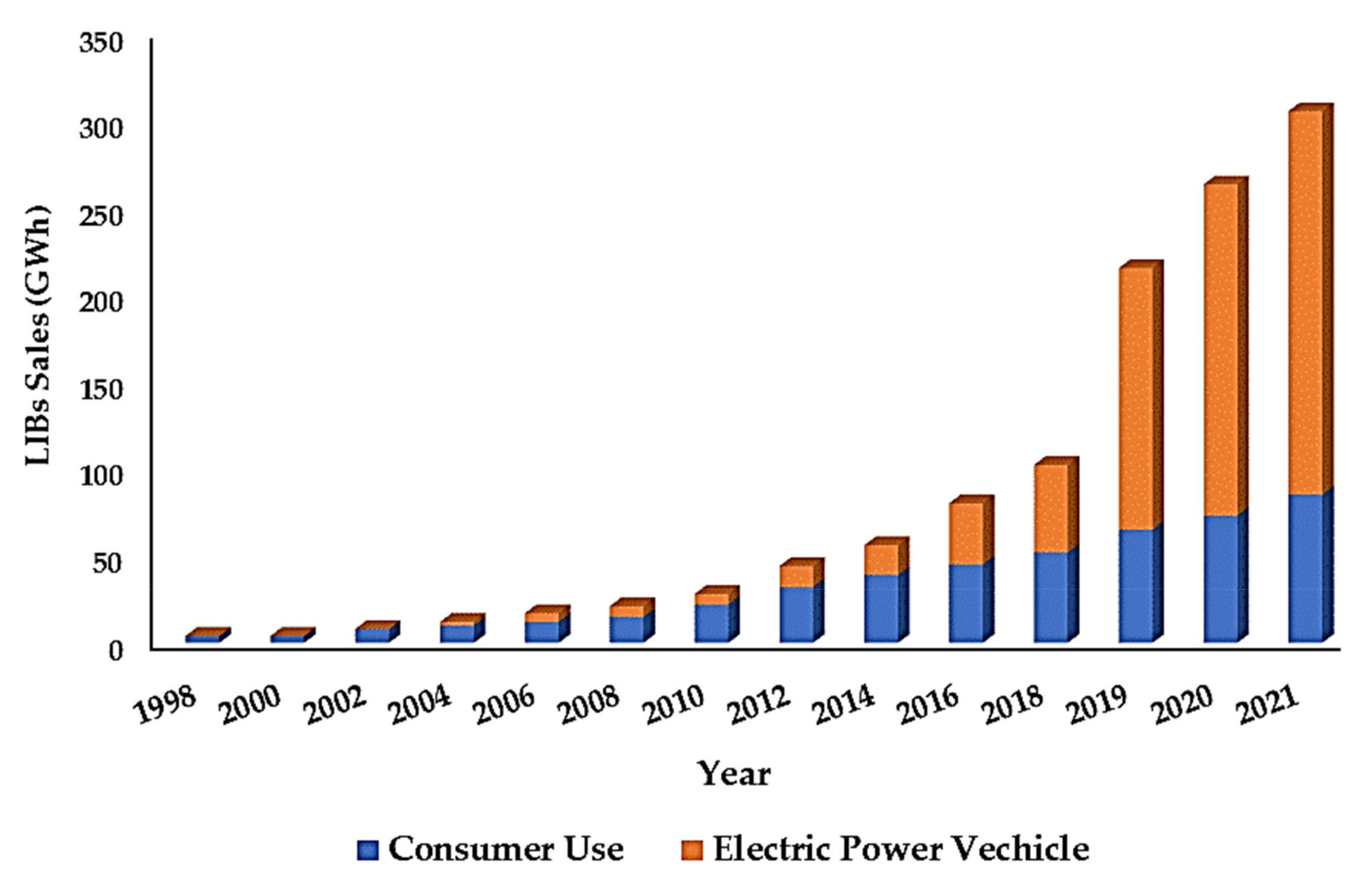
1. Introduction
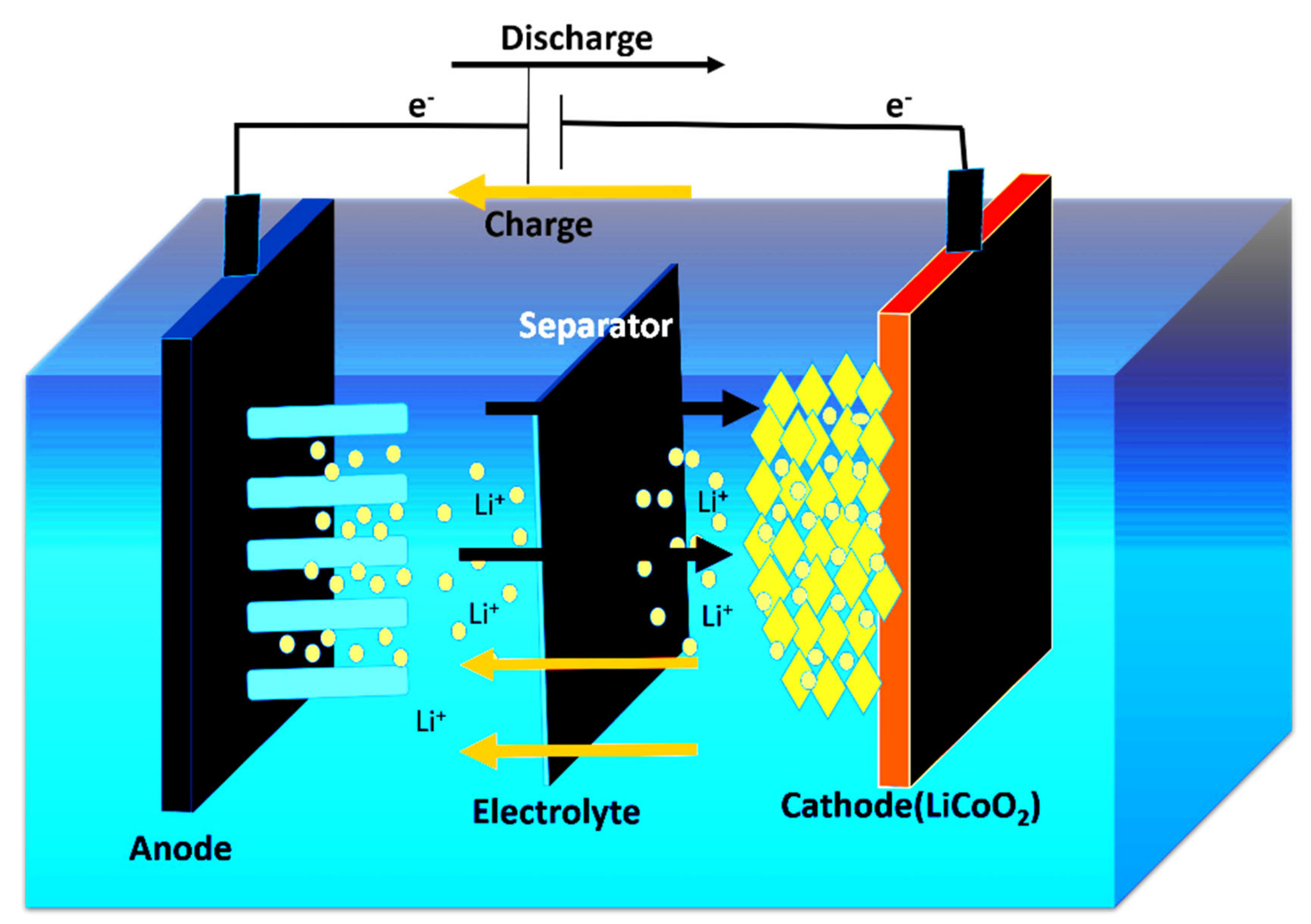
2. Electrochemistry of LIBs
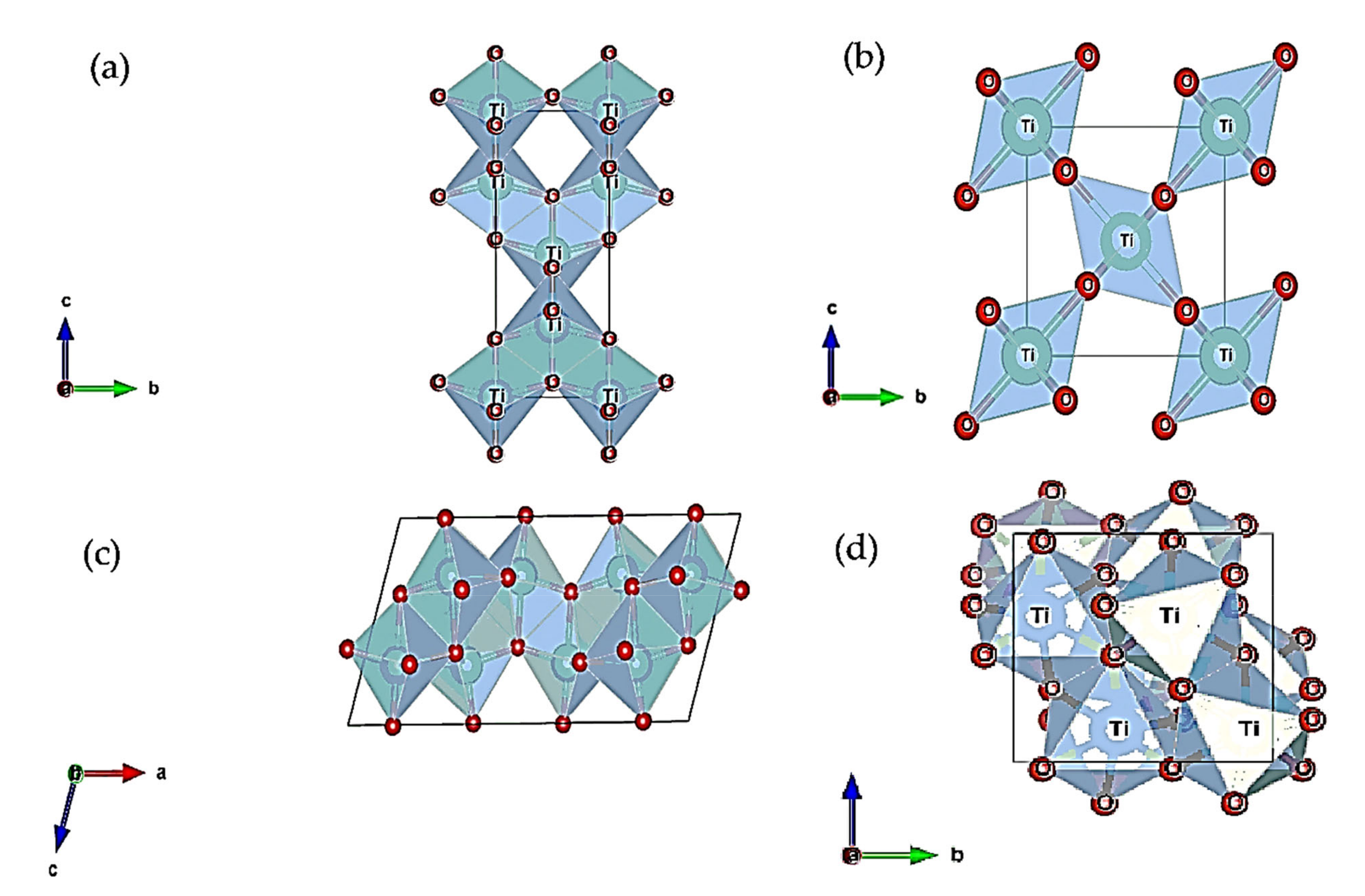
3. Different TiO2 Polymorphs
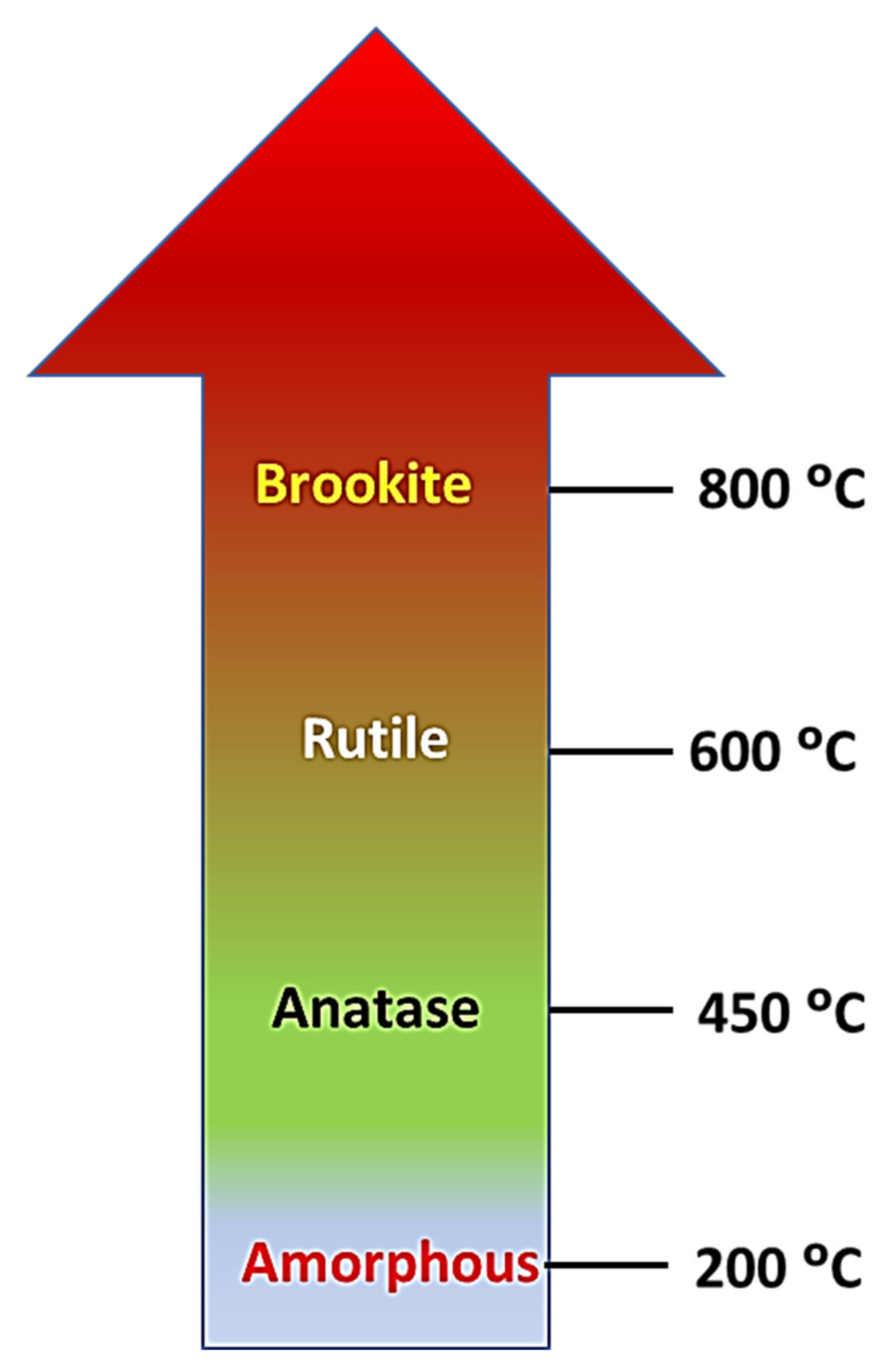
Influence of Experimental Conditions on TiO2 Crystal Structures
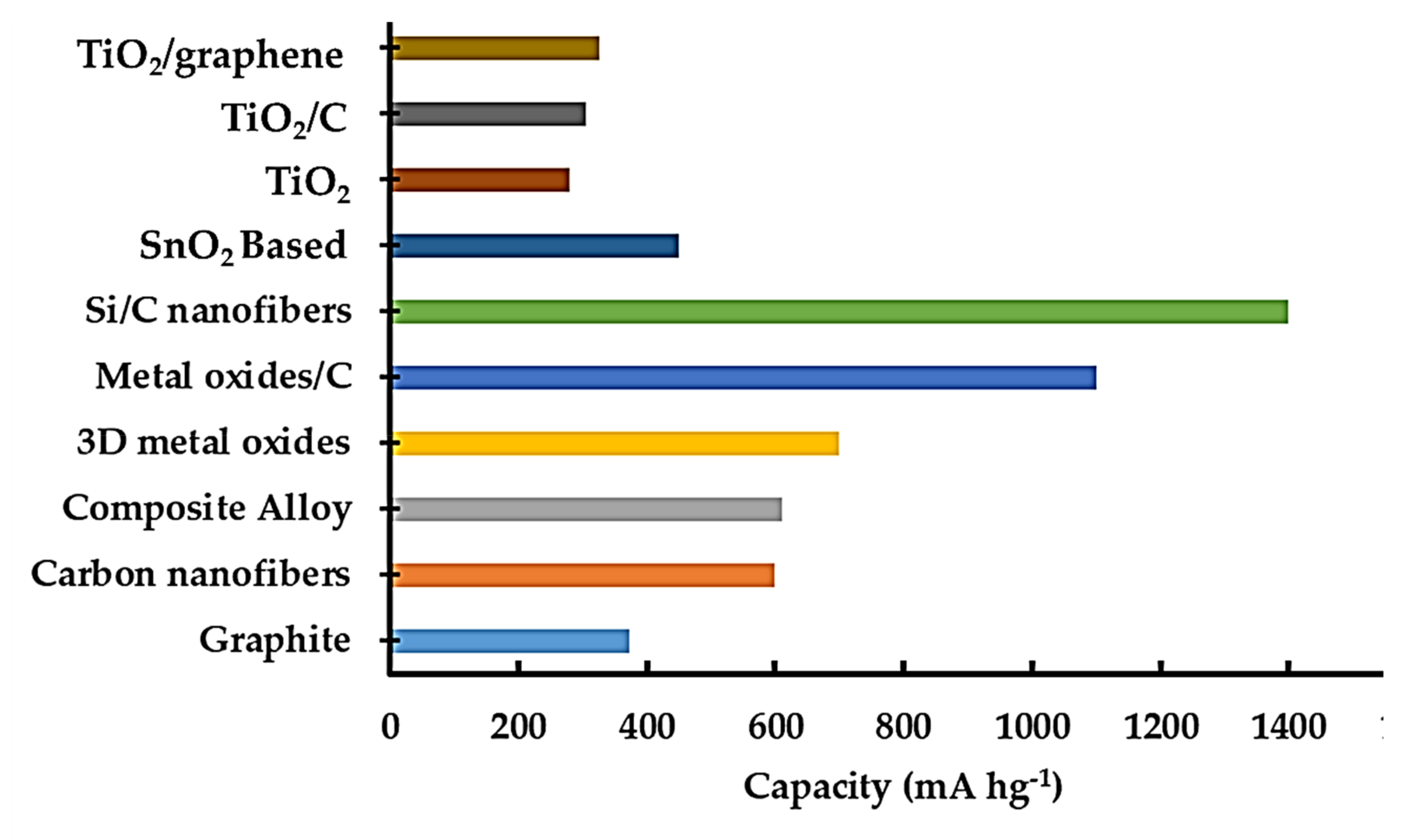
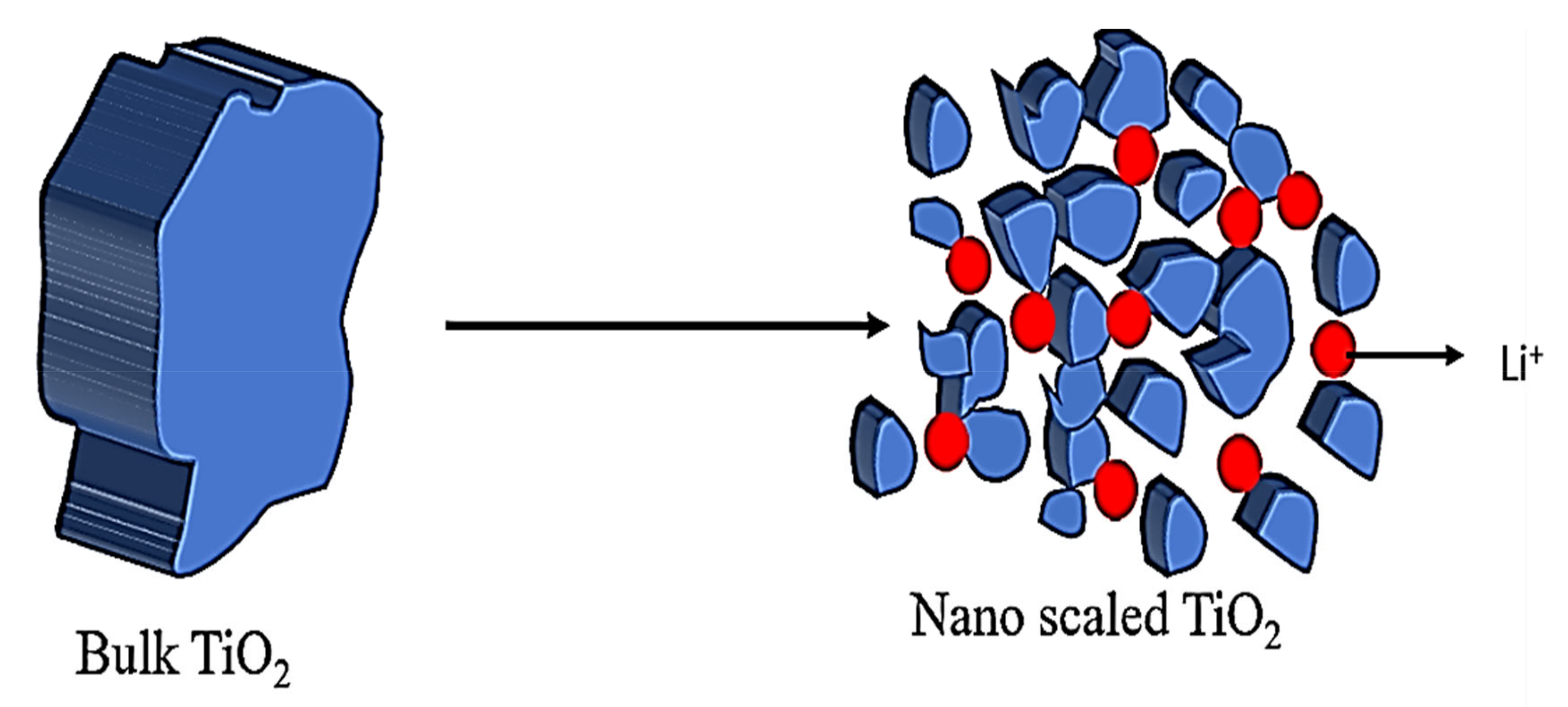
4. Different Nanostructures
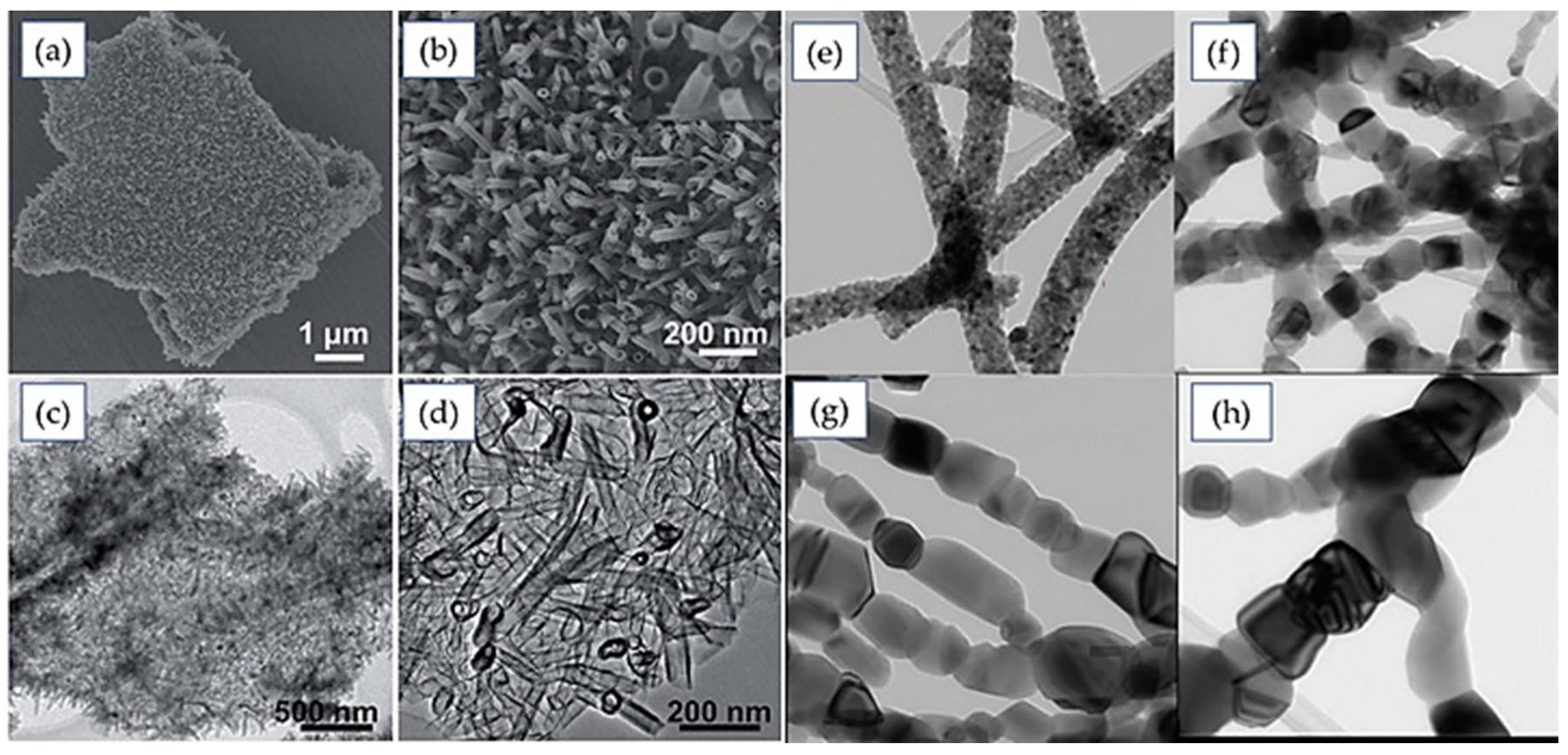
4.1. One-Dimensional Structure
4.2. Two-Dimensional Structures
4.3. Three-Dimensional Structure
5. Different Preparation Techniques
5.1. Solvothermal
5.2. Hydrothermal
5.3. Hydrolysis
5.4. Electrospinning
5.5. Anodization
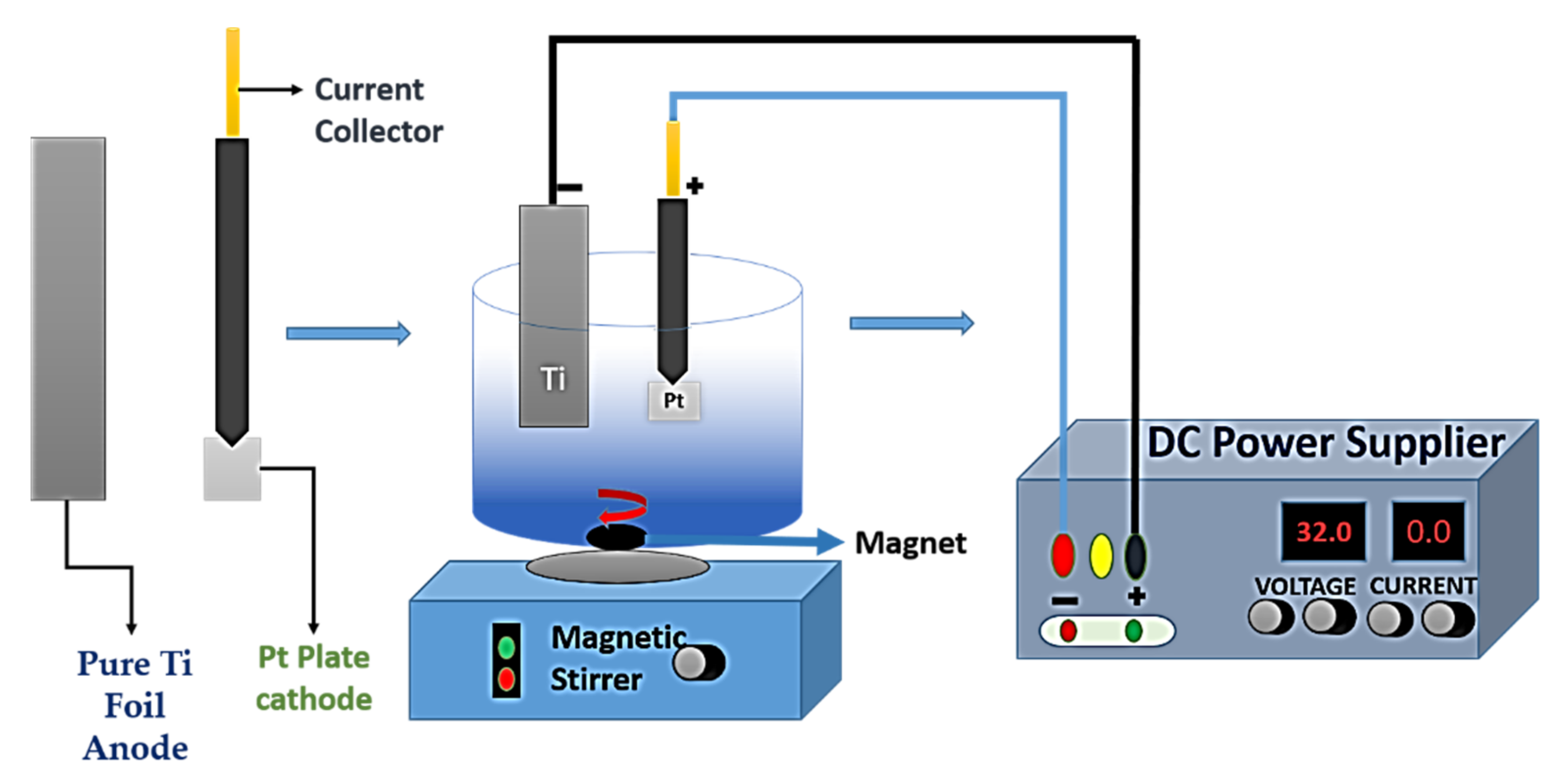
6. Nanostructured TiO2 by Electrochemical Anodization
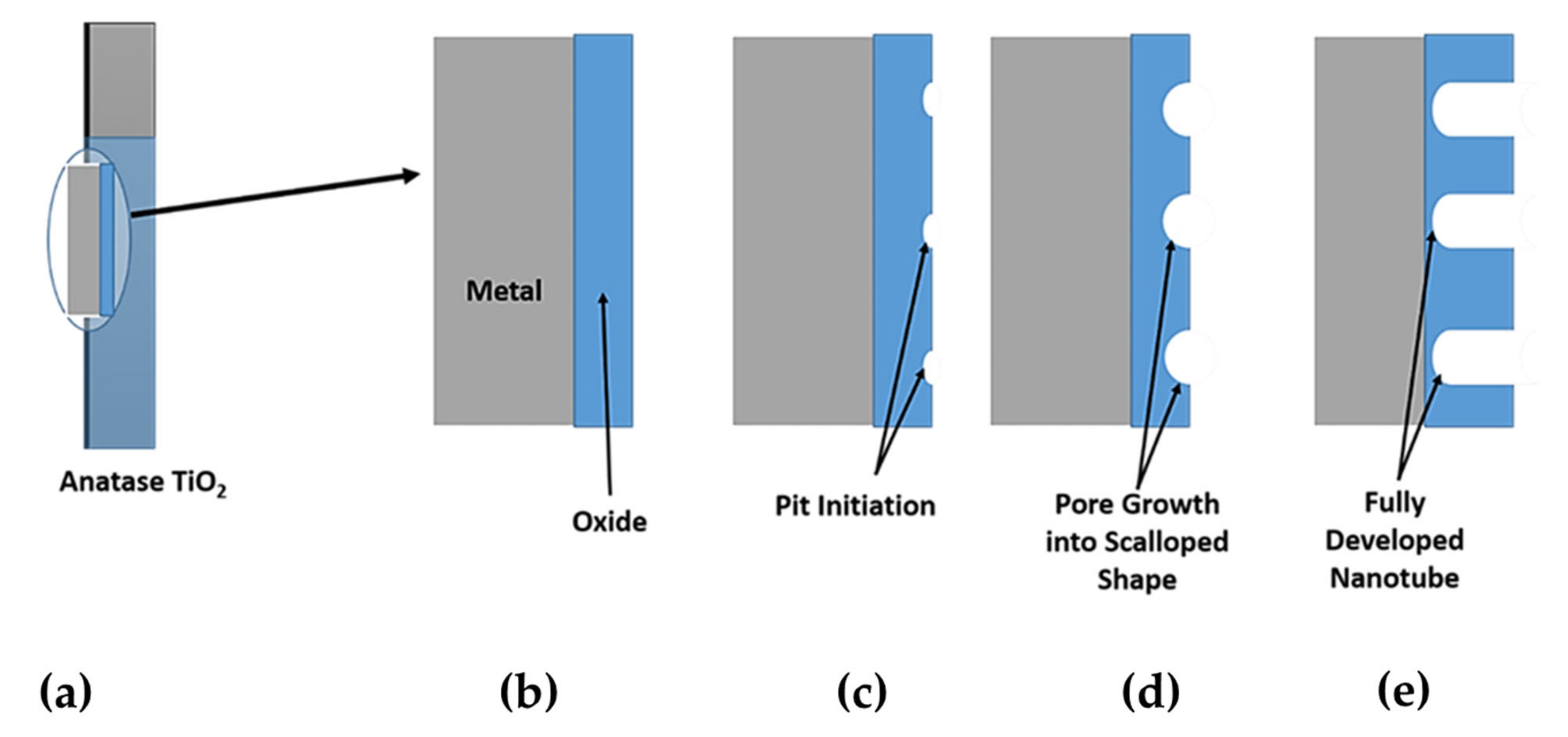
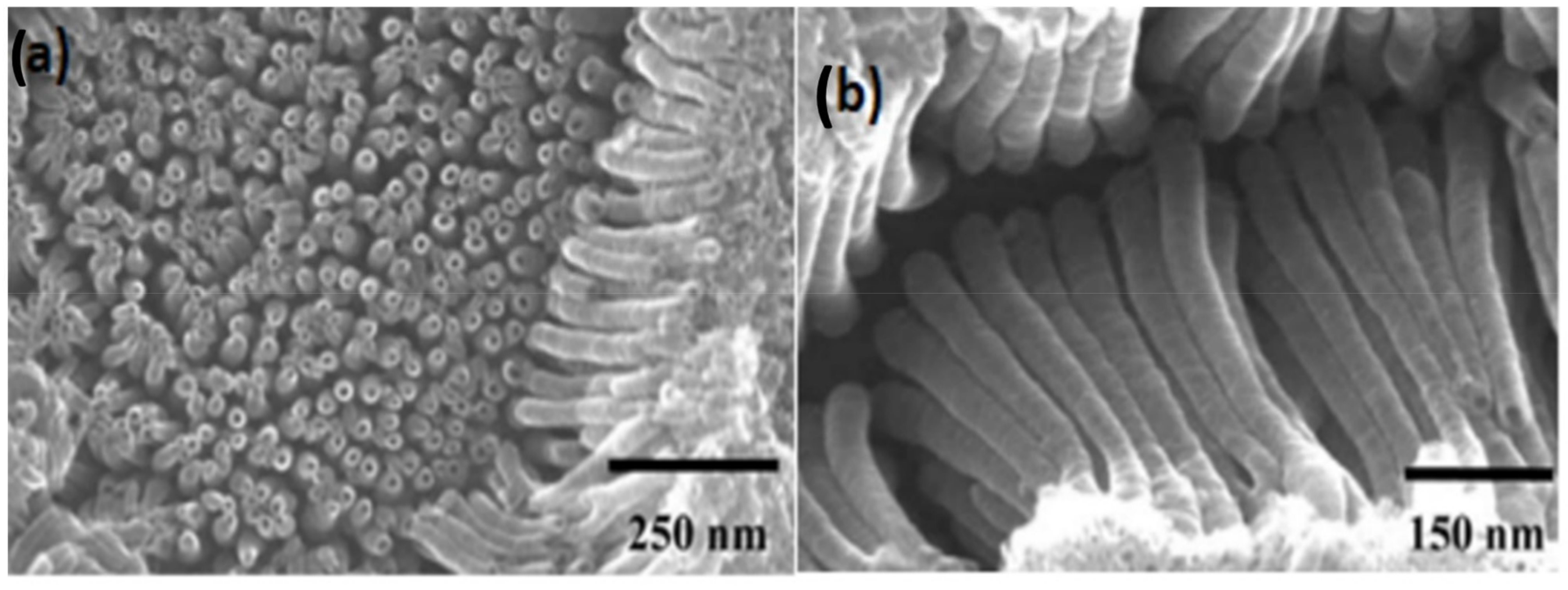
6.1. Anodization Process
6.2. Anodizing Parameters
6.2.1. Voltage
6.2.2. Electrolyte Composition
6.2.3. Electrolyte pH
6.2.4. Current Density
6.2.5. Electrolyte Temperature
6.2.6. Time
6.2.7. Counter Electrode
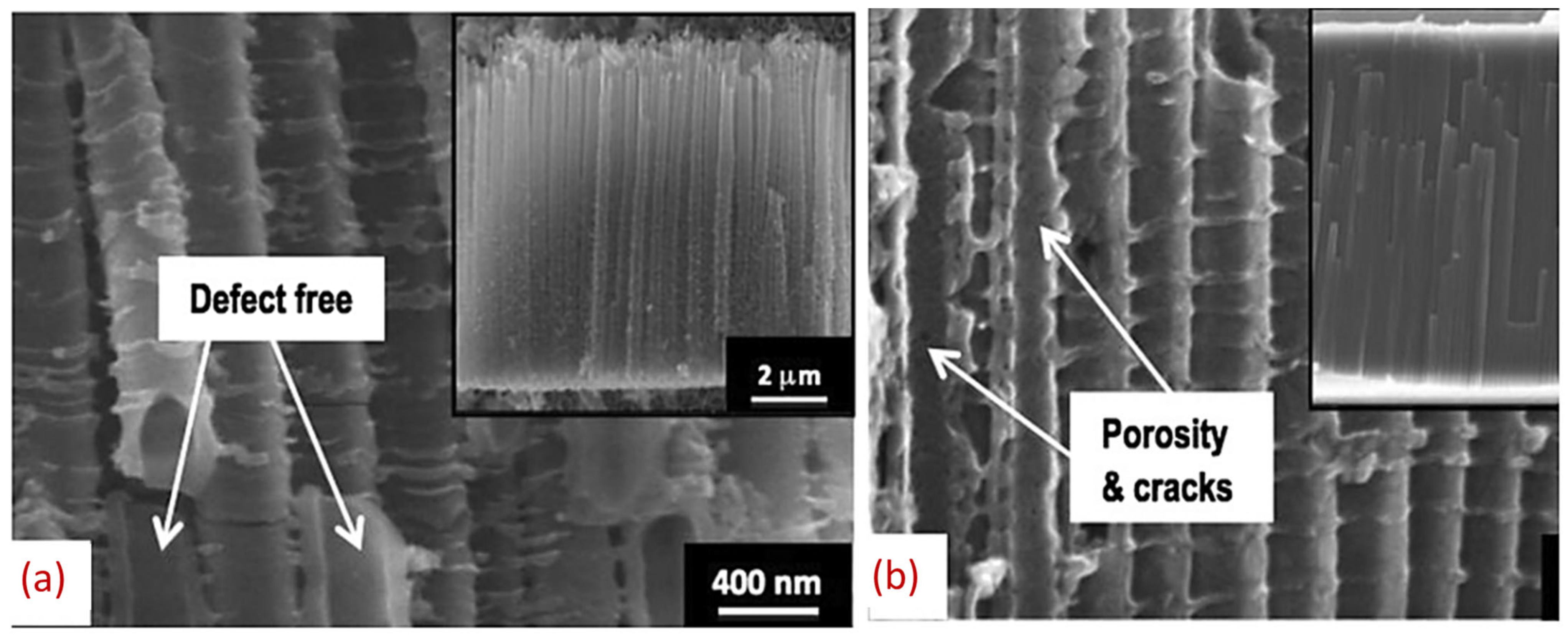
7. Anodized TiO2 as a Promising Anode for LIBs
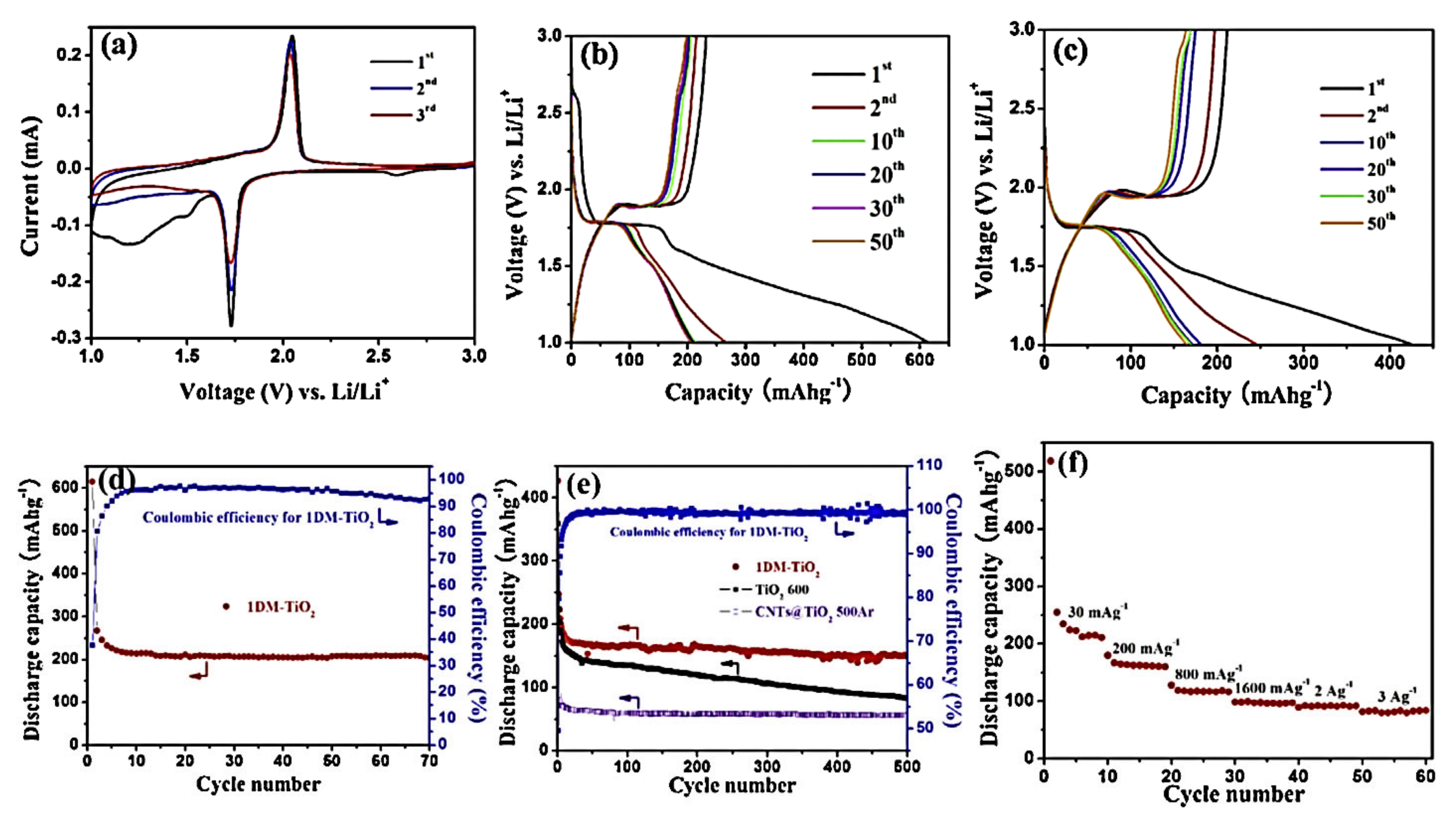
7.1. Morphological Impacts of Nanotubes on Electrochemical Performances
7.2. Impact of Nanotubes Exposed Energy Facets on Electrochemical Performances
7.3. Doping
7.3.1. Self-Doping by Annealing
7.3.2. Electrochemical Self-Doping
7.3.3. Doping by Foreign Materials
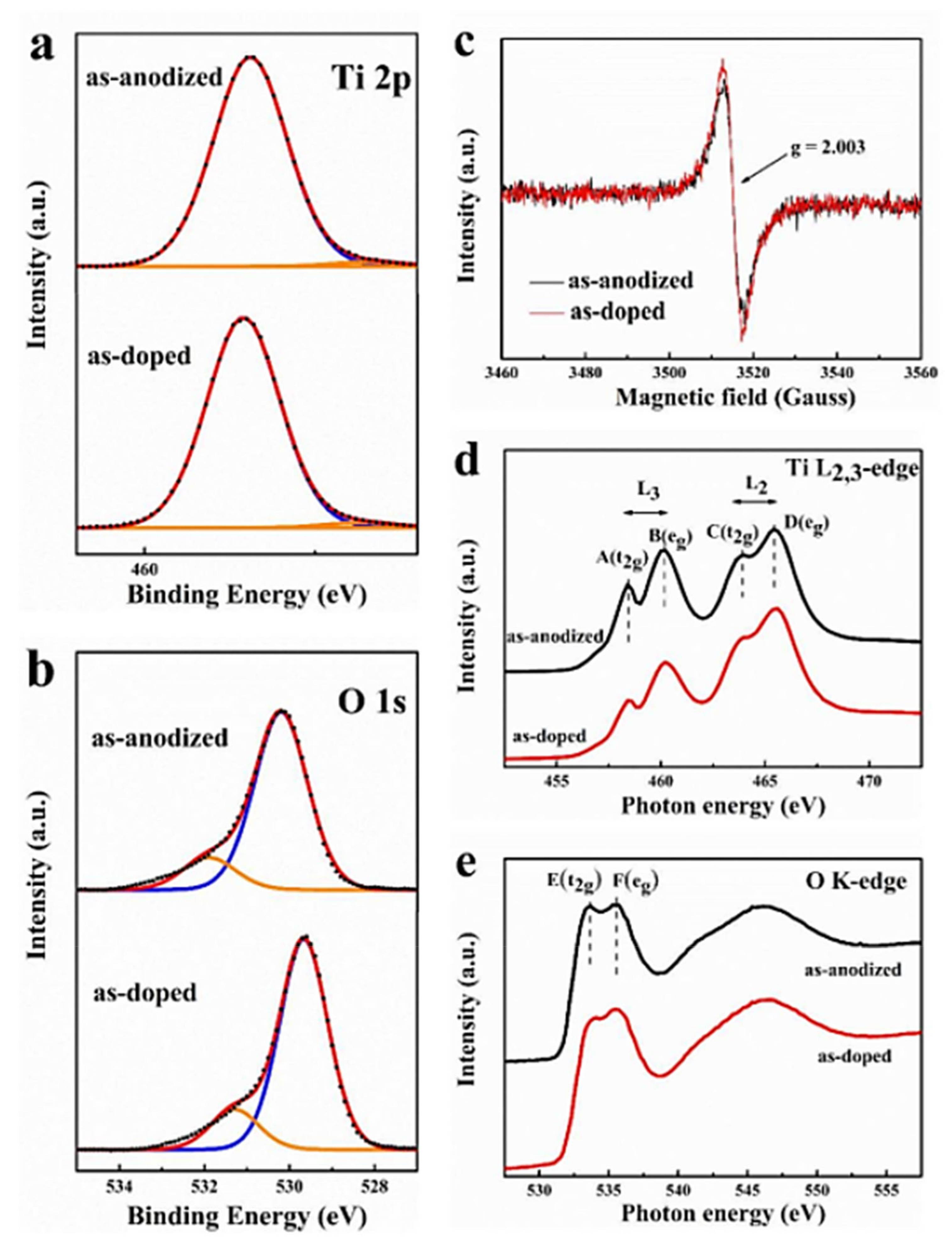
7.3.4. Amorphous and Anatase TiO2
8. Conclusions and Outlooks
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rahman, M.A.; Kim, J.; Hossain, S. Recent Advances of Energy Storage Technologies for Grid: A Comprehensive Review. Energy Storage 2021, e322. [Google Scholar] [CrossRef]
- Rodrigues, M.-T.F.; Babu, G.; Gullapalli, H.; Kalaga, K.; Sayed, F.N.; Kato, K.; Joyner, J.; Ajayan, P.M. A materials perspective on Li-ion batteries at extreme temperatures. Nat. Energy 2017, 2, 1–14. [Google Scholar] [CrossRef]
- Jabbour, L.; Bongiovanni, R.; Chaussy, D.; Gerbaldi, C.; Beneventi, D. Cellulose-based Li-ion batteries: A review. Cellulose 2013, 20, 1523–1545. [Google Scholar] [CrossRef]
- Bredas, J.-L.; Buriak, J.M.; Caruso, F.; Choi, K.-S.; Korgel, B.A.; Palacin, M.R.; Persson, K.; Reichmanis, E.; Schüth, F.; Seshadri, R.; et al. An electrifying choice for the 2019 chemistry Nobel Prize: Goodenough, Whittingham, and Yoshino. ACS Publ. 2019, 31, 8577–8581. [Google Scholar] [CrossRef]
- Zhang, J.; Terrones, M.; Park, C.R.; Mukherjee, R.; Monthioux, M.; Koratkar, N.; Kim, Y.S.; Hurt, R.; Frackowiak, E.; Enoki, T. Carbon science in 2016: Status, challenges and perspectives. Carbon N. Y. 2016, 98, 708–732. [Google Scholar] [CrossRef]
- Yang, Z.; Choi, D.; Kevin, S.; Rosso, K.M.; Wang, D.; Zhang, J.; Graff, G.; Liu, J. Nanostructures and lithium electrochemical reactivity of lithium titanites and titanium oxides: A review. J. Power Sources 2009, 192, 588–598. [Google Scholar] [CrossRef]
- Chandran, R.; Chevvaa, H.; Zeng, Z.; Liu, Y.; Zhang, W.; Wei, J.; LaJeunesse, D. Solid-state synthesis of silver nanowires using biopolymer thin films. Mater. Today Nano 2018, 1, 22–28. [Google Scholar] [CrossRef]
- Rahman, M.A.; Rahman, M.M.; Song, G. A review on binder-free NiO-Ni foam as anode of high performance lithium-ion batteries. Energy Storage 2021, 4, e278. [Google Scholar] [CrossRef]
- Castelvecchi, D.; Stoye, E. Chemistry Nobel honours world-changing batteries. Nature 2019, 574, 308–309. [Google Scholar] [CrossRef] [PubMed]
- Alva, G.; Lin, Y.; Fang, G. An overview of thermal energy storage systems. Energy 2018, 144, 341–378. [Google Scholar] [CrossRef]
- Thackeray, M.M.; Wolverton, C.; Isaacs, E.D. Electrical energy storage for transportation—approaching the limits of, and going beyond, lithium-ion batteries. Energy Environ. Sci. 2012, 5, 7854–7863. [Google Scholar] [CrossRef]
- Yoshino, A. The birth of the lithium-ion battery. Angew. Chem. Int. Ed. 2012, 51, 5798–5800. [Google Scholar] [CrossRef]
- Deshwal, D.; Sangwan, P.; Dahiya, N. Economic Analysis of Lithium Ion Battery Recycling in India. Wirel. Pers. Commun. 2022, 1–24. [Google Scholar] [CrossRef]
- Yariv, O.; Hirshberg, D.; Zinigrad, E.; Meitav, A.; Aurbach, D.; Jiang, M.; Powell, B.R. Carbon negative electrodes for Li-ion batteries: The effect of solutions and temperatures. J. Electrochem. Soc. 2014, 161, A1422. [Google Scholar] [CrossRef]
- Yaakov, D.; Gofer, Y.; Aurbach, D.; Halalay, I.C. On the study of electrolyte solutions for Li-ion batteries that can work over a wide temperature range. J. Electrochem. Soc. 2010, 157, A1383. [Google Scholar] [CrossRef]
- Goriparti, S.; Miele, E.; De Angelis, F.; Di Fabrizio, E.; Zaccaria, R.P.; Capiglia, C. Review on recent progress of nanostructured anode materials for Li-ion batteries. J. Power Sources 2014, 257, 421–443. [Google Scholar] [CrossRef]
- Uhlmann, C.; Illig, J.; Ender, M.; Schuster, R.; Ivers-Tiffée, E. In situ detection of lithium metal plating on graphite in experimental cells. J. Power Sources 2015, 279, 428–438. [Google Scholar] [CrossRef]
- Bandhauer, T.M.; Garimella, S.; Fuller, T.F. A critical review of thermal issues in lithium-ion batteries. J. Electrochem. Soc. 2011, 158, R1. [Google Scholar] [CrossRef]
- Rahman, M.; Wen, C. A study of the capacity fade of porous NiO/Ni foam as negative electrode for lithium-ion batteries. Ionics 2016, 22, 173–184. [Google Scholar] [CrossRef]
- Rahman, M.A.; Song, G.; Bhatt, A.I.; Wong, Y.C.; Wen, C. Nanostructured silicon anodes for high-performance lithium-ion batteries. Adv. Funct. Mater. 2016, 26, 647–678. [Google Scholar] [CrossRef]
- Watanabe, K.; Kikuoka, T.; Kumagai, N. Physical and electrochemical characteristics of nickel hydroxide as a positive material for rechargeable alkaline batteries. J. Appl. Electrochem. 1995, 25, 219–226. [Google Scholar] [CrossRef]
- Lacerda, V.G.; Mageste, A.B.; Santos, I.J.B.; Da Silva, L.H.M.; Da Silva, M.d.C.H. Separation of Cd and Ni from Ni–Cd batteries by an environmentally safe methodology employing aqueous two-phase systems. J. Power Sources 2009, 193, 908–913. [Google Scholar] [CrossRef]
- Rahman, M.; Wong, Y.C.; Song, G.; Wen, C. A review on porous negative electrodes for high performance lithium-ion batteries. J. Porous Mater. 2015, 22, 1313–1343. [Google Scholar] [CrossRef]
- Serrao, L.; Chehab, Z.; Guezennee, Y.; Rizzoni, G. An aging model of Ni-MH batteries for hybrid electric vehicles. In Proceedings of the IEEE Vehicle Power and Propulsion Conference, Chicago, IL, USA, 7 September 2005; p. 8. [Google Scholar] [CrossRef]
- Antony, R.P.; Mathews, T.; Dasgupta, A.; Dash, S.; Tyagi, A.K.; Raj, B. Rapid breakdown anodization technique for the synthesis of high aspect ratio and high surface area anatase TiO2 nanotube powders. J. Solid State Chem. 2011, 184, 624–632. [Google Scholar] [CrossRef]
- Motola, M.; Hromadko, L.; Prikryl, J.; Sopha, H.; Krbal, M.; Macak, J.M. Intrinsic properties of high-aspect ratio single-and double-wall anodic TiO2 nanotube layers annealed at different temperatures. Electrochim. Acta 2020, 352, 136479. [Google Scholar] [CrossRef]
- Jeon, H.; Yeon, D.; Lee, T.; Park, J.; Hwang, S.; Ryou, M.-H.; Lee, Y.M. Aqueous Ceramic Coating upon Hydrophobic Polyethylene Lithium-Ion Battery Separators through Use of an Anionic Surfactant. In Proceedings of the 229th ECS Meeting, San Diego, CA, USA, 29 May–2 June 2016; p. 293. [Google Scholar] [CrossRef]
- Amanor-Boadu, J.M.; Guiseppi-Elie, A.; Sánchez-Sinencio, E. Search for optimal pulse charging parameters for Li-ion polymer batteries using Taguchi orthogonal arrays. IEEE Trans. Ind. Electron. 2018, 65, 8982–8992. [Google Scholar] [CrossRef]
- Choi, J.W.; Aurbach, D. Promise and reality of post-lithium-ion batteries with high energy densities. Nat. Rev. Mater. 2016, 1, 1–16. [Google Scholar] [CrossRef]
- Song, Y.; Jiao, S.; Tu, J.; Wang, J.; Liu, Y.; Jiao, H.; Mao, X.; Guoa, Z.; Frayb, D.J. A long-life rechargeable Al ion battery based on molten salts. J. Mater. Chem. A 2017, 5, 1282–1291. [Google Scholar] [CrossRef]
- Bai, L.; Fang, F.; Zhao, Y.; Liu, Y.; Li, J.; Huang, G.; Sun, H. A sandwich structure of mesoporous anatase TiO2 sheets and reduced graphene oxide and its application as lithium-ion battery electrodes. RSC Adv. 2014, 4, 43039–43046. [Google Scholar] [CrossRef]
- Shimizu, Y.; Uemura, K.; Matsuda, H.; Miura, N.; Yamazoe, N. Bi-Functional Oxygen Electrode Using Large Surface Area La1− xCax CoO3 for Rechargeable Metal-Air Battery. J. Electrochem. Soc. 1990, 137, 3430. [Google Scholar] [CrossRef]
- Howard, C.J.; Sabine, T.M.; Dickson, F. Structural and thermal parameters for rutile and anatase. Acta Crystallogr. Sect. B Struct. Sci. 1991, 47, 462–468. [Google Scholar] [CrossRef]
- Wagemaker, M.; Van Well, A.A.; Kearley, G.J.; Mulder, F.M. The life and times of lithium in anatase TiO2. Solid State Ion. 2004, 175, 191–193. [Google Scholar] [CrossRef]
- Cava, R.J.; Murphy, D.W.; Zahurak, S.; Santoro, A.; Roth, R.S. The crystal structures of the lithium-inserted metal oxides Li0.5TiO2 anatase, LiTi2O4 spinel, and Li2Ti2O4. J. Solid State Chem. 1984, 53, 64–75. [Google Scholar] [CrossRef]
- Sudant, G.; Baudrin, E.; Larcher, D.; Tarascon, J.-M. Electrochemical lithium reactivity with nanotextured anatase-type TiO2. J. Mater. Chem. 2005, 15, 1263–1269. [Google Scholar] [CrossRef]
- Li, J.; Tang, Z.; Zhang, Z. Preparation and novel lithium intercalation properties of titanium oxide nanotubes. Electrochem. Solid State Lett. 2005, 8, A316. [Google Scholar] [CrossRef]
- Wagemaker, M.; Borghols, W.J.H.; Mulder, F.M. Large impact of particle size on insertion reactions. a case for anatase LixTiO2. J. Am. Chem. Soc. 2007, 129, 4323–4327. [Google Scholar] [CrossRef]
- Kavan, L.; Kalbáč, M.; Zukalová, M.; Exnar, I.; Lorenzen, V.; Nesper, R.; Graetzel, M. Lithium storage in nanostructured TiO2 made by hydrothermal growth. Chem. Mater. 2004, 16, 477–485. [Google Scholar] [CrossRef]
- Zukalová, M.; Kalbác, M.; Kavan, L.; Exnar, I.; Graetzel, M. Pseudocapacitive lithium storage in TiO2 (B). Chem. Mater. 2005, 17, 1248–1255. [Google Scholar] [CrossRef]
- Mattesini, M.; De Almeida, J.S.; Dubrovinsky, L.; Dubrovinskaia, N.; Johansson, B.; Ahuja, R. High-pressure and high-temperature synthesis of the cubic TiO2 polymorph. Phys. Rev. B 2004, 70, 212101. [Google Scholar] [CrossRef]
- Zhu, S.-C.; Xie, S.-H.; Liu, Z.-P. Nature of rutile nuclei in anatase-to-rutile phase transition. J. Am. Chem. Soc. 2015, 137, 11532–11539. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, Z.; Huang, J.; Lim, L.Y.; Li, W.; Deng, J.; Gong, D.; Tang, Y.; Lai, Y.; Chen, Z. Titanate and titania nanostructured materials for environmental and energy applications: A review. RSC Adv. 2015, 5, 79479–79510. [Google Scholar] [CrossRef]
- Yan, X.; Wang, D.Z.; He, P.M.; Hou, P.Z.; Xia, T.; Liu, D.G.; Chen, P.X. TiO2 nanomaterials as anode materials for lithium-ion rechargeable batteries. Energy Technol. 2015, 3, 801–814. [Google Scholar] [CrossRef]
- Wagemaker, M.; van de Krol, R.; Kentgens, A.P.M.; Van Well, A.A.; Mulder, F.M. Two phase morphology limits lithium diffusion in TiO2 (anatase): A 7Li MAS NMR study. J. Am. Chem. Soc. 2001, 123, 11454–11461. [Google Scholar] [CrossRef] [PubMed]
- Koudriachova, M.V.; Harrison, N.M.; de Leeuw, S.W. Effect of diffusion on lithium intercalation in titanium dioxide. Phys. Rev. Lett. 2001, 86, 1275. [Google Scholar] [CrossRef]
- Koudriachova, M.V.; Harrison, N.M.; de Leeuw, S.W. Diffusion of Li-ions in rutile. An ab initio study. Solid State Ion. 2003, 157, 35–38. [Google Scholar] [CrossRef]
- Hu, Y.; Kienle, L.; Guo, Y.; Maier, J. High lithium electroactivity of nanometer-sized rutile TiO2. Adv. Mater. 2006, 18, 1421–1426. [Google Scholar] [CrossRef]
- Baudrin, E.; Cassaignon, S.; Koelsch, M.; Jolivet, J.-P.; Dupont, L.; Tarascon, J.-M. Structural evolution during the reaction of Li with nano-sized rutile type TiO2 at room temperature. Electrochem. Commun. 2007, 9, 337–342. [Google Scholar] [CrossRef]
- Cromer, D.T.; Herrington, K. The structures of anatase and rutile. J. Am. Chem. Soc. 1955, 77, 4708–4709. [Google Scholar] [CrossRef]
- Lee, D.; Park, J.; Jin Choi, K.; Choi, H.; Kim, D. Preparation of Brookite-Type TiO2/Carbon Nanocomposite Electrodes for Application to Li Ion Batteries. Eur. J. Inorg. Chem. 2008, 6, 878–882. [Google Scholar] [CrossRef]
- Ibrahim, S.A.; Sreekantan, S. Effect of pH on TiO2 nanoparticles via sol-gel method. Adv. Mater. Res. 2011, 173, 184–189. [Google Scholar] [CrossRef]
- Chen, Z.; Zhao, G.; Li, H.; Han, G.; Song, B. Effects of water amount and pH on the Crystal Behavior of a TiO2 nanocrystalline derived from a sol-gel process at a low temperature. J. Am. Ceram. Soc. 2009, 92, 1024–1029. [Google Scholar] [CrossRef]
- Molea, A.; Popescu, V.; Rowson, N.A.; Dinescu, d.A.M. Influence of pH on the formulation of TiO2 nano-crystalline powders with high photocatalytic activity. Powder Technol. 2014, 253, 22–28. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, B.; Cui, H.; An, H.; Pan, Y.; Zhai, J. Synthesis of crystal-controlled TiO2 nanorods by a hydrothermal method: Rutile and brookite as highly active photocatalysts. J. Phys. Chem. C 2015, 119, 16905–16912. [Google Scholar] [CrossRef]
- Bakri, A.S.; Sahdan, M.Z.; Adriyanto, F.; Raship, N.A.; Said, N.D.M.; Abdullah, S.A.; Rahim, M.S. Effect of annealing temperature of titanium dioxide thin films on structural and electrical properties. AIP Conf. Proc. 2017, 1788, 030030. [Google Scholar] [CrossRef]
- Alsawat, M.; Altalhi, T.; Shapter, J.G.; Losic, D. Influence of dimensions, inter-distance and crystallinity of titania nanotubes (TNTs) on their photocatalytic activity. Catal. Sci. Technol. 2014, 4, 2091–2098. [Google Scholar] [CrossRef]
- Rahman, M.; Wen, C. Nanogravel structured NiO/Ni foam as electrode for high-performance lithium-ion batteries. Ionics 2015, 21, 2709–2723. [Google Scholar] [CrossRef]
- Sarker, B.; Rahman, M.A.; Rahman, M.M.; Islam, M.S. Fabrication of Ni-NiO Foams by Powder Metallurgy Technique and Study of Bulk Crushing Strength. Trends Sci. 2021, 18, 683. [Google Scholar] [CrossRef]
- Rahman, M.A.; Zhu, X.; Wen, C. Fabrication of nanoporous Ni by chemical dealloying Al from Ni–Al alloys for lithium-ion batteries. Int. J. Electrochem. Sci. 2015, 10, 3767–3783. [Google Scholar]
- Daghrir, R.; Drogui, P.; Robert, D. Modified TiO2 for environmental photocatalytic applications: A review. Ind. Eng. Chem. Res. 2013, 52, 3581–3599. [Google Scholar] [CrossRef]
- Zhou, W.; Liu, H.; Boughton, R.I.; Du, G.; Lin, J.; Wanga, J.; Liua, D. One-dimensional single-crystalline Ti-O based nanostructures: Properties, synthesis, modifications and applications. J. Mater. Chem. 2010, 20, 5993–6008. [Google Scholar] [CrossRef]
- Tian, J.; Zhao, Z.; Kumar, A.; Boughton, R.I.; Liu, H. Recent progress in design, synthesis, and applications of one-dimensional TiO2 nanostructured surface heterostructures: A review. Chem. Soc. Rev. 2014, 43, 6920–6937. [Google Scholar] [CrossRef]
- Sakamoto, M.; Majima, T. Photochemistry for the synthesis of noble metal nanoparticles. Bull. Chem. Soc. Jpn. 2010, 83, 1133–1154. [Google Scholar] [CrossRef]
- Lu, Q.; Lu, Z.; Lu, Y.; Lv, L.; Ning, Y.; Yu, H.; Hou, Y.; Yin, Y. Photocatalytic synthesis and photovoltaic application of Ag-TiO2 nanorod composites. Nano Lett. 2013, 13, 5698–5702. [Google Scholar] [CrossRef]
- Wang, G.; He, X.; Xu, G.; Chen, L.; Zhu, Y.; Zhang, X.; Wang, L. Detection of T4 polynucleotide kinase activity with immobilization of TiO2 nanotubes and amplification of Au nanoparticles. Biosens. Bioelectron. 2013, 43, 125–130. [Google Scholar] [CrossRef]
- Pu, Y.C.; Wang, G.; Chang, K.D.; Ling, Y.; Lin, Y.K.; Fitzmorris, B.C.; Liu, C.M.; Lu, X.; Tong, Y.; Zhang, J.Z.; et al. Au nanostructure-decorated TiO2 nanowires exhibiting photoactivity across entire UV-visible region for photoelectrochemical water splitting. Nano Lett. 2013, 13, 3817–3823. [Google Scholar] [CrossRef]
- Wei, J.; Liu, J.X.; Dang, Y.C.; Xu, K.; Zhou, Y. A review of nanostructured TiO2 application in Li-Ion batteries. Adv. Mater. Res. 2013, 750–752, 301–306. [Google Scholar] [CrossRef]
- Tian, Q.; Zhang, Z.; Yang, L.; Hirano, S.I. Facile fabrication of one-dimensional mesoporous titanium dioxide composed of nanocrystals for lithium storage. Electrochim. Acta 2014, 138, 155–162. [Google Scholar] [CrossRef]
- Tang, Y.; Liu, Z.; Lü, X.; Wang, B.; Huang, F. TiO2 nanotubes grown on graphene sheets as advanced anode materials for high rate lithium ion batteries. RSC Adv. 2014, 4, 36372–36376. [Google Scholar] [CrossRef]
- Tammawat, P.; Meethong, N. Synthesis and characterization of stable and binder-free electrodes of TiO2 nanofibers for li-ion batteries. J. Nanomater. 2013, 2013, 1. [Google Scholar] [CrossRef]
- Armstrong, A.R.; Armstrong, G.; Canales, J.; Bruce, P.G. TiO2-B nanowires as negative electrodes for rechargeable lithium batteries. J. Power Sources 2005, 146, 501–506. [Google Scholar] [CrossRef]
- Xia, H.R.; Li, J.; Peng, C.; Sun, W.T.; Li, L.W.; Peng, L.M. Floating growth of large-scale freestanding TiO2 nanorod films at the gas-liquid interface for additive-free li-ion battery applications. ACS Appl. Mater. Interfaces 2014, 6, 17376–17383. [Google Scholar] [CrossRef]
- Kubiak, A.; Wojciechowska, W.; Kurc, B.; Pigłowska, M.; Synoradzki, K.; Gabała, E.; Moszyński, D.; Szybowicz, M.; Siwińska-Ciesielczyk, K.; Jesionowski, T. Highly crystalline TiO2-MoO3 composite materials synthesized via a template-assisted microwave method for electrochemical application. Crystals 2020, 10, 493. [Google Scholar] [CrossRef]
- Hu, J.; Wang, H.; Qin, C.; Li, Y.; Yang, Y. Fabrication of TiO2@ C/N composite nanofibers and application as stable lithium-ion battery anode. Mater. Lett. 2020, 279, 128491. [Google Scholar] [CrossRef]
- Pineda-Aguilar, N.; Sánchez-Domínguez, M.; Sánchez-Cervantes, E.M.; Garza-Tovar, L.L. Preparation of TiO2-(B)/SnO2 nanostructured composites and its performance as anodes for lithium-ion batteries. J. Mater. Res. 2020, 35, 2491–2505. [Google Scholar] [CrossRef]
- Chen, J.S.; Liu, H.; Qiao, S.Z.; Lou, X.W.D. Carbon-supported ultra-thin anatase TiO2 nanosheets for fast reversible lithium storage. J. Mater. Chem. 2011, 21, 5687–5692. [Google Scholar] [CrossRef]
- Chen, J.S.; Lou, X.W. Anatase TiO2 nanosheet: An ideal host structure for fast and efficient lithium insertion/extraction. Electrochem. Commun. 2009, 11, 2332–2335. [Google Scholar] [CrossRef]
- Liu, Y.; Ding, C.; Yan, X.; Xie, P.; Xu, B.; Chen, L.; Liu, Y.; Liu, C.; Yu, Y.; Lin, Y. Interface-strain-confined synthesis of amorphous TiO2 mesoporous nanosheets with stable pseudocapacitive lithium storage. Chem. Eng. J. 2021, 420, 129894. [Google Scholar] [CrossRef]
- Jiang, S.; Wang, R.; Pang, M.; Wang, H.; Zeng, S.; Yue, X.; Ni, L.; Qiu, S.; Zhang, Z. Hierarchical composites of ultrathin carbon self-coated TiO2 nanosheets on reduced graphene oxide with enhanced lithium storage capability. Chem. Eng. J. 2015, 280, 614–622. [Google Scholar] [CrossRef]
- Tian, Q.; Chen, Y.; Zhang, F.; Zhang, W.; Sui, Z.; Yang, L. Hierarchical carbon-riveted 2D@0D TiO2 nanosheets@SnO2 nanoparticles composite for a improved lithium-ion battery anode. Appl. Surf. Sci. 2020, 511, 145625. [Google Scholar] [CrossRef]
- Mei, J.; Zhang, Y.; Liao, T.; Peng, X.; Ayoko, G.A.; Sun, Z. Black phosphorus nanosheets promoted 2D-TiO2−2D heterostructured anode for high-performance lithium storage. Energy Storage Mater. 2019, 19, 424–431. [Google Scholar] [CrossRef]
- Lopez, J.; Gonzalez, R.; Ayala, J.; Cantu, J.; Castillo, A.; Parsons, J.; Myers, J.; Lodge, T.P.; Alcoutlabi, M. Centrifugally spun TiO2/C composite fibers prepared from TiS2/PAN precursor fibers as binder-free anodes for LIBS. J. Phys. Chem. Solids 2021, 149, 109795. [Google Scholar] [CrossRef]
- Huang, H.; Fang, J.; Xia, Y.; Tao, X.; Gan, Y.; Du, J.; Zhua, W.; Zhang, W. Construction of sheet-belt hybrid nanostructures from one-dimensional mesoporous TiO2(B) nanobelts and graphene sheets for advanced lithium-ion batteries. J. Mater. Chem. A 2013, 1, 2495–2500. [Google Scholar] [CrossRef]
- Liang, Y.; Xiong, X.; Xu, Z.; Xia, Q.; Wan, L.; Liu, R.; Chen, G.; Chou, S.-L. Ultrathin 2D Mesoporous TiO2/rGO Heterostructure for High-Performance Lithium Storage. Small 2020, 16, 2000030. [Google Scholar] [CrossRef]
- Pan, L.; Liu, Y.; Xie, X.; Ye, X.; Zhu, X. Multi-dimensionally ordered, multi-functionally integrated r-GO@TiO2(B)@Mn3O4 yolk–membrane–shell superstructures for ultrafast lithium storage. Nano Res. 2016, 9, 2057–2069. [Google Scholar] [CrossRef]
- Ding, S.; Chen, J.S.; Wang, Z.; Cheah, Y.L.; Madhavi, S.; Hub, X.; Lou, X.W. TiO2 hollow spheres with large amount of exposed (001) facets for fast reversible lithium storage. J. Mater. Chem. 2011, 21, 1677–1680. [Google Scholar] [CrossRef]
- Wu, F.; Wang, Z.; Li, X.; Guo, H. Simple preparation of petal-like TiO2 nanosheets as anode materials for lithium-ion batteries. Ceram. Int. 2014, 40, 16805–16810. [Google Scholar] [CrossRef]
- Liu, J.; Chen, J.S.; Wei, X.; Lou, X.W.; Liu, X.W. Sandwich-like, stacked ultrathin titanate nanosheets for ultrafast lithium storage. Adv. Mater. 2011, 23, 998–1002. [Google Scholar] [CrossRef]
- Chen, B.; Liu, E.; He, F.; Shi, C.; He, C.; Li, J.; Zhao, N. 2D sandwich-like carbon-coated ultrathin TiO2@defect-rich MoS2 hybrid nanosheets: Synergistic-effect-promoted electrochemical performance for lithium ion batteries. Nano Energy 2016, 26, 541–549. [Google Scholar] [CrossRef]
- Cai, Y.; Wang, H.-E.; Huang, S.-Z.; Yuen, M.F.; Cai, H.-H.; Wang, C.; Yua, Y.; Li, Y.; Zhang, W.-J.; Su, B.-L. Porous TiO2 urchins for high performance Li-ion battery electrode: Facile synthesis, characterization and structural evolution. Electrochim. Acta 2016, 210, 206–214. [Google Scholar] [CrossRef]
- Shin, J.Y.; Samuelis, D.; Maier, J. Sustained lithium-storage performance of hierarchical, nanoporous anatase TiO2 at high rates: Emphasis on interfacial storage phenomena. Adv. Funct. Mater. 2011, 21, 3464–3472. [Google Scholar] [CrossRef]
- Xie, F.; Li, Y.; Dou, J.; Wu, J.; Wei, M. Facile synthesis of SnO2 coated urchin-like TiO2 hollow microspheres as efficient scattering layer for dye-sensitized solar cells. J. Power Sources 2016, 336, 143–149. [Google Scholar] [CrossRef]
- Lewis, C.S.; Li, Y.R.; Wang, L.; Li, J.; Stach, E.A.; Takeuchi, K.J.; Marschilok, A.C.; Takeuchi, E.S.; Wong, S.S. Correlating Titania Nanostructured Morphologies with Performance as Anode Materials for Lithium-Ion Batteries. ACS Sustain. Chem. Eng. 2016, 4, 6299–6312. [Google Scholar] [CrossRef]
- Lui, G.; Li, G.; Wang, X.; Jiang, G.; Lin, E.; Fowler, M.; Yua, A.; Chen, Z. Flexible, three-dimensional ordered macroporous TiO2 electrode with enhanced electrode–electrolyte interaction in high-power Li-ion batteries. Nano Energy 2016, 24, 72–77. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.; Yang, L.; Wang, K.; Lou, X.; Cai, B. Template-free synthesis of homogeneous yolk-shell TiO2 hierarchical microspheres for high performance lithium ion batteries. J. Power Sources 2014, 262, 72–78. [Google Scholar] [CrossRef]
- Chang, Y.C.; Peng, C.W.; Chen, P.C.; Lee, C.Y.; Chiu, H.T. Bio-ingredient assisted formation of porous TiO2 for Li-ion battery electrodes. RSC Adv. 2015, 5, 34949–34955. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, C.; Xie, A.; Li, C.; Song, J.; Shen, Y. Novel template-free synthesis of hollow@porous TiO2 superior anode materials for lithium ion battery. J. Mater. Sci. 2016, 51, 3448–3453. [Google Scholar] [CrossRef]
- Wang, X.; Meng, Q.; Wang, Y.; Liang, H.; Bai, Z.; Wang, K.; Lou, X.; Cai, B.; Yang, L. TiO2 hierarchical hollow microspheres with different size for application as anodes in high-performance lithium storage. Appl. Energy 2016, 175, 488–494. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, X.; Sun, H.; Ahmad, M. 3D anatase TiO2 hollow microspheres assembled with high-energy {001} facets for lithium-ion batteries. RSC Adv. 2012, 2, 7901–7905. [Google Scholar] [CrossRef]
- Hasegawa, G.; Sato, T.; Kanamori, K.; Nakanishi, K.; Abe, T. Synthesis and electrochemical performance of hierarchically porous N-doped TiO2 for Li-ion batteries. New J. Chem. 2014, 38, 1380–1384. [Google Scholar] [CrossRef]
- Di Lupo, F.; Tuel, A.; Mendez, V.; Francia, C.; Meligrana, G.; Bodoardo, S.; Gerbaldia, C. Mesoporous TiO2 nanocrystals produced by a fast hydrolytic process as high-rate long-lasting Li-ion battery anodes. Acta Mater. 2014, 69, 60–67. [Google Scholar] [CrossRef]
- Jiang, C.; Honma, I.; Kudo, T.; Zhou, H. Nanocrystalline rutile TiO2 electrode for high-capacity and high-rate lithium storage. Electrochem. Solid-State Lett. 2007, 10, A127. [Google Scholar] [CrossRef]
- Jamnik, J.; Maier, J. Nanocrystallinity effects in lithium battery materials Aspects of nano-ionics. Part IV. Phys. Chem. Chem. Phys. 2003, 5, 5215–5220. [Google Scholar] [CrossRef]
- Borghols, W.J.H.; Wagemaker, M.; Lafont, U.; Kelder, E.M.; Mulder, F.M. Impact of nanosizing on lithiated rutile TiO2. Chem. Mater. 2008, 20, 2949–2955. [Google Scholar] [CrossRef]
- Liang, J.; Wang, Z.; Li, Z.; Wang, X.; Yu, K. Fabrication of nanostructured TiO2 using a solvothermal reaction for lithium-ion batteries. Nanomater. Nanotechnol. 2016, 6, 15. [Google Scholar] [CrossRef][Green Version]
- Jin, J.; Huang, S.-Z.; Li, Y.; Tian, H.; Wang, H.-E.; Yu, Y.; Chen, L.-H.; Hasan, T.; Su, B.-L. Hierarchical nanosheet-constructed yolk–shell TiO2 porous microspheres for lithium batteries with high capacity, superior rate and long cycle capability. Nanoscale 2015, 7, 12979–12989. [Google Scholar] [CrossRef]
- Ding, S.; Chen, J.S.; Luan, D.; Boey, F.Y.C.; Madhavi, S.; Lou, X.W.D. Graphene-supported anatase TiO2 nanosheets for fast lithium storage. Chem. Commun. 2011, 47, 5780–5782. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, L.; Li, W.; Yu, A.; Wu, P. Carbon-coated mesoporous TiO2 nanocrystals grown on graphene for lithium-ion batteries. ACS Appl. Mater. Interfaces 2015, 7, 10395–10400. [Google Scholar] [CrossRef]
- Goriparti, S.; Miele, E.; Prato, M.; Scarpellini, A.; Marras, S.; Monaco, S.; Toma, A.; Messina, G.C.; Alabastri, A.; De Angelis, F.; et al. Direct synthesis of carbon-doped TiO2–bronze nanowires as anode materials for high performance lithium-ion batteries. ACS Appl. Mater. Interfaces 2015, 7, 25139–25146. [Google Scholar] [CrossRef]
- Wang, W.; Sa, Q.; Chen, J.; Wang, Y.; Jung, H.; Yin, Y. Porous TiO2/C nanocomposite shells as a high-performance anode material for lithium-ion batteries. ACS Appl. Mater. Interfaces 2013, 5, 6478–6483. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, Y.; Xiong, B.; Zhao, Y.; Huang, X.; Shao, Z. Fast lithium-ion insertion of TiO2 nanotube and graphene composites. Electrochim. Acta 2013, 88, 847–857. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, M.; Zhang, W.F. Preparation and electrochemical characterization of TiO2 nanowires as an electrode material for lithium-ion batteries. Electrochim. Acta 2008, 53, 7863–7868. [Google Scholar] [CrossRef]
- Gao, X.; Zhu, H.; Pan, G.; Ye, S.; Lan, Y.; Wu, F.; Son, D. Preparation and electrochemical characterization of anatase nanorods for lithium-inserting electrode material. J. Phys. Chem. B 2004, 108, 2868–2872. [Google Scholar] [CrossRef]
- Zuniga, L.; Agubra, V.; Flores, D.; Campos, H.; Villareal, J.; Alcoutlabi, M. Multichannel hollow structure for improved electrochemical performance of TiO2/Carbon composite nanofibers as anodes for lithium ion batteries. J. Alloys Compd. 2016, 686, 733–743. [Google Scholar] [CrossRef]
- Thirugunanam, L.; Kaveri, S.; Etacheri, V.; Ramaprabhu, S.; Dutta, M.; Pol, V.G. Electrospun nanoporous TiO2 nanofibers wrapped with reduced graphene oxide for enhanced and rapid lithium-ion storage. Mater. Charact. 2017, 131, 64–71. [Google Scholar] [CrossRef]
- Shannon, R.D. Phase Transformation Studies in TiO2 Supporting Different Defect Mechanisms in Vacuum-Reduced and Hydrogen-Reduced Rutile. J. Appl. Phys. 1964, 35, 3414–3416. [Google Scholar] [CrossRef]
- Wei, W.; Oltean, G.; Tai, C.W.; Edström, K.; Björefors, F.; Nyholm, L. High energy and power density TiO2 nanotube electrodes for 3D Li-ion microbatteries. J. Mater. Chem. A 2013, 1, 8160–8169. [Google Scholar] [CrossRef]
- Madian, M.; Giebeler, L.; Klose, M.; Jaumann, T.; Uhlemann, M.; Gebert, A.; Oswald, S.; Ismail, N.; Eychmuller, A.; Eckert, J. Self-organized TiO2/CoO nanotubes as potential anode materials for lithium ion batteries. ACS Sustain. Chem. Eng. 2015, 3, 909–919. [Google Scholar] [CrossRef]
- Tao, H.-C.; Fan, L.-Z.; Yan, X.; Qu, X. In situ synthesis of TiO2–graphene nanosheets composites as anode materials for high-power lithium ion batteries. Electrochim. Acta 2012, 69, 328–333. [Google Scholar] [CrossRef]
- Cao, F.-F.; Guo, Y.-G.; Zheng, S.-F.; Wu, X.-L.; Jiang, L.-Y.; Bi, R.-R.; Wan, L.-J.; Maier, J. Symbiotic coaxial nanocables: Facile synthesis and an efficient and elegant morphological solution to the lithium storage problem. Chem. Mater. 2010, 22, 1908–1914. [Google Scholar] [CrossRef]
- Xin, X.; Zhou, X.; Wu, J.; Yao, X.; Liu, Z. Scalable synthesis of TiO2/graphene nanostructured composite with high-rate performance for lithium ion batteries. ACS Nano 2012, 6, 11035–11043. [Google Scholar] [CrossRef]
- Xiao, L.; Cao, M.; Mei, D.; Guo, Y.; Yao, L.; Qu, D.; Deng, B. Preparation and electrochemical lithium storage features of TiO2 hollow spheres. J. Power Sources 2013, 238, 197–202. [Google Scholar] [CrossRef]
- Saravanan, K.; Ananthanarayanan, K.; Balaya, P. Mesoporous TiO2 with high packing density for superior lithium storage. Energy Environ. Sci. 2010, 3, 939–948. [Google Scholar] [CrossRef]
- Kim, M.; Lee, J.; Lee, S.; Seo, S.; Bae, C.; Shin, H. Nanotubular Heterostructure of Tin Dioxide/Titanium Dioxide as a Binder-Free Anode in Lithium-Ion Batteries. ChemSusChem 2015, 8, 2363–2371. [Google Scholar] [CrossRef]
- Raja, K.S.; Misra, M. Ordered arrays of Ti-Mn oxide nanotubes for high capacity Li-ion battery. ECS Trans. 2011, 33, 31. [Google Scholar] [CrossRef]
- Madian, M.; Klose, M.; Jaumann, T.; Gebert, A.; Oswald, S.; Ismail, N.; Eychmüller, A.; Eckert, J.; Giebeler, L. Anodically fabricated TiO2–SnO2 nanotubes and their application in lithium ion batteries. J. Mater. Chem. A 2016, 4, 5542–5552. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, H.; Xie, Y.; Guo, J. Ultralong life lithium-ion battery anode with superior high-rate capability and excellent cyclic stability from mesoporous Fe2O3@TiO2 core–shell nanorods. J. Mater. Chem. A 2014, 2, 3912–3918. [Google Scholar] [CrossRef]
- Madian, M.; Eychmüller, A.; Giebeler, L. Current advances in TiO2 -based nanostructure electrodes for high performance lithium ion batteries. Batteries 2018, 4, 7. [Google Scholar] [CrossRef]
- Quan, X.; Yang, S.; Ruan, X.; Zhao, H. Preparation of titania nanotubes and their environmental applications as electrode. Environ. Sci. Technol. 2005, 39, 3770–3775. [Google Scholar] [CrossRef]
- Ruan, C.; Paulose, M.; Varghese, O.K.; Mor, G.K.; Grimes, C.A. Fabrication of highly ordered TiO2 nanotube arrays using an organic electrolyte. J. Phys. Chem. B 2005, 109, 15754–15759. [Google Scholar] [CrossRef]
- Mor, G.K.; Varghese, O.K.; Paulose, M.; Mukherjee, N.; Grimes, C.A. Fabrication of tapered, conical-shaped titania nanotubes. J. Mater. Res. 2003, 18, 2588–2593. [Google Scholar] [CrossRef]
- Gong, D.; Varghese, O.K.; Hu, W.; Singh, R.S.; Chen, Z.; Dickey, E.C. RAPID COMMUNICATIONS Titanium oxide nanotube arrays prepared by anodic oxidation. J. Mater. Res. 2001, 16, 3331–3334. [Google Scholar] [CrossRef]
- Liu, G.; Hoivik, N.; Wang, K. Small diameter TiO2 nanotubes with enhanced photoresponsivity. Electrochem. Commun. 2013, 28, 107–110. [Google Scholar] [CrossRef]
- Bhardwaj, S.; Rana, T.; Laha, P.; Barman, A.; Biswas, S. Study of Titanium Dioxide Nanotube Array for the Application in Dye-Sensitized Solar Cells. Int. J. Mater. Mech. Manuf. 2014, 2, 47–50. [Google Scholar] [CrossRef][Green Version]
- Li, Q.; Deng, L.; Kim, J.-K.; Zhu1, Y.Q.; Holmes, S.M.; Perez-Page, M.; Eichhorn, S.J. Growth of carbon nanotubes on electrospun cellulose fibers for high performance supercapacitors. J. Electrochem. Soc. 2017, 164, A3220. [Google Scholar] [CrossRef]
- Liao, J.J.; Lin, S.W.; Pan, N.Q.; Cao, X.K.; Li, J.B. Facile fabrication of open-ended high aspect-ratio anodic TiO2 nanotube films and their applications. Key Eng. Mater. 2012, 512, 1659–1662. [Google Scholar] [CrossRef]
- Beranek, R.; Hildebrand, H.; Schmuki, P. Self-organized porous titanium oxide prepared in H2SO4/HF electrolytes. Electrochem. Solid-State Lett. 2003, 6, B12. [Google Scholar] [CrossRef]
- Park, I.S.; Woo, T.G.; Lee, M.H.; Ahn, S.G.; Park, M.S.; Bae, T.S.; Seol, K.W. Effects of anodizing voltage on the anodized and hydrothermally treated titanium surface. Met. Mater. Int. 2006, 12, 505–511. [Google Scholar] [CrossRef]
- Kulkarni, M.; Mazare, A.; Schmuki, P.; Iglic, A. Influence of anodization parameters on morphology of TiO2 nanostructured surfaces. Adv. Mater. Lett. 2016, 7, 23–28. [Google Scholar] [CrossRef]
- Zwilling, V.; Aucouturier, M.; Darque-Ceretti, E. Anodic Oxidation of Titanium and TA6V Alloy in Chromic media. An Electrochemical Approach. Electrochim. Acta 1999, 45, 921–929. [Google Scholar] [CrossRef]
- Kelly, J.J. The influence of fluoride ions on the passive dissolution of titanium. Electrochim. Acta 1979, 24, 1273–1282. [Google Scholar] [CrossRef]
- Macak, J.M.; Tsuchiya, H.; Taveira, L.; Aldabergerova, S.; Schmuki, P. Smooth anodic TiO2 nanotubes. Angew. Chem.-Int. Ed. 2005, 44, 7463–7465. [Google Scholar] [CrossRef] [PubMed]
- Acevedo-Peña, P.; Lartundo-Rojas, L.; González, I. Effect of water and fluoride content on morphology and barrier layer properties of TiO2 nanotubes grown in ethylene glycol-based electrolytes. J. Solid State Electrochem. 2013, 17, 2939–2947. [Google Scholar] [CrossRef]
- Rahman, M.A.; Wang, X.; Wen, C. Enhanced electrochemical performance of Li-ion batteries with nanoporous titania as negative electrodes. J. Energy Chem. 2015, 24, 157–170. [Google Scholar] [CrossRef]
- Ottone, C.; Lamberti, A.; Fontana, M.; Cauda, V. Wetting behavior of hierarchical oxide nanostructures: TiO2 nanotubes from anodic oxidation decorated with ZnO nanostructures. J. Electrochem. Soc. 2014, 161, D484. [Google Scholar] [CrossRef]
- Richter, C.; Panaitescu, E.; Willey, R.; Menon, L. Titania nanotubes prepared by anodization in fluorine-free acids. J. Mater. Res. 2007, 22, 1624–1631. [Google Scholar] [CrossRef]
- Hahn, R.; Macak, J.M.; Schmuki, P. Rapid anodic growth of TiO2 and WO3 nanotubes in fluoride free electrolytes. Electrochem. Commun. 2007, 9, 947–952. [Google Scholar] [CrossRef]
- Nguyen, Q.A.; Bhargava, Y.V.; Devine, T.M. Titania nanotube formation in chloride and bromide containing electrolytes. Electrochem. Commun. 2008, 10, 471–475. [Google Scholar] [CrossRef]
- Raja, K.S.; Gandhi, T.; Misra, M. Effect of water content of ethylene glycol as electrolyte for synthesis of ordered titania nanotubes. Electrochem. Commun. 2007, 9, 1069–1076. [Google Scholar] [CrossRef]
- Yoriya, S.; Kittimeteeworakul, W.; Punprasert, N. Effect of anodization parameters on morphologies of TiO2 nanotube arrays and their surface properties. J. Chem. Chem. Eng. 2012, 6, 686. [Google Scholar]
- Balaur, E.; Macak, J.M.; Tsuchiya, H.; Schmuki, P. Wetting behaviour of layers of TiO2 nanotubes with different diameters. J. Mater. Chem. 2005, 15, 4488–4491. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, B.; Li, J.; Gan, X.; Bai, J.; Cai, W. Preparation of short, robust and highly ordered TiO2 nanotube arrays and their applications as electrode. Appl. Catal. B Environ. 2009, 92, 326–332. [Google Scholar] [CrossRef]
- Varghese, O.K.; Gong, D.; Paulose, M.; Ong, K.G.; Grimes, C.A. Hydrogen sensing using titania nanotubes. Sens. Actuators B Chem. 2003, 93, 338–344. [Google Scholar] [CrossRef]
- Kang, S.H.; Kim, J.-Y.; Kim, H.S.; Sung, Y.-E. Formation and mechanistic study of self-ordered TiO2 nanotubes on Ti substrate. J. Ind. Eng. Chem. 2008, 14, 52–59. [Google Scholar] [CrossRef]
- Joseph, S.; David, T.M.; Ramesh, C.; Sagayaraj, P. The Role of Electrolyte PH in Enhancing the Surface Morphology of TiO2 Nanotube Arrays Grown on Ti Substrate. Int. J. Sci. Eng. Res. 2014, 5, 85–91. [Google Scholar]
- Chin, L.Y.; Zainal, Z.; Hussein, M.Z.; Tee, T.W. Fabrication of highly ordered TiO2 nanotubes from fluoride containing aqueous electrolyte by anodic oxidation and their photoelectrochemical response. J. Nanosci. Nanotechnol. 2011, 11, 4900–4909. [Google Scholar] [CrossRef]
- Tsuchiya, H.; Macak, J.M.; Ghicov, A.; Räder, A.S.; Taveira, L.; Schmuki, P. Characterization of electronic properties of TiO2 nanotube films. Corros. Sci. 2007, 49, 203–210. [Google Scholar] [CrossRef]
- Joo, S.; Muto, I.; Hara, N. In situ ellipsometric analysis of growth processes of anodic TiO2 nanotube films. J. Electrochem. Soc. 2008, 155, C154. [Google Scholar] [CrossRef]
- Tsuchiya, H.; Macak, J.M.; Taveira, L.; Balaur, E.; Ghicov, A.; Sirotna, K.; Schmuki, P. Self-organized TiO2 nanotubes prepared in ammonium fluoride containing acetic acid electrolytes. Electrochem. Commun. 2005, 7, 576–580. [Google Scholar] [CrossRef]
- Roman, I.; Trusca, R.D.; Soarea, M.-L.; Fratila, C.; Krasicka-Cydzik, E.; Stand, M.-S.; Dinischiotud, A. Titanium dioxide nanotube films: Preparation, characterization and electrochemical biosensitivity towards alkaline phosphatase. Mater. Sci. Eng. C 2014, 37, 374–382. [Google Scholar] [CrossRef]
- Khudhair, D.; Bhatti, A.; Li, Y.; Hamedani, H.A.; Garmestani, H.; Hodgson, P.; Nahavandia, S. Anodization parameters influencing the morphology and electrical properties of TiO2 nanotubes for living cell interfacing and investigations. Mater. Sci. Eng. C 2016, 59, 1125–1142. [Google Scholar] [CrossRef]
- Mor, G.K.; Shankar, K.; Paulose, M.; Varghese, O.K.; Grimes, C.A. Enhanced photocleavage of water using titania nanotube arrays. Nano Lett. 2005, 5, 191–195. [Google Scholar] [CrossRef]
- Indira, K.; Ningshen, S.; Mudali, U.K.; Rajendran, N. Effect of anodization temperature on the surface morphology of anodized titanium. In Thin Film; Nanomater: Noida, India, 2011; pp. 63–66. [Google Scholar]
- Chen, J.; Lin, J.; Chen, X. Self-assembled TiO2 nanotube arrays with U-shaped profile by controlling anodization temperature. J. Nanomater. 2010, 2010, 1–4. [Google Scholar]
- Li, Y.; Ma, Q.; Han, J.; Ji, L.; Wang, J.; Chen, J.; Wang, Y. Controllable preparation, growth mechanism and the properties research of TiO2 nanotube arrays. Appl. Surf. Sci. 2014, 297, 103–108. [Google Scholar] [CrossRef]
- Regonini, D.; Clemens, F.J. Anodized TiO2 nanotubes: Effect of anodizing time on film length, morphology and photoelectrochemical properties. Mater. Lett. 2015, 142, 97–101. [Google Scholar] [CrossRef]
- So, S.; Lee, K.; Schmuki, P. Ultrafast growth of highly ordered anodic TiO2 nanotubes in lactic acid electrolytes. J. Am. Chem. Soc. 2012, 134, 11316–11318. [Google Scholar] [CrossRef]
- Asmatulu, R.; Karthikeyan, A.; Bell, D.C.; Ramanathan, S.M.; Aziz, J. Synthesis and variable temperature electrical conductivity studies of highly ordered TiO2 nanotubes. J. Mater. Sci. 2009, 44, 4613–4616. [Google Scholar] [CrossRef][Green Version]
- Sreekantan, S.; Saharudin, K.A.; Wei, L.C. Formation of TiO2 nanotubes via anodization and potential applications for photocatalysts, biomedical materials, and photoelectrochemical cell. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2011; Volume 21, p. 12002. [Google Scholar]
- Allam, N.K.; Grimes, C.A. Effect of cathode material on the morphology and photoelectrochemical properties of vertically oriented TiO2 nanotube arrays. Sol. Energy Mater. Sol. Cells 2008, 92, 1468–1475. [Google Scholar] [CrossRef]
- Yoriya, S. Effect of inter-electrode spacing on electrolyte properties and morphologies of anodic TiO2 nanotube array films. Int. J. Electrochem. Sci. 2012, 7, 9454–9464. [Google Scholar]
- Rahman, M.A.; Wong, Y.C.; Song, G.; Zhu, D.M.; Wen, C. Improvement on electrochemical performances of nanoporous titania as anode of lithium-ion batteries through annealing of pure titanium foils. J. Energy Chem. 2018, 27, 250–263. [Google Scholar] [CrossRef]
- Park, H.; Kim, H.-G.; Choi, W.-Y. Characterizations of highly ordered TiO2 nanotube arrays obtained by anodic oxidation. Trans. Electr. Electron. Mater. 2010, 11, 112–115. [Google Scholar] [CrossRef][Green Version]
- Wang, Y.; Wen, C.; Hodgson, P.; Li, Y. Biocompatibility of TiO2 nanotubes with different topographies. J. Biomed. Mater. Res. Part A 2014, 102, 743–751. [Google Scholar] [CrossRef] [PubMed]
- Ryu, W.-H.; Nam, D.-H.; Ko, Y.-S.; Kim, R.-H.; Kwon, H.-S. Electrochemical performance of a smooth and highly ordered TiO2 nanotube electrode for Li-ion batteries. Electrochim. Acta 2012, 61, 19–24. [Google Scholar] [CrossRef]
- Fröschl, T.; Hormann, U.; Kubiak, P.; Kucerova, G.; Pfanzelt, M.; Weiss, C.K.; Behm, R.J.; Husing, N.; Kaiser, U.; Landfester, K.; et al. High surface area crystalline titanium dioxide: Potential and limits in electrochemical energy storage and catalysis. Chem. Soc. Rev. 2012, 41, 5313–5360. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Wei, M.; Qi, Z.; Kudo, T.; Honma, I.; Zhou, H. Particle size dependence of the lithium storage capability and high rate performance of nanocrystalline anatase TiO2 electrode. J. Power Sources 2007, 166, 239–243. [Google Scholar] [CrossRef]
- Zhu, K.; Wang, Q.; Kim, J.-H.; Pesaran, A.A.; Frank, A.J. Pseudocapacitive lithium-ion storage in oriented anatase TiO2 nanotube arrays. J. Phys. Chem. C 2012, 116, 11895–11899. [Google Scholar] [CrossRef]
- Kim, J.-H.; Zhu, K.; Kim, J.Y.; Frank, A.J. Tailoring oriented TiO2 nanotube morphology for improved Li storage kinetics. Electrochim. Acta 2013, 88, 123–128. [Google Scholar] [CrossRef]
- González, J.R.; Alcántara, R.; Ortiz, G.F.; Nacimiento, F.; Tirado, J.L. Controlled growth and application in lithium and sodium batteries of high-aspect-ratio, self-organized titania nanotubes. J. Electrochem. Soc. 2013, 160, A1390. [Google Scholar] [CrossRef]
- Roy, P.; Berger, S.; Schmuki, P. TiO2 nanotubes: Synthesis and applications. Angew. Chem. Int. Ed. 2011, 50, 2904–2939. [Google Scholar] [CrossRef]
- Lee, K.; Mazare, A.; Schmuki, P. One-dimensional titanium dioxide nanomaterials: Nanotubes. Chem. Rev. 2014, 114, 9385–9454. [Google Scholar] [CrossRef]
- Albu, S.P.; Ghicov, A.; Aldabergenova, S.; Drechsel, P.; LeClere, D.; Thompson, G.E.; Macak, J.M.; Schmuki, P. Formation of double-walled TiO2 nanotubes and robust anatase membranes. Adv. Mater. 2008, 20, 4135–4139. [Google Scholar]
- Paul, N.; Brumbarov, J.; Paul, A.; Chen, Y.; Moulin, J.-F.; Müller-Buschbaum, P.; Kunze-Liebhäuser, J.; Gilles, R. GISAXS and TOF-GISANS studies on surface and depth morphology of self-organized TiO2 nanotube arrays: Model anode material in Li-ion batteries. J. Appl. Crystallogr. 2015, 48, 444–454. [Google Scholar] [CrossRef]
- Zhou, H.; Zhang, Y. Electrochemically self-doped TiO2 nanotube arrays for supercapacitors. J. Phys. Chem. C 2014, 118, 5626–5636. [Google Scholar] [CrossRef]
- Tighineanu, A.; Ruff, T.; Albu, S.; Hahn, R.; Schmuki, P. Conductivity of TiO2 nanotubes: Influence of annealing time and temperature. Chem. Phys. Lett. 2010, 494, 260–263. [Google Scholar] [CrossRef]
- Pan, D.; Huang, H.; Wang, X.; Wang, L.; Liao, H.; Li, Z.; Wu, M. C-axis preferentially oriented and fully activated TiO2 nanotube arrays for lithium ion batteries and supercapacitors. J. Mater. Chem. A 2014, 2, 11454–11464. [Google Scholar] [CrossRef]
- Liu, S.; Yu, J.; Jaroniec, M. Anatase TiO2 with dominant high-energy {001} facets: Synthesis, properties, and applications. Chem. Mater. 2011, 23, 4085–4093. [Google Scholar] [CrossRef]
- Lee, S.; Park, I.J.; Kim, D.H.; Seong, W.M.; Kim, D.W.; Han, G.S.; Kim, J.Y.; Jung, H.S.; Hong, K.S. Crystallographically preferred oriented TiO2 nanotube arrays for efficient photovoltaic energy conversion. Energy Environ. Sci. 2012, 5, 7989–7995. [Google Scholar] [CrossRef]
- Seong, W.M.; Kim, D.H.; Park, I.J.; Park, G.D.; Kang, K.; Lee, S.; Hong, K.S. Roughness of Ti substrates for control of the preferred orientation of TiO2 nanotube arrays as a new orientation factor. J. Phys. Chem. C 2015, 119, 13297–13305. [Google Scholar] [CrossRef]
- Lu, Z.; Yip, C.; Wang, L.; Huang, H.; Zhou, L. Hydrogenated TiO2 nanotube arrays as high-rate anodes for lithium-ion microbatteries. Chempluschem 2012, 77, 991–1000. [Google Scholar] [CrossRef]
- Brumbarov, J.; Vivek, J.P.; Leonardi, S.; Valero-Vidal, C.; Portenkirchner, E.; Kunze-Liebhäuser, J. Oxygen deficient, carbon coated self-organized TiO 2 nanotubes as anode material for Li-ion intercalation. J. Mater. Chem. A 2015, 3, 16469–16477. [Google Scholar] [CrossRef]
- Shin, J.-Y.; Joo, J.H.; Samuelis, D.; Maier, J. Oxygen-deficient TiO2− δ nanoparticles via hydrogen reduction for high rate capability lithium batteries. Chem. Mater. 2012, 24, 543–551. [Google Scholar] [CrossRef]
- Auer, A.; Portenkirchner, E.; Götsch, T.; Valero-Vidal, C.; Penner, S.; Kunze-Liebhäuser, J. Preferentially oriented TiO2 nanotubes as anode material for Li-ion batteries: Insight into Li-ion storage and lithiation kinetics. ACS Appl. Mater. Interfaces 2017, 9, 36828–36836. [Google Scholar] [CrossRef]
- Kashani, H.; Gharibi, H.; Javadian, S.; Kakemam, J. Study of counter electrodes as an effective controlling factor of crystal orientation of TiO2 nanoarrays used as the anode in lithium-ion batteries. New J. Chem. 2017, 41, 12442–12450. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, J.; Huang, H.; Wu, Z.; Zhang, Z. Surface-modification and characterization of H-titanate nanotube. Colloids Surf. A Physicochem. Eng. Asp. 2008, 317, 270–276. [Google Scholar] [CrossRef]
- Li, G.; Li, L.; Boerio-Goates, J.; Woodfield, B.F. High purity anatase TiO2 nanocrystals: Near room-temperature synthesis, grain growth kinetics, and surface hydration chemistry. J. Am. Chem. Soc. 2005, 127, 8659–8666. [Google Scholar] [CrossRef]
- Di Paola, A.; Bellardita, M.; Palmisano, L. Brookite, the least known TiO2 photocatalyst. Catalysts 2013, 3, 36–73. [Google Scholar] [CrossRef]
- Macklin, W.J.; Neat, R.J. Performance of titanium dioxide-based cathodes in a lithium polymer electrolyte cell. Solid State Ion. 1992, 53, 694–700. [Google Scholar] [CrossRef]
- Kyeremateng, N.A.; Vacandio, F.; Sougrati, M.-T.; Martinez, H.; Jumas, J.-C.; Knauth, P.; Djenizian, T. Effect of Sn-doping on the electrochemical behaviour of TiO2 nanotubes as potential negative electrode materials for 3D Li-ion micro batteries. J. Power Sources 2013, 224, 269–277. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, Y. Recent Progress of TiO2-Based Anodes for Li Ion Batteries. J. Nanomater. 2016, 2016, 21–35. [Google Scholar] [CrossRef]
- Duan, J.; Hou, H.; Liu, X.; Yan, C.; Liu, S.; Meng, R.; Hao, Z.; Yao, Y.; Liao, Q. In situ Ti3+-doped TiO2 nanotubes anode for lithium ion battery. J. Porous Mater. 2016, 23, 837–843. [Google Scholar] [CrossRef]
- Kim, H.S.; Yu, S.-H.; Sung, Y.-E.; Kang, S.H. Carbon treated self-ordered TiO2 nanotube arrays with enhanced lithium-ion intercalation performance. J. Alloys Compd. 2014, 597, 275–281. [Google Scholar] [CrossRef]
- Choi, J.; Park, H.; Hoffmann, M.R. Effects of single metal-ion doping on the visible-light photoreactivity of TiO2. J. Phys. Chem. C 2010, 114, 783–792. [Google Scholar] [CrossRef]
- Lin, S.-H.; Ou, C.-C.; Su, M.-D.; Yang, C.-S. Photo-catalytic behavior of vanadia incorporated titania nanoparticles. Catal. Sci. Technol. 2013, 3, 2081–2091. [Google Scholar] [CrossRef]
- Zhang, M.; Yin, K.; Hood, Z.D.; Bi, Z.; Bridges, C.A.; Dai, S.; Meng, Y.S.; Paranthaman, M.P.; Chi, M. In situ TEM observation of the electrochemical lithiation of N-doped anatase TiO2 nanotubes as anodes for lithium-ion batteries. J. Mater. Chem. A 2017, 5, 20651–20657. [Google Scholar] [CrossRef]
- Mole, F.; Wang, J.; Clayton, D.A.; Xu, C.; Pan, S. Highly conductive nanostructured C-TiO2 electrodes with enhanced electrochemical stability and double layer charge storage capacitance. Langmuir 2012, 28, 10610–10619. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, S.; Huang, K.; Fang, D.; Zhuang, S. Electrochemical properties of freestanding TiO2 nanotube membranes annealed in Ar for lithium anode material. J. Solid State Electrochem. 2012, 16, 723–729. [Google Scholar] [CrossRef]
- Wang, G.; Yang, Y.; Han, D.; Li, Y. Oxygen defective metal oxides for energy conversion and storage. Nano Today 2017, 13, 23–39. [Google Scholar] [CrossRef]
- Liao, A.-Z.; Wang, C.-W.; Chen, J.-B.; Zhang, X.-Q.; Li, Y.; Wang, J. Remarkably improved field emission of TiO2 nanotube arrays by annealing atmosphere engineering. Mater. Res. Bull. 2015, 70, 988–994. [Google Scholar] [CrossRef]
- Qiu, J.; Terrones, M.; Park, C.R.; Mukherjee, R.; Monthioux, M.; Koratkar, N.; Kim, Y.S.; Hurt, R.; Frackowiak, E.; Enoki, T. Hydrogenation synthesis of blue TiO2 for high-performance lithium-ion batteries. J. Phys. Chem. C 2014, 118, 8824–8830. [Google Scholar] [CrossRef]
- Wang, G.; Wang, H.; Ling, Y.; Tang, Y.; Yang, X.; Fitzmorris, R.C.; Wang, C.; Zhang, J.Z.; Li, Y. Hydrogen-treated TiO2 nanowire arrays for photoelectrochemical water splitting. Nano Lett. 2011, 11, 3026–3033. [Google Scholar] [CrossRef]
- Savva, A.I.; Smith, K.A.; Lawson, M.; Croft, S.R.; Weltner, A.E.; Jones, C.D.; Bull, H.; Simmonds, P.J.; Lia, L.; Xiong, H. Defect generation in TiO2 nanotube anodes via heat treatment in various atmospheres for lithium-ion batteries. Phys. Chem. Chem. Phys. 2018, 20, 22537–22546. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, C.; Li, H.; Wang, J.; Li, M.; Chen, X. Enhanced performance of lithium ion batteries from self-doped TiO2 nanotube anodes via an adjustable electrochemical process. Electrochim. Acta 2019, 326, 134972. [Google Scholar] [CrossRef]
- Liao, W.; Yang, J.; Zhou, H.; Murugananthan, M.; Zhang, Y. Electrochemically self-doped TiO2 nanotube arrays for efficient visible light photoelectrocatalytic degradation of contaminants. Electrochim. Acta 2014, 136, 310–317. [Google Scholar] [CrossRef]
- Fabregat-Santiago, F.; Barea, E.M.; Bisquert, J.; Mor, G.K.; Shankar, K.; Grimes, C.A. High carrier density and capacitance in TiO2 nanotube arrays induced by electrochemical doping. J. Am. Chem. Soc. 2008, 130, 11312–11316. [Google Scholar] [CrossRef]
- Meekins, B.H.; Kamat, P.V. Got TiO2 nanotubes? Lithium ion intercalation can boost their photoelectrochemical performance. ACS Nano 2009, 3, 3437–3446. [Google Scholar] [CrossRef]
- Idigoras, J.; Berger, T.; Anta, J.A. Modification of mesoporous TiO2 films by electrochemical doping: Impact on photoelectrocatalytic and photovoltaic performance. J. Phys. Chem. C 2013, 117, 1561–1570. [Google Scholar] [CrossRef]
- Xu, C.; Song, Y.; Lu, L.; Cheng, C.; Liu, D.; Fang, X.; Chen, X.; Zhu, X.; Li, D. Electrochemically hydrogenated TiO2 nanotubes with improved photoelectrochemical water splitting performance. Nanoscale Res. Lett. 2013, 8, 1–7. [Google Scholar] [CrossRef]
- Song, W.; Luo, H.; Hanson, K.; Concepcion, J.J.; Brennaman, M.K.; Meyer, T.J. Visualization of cation diffusion at the TiO2 interface in dye sensitized photoelectrosynthesis cells (DSPEC). Energy Environ. Sci. 2013, 6, 1240–1248. [Google Scholar] [CrossRef]
- Li, H.; Chen, Z.; Tsang, C.K.; Li, Z.; Ran, X.; Lee, C.; Nie, B.; Zheng, L.; Hung, T.; Lu, J.; et al. Electrochemical doping of anatase TiO2 in organic electrolytes for high-performance supercapacitors and photocatalysts. J. Mater. Chem. A 2014, 2, 229–236. [Google Scholar] [CrossRef]
- Zhu, W.-D.; Wang, C.-W.; Chen, J.-B.; Li, Y.; Wang, J. Enhanced field emission from Ti3+ self-doped TiO2 nanotube arrays synthesized by a facile cathodic reduction process. Appl. Surf. Sci. 2014, 301, 525–529. [Google Scholar] [CrossRef]
- Li, Z.; Ding, Y.; Kang, W.; Li, C.; Lina, D.; Wang, X.; Chen, Z.; Wu, M.; Pan, D. Reduction mechanism and capacitive properties of highly electrochemically reduced TiO2 nanotube arrays. Electrochim. Acta 2015, 161, 40–47. [Google Scholar] [CrossRef]
- Ghicov, A.; Yamamoto, M.; Schmuki, P. Lattice widening in Niobium-doped TiO2 nanotubes: Efficient ion intercalation and swift electrochromic contrast. Angew. Chem. Int. Ed. 2008, 47, 7934–7937. [Google Scholar] [CrossRef] [PubMed]
- Zhi, J.; Cui, H.; Wang, Z.; Huang, F. Surface confined titania redox couple for ultrafast energy storage. Mater. Horiz. 2018, 5, 691–698. [Google Scholar] [CrossRef]
- Zhang, Y.; Du, F.; Yan, X.; Jin, Y.; Zhu, K.; Wang, X.; Li, H.; Chen, G.; Wang, C.; Wei, Y. Improvements in the electrochemical kinetic properties and rate capability of anatase titanium dioxide nanoparticles by nitrogen doping. ACS Appl. Mater. Interfaces 2014, 6, 4458–4465. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.; Bridges, C.A.; Unocic, R.R.; Paranthaman, M.P. Mesoporous TiO2 spheres with a nitridated conducting layer for lithium-ion batteries. J. Mater. Sci. 2013, 48, 5125–5131. [Google Scholar] [CrossRef]
- Weibel, A.; Bouchet, R.; Savin, S.L.P.; Chadwick, A.V.; Lippens, P.E.; Womes, M.; Knauth, P. Local Atomic and Electronic Structure in Nanocrystalline Sn-Doped Anatase TiO2. Chem. Phys. Chem. 2006, 7, 2377–2383. [Google Scholar] [CrossRef]
- Fresno, F.; Tudela, D.; Coronado, J.M.; Soria, J. Synthesis of Ti1−xSnxO2 nanosized photocatalysts in reverse microemulsions. Catal. Today 2009, 143, 230–236. [Google Scholar] [CrossRef]
- Wang, Y.B.; Smarsly, M.; Djerdj, I. Niobium doped TiO2 with mesoporosity and its application for lithium insertion. Chem. Mater. 2010, 22, 6624–6631. [Google Scholar] [CrossRef]
- Salian, G.D.; Koo, B.M.; Lefevre, C.; Cottineau, T.; Lebouin, C.; Tesfaye, A.T.; Knauth, P.; Keller, V.; Djenizian, T. Niobium Alloying of Self-Organized TiO2 Nanotubes as an Anode for Lithium-Ion Microbatteries. Adv. Mater. Technol. 2018, 3, 1700274. [Google Scholar] [CrossRef]
- Ivanov, S.; Cheng, L.; Wulfmeier, H.; Albrecht, D.; Fritze, H.; Bunda, A. Electrochemical behavior of anodically obtained titania nanotubes in organic carbonate and ionic liquid based Li ion containing electrolytes. Electrochim. Acta 2013, 104, 228–235. [Google Scholar] [CrossRef]
- Li, H.; Martha, S.K.; Unocic, R.R.; Luo, H.; Dai, S.; Qu, J. High cyclability of ionic liquid-produced TiO2 nanotube arrays as an anode material for lithium-ion batteries. J. Power Sources 2012, 218, 88–92. [Google Scholar] [CrossRef]
- Kirchgeorg, R.; Kallert, M.; Liu, N.; Hahn, R.; Killian, M.S.; Schmuki, P. Key factors for an improved lithium ion storage capacity of anodic TiO2 nanotubes. Electrochim. Acta 2016, 198, 56–65. [Google Scholar] [CrossRef]
- Gao, Q.; Gu, M.; Nie, A.; Mashayek, F.; Wang, C.; Odegard, G.M.; Shahbazian-Yassar, R. Direct evidence of lithium-induced atomic ordering in amorphous TiO2 nanotubes. Chem. Mater. 2014, 26, 1660–1669. [Google Scholar] [CrossRef]
- Xiong, H.; Yildirim, H.; Shevchenko, E.V.; Prakapenka, V.B.; Koo, B.; Slater, M.D.; Balasubramanian, M.; Sankaranarayanan, S.K.R.S.; Greeley, J.P.; Tepavcevic, S.; et al. Self-improving anode for lithium-ion batteries based on amorphous to cubic phase transition in TiO2 nanotubes. J. Phys. Chem. C 2012, 116, 3181–3187. [Google Scholar] [CrossRef]
- Borghols, W.J.H.; Lützenkirchen-Hecht, D.; Haake, U.; Chan, W.; Lafont, U.; Kelder, E.M.; van Eck, E.R.H.; Kentgens, A.P.M.; Mulder, F.M.; Wagemaker, M. Lithium storage in amorphous TiO2 nanoparticles. J. Electrochem. Soc. 2010, 157, A582. [Google Scholar] [CrossRef]
- Chen, J.S.; Tan, Y.L.; Li, C.M.; Cheah, Y.L.; Luan, D.; Madhavi, S.; Boey, F.Y.C.; Archer, L.A.; Lou, X.W. Constructing hierarchical spheres from large ultrathin anatase TiO2 nanosheets with nearly 100% exposed (001) facets for fast reversible lithium storage. J. Am. Chem. Soc. 2010, 132, 6124–6130. [Google Scholar] [CrossRef]
- Guan, D.; Cai, C.; Wang, Y. Amorphous and crystalline TiO2 nanotube arrays for enhanced Li-Ion intercalation properties. J. Nanosci. Nanotechnol. 2011, 11, 3641–3650. [Google Scholar] [CrossRef]
- Wei, Z.; Liu, Z.; Jiang, R.; Bian, C.; Huang, T.; Yu, A. TiO2 nanotube array film prepared by anodization as anode material for lithium ion batteries. J. Solid State Electrochem. 2010, 14, 1045–1050. [Google Scholar] [CrossRef]
- Kartini, E.; Genardy, C.T. The future of all solid state battery. In Proceedings of the International Conference on Advanced Materials and Technology, Bogor, Indonesia, 8–9 October 2019; p. 012038. [Google Scholar]
- Sun, Y.-K. Promising All-Solid-State Batteries for Future Electric Vehicles. ACS Energy Lett. 2020, 5, 3221–3223. [Google Scholar] [CrossRef]
- Sugiawati, V.A.; Vacandio, F.; Djenizian, T. All-Solid-State Lithium Ion Batteries Using Self-Organized TiO2 Nanotubes Grown from Ti-6Al-4V Alloy. Molecules 2020, 25, 2121. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, Y.; Fu, H.; Wu, Y.; Zhang, D.; Wen, J.; Huang, L.; Dai, Y.; Huang, Y.; Luo, W. TiO2 Nanofiber-Modified Lithium Metal Composite Anode for Solid-State Lithium Batteries. ACS Appl. Mater. Interfaces 2021, 13, 28398–28404. [Google Scholar] [CrossRef]

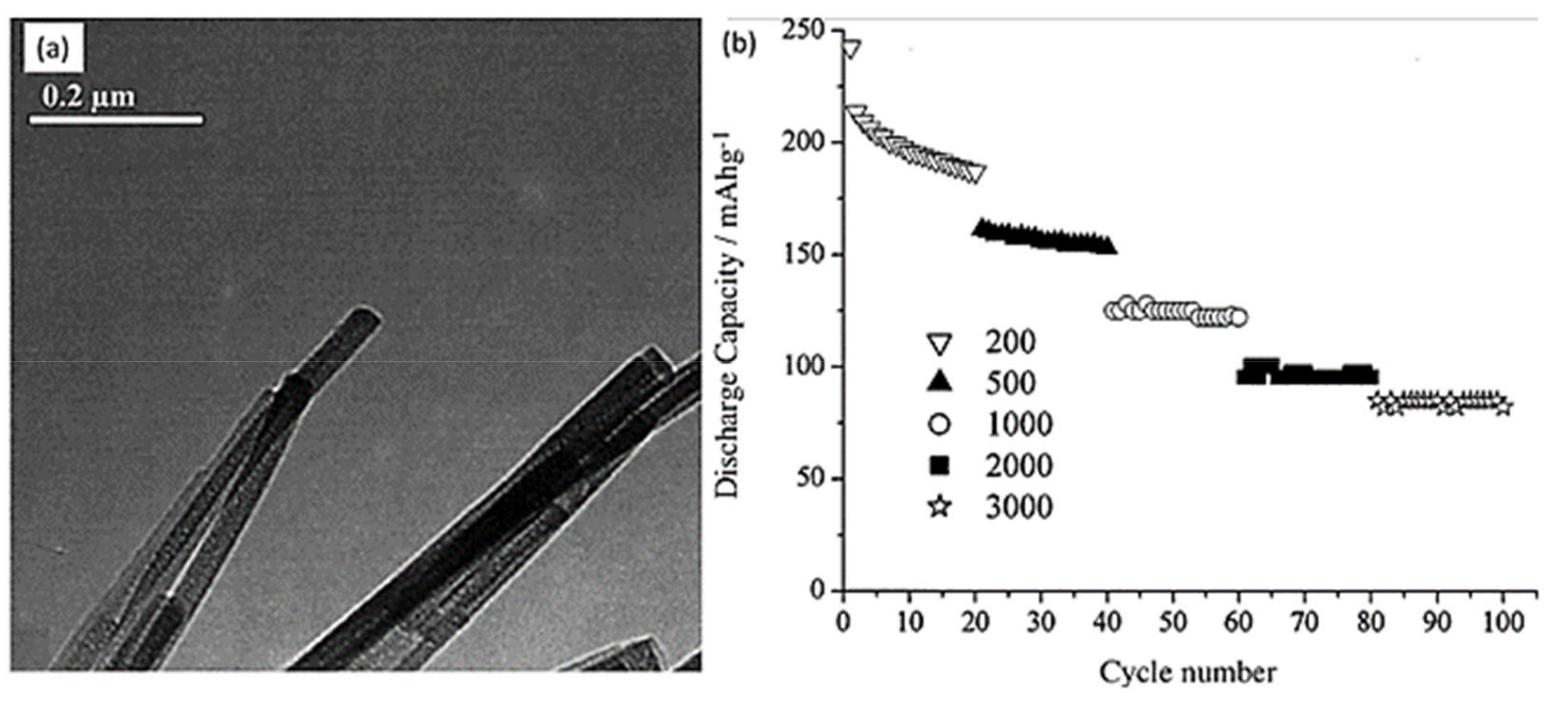
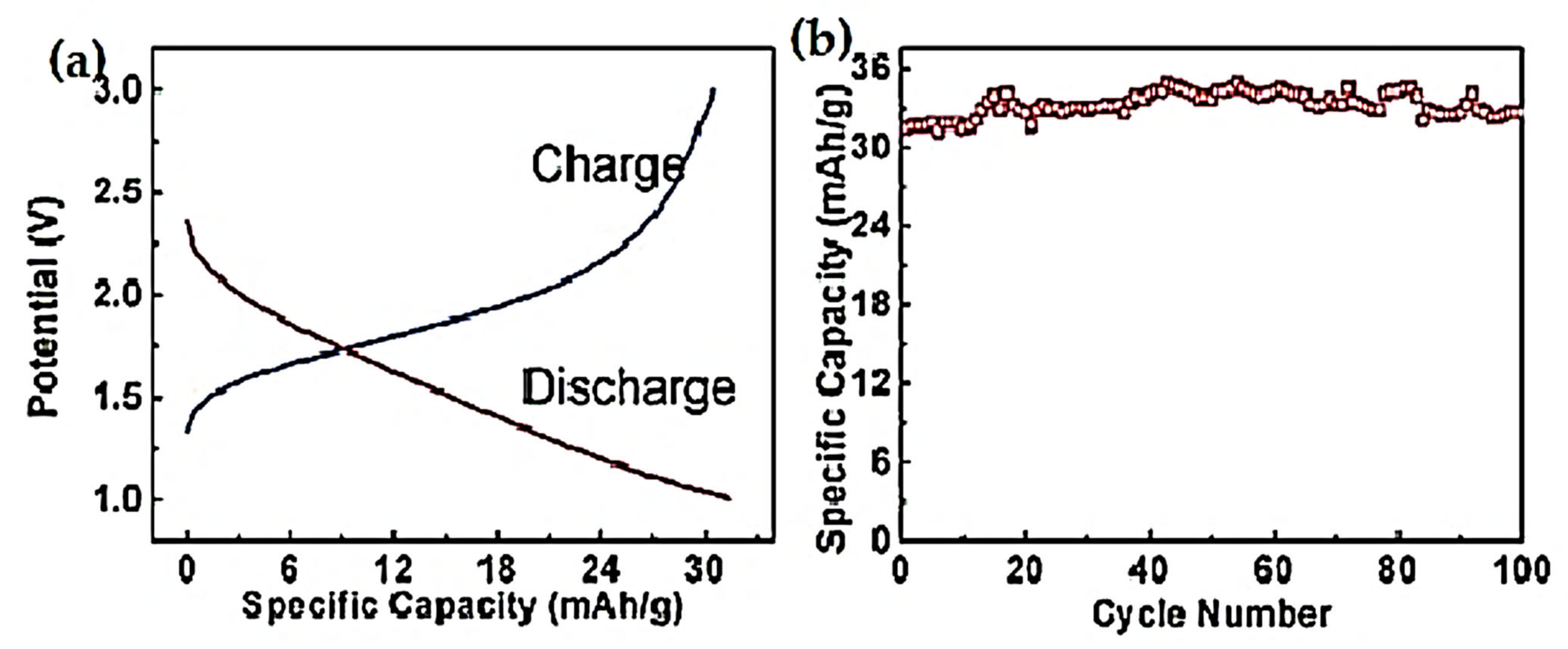
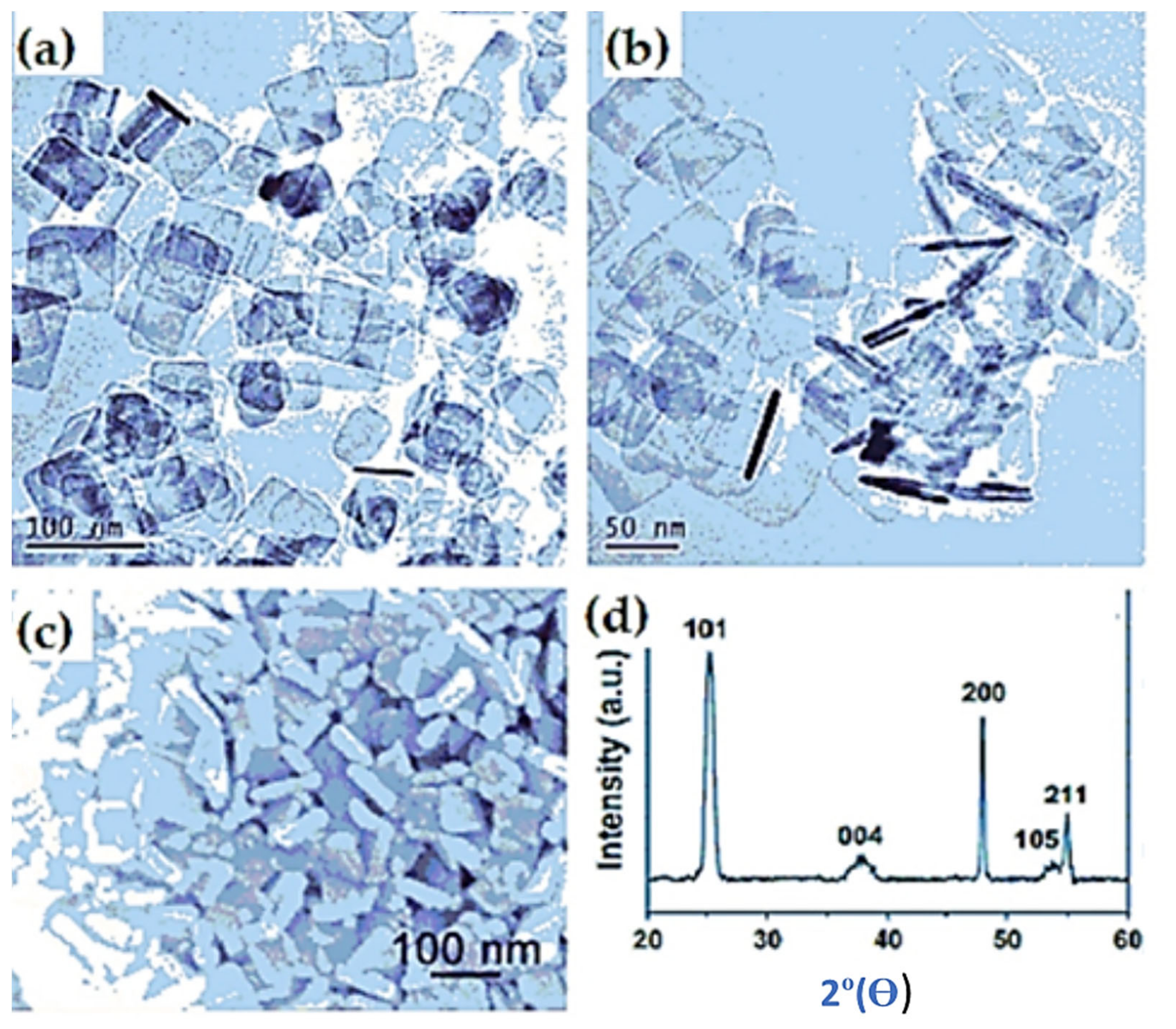
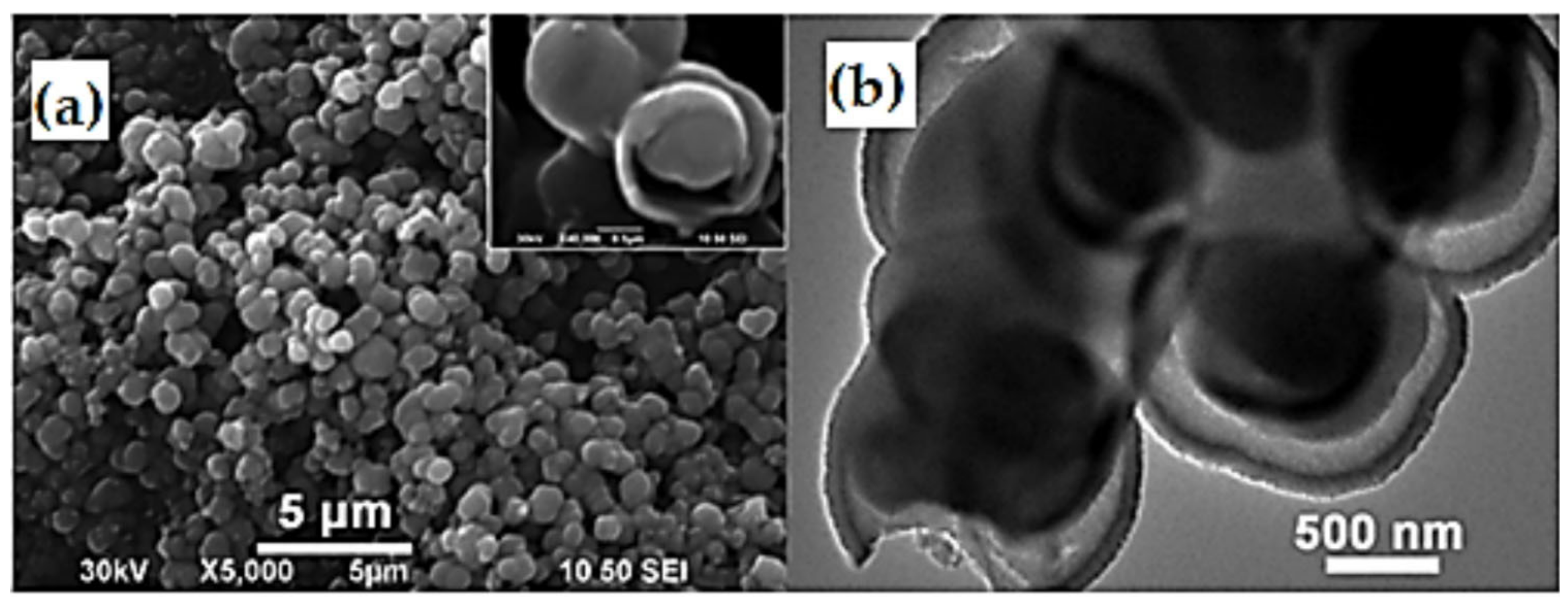

| Preparation Technique | Nanostructures | No of Reversible Cycles | C Rate | Reversible Capacity after 20 Cycles |
|---|---|---|---|---|
| Solvothermal | Anatase Nanoparticles [106] | 100 | 0.2C | 196 mAhg−1 |
| Anatase/Graphene nanosheets [108] | 120 | 1C | 161 mAhg−1 | |
| C-coated mesoporous | ||||
| TiO2@graphenenanosheets [109] | 100 | 0.2 Ag−1 | 111 mAhg−1 | |
| Hierarchical nanosheets [107] | 700 | 1C | 225 mAhg−1 | |
| Hydrothermal | TiO2@C hollow spheres [111] | 300 | 2C | 170 mAhg−1 |
| C-doped TiO2 nanowires [110] | 1000 | 0.1C | 306 mAhg−1 | |
| TiO2 nanotube@graphene [112] | 50 | 10 mAg−1 | 357 mAhg−1 | |
| Hydrolysis | TiO2 nanoparticles@graphene [120] | 400 | 2C | 70 mAhg−1 |
| Core-shell CNTs/TiO2 [121] | 100 | 5 Ag−1 | 240 mAhg−1 | |
| Anatase Nanorods [114] | 30 | 5 Ag−1 | 206 mAhg−1 | |
| Anatase Nanowires [113] | 40 | 50 mAg−1 | 280 mAhg−1 | |
| TiO2/graphene [122] | 100 | 140 mAg−1 | 230 mAhg−1 | |
| Electrospinning | TiO2 Nanofibers [71] | 50 | 0.3C | 170 mAhg−1 |
| TiO2/C hollow nanofibers [115] | 100 | 100 mAg−1 | 229 mAhg−1 | |
| Graphene wrapped TiO2 nanofibers [116] | 35 | 0.1 mAg−1 | 200 mAhg−1 | |
| Template Assisted | TiO2 nanoporous hollow spheres [123] | 50 | 33.5 mAg−1 | 230 mAhg−1 |
| Mesoporous TiO2 nanoparticles [124] | 30 | 0.2C | 268 mAhg−1 | |
| Anodization | TiO2 Nanotubes [117] | 1000 | 0.1C | 140 mAhg−1 |
| 3D Free Standing TiO2 nanotubes [118] | 500 | 0.05 mAcm−2 | 184 mAhg−1 | |
| SnO2/TiO2 Nanotubes [125] | 50 | 0.1C | 250 mAhg−1 | |
| Ti-Mn-O Nanotubes [126] | 30 | 175 mAg−1 | 474 mAhg−1 | |
| CoO/TiO2 Nanotubes [119] | 100 | 10 μAhcm−2 | 600 μAhcm−1 | |
| TiO2-SnO2 Nanotubes [127] | 400 | 504 μAhcm−2 | 405 μAhcm−1 | |
| CoO/TiO2 Nanotubes [127] | 90 | 50 μAhcm−2 | 450 μAhcm−1 | |
| Thermal Treatment | Fe2O3 nanorods-TiO2 [128] | 1000 | 1 Ag−1 | 860 mAhg−1 |
| Commercial | Rutile nanoparticles [103] | 20 | 0.05 Ag−1 | 207 mAhg−1 |
| Voltage (V) | Time | Electrolyte | Average Diameter | Length | |
|---|---|---|---|---|---|
| 20 | 60 min | 1 M Na2SO4 + | [144] | ||
| 0.5 wt.% NH4 + | 50–80 nm | Negligible | |||
| 1 wt.% NH4 | 20–30 nm | Negligible | |||
| 3 wt.% NH4 | 75–100 nm | 670–730 nm | |||
| 5 wt.% NH4 | 75–100 nm | 650–680 nm | |||
| 1 M (NH4)2SO4 + | |||||
| 0.5 wt.% NH4 | 80–100 nm | Negligible | |||
| 1 wt.% NH4 | 90–130 nm | Negligible | |||
| 3 wt.% NH4 | 110–130 nm | 450–480 nm | |||
| 5 wt.% NH4 | 60–90 nm | 300–340 nm | |||
| 32 | 120 min | 0.5 wt.% NH4F + | [145] | ||
| 1 M (NH4)2SO4 + | |||||
| EG 5 vol% | 14.7 ± 8.2 nm | ||||
| 10 vol% | 12.8 ± 6.8 nm | ||||
| 30 vol% | 11 ± 5.5 nm | ||||
| 50 vol% | 26.7 ± 13.6 nm | ||||
| 32 | 120 min | 0.4 wt.% NH4F + 1 M (NH4)2SO4 + EG 5 vol% | 15.70 ± 17.70 nm | [173] | |
| 18.75 ± 15.40 nm | |||||
| (Pre heat treated Ti foil) | |||||
| 20 | 18 h | 0.5 wt.% NaF + 0.5 M Na2SO4 + 0.5 M H3PO4 + 0.2 M Na3C6H5O7 + NaOH with pH = 1 pH = 3 pH = 4.2 pH = 5 | [155] | ||
| 110 nm | 1 μm | ||||
| 110 nm | 1.5 μm | ||||
| 110 nm | 2.6 μm | ||||
| 110 nm | 3 μm | ||||
| 40 | 70 h | DMSO electrolyte | 120 nm | [146] | |
| with | |||||
| 1 wt% HF | 53 μm | ||||
| 2 wt% HF | 13 μm | ||||
| 4 wt% HF | 53 μm | ||||
| 6 wt% HF | 52 μm | ||||
| 60 | 20 min 30 min 40 min | EG containing 0.3 wt.% NH4F and 2 vol% H2O | 120 nm | 18 μm 25.2 μm 30.8 μm | [174] |
| 30 | 3 h | EG containing 0.25 wt.% NH4F and 10 wt.% H2O | 100 nm | 4 μm | [175] |
| 20 | 45 min | EG containing 3 wt.% NH4F and 2 vol% H2O | 40 nm | 1.2 μm | [138] |
| 60 | 3 h | EG containing 0.3 wt.% NH4F and 2 vol% H2O | 120 nm | 46 μm | [137] |
| 50 | 30 min | EG containing NH4F and DI water | 34 nm | 100 nm | [135] |
| 52 | 44 nm | 100 nm | |||
| 55 | 53 nm | 100 nm | |||
| 57 | 58 nm | 200 nm | |||
| 25 | 2 h | EG containing 0.27 M NH4F and 0.2 wt.% H2O | 58.25 nm | 1.04 μm | [136] |
| 30 | 70.29 nm | 3.32 μm | |||
| 35 | 91.24 nm | 3.68 μm | |||
| 40 | 77.33 nm | 3.21 μm | |||
| 45 | 96.98 nm | 4.27 μm | |||
| 50 | 119.68 nm | 8.35 μm | |||
| 40 | 19 h | EG containing 0.27 M NH4F, 1.5 wt.% H2O, 0.05% HF aqueous solution | 75 nm | 1.3 μm | [170] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paul, S.; Rahman, M.A.; Sharif, S.B.; Kim, J.-H.; Siddiqui, S.-E.-T.; Hossain, M.A.M. TiO2 as an Anode of High-Performance Lithium-Ion Batteries: A Comprehensive Review towards Practical Application. Nanomaterials 2022, 12, 2034. https://doi.org/10.3390/nano12122034
Paul S, Rahman MA, Sharif SB, Kim J-H, Siddiqui S-E-T, Hossain MAM. TiO2 as an Anode of High-Performance Lithium-Ion Batteries: A Comprehensive Review towards Practical Application. Nanomaterials. 2022; 12(12):2034. https://doi.org/10.3390/nano12122034
Chicago/Turabian StylePaul, Sourav, Md. Arafat Rahman, Sazzad Bin Sharif, Jin-Hyuk Kim, Safina-E-Tahura Siddiqui, and Md. Abu Mowazzem Hossain. 2022. "TiO2 as an Anode of High-Performance Lithium-Ion Batteries: A Comprehensive Review towards Practical Application" Nanomaterials 12, no. 12: 2034. https://doi.org/10.3390/nano12122034
APA StylePaul, S., Rahman, M. A., Sharif, S. B., Kim, J.-H., Siddiqui, S.-E.-T., & Hossain, M. A. M. (2022). TiO2 as an Anode of High-Performance Lithium-Ion Batteries: A Comprehensive Review towards Practical Application. Nanomaterials, 12(12), 2034. https://doi.org/10.3390/nano12122034










