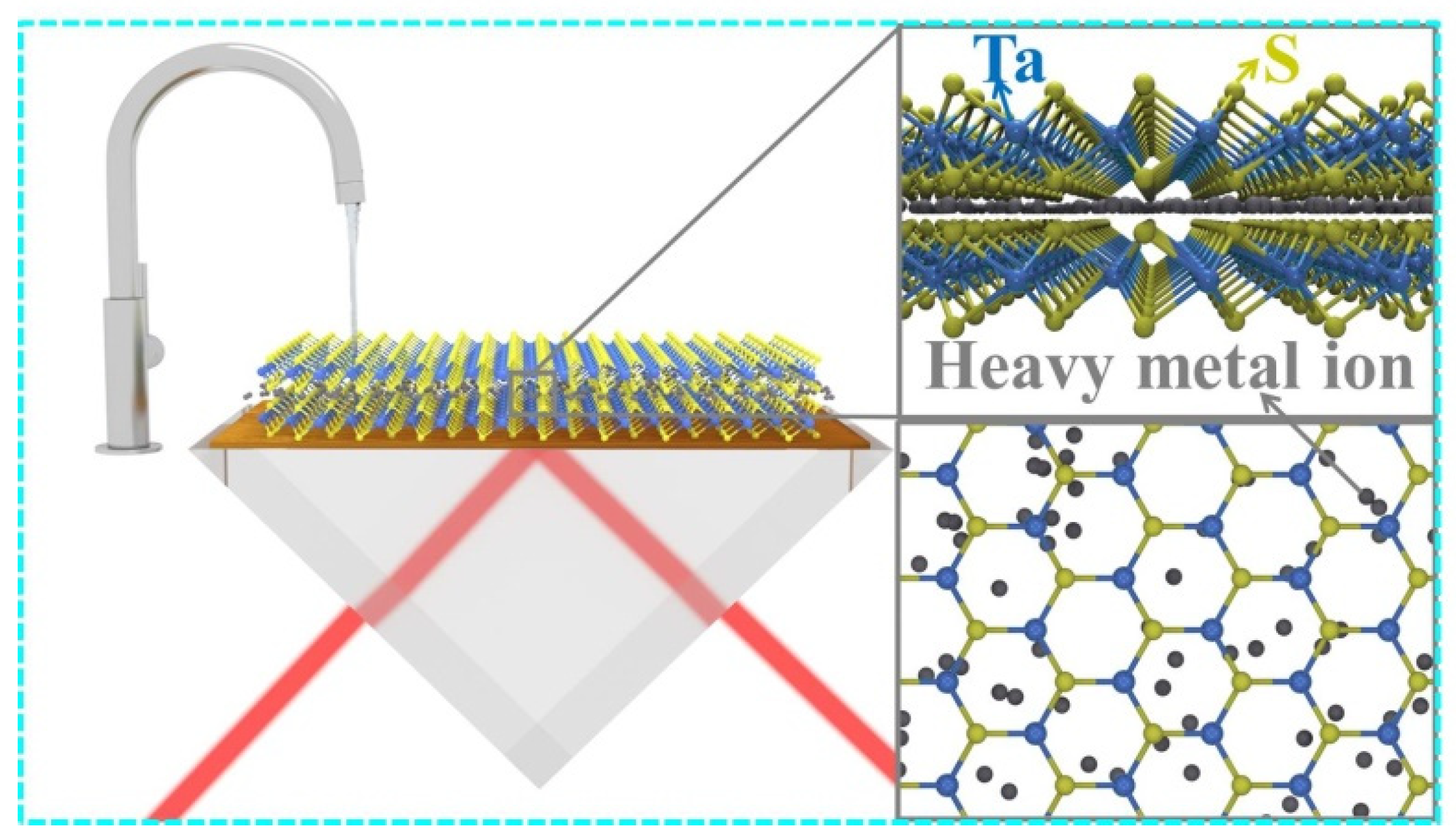
High Quality TaS2 Nanosheet SPR Biosensors Improved Sensitivity and the Experimental Demonstration for the Detection of Hg2+
Abstract
:1. Introduction
2. Experiment
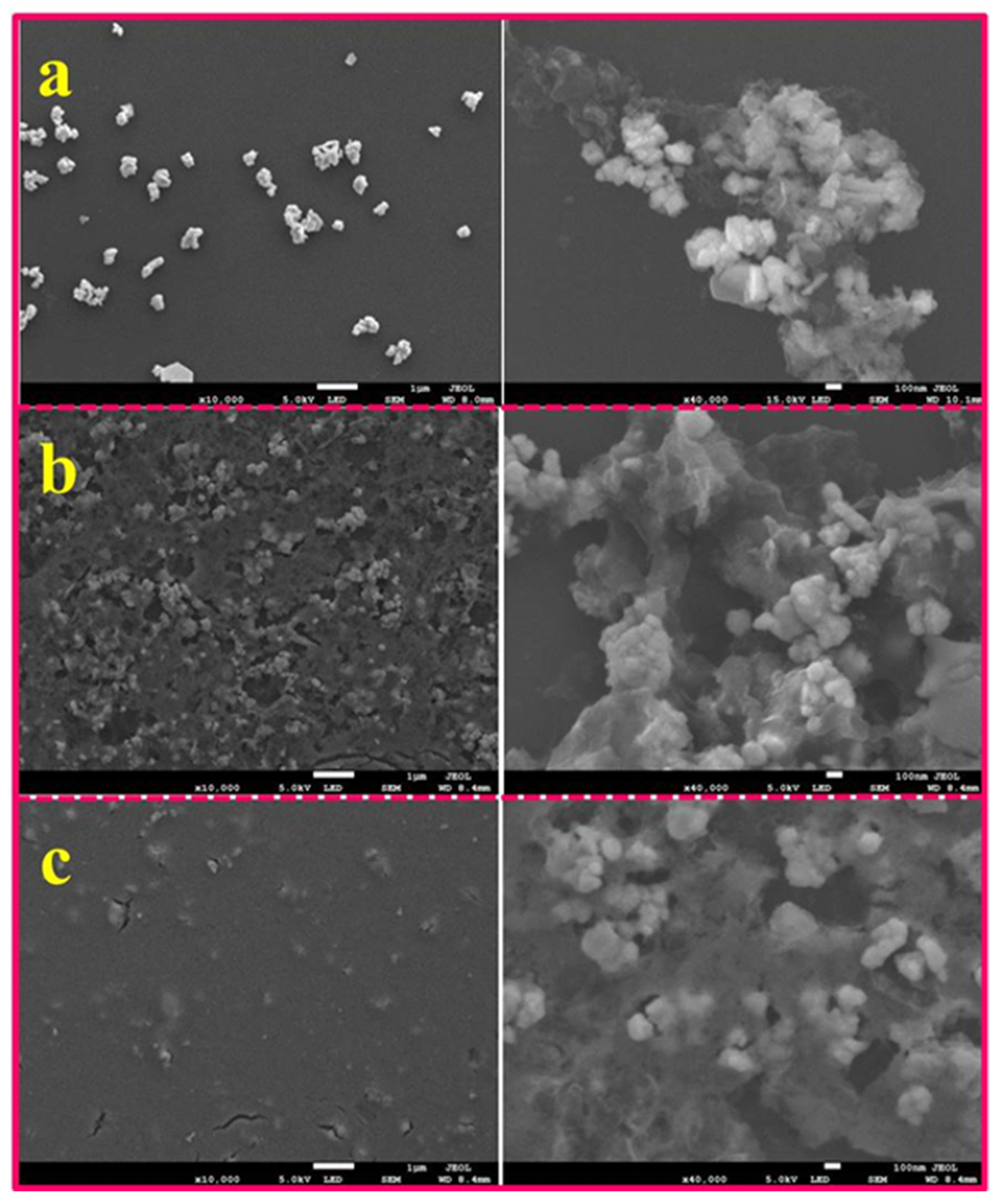
2.1. Synthesis of Superstructure TaS2 Nanoflakes
2.2. Assembling of TaS2 Nanoflakes
2.3. Mercury Ion Detection
3. Results and Discussion
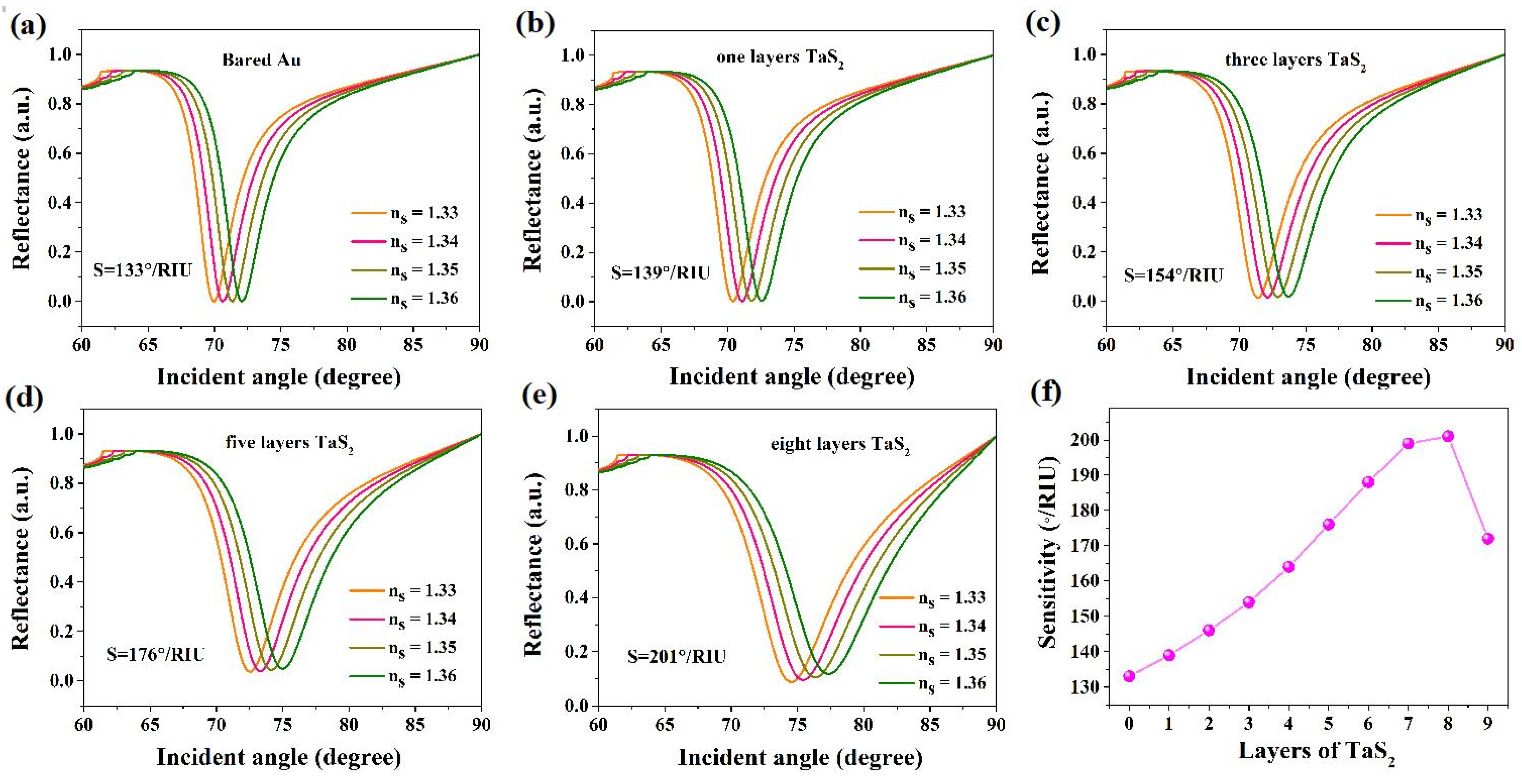
3.1. Numerical Simulation
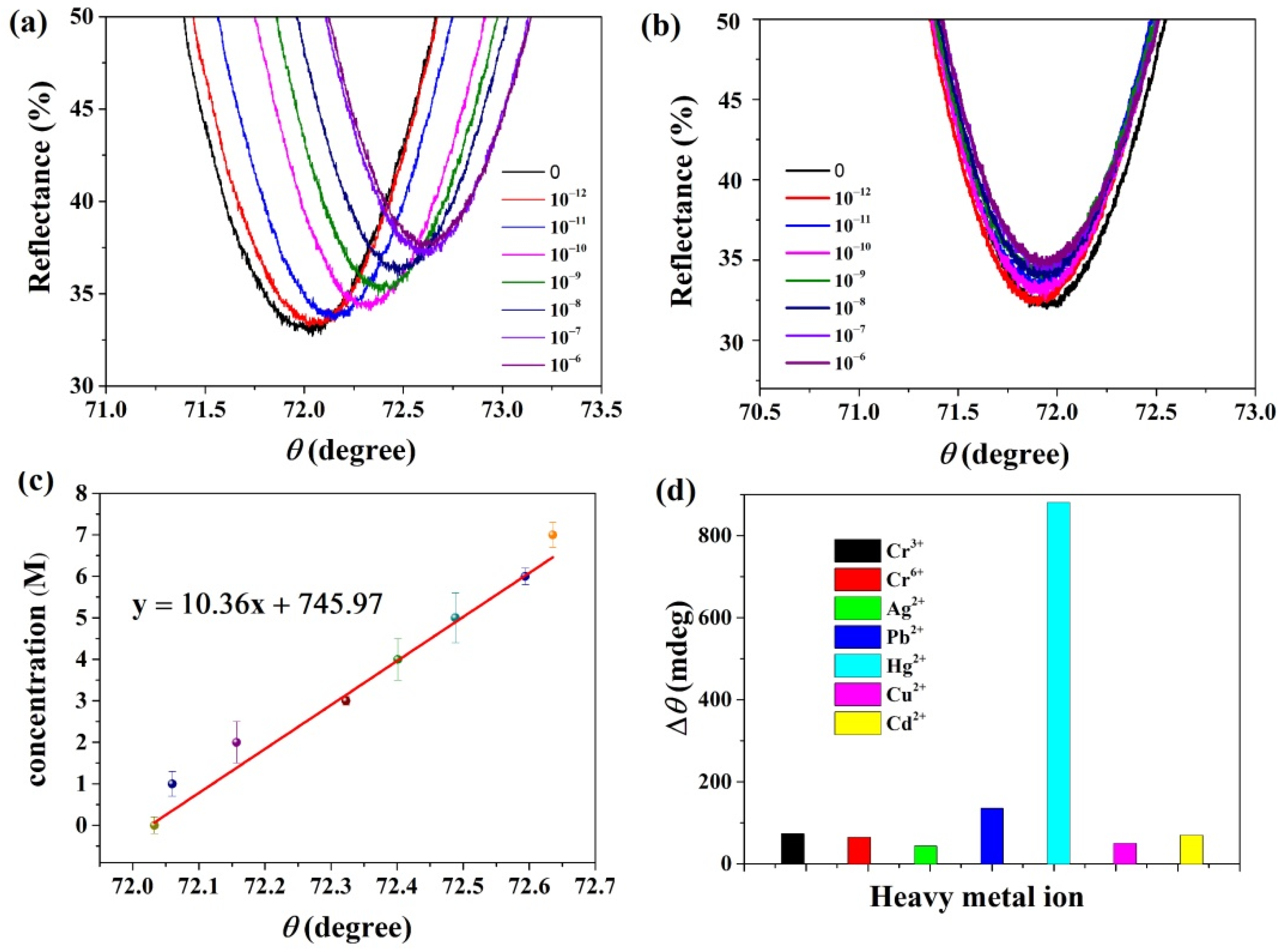
3.2. Application of TaS2 Based SPR Biosensors in Heavy Metal Ion Detection
3.3. Study of the Mechanism of High Sensitivity of These SPR Biosensors
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bolisetty, S.; Mezzenga, R. Amyloid-carbon hybrid membranes for universal water purification. Nat. Nanotechnol. 2016, 11, 365. [Google Scholar] [CrossRef]
- Lamborg, C.H.; Hammerschmidt, C.R.; Bowman, K.L.; Swarr, G.J.; Munson, K.M.; Ohnemus, D.C.; Lam, P.J.; Heimbuerger, L.-E.; Rijkenberg, M.J.A.; Saito, M.A. A global ocean inventory of anthropogenic mercury based on water column measurements. Nature 2014, 512, 65. [Google Scholar] [CrossRef] [PubMed]
- Gu, B.; Zhou, Y.; Zhang, X.; Liu, X.; Zhang, Y.; Marks, R.; Zhang, H.; Liu, X.; Zhang, Q. Thiazole derivative-modified upconversion nanoparticles for Hg2+ detection in living cells. Nanoscale 2016, 8, 276–282. [Google Scholar] [CrossRef]
- Kosik-Bogacka, D.; Lanocha-Arendarczyk, N.; Kot, K.; Malinowski, W.; Szymanski, S.; Sipak-Szmigiel, O.; Pilarczyk, B.; Tomza-Marciniak, A.; Podlasinska, J.; Tomska, N.; et al. Concentrations of mercury (Hg) and selenium (Se) in afterbirth and their relations with various factors. Environ. Geochem. Health 2018, 40, 1683–1695. [Google Scholar] [CrossRef]
- Mao, S.; Chang, J.; Pu, H.; Lu, G.; He, Q.; Zhang, H.; Chen, J. Two-dimensional nanomaterial-based field-effect transistors for chemical and biological sensing. Chem. Soc. Rev. 2017, 46, 6872–6904. [Google Scholar] [CrossRef] [PubMed]
- Ai, K.; Ruan, C.; Shen, M.; Lu, L. MoS2 Nanosheets with Widened Interlayer Spacing for High-Efficiency Removal of Mercury in Aquatic Systems. Adv. Funct. Mater. 2016, 26, 5542–5549. [Google Scholar] [CrossRef]
- Geng, F.; Jiang, X.; Wang, Y.; Shao, C.; Wang, K.; Qu, P.; Xu, M. DNA-based dual fluorescence signals on and ratiometric mercury sensing in fetal calf serum with simultaneous excitation. Sens. Actuators B Chem. 2018, 260, 793–799. [Google Scholar] [CrossRef]
- Huang, J.; Gao, X.; Jia, J.; Kim, J.-K.; Li, Z. Graphene Oxide-Based Amplified Fluorescent Biosensor for Hg2+ Detection through Hybridization Chain Reactions. Anal. Chem. 2014, 86, 3209–3215. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Tang, A.; Liao, S.; Chen, P.; Wu, Z.; Shen, G.; Yu, R. Oligonucleotide probes applied for sensitive enzyme-amplified electrochemical assay of mercury(II) ions. Biosens. Bioelectron. 2011, 26, 3320–3324. [Google Scholar] [CrossRef]
- Kopysc, E.; Pyrzynska, K.; Garbos, S.; Bulska, E. Determination of mercury by cold-vapor atomic absorption spectrometry with preconcentration on a gold-trap. Anal. Sci. 2000, 16, 1309–1312. [Google Scholar] [CrossRef] [Green Version]
- Karunasagar, D.; Arunachalam, J.; Gangadharan, S. Development of a ‘collect and punch’ cold vapour inductively coupled plasma mass spectrometric method for the direct determination of mercury at nanograms per litre levels. J. Anal. At. Spectrom. 1998, 13, 679–682. [Google Scholar] [CrossRef]
- Elgazali, A.A.S.; Gajdosechova, Z.; Abbas, Z.; Lombi, E.; Scheckel, K.G.; Donner, E.; Fiedler, H.; Feldmann, J.; Krupp, E.M. Reactive gaseous mercury is generated from chloralkali factories resulting in extreme concentrations of mercury in hair of workers. Sci. Rep. 2018, 8, 3675. [Google Scholar] [CrossRef] [Green Version]
- Kabashin, A.V.; Evans, P.; Pastkovsky, S.; Hendren, W.; Wurtz, G.A.; Atkinson, R.; Pollard, R.; Podolskiy, V.A.; Zayats, A.V. Plasmonic nanorod metamaterials for biosensing. Nat. Mater. 2009, 8, 867–871. [Google Scholar] [CrossRef]
- Sreekanth, K.V.; Alapan, Y.; ElKabbash, M.; Ilker, E.; Hinczewski, M.; Gurkan, U.A.; de Luca, A.; Strangi, G. Extreme sensitivity biosensing platform based on hyperbolic metamaterials. Nat. Mater. 2016, 15, 621–627. [Google Scholar] [CrossRef] [Green Version]
- Garoli, D.; Calandrini, E.; Giovannini, G.; Hubarevich, A.; Caligiuri, V.; de Angelis, F. Nanoporous gold metamaterials for high sensitivity plasmonic sensing. Nanoscale Horiz. 2019, 4, 1153–1157. [Google Scholar] [CrossRef]
- Yan, R.; Wang, T.; Yue, X.; Wang, H.; Zhang, Y.-H.; Xu, P.; Wang, L.; Wang, Y.; Zhang, J. Highly sensitive plasmonic nanorod hyperbolic metamaterial biosensor. Photon. Res. 2022, 10, 84–95. [Google Scholar] [CrossRef]
- Wei, Q.; Nagi, R.; Sadeghi, K.; Feng, S.; Yan, E.; Ki, S.J.; Caire, R.; Tseng, D.; Ozcan, A. Detection and Spatial Mapping of Mercury Contamination in Water Samples Using a Smart-Phone. ACS Nano 2014, 8, 1121–1129. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.; Pei, X.; Cheng, Y.; Zhang, C.; Zhang, J.; Yan, Y.; Ding, C.; Xian, Y. Black Phosphorus Quantum Dots as the Ratiometric Fluorescence Probe for Trace Mercury Ion Detection Based on Inner Filter Effect. ACS Sens. 2017, 2, 576–582. [Google Scholar] [CrossRef]
- Berger, C.; Song, Z.; Li, X.; Wu, X.; Brown, N.; Naud, C.; Mayou, D.; Li, T.; Hass, J.; Marchenkov, A.N.; et al. Electronic confinement and coherence in patterned epitaxial graphene. Science 2006, 312, 1191–1196. [Google Scholar] [CrossRef] [Green Version]
- Xue, T.; Cui, X.; Chen, J.; Liu, C.; Wang, Q.; Wang, H.; Zheng, W. A Switch of the Oxidation State of Graphene Oxide on a Surface Plasmon Resonance Chip. ACS Appl. Mater. Interfaces 2013, 5, 2096–2103. [Google Scholar] [CrossRef]
- Han, S.-T.; Peng, H.; Sun, Q.; Venkatesh, S.; Chung, K.-S.; Lau, S.C.; Zhou, Y.; Roy, V.A.L. An Overview of the Development of Flexible Sensors. Adv. Mater. 2017, 29, 1700375. [Google Scholar] [CrossRef] [PubMed]
- Xue, T.; Qi, K.; Hu, C. Novel SPR sensing platform based on superstructure MoS2 nanosheets for ultrasensitive detection of mercury ion. Sens. Actuators B Chem. 2019, 284, 589–594. [Google Scholar] [CrossRef]
- Yuting, Z.; Shuaiwen, G.; Leiming, W.; Jiaqi, Z.; Yuanjiang, X.; Xiaoyu, D. GeSe nanosheets modified surface plasmon resonance sensors for enhancing sensitivity. Nanophotonics 2020, 9, 327–336. [Google Scholar]
- Xue, T.; Yu, S.; Zhang, X.; Zhang, X.; Wang, L.; Bao, Q.; Chen, C.; Zheng, W.; Cui, X. R6G molecule induced modulation of the optical properties of reduced graphene oxide nanosheets for use in ultrasensitive SPR sensing. Sci. Rep. 2016, 6, 21254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loh, K.P.; Bao, Q.; Eda, G.; Chhowalla, M. Graphene oxide as a chemically tunable platform for optical applications. Nat. Chem. 2010, 2, 1015–1024. [Google Scholar] [CrossRef]
- Tan, C.; Lai, Z.; Zhang, H. Ultrathin Two-Dimensional Multinary Layered Metal Chalcogenide Nanomaterials. Adv. Mater. 2017, 29, 1701392. [Google Scholar] [CrossRef]
- Zhou, G.; Chang, J.; Pu, H.; Shi, K.; Mao, S.; Sui, X.; Ren, R.; Cui, S.; Chen, J. Ultrasensitive Mercury Ion Detection Using DNA-Functionalized Molybdenum Disulfide Nanosheet/Gold Nanoparticle Hybrid Field Effect Transistor Device. ACS Sens. 2016, 1, 295–302. [Google Scholar] [CrossRef]
- Gooding, J.J.; Gaus, K. Single-Molecule Sensors: Challenges and Opportunities for Quantitative Analysis. Angew. Chem. Int. Ed. 2016, 55, 11354–11366. [Google Scholar] [CrossRef]
- Li, H.; Tan, Y.; Liu, P.; Guo, C.; Luo, M.; Han, J.; Lin, T.; Huang, F.; Chen, M. Atomic-Sized Pores Enhanced Electrocatalysis of TaS2 Nanosheets for Hydrogen Evolution. Adv. Mater. 2016, 28, 8945–8949. [Google Scholar] [CrossRef]
- Sanders, C.E.; Dendzik, M.; Ngankeu, A.S.; Eich, A.; Bruix, A.; Bianchi, M.; Miwa, J.A.; Hammer, B.; Khajetoorians, A.A.; Hofmann, P. Crystalline and electronic structure of single-layer TaS2. Phys. Rev. B 2016, 94, 081404. [Google Scholar] [CrossRef] [Green Version]
- Ma, L.; Ye, C.; Yu, Y.; Lu, X.F.; Niu, X.; Kim, S.; Feng, D.; Tomanek, D.; Son, Y.-W.; Chen, X.H.; et al. A metallic mosaic phase and the origin of Mott-insulating state in 1T-TaS2. Nat. Commun. 2016, 7, 10956. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; Chen, Y.; Lin, J.; Wang, X.; Zeng, Q.; Zhou, J.; Zheng, L.; Wang, H.; He, Y.; He, H.; et al. Controlled Synthesis of Atomically Thin 1T-TaS2 for Tunable Charge Density Wave Phase Transitions. Chem. Mater. 2016, 28, 7613–7618. [Google Scholar] [CrossRef]
- Yoshida, M.; Zhang, Y.; Ye, J.; Suzuki, R.; Imai, Y.; Kimura, S.; Fujiwara, A.; Iwasa, Y. Controlling charge-density-wave states in nano-thick crystals of 1T-TaS2. Sci. Rep. 2014, 4, 7302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, Y.; Wang, Y.; Ni, J.; Shi, L.; Shi, S.; Tang, W. First principles study of structural, vibrational and electronic properties of graphene-like MX2 (M=Mo, Nb, W, Ta; X=S, Se, Te) monolayers. Phys. B Condens. Matter 2011, 406, 2254–2260. [Google Scholar] [CrossRef]
- Yang, Y.; Fang, S.; Fatemi, V.; Ruhman, J.; Navarro-Moratalla, E.; Watanabe, K.; Taniguchi, T.; Kaxiras, E.; Jarillo-Herrero, P. Enhanced superconductivity upon weakening of charge density wave transport in 2H-TaS2 in the two-dimensional limit. Phys. Rev. B 2018, 98, 035203. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Peng, J.; Yu, Z.; Zhou, Y.; Guo, Y.; Li, Z.; Lin, Y.; Ruan, K.; Wu, C.; Xie, Y. Acid-Assisted Exfoliation toward Metallic Sub-nanopore TaS2 Monolayer with High Volumetric Capacitance. J. Am. Chem. Soc. 2018, 140, 493–498. [Google Scholar] [CrossRef]
- Zeng, Z.; Tan, C.; Huang, X.; Bao, S.; Zhang, H. Growth of noble metal nanoparticles on single-layer TiS2 and TaS2 nanosheets for hydrogen evolution reaction. Energy Environ. Sci. 2014, 7, 797–803. [Google Scholar] [CrossRef]
- Zeng, S.; Hu, S.; Xia, J.; Anderson, T.; Dinh, X.-Q.; Meng, X.-M.; Coquet, P.; Yong, K.-T. Graphene-MoS2 hybrid nanostructures enhanced surface plasmon resonance biosensors. Sens. Actuators B Chem. 2015, 207, 801–810. [Google Scholar] [CrossRef]
- Polyanskiy, M.M. Refractive Index Database. Johnson and Christy 1972. Available online: http://refractiveindex.info (accessed on 4 April 2022).
- Gupta, B.D.; Sharma, A.K. Sensitivity evaluation of a multi-layered surface plasmon resonance-based fiber optic sensor: A theoretical study. Sens. Actuators B Chem. 2005, 107, 40–46. [Google Scholar] [CrossRef]
- Van Soest, J.P. Optical and Electronic Reflectivity of TaS2. 2018. Available online: https://hdl.handle.net/1887/64692 (accessed on 4 April 2022).
- Albertini, O.R.; Liu, A.Y.; Calandra, M. Effect of electron doping on lattice instabilities in single-layer 1H-TaS2. Phys. Rev. B 2017, 95, 235121. [Google Scholar] [CrossRef] [Green Version]
- Hansen, W.N. Electric fields produced by propagation of plane coherent electromagnetic radiation in a stratified medium. J. Opt. Soc. Am. 1968, 58, 380–390. [Google Scholar] [CrossRef]
- Maharana, P.K.; Jha, R. Chalcogenide prism and graphene multilayer based surface plasmon resonance affinity biosensor for high performance. Sens. Actuators B Chem. 2012, 169, 161–166. [Google Scholar] [CrossRef]
- Zeng, S.; Baillargeat, D.; Ho, H.-P.; Yong, K.-T. Nanomaterials enhanced surface plasmon resonance for biological and chemical sensing applications. Chem. Soc. Rev. 2014, 43, 3426–3452. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.-B.; Li, Y.-L.; Zhong, G.-H.; Zeng, Z.; Qin, X.-Y. Anisotropic properties of TaS2. Chin. Phys. 2007, 16, 3809–3814. [Google Scholar]
- Reshak, A.H.; Auluck, S. Full-potential calculations of the electronic and optical properties for 1T and 2H phases of TaS2 and TaSe2. Phys. B Condens. Matter 2005, 358, 158–165. [Google Scholar] [CrossRef]
- Sharma, S.; Auluck, S.; Khan, M.A. Optical properties of 1T and 2H phases of TaS2 and TaSe2. Pramana 2000, 54, 431–440. [Google Scholar] [CrossRef]
- Meshginqalam, B.; Ahmadi, M.T.; Ismail, R.; Sabatyan, A. Graphene/Graphene Oxide-Based Ultrasensitive Surface Plasmon Resonance Biosensor. Plasmonics 2017, 12, 1991–1997. [Google Scholar] [CrossRef]
- Choi, S.H.; Kim, Y.L.; Byun, K.M. Graphene-on-silver substrates for sensitive surface plasmon resonance imaging biosensors. Opt. Express 2011, 19, 458–466. [Google Scholar] [CrossRef]
- Nigam, A.; Goel, N.; Bhat, T.N.; Rahman, M.T.; Dolmanan, S.B.; Qiao, Q.; Tripathy, S.; Kumar, M. Real time detection of Hg2+ ions using MoS2 functionalized AlGaN/GaN high electron mobility transistor for water quality monitoring. Sens. Actuators B Chem. 2020, 309, 127832. [Google Scholar] [CrossRef]
- Wu, L.; Zhu, J.; Gan, S.; Ma, Q.; Dai, X.; Xiang, Y. Application of Few-Layer Transition Metal Dichalcogenides to Detect the Refractive Index Variation in Lossy-Mode Resonance Sensors with High Figure of Merit. IEEE Sens. J. 2019, 19, 5030–5034. [Google Scholar] [CrossRef]
- Zhu, J.; Ke, Y.; Dai, J.; You, Q.; Wu, L.; Li, J.; Guo, J.; Xiang, Y.; Dai, X. Topological insulator overlayer to enhance the sensitivity and detection limit of surface plasmon resonance sensor. Nanophotonics 2020, 9, 1941–1951. [Google Scholar] [CrossRef] [Green Version]
- Zuo, X.; Zhang, H.; Zhu, Q.; Wang, W.; Feng, J.; Chen, X. A dual-color fluorescent biosensing platform based on WS2 nanosheet for detection of Hg2+ and Ag+. Biosens. Bioelectron. 2016, 85, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Thompson, M.; Ellison, S.L.R.; Wood, R. Harmonized guidelines for single-laboratory validation of methods of analysis—(IUPAC technical report). Pure Appl. Chem. 2002, 74, 835–855. [Google Scholar] [CrossRef]
- Xuan, F.; Luo, X.; Hsing, I.M. Conformation-Dependent Exonuclease III Activity Mediated by Metal Ions Reshuffling on Thymine-Rich DNA Duplexes for an Ultrasensitive Electrochemical Method for Hg2+ Detection. Anal. Chem. 2013, 85, 4586–4593. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Wu, J.; Li, M.; Ju, H. Highly sensitive electrochemical detection of mercury (II) via single ion-induced three-way junction of DNA. Electrochem. Commun 2015, 59, 77–80. [Google Scholar] [CrossRef]
- Shahat, A.; Elsalam, S.A.; Herrero-Martinez, J.M.; Simo-Alfonso, E.F.; Ramis-Ramos, G. Optical recognition and removal of Hg(II) using a new self-chemosensor based on a modified amino-functionalized Al-MOF. Sens. Actuators B Chem. 2017, 253, 164–172. [Google Scholar] [CrossRef]
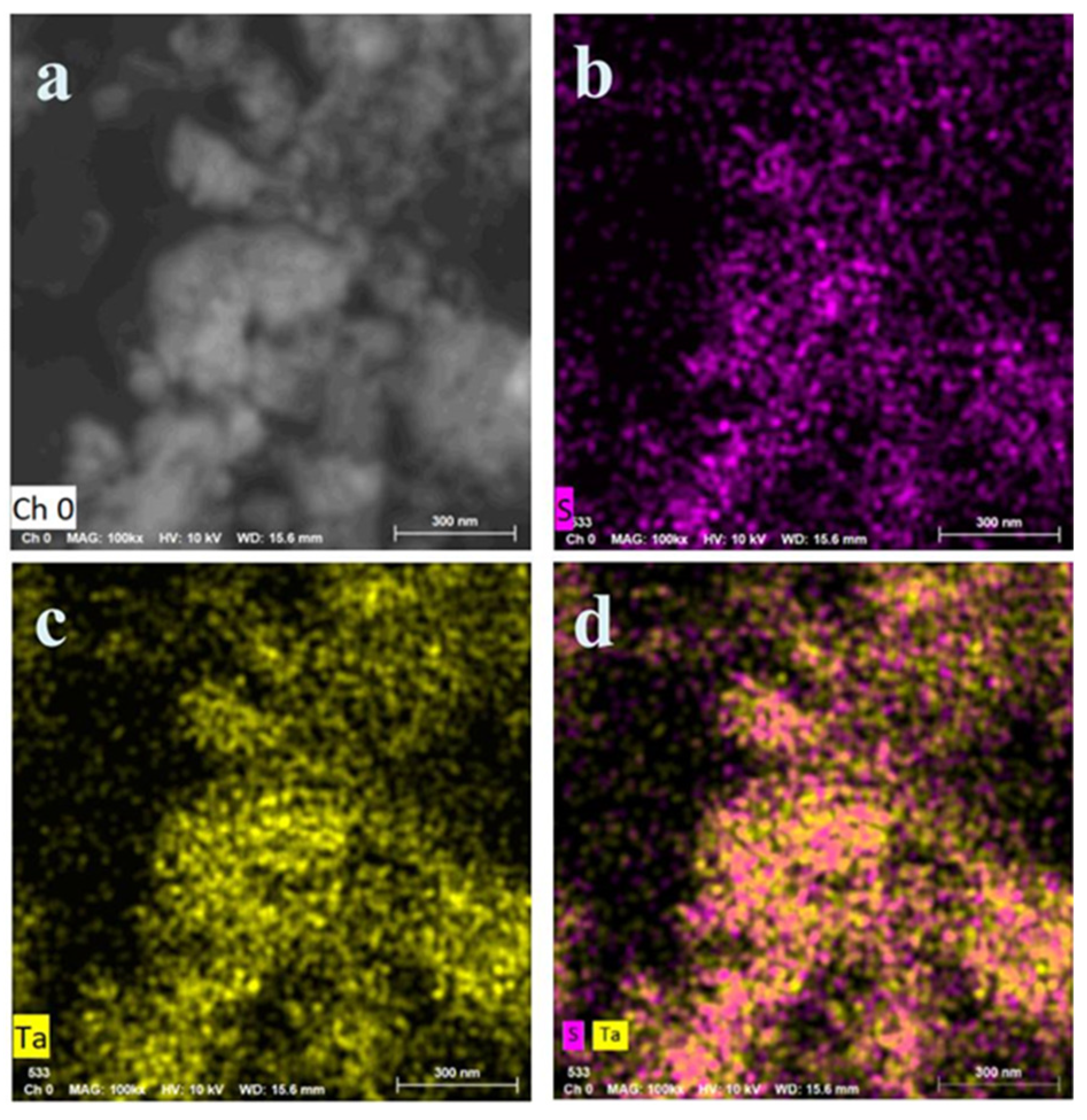
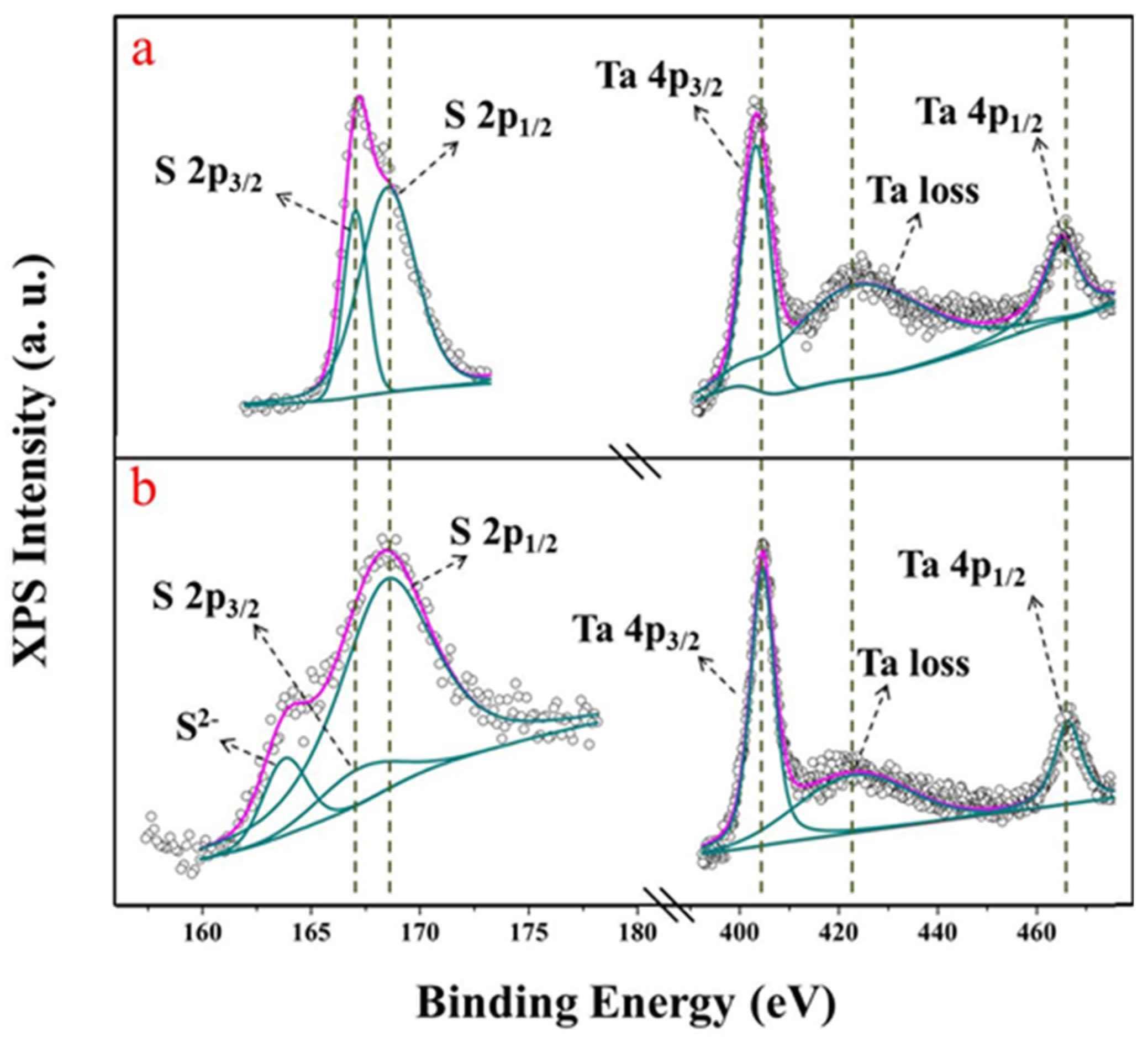
| 2D Materials | Optimal Sensitivity | Maximum Sensitivity | Detection Limit | Ref. |
|---|---|---|---|---|
| Graphene oxide | 91.1°/RIU | 2715 nm/RIU | 10−11 mol/L | [49,50] |
| MoS2 | 49.2°/RIU | 0.64 μA/ppb | 11.52 × 10−3 ppb | [51,52] |
| MoSe2 | 50.4°/RIU | 2524 nm/RIU | 3.5 nM | [53] |
| WS2 | 48.6°/RIU | 2459 nm/RIU | 3.3 nM | [54] |
| TaS2 | 201°/RIU | 10.36°/μM | 1 pM | Our work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, Y.; Liao, Y.; Cai, H. High Quality TaS2 Nanosheet SPR Biosensors Improved Sensitivity and the Experimental Demonstration for the Detection of Hg2+. Nanomaterials 2022, 12, 2075. https://doi.org/10.3390/nano12122075
Jia Y, Liao Y, Cai H. High Quality TaS2 Nanosheet SPR Biosensors Improved Sensitivity and the Experimental Demonstration for the Detection of Hg2+. Nanomaterials. 2022; 12(12):2075. https://doi.org/10.3390/nano12122075
Chicago/Turabian StyleJia, Yue, Yunlong Liao, and Houzhi Cai. 2022. "High Quality TaS2 Nanosheet SPR Biosensors Improved Sensitivity and the Experimental Demonstration for the Detection of Hg2+" Nanomaterials 12, no. 12: 2075. https://doi.org/10.3390/nano12122075





