Electrochemical Properties of an Sn-Doped LATP Ceramic Electrolyte and Its Derived Sandwich-Structured Composite Solid Electrolyte
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of an LATP-xSn Ceramic Solid Electrolyte
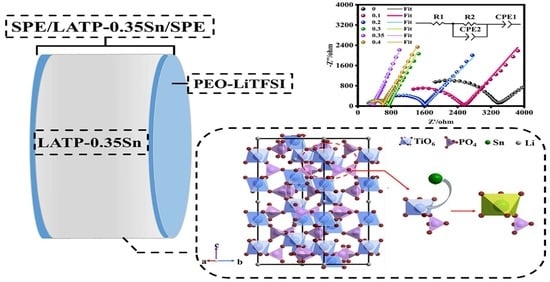
2.3. Preparation of a Composite Solid Electrolyte
2.4. Preparation of Electrodes and Assembly of the Battery
2.5. Materials Characterization
2.6. Electrochemical Performance Test
3. Results
3.1. Influences of Sn Doping on the Structural Properties and Electrochemical Performance of an LATP-xSn Solid Electrolyte
3.2. Electrochemical Performance of the Composite Solid Electrolyte
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mamidi, S.; Gangadharan, A.; Sharma, C.S. Graphite coated pyrolyzed filter paper as a low-cost binder-free and freestanding anode for practical lithium-ion battery application. Electrochim. Acta 2019, 310, 222–229. [Google Scholar] [CrossRef]
- Gangadharan, A.; Mamidi, S.; Sharma, C.S.; Rao, T.N. Urea-modified candle soot for enhanced anodic performance for fast-charging lithium-ion battery application. Mater. Today Commun. 2020, 23, 100926. [Google Scholar] [CrossRef]
- Mamidi, S.; Pandey, A.K.; Pathak, A.D.; Rao, T.N.; Sharma, C.S. Pencil lead powder as a cost-effective and high-performancegraphite-silica composite anode for high performance lithium-ion batteries. J. Alloys Compd. 2021, 87, 159719. [Google Scholar] [CrossRef]
- Zheng, F.; Kotobuki, M.; Song, S.; Lai, M.O.; Lu, L. Review on solid electrolytes for all-solid-state lithium-ion batteries. J. Power Sources 2018, 389, 198–213. [Google Scholar] [CrossRef]
- Zhang, B.; Tan, R.; Yang, L.; Zheng, J.; Zhang, K.; Mo, S.; Lin, Z.; Pan, F. Mechanisms and properties of ion-transport in inorganic solid electrolytes. Energy Storage Mater. 2018, 10, 139–159. [Google Scholar] [CrossRef]
- Luo, C.; Ji, X.; Chen, J.; Gaskell, K.J.; He, X.; Liang, Y.; Jiang, J.; Wang, C. Solid-State Electrolyte Anchored with a Carboxylated Azo Compound for All-Solid-State Lithium Batteries. Angew. Chem. Int. Ed. 2018, 57, 8567–8571. [Google Scholar] [CrossRef] [PubMed]
- Rai, K.; Kundu, S. Fabrication and performances of high lithium-ion conducting solid electrolytes based on NASICON Li1.3Al0.3Ti1.7−xZrx(PO4)3 (0 ≤ x ≤ 0.2). Ceram. Int. 2020, 46, 23695–23705. [Google Scholar] [CrossRef]
- Huang, B.; Xu, B.; Li, Y.; Zhou, W.; You, Y.; Zhon, S.; Wang, C.A.; Goodenough, J.B. Li-Ion Conduction and Stability of Perovskite Li3/8Sr7/16Hf1/4Ta3/4O3. ACS Appl. Mater. Interfaces 2016, 8, 14552–14557. [Google Scholar] [CrossRef] [PubMed]
- Bachman, J.C.; Muy, S.; Grimaud, A.; Chang, H.H.; Pour, N.; Lux, S.F.; Paschos, O.; Maglia, F.; Lupart, S.; Lamp, P.; et al. Inorganic Solid-State Electrolytes for Lithium Batteries: Mechanisms and Properties Governing Ion Conduction. Chem. Rev. 2016, 116, 140–162. [Google Scholar] [CrossRef] [PubMed]
- Kotobuki, M.; Koishi, M. Influence of precursor calcination temperature on sintering and conductivity of Li1.5Al0.5Ti1.5(PO4)3 ceramics. J. Asian Ceram. Soc. 2019, 7, 69–74. [Google Scholar] [CrossRef] [Green Version]
- Waetzig, K.; Rost, A.; Heubner, C.; Coeler, M.; Nikolowski, K.; Wolter, M.; Schilm, J. Synthesis and sintering of Li1.3Al0.3Ti1.7(PO4)3 (LATP) electrolyte for ceramics with improved Li+ conductivity. J. Alloys Compd. 2020, 818, 153237. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, Q.; Zhao, Y.; Wang, B.; Kaghazchi, P.; Adair, K.R.; Li, R.; Zhang, C.; Liu, J.; Kuo, L.Y.; et al. Stabilizing the Interface of NASICON Solid Electrolyte against Li Metal with Atomic Layer Deposition. ACS Appl. Mater. Interfaces 2018, 10, 31240–31248. [Google Scholar] [CrossRef] [PubMed]
- Hitz, G.T.; McOwen, D.W.; Zhang, L.; Ma, Z.; Fu, Z.; Wen, Y.; Gong, Y.; Dai, J.; Hamann, T.R.; Hu, L.; et al. High-rate lithium cycling in a scalable trilayer Li-garnet-electrolyte architecture. Mater. Today 2019, 22, 50–57. [Google Scholar] [CrossRef]
- Hu, Z.; Sheng, J.; Chen, J.; Sheng, G.; Li, Y.; Fu, X.Z.; Wang, L.; Sun, R.; Wong, C.P. Enhanced Li ion conductivity in Ge-doped Li0.33La0.56TiO3 perovskite solid electrolytes for all-solid-state Li-ion batteries. New J. Chem. 2018, 42, 9074–9079. [Google Scholar] [CrossRef]
- Deng, Y.; Eames, C.; Fleutot, B.; David, R.; Chotard, J.N.; Suard, E.; Masquelier, C.; Islam, M.S. Enhancing the Lithium Ion Conductivity in Lithium Superionic Conductor (LISICON) Solid Electrolytes through a Mixed Polyanion Effect. ACS Appl. Mater. Interfaces 2017, 9, 7050–7058. [Google Scholar] [CrossRef] [Green Version]
- Sepúlveda, A.; Criscuolo, F.; Put, B.; Vereecken, P.M. Effect of high temperature LiPON electrolyte in all solid state batteries. Solid State Ion. 2019, 337, 24–32. [Google Scholar] [CrossRef]
- Hartley, G.O.; Jin, L.; Bergner, B.J.; Jolly, D.S.; Rees, G.J.; Zekoll, S.; Ning, Z.; Pateman, A.T.R.; Holc, C.; Adamson, P.; et al. Is Nitrogen Present in Li3N·P2S5 Solid Electrolytes Produced by Ball Milling? Chem. Mater. 2019, 31, 9993–10001. [Google Scholar] [CrossRef] [Green Version]
- Lau, J.; DeBlock, R.H.; Butts, D.M.; Ashby, D.S.; Choi, C.S.; Dunn, B.S. Sulfide Solid Electrolytes for Lithium Battery Applications. Adv. Energy Mater. 2018, 8, 1800933. [Google Scholar] [CrossRef] [Green Version]
- Hanghofer, I.; Redhammer, G.J.; Rohde, S.; Hanzu, I.; Senyshyn, A.; Wilkening, H.M.R.; Rettenwander, D. Untangling the Structure and Dynamics of Lithium-Rich Anti-Perovskites Envisaged as Solid Electrolytes for Batteries. Chem. Mater. 2018, 30, 8134–8144. [Google Scholar] [CrossRef]
- Lu, X.; Wu, Y.; Yang, D.; Wang, R. Increased ionic conductivity of a NASICON lithium ion conductor under the influence of mesoporous materials. J. Alloys Compd. 2019, 794, 585–593. [Google Scholar] [CrossRef]
- Mertens, A.; Yu, S.; Schön, N.; Gunduz, D.C.; Tempel, H.; Schierholz, R.; Hausen, F.; Kungl, H.; Granwehr, J.; Eichel, R.A. Superionic bulk conductivity in Li1.3Al0.3Ti1.7(PO4)3 solid electrolyte. Solid State Ion. 2017, 309, 180–186. [Google Scholar] [CrossRef]
- Anantharamulu, N.; Rao, K.K.; Rambabu, G.; Kumar, B.V.; Radha, V.; Vithal, M. A wide-ranging review on Nasicon type materials. J. Mater. Sci. 2011, 46, 2821–2837. [Google Scholar] [CrossRef]
- Arbi, K.; Lazarraga, M.G.; Chehimi, D.B.H.; Ayadi-Trabelsi, M.; Rojo, J.M.; Sanz, J. Lithium Mobility in Li1.2Ti1.8R0.2(PO4)3 Compounds (R = Al, Ga, Sc, In) as Followed by NMR and Impedance Spectroscopy. Chem. Mater. 2004, 16, 255–262. [Google Scholar] [CrossRef]
- Shang, X.; Cheng, S.; Zhang, J.; Wang, Y. Comparison of a high lithium-ion conductivity solid electrolyte prepared by two methods. IOP Conf. Ser. Mater. Sci. Eng. 2017, 242, 012046. [Google Scholar] [CrossRef]
- Kothari, D.H.; Kanchan, D.K.; Sharma, P. Electrical properties of Li-based NASICON compounds doped with yttrium oxide. Ionics 2014, 20, 1385–1390. [Google Scholar] [CrossRef]
- Liang, Y.; Peng, C.; Kamiike, Y.; Kuroda, K.; Okido, M. Gallium doped NASICON type LiTi2(PO4)3 thin-film grown on graphite anode as solid electrolyte for all solid state lithium batteries. J. Alloys Compd. 2019, 775, 1147–1155. [Google Scholar] [CrossRef]
- Rao, R.P.; Maohua, C.; Adams, S. Preparation and characterization of NASICON type Li+ ionic conductors. J. Solid State Electrochem. 2012, 16, 3349–3354. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, L.; Zhao, B.; Zhang, L.; Xiao, X.; Yan, H.; Xu, G.; Ma, L.; Liu, Y. Transport and interface characteristics of Te-doped NASICON solid electrolyte Li1.3Al0.3Ti1.7(PO4)3. Electrochim. Acta 2021, 399, 139367. [Google Scholar] [CrossRef]
- Zhu, J.; Xiang, Y.; Zhao, J.; Wang, H.; Li, Y.; Zheng, B.; He, H.; Zhang, Z.; Huang, J.; Yang, Y. Insights into the Local Structure, Microstructure and Ionic Conductivity of Silicon Doped NASICON-type Solid Electrolyte Li1.3Al0.3Ti1.7P3O12. Energy Storage Mater. 2021, 44, 190–196. [Google Scholar] [CrossRef]
- Vijayan, L.; Govindaraj, G. Structural and electrical properties of high-energy ball-milled NASICON type Li1.3Ti1.7Al0.3(PO4)2.9(VO4)0.1 ceramics. J. Phys. Chem. Solids 2011, 72, 613–619. [Google Scholar] [CrossRef]
- Li, S.; Huang, Z.; Xiao, Y.; Sun, C. Chlorine-doped Li1.3Al0.3Ti1.7(PO4)3 as an electrolyte for solid lithium metal batteries. Mater. Chem. Front. 2021, 5, 5336–5343. [Google Scholar] [CrossRef]
- Jeong, H.; Na, D.; Baek, J.; Kim, S.; Mamidi, S.; Lee, C.R.; Seo, H.K.; Seo, I. Synthesis of Superionic Conductive Li1+x+yAlxSiyTi2−xP3−yO12 Solid Electrolytes. Nanomaterials 2022, 12, 1158. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.V.; Veeraiah, V.; Rao, A.V.P.; Babu, B.K.; Brahmayya, M. Spectroscopic characterization and conductivity of Sn-substituted LiTi2(PO4)3. Res. Chem. Intermed. 2014, 41, 4327–4337. [Google Scholar]
- Roose, B.; Baena, J.P.C.; Gödel, K.C.; Graetzel, M.; Hagfeldt, A.; Steiner, U.; Abate, A. Mesoporous SnO2 electron selective contact enables UV-stable perovskite solar cells. Nano Energy 2016, 30, 517–522. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Teng, G.; Yang, J.; Tan, R.; Duan, Y.; Zheng, J.; Pan, F. Sn(II,IV) steric and electronic structure effects enableself-selective doping on Fe/Si-sites of Li2FeSiO4 nanocrystals for high performance lithium ion batteries. J. Mater. Chem. A 2015, 3, 24437–24445. [Google Scholar] [CrossRef]
- Park, G.D.; Lee, J.K.; Kang, Y.C. Design and synthesis of Janus-structured mutually doped SnO2-Co3O4 hollow nanostructures as superior anode materials for lithium-ion batteries. J. Mater. Chem. A 2017, 5, 25319–25327. [Google Scholar] [CrossRef]
- Mehraz, S.; Kongsong, P.; Taleb, A.; Dokhane, N.; Sikong, L. Large scale and facile synthesis of Sn doped TiO2 aggregates using hydrothermal synthesis. Sol. Energy Mater. Sol. Cells 2019, 189, 254–262. [Google Scholar] [CrossRef]
- Lim, Y.J.; Kim, H.W.; Lee, S.S.; Kim, H.J.; Kim, J.K.; Jung, Y.G.; Kim, Y. Ceramic-Based Composite Solid Electrolyte for Lithium-Ion Batteries. Chempluschem 2015, 80, 1100–1103. [Google Scholar] [CrossRef]
- Zou, Y.; Zheng, H.; Wu, S.; Hei, Z.; Liu, H.; Duan, H. Improving Li/garnet interface by amorphous SnO2 interlayerdeposited via sol–gel method. Mater. Lett. 2021, 297, 129959. [Google Scholar] [CrossRef]
- Chen, L.; Li, Y.; Li, S.P.; Fan, L.Z.; Nan, C.W.; Goodenough, J.B. PEO/garnet composite electrolytes for solid-state lithium batteries: From “ceramic-in-polymer” to “polymer-in-ceramic”. Nano Energy 2018, 46, 176–184. [Google Scholar] [CrossRef]
- Jiang, Y.; Yan, X.; Ma, Z.; Mei, P.; Xiao, W.; You, Q.; Zhang, Y. Development of the PEO Based Solid Polymer Electrolytes for All-Solid State Lithium Ion Batteries. Polymers 2018, 10, 1237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, Q.; Han, Y.; Wang, H.; Xiong, S.; Li, Y.; Liu, S.; Xie, K. New Class of LAGP-Based Solid Polymer Composite Electrolyte for Efficient and Safe Solid-State Lithium Batteries. ACS Appl. Mater. Interfaces 2017, 9, 41837–41844. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Liao, X.; Zhang, H.; Shi, L.; Wang, P.; Cheng, Q.; Borovilas, J.; Li, Z.; Huang, W.; Fu, Z.; et al. Nacre-Inspired Composite Electrolytes for Load-Bearing Solid-State Lithium-Metal Batteries. Adv. Mater. 2020, 32, 1905517. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Liu, J.; Gong, Y.; Wilkinsone, D.P.; Zhang, J. Recent advances in all-solid state rechargeable lithium batteries. Nano Energy 2017, 33, 363–386. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.G.; Son, B.; Mukherjee, S.; Schuppert, N.; Bates, A.; Kwon, O.; Choi, M.J.; Chung, H.Y.; Park, S. A review of lithium and non-lithium based solid state batteries. J. Power Sources 2015, 282, 299–322. [Google Scholar] [CrossRef]
- Cheng, Z.; Liu, T.; Zhao, B.; Shen, F.; Jin, H.; Han, X. Recent advances in organic-inorganic composite solid electrolytes for all-solid-state lithium batteries. Energy Storage Mater. 2021, 34, 388–416. [Google Scholar] [CrossRef]
- Zhai, H.; Xu, P.; Ning, M.; Cheng, Q.; Mandal, J.; Yang, Y. A Flexible Solid Composite Electrolyte with Vertically Aligned and Connected Ion-Conducting Nanoparticles for Lithium Batteries. Nano Lett. 2017, 17, 3182–3187. [Google Scholar] [CrossRef]
- Yang, J.; Huang, Z.; Zhang, P.; Liu, G.; Xu, X.; Yao, X. Titanium Dioxide Doping toward High-Lithium-Ion-Conducting Li1.5Al0.5Ge1.5(PO4)3 Glass-Ceramics for All-Solid-State Lithium Batteries. ACS Appl. Energy Mater. 2019, 2, 7299–7305. [Google Scholar] [CrossRef]
- Santagneli, S.H.; Baldacim, H.V.A.; Ribeiro, S.J.L.; Kundu, S.; Rodrigues, A.C.M.; Doerenkamp, C.; Eckert, H. Preparation, Structural Characterization, and Electrical Conductivity of Highly Ion-Conducting Glasses and Glass Ceramics in the System Li1+xAlxSnyGe2−(x+y)(PO4)3. J. Phys. Chem. C 2016, 120, 14556–14567. [Google Scholar] [CrossRef]
- Pérez-Estébanez, M.; Isasi-Marín, J.; Díaz-Guerra, C.; Rivera-Calzada, A.; León, C.; Santamaría, J. Influence of chromium content on the optical and electrical properties of Li1+xCrxTi2−x(PO4)3. Solid State Ion. 2013, 241, 36–45. [Google Scholar] [CrossRef]
- Burba, C.M.; Frech, R. Vibrational spectroscopic study of lithium intercalation into LiTi2(PO4)3. Solid State Ion. 2006, 177, 1489–1494. [Google Scholar] [CrossRef]
- Xu, Q.; Tsai, C.L.; Song, D.; Basak, S.; Kungl, H.; Tempel, H.; Hausen, F.; Yu, S.; Eichel, R.A. Insights into the reactive sintering and separated specific grain/grain boundary conductivities of Li1.3Al0.3Ti1.7(PO4)3. J. Power Sources 2021, 492, 229631. [Google Scholar] [CrossRef]
- Ohta, S.; Kobayashi, T.; Asaoka, T. High lithium ionic conductivity in the garnet-type oxide Li7−XLa3(Zr2−X, NbX)O12 (X = 0–2). J. Power Sources 2011, 196, 3342–3345. [Google Scholar] [CrossRef]
- DeWees, R.; Wang, H. Synthesis and Properties of NASICON-type LATP and LAGP Solid Electrolytes. ChemSusChem 2019, 12, 3713–3725. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Xu, Y.; Zhang, B.; Sun, X.; Chen, Y.; Jin, Y. Unique rhombus-like precursor for synthesis of Li1.3Al0.3Ti1.7(PO4)3 solid electrolyte with high ionic conductivity. Chem. Eng. J. 2018, 345, 483–491. [Google Scholar] [CrossRef]
- Cortés-Adasme, E.; Vega, M.; Martin, I.R.; Llanos, J. Synthesis and characterization of SrSnO3 doped with Er3+ for up-conversion luminescence temperature sensors. RSC Adv. 2017, 7, 46796–46802. [Google Scholar] [CrossRef] [Green Version]
- Cai, Z.; Huang, Y.; Zhu, W.; Xiao, R. Increase in ionic conductivity of NASICON-type solid electrolyte Li1.5Al0.5Ti1.5(PO4)3 by Nb2O5 doping. Solid State Ion. 2020, 354, 113359. [Google Scholar] [CrossRef]
- Dermenci, K.B.; Buluc, A.F.; Turan, S. The effect of limonite addition on the performance of Li7La3Zr2O12. Ceram. Int. 2019, 45, 21401–21408. [Google Scholar] [CrossRef]
- Song, S.; Lu, J.; Zheng, F.; Duong, H.M.; Lu, L. A facile strategy to achieve high conduction and excellent chemical stability of lithium solid electrolytes. RSC Adv. 2015, 5, 6588–6594. [Google Scholar] [CrossRef]
- Lee, S.; Jung, S.; Yang, S.; Lee, J.H.; Shin, H.; Kim, J.; Park, S. Revisiting the LiPON/Li thin film as a bifunctional interlayer for NASICON solid electrolyte-based lithium metal batteries. Appl. Surf. Sci. 2022, 586, 152790. [Google Scholar] [CrossRef]
- Ito, S.; Nakakita, M.; Aihara, Y.; Uehara, T.; Machida, N. A synthesis of crystalline Li7P3S11 solid electrolyte from 1,2-dimethoxyethane solvent. J. Power Sources 2014, 271, 342–345. [Google Scholar] [CrossRef]
- Lü, X.; Howard, J.W.; Chen, A.; Zhu, J.; Li, S.; Wu, G.; Dowden, P.; Xu, H.; Zhao, Y.; Ji, Q. Antiperovskite Li3OCl Superionic Conductor Films for Solid-State Li-Ion Batteries. Adv. Sci. 2016, 3, 1500359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Y.; Liu, K.; Xiong, C.; Wu, M.; Zhao, T. A composite solid electrolyte with an asymmetric ceramic framework for dendrite-free all-solid-state Li metal batteries. J. Mater. Chem. A 2021, 9, 9665–9674. [Google Scholar] [CrossRef]
- Peng, J.; Xiao, Y.; Clarkson, D.A.; Greenbaum, S.G.; Zawodzinski, T.A.; Chen, X.C. A Nuclear Magnetic Resonance Study of Cation and Anion Dynamics in Polymer-Ceramic Composite Solid Electrolytes. ACS Appl. Polym. Mater. 2020, 2, 1180–1189. [Google Scholar] [CrossRef]
- Zhao, E.; Guo, Y.; Xin, Y.; Xu, G.; Guo, X. Enhanced electrochemical properties and interfacial stability of poly(ethylene oxide) solid electrolyte incorporating nanostructured Li1.3Al0.3Ti1.7(PO4)3 fillers for all solid state lithium-ion batteries. Int. J. Energy Res. 2021, 45, 6876–6887. [Google Scholar] [CrossRef]
- Jin, Y.; Liu, C.; Zong, X.; Li, D.; Fu, M.; Tan, S.; Xiong, Y.; Wei, J. Interface engineering of Li1.3Al0.3Ti1.7(PO4)3 ceramic electrolyte via multifunctional interfacial layer for all-solid-state lithium batteries. J. Power Sources 2020, 460, 228125. [Google Scholar] [CrossRef]
- He, J.; Manthiram, A. 3D CoSe@C Aerogel as a Host for Dendrite-Free LithiumMetal Anode and Efficient Sulfur Cathode in Li–S Full Cells. Adv. Energy Mater. 2020, 10, 2002654. [Google Scholar] [CrossRef]








| x | a = b (Å) | c (Å) | Density (g/cm3) | Rp | Rwp | Rexp |
|---|---|---|---|---|---|---|
| 0 | 8.509 | 20.864 | 2.92 | 9.8 | 13.5 | 10.3 |
| 0.1 | 8.515 | 20.884 | 2.78 | 10.1 | 14.2 | 9.2 |
| 0.2 | 8.522 | 20.919 | 2.95 | 11.2 | 13.9 | 10.9 |
| 0.3 | 8.526 | 20.939 | 3.31 | 9.3 | 13.1 | 10.2 |
| 0.35 | 8.528 | 20.944 | 3.32 | 10.3 | 14.0 | 11.5 |
| 0.4 | 8.525 | 20.973 | 3.11 | 9.4 | 13.3 | 10.5 |
| x | Rg (Ω) | Rgb (Ω) | σg (mS/cm) | σgb (mS/cm) | σt (mS/cm) | Ea (eV) | Relative Density (%) |
|---|---|---|---|---|---|---|---|
| 0 | 653 | 2798 | 0.267 | 0.0624 | 0.0505 | 0.32 | 87.5 |
| 0.1 | 580 | 1956 | 0.277 | 0.0821 | 0.0633 | 0.28 | 88.2 |
| 0.2 | 375.2 | 1237.4 | 0.402 | 0.122 | 0.0939 | 0.27 | 89.4 |
| 0.3 | 232 | 498.8 | 0.595 | 0.277 | 0.189 | 0.26 | 90.1 |
| 0.35 | 218.5 | 139.6 | 0.772 | 1.21 | 0.471 | 0.23 | 91.8 |
| 0.4 | 339.5 | 253.9 | 0.556 | 0.742 | 0.322 | 0.24 | 92.0 |
| Electrolytes | |||||
|---|---|---|---|---|---|
| SPE/LATP-0Sn/SPE | 4.79 | 2.03 | 1039.8 | 1084.1 | 0.27 |
| SPE/LATP-0.35Sn/SPE | 9.72 | 4.73 | 358.2 | 372.3 | 0.38 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, A.; Wang, R.; Yao, M.; Cao, J.; Li, M.; Yang, C.; Liu, F.; Ma, J. Electrochemical Properties of an Sn-Doped LATP Ceramic Electrolyte and Its Derived Sandwich-Structured Composite Solid Electrolyte. Nanomaterials 2022, 12, 2082. https://doi.org/10.3390/nano12122082
Xu A, Wang R, Yao M, Cao J, Li M, Yang C, Liu F, Ma J. Electrochemical Properties of an Sn-Doped LATP Ceramic Electrolyte and Its Derived Sandwich-Structured Composite Solid Electrolyte. Nanomaterials. 2022; 12(12):2082. https://doi.org/10.3390/nano12122082
Chicago/Turabian StyleXu, Aihong, Ruoming Wang, Mengqin Yao, Jianxin Cao, Mengjun Li, Chunliang Yang, Fei Liu, and Jun Ma. 2022. "Electrochemical Properties of an Sn-Doped LATP Ceramic Electrolyte and Its Derived Sandwich-Structured Composite Solid Electrolyte" Nanomaterials 12, no. 12: 2082. https://doi.org/10.3390/nano12122082
APA StyleXu, A., Wang, R., Yao, M., Cao, J., Li, M., Yang, C., Liu, F., & Ma, J. (2022). Electrochemical Properties of an Sn-Doped LATP Ceramic Electrolyte and Its Derived Sandwich-Structured Composite Solid Electrolyte. Nanomaterials, 12(12), 2082. https://doi.org/10.3390/nano12122082