Formamidinium Lead Halide Perovskite Nanocomposite Scintillators
Abstract
:1. Introduction
2. Results and Discussion
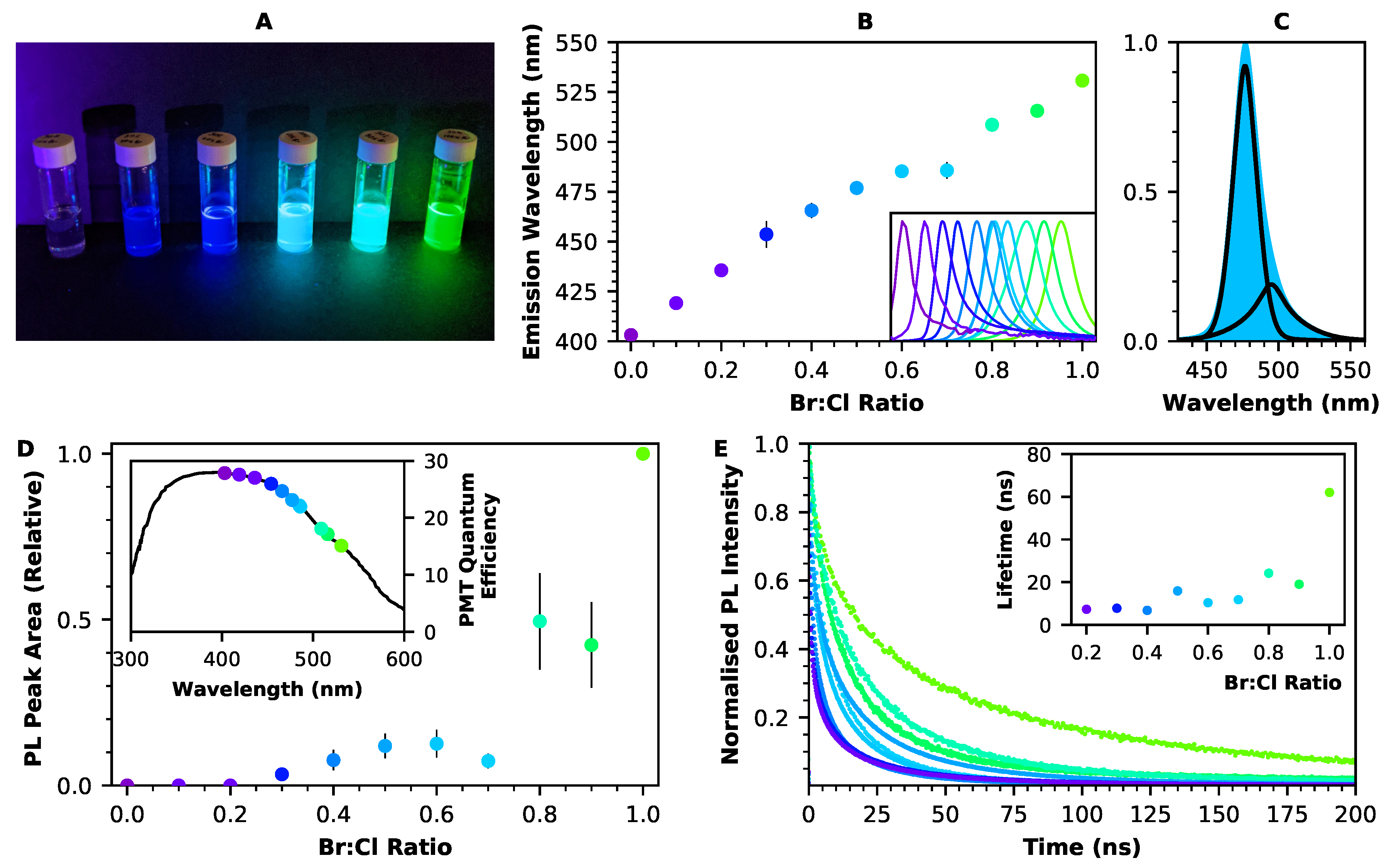
2.1. Optical Properties of Perovskite Nanocrystals
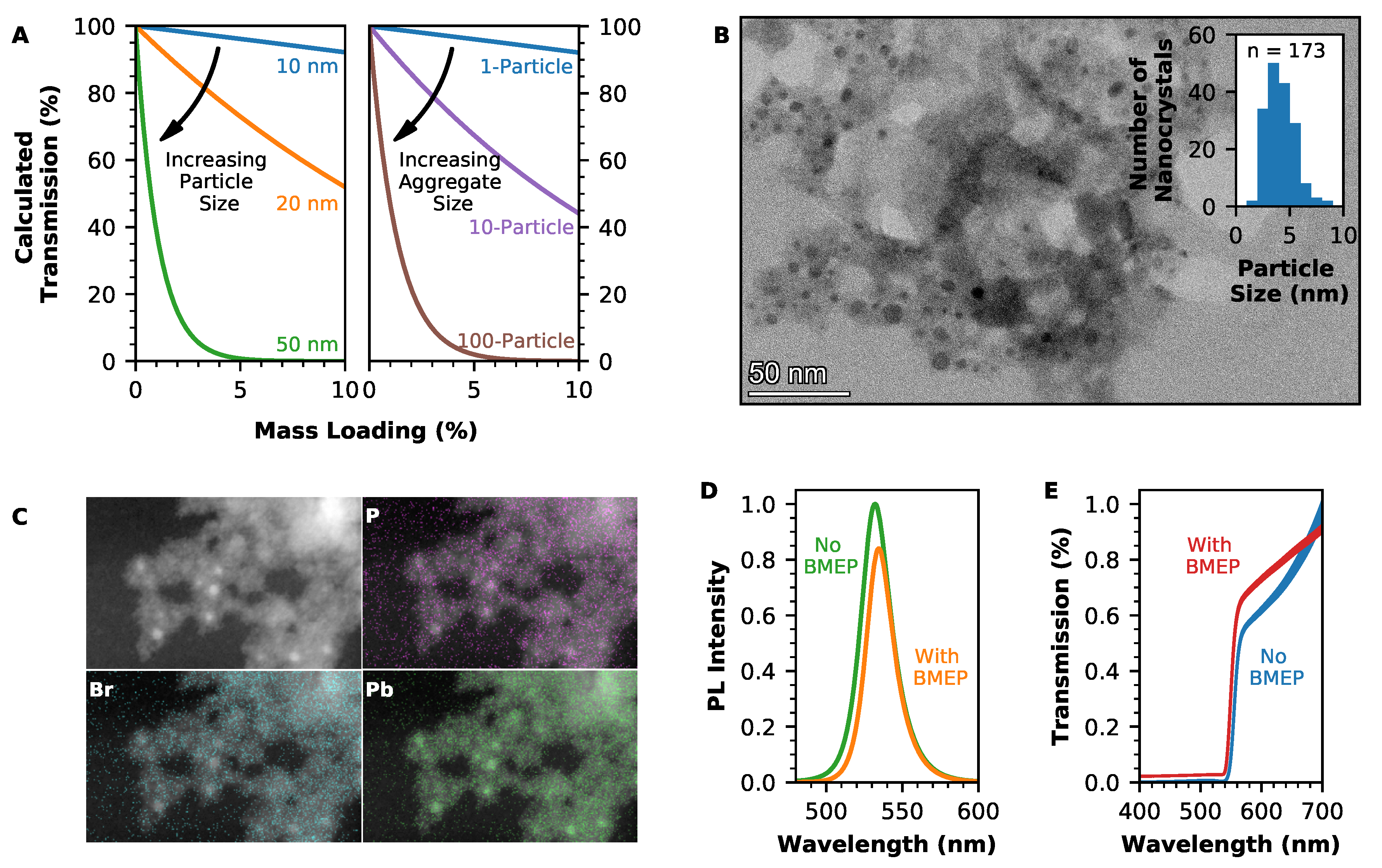
2.2. Optimising Transmission through Nanocomposite Scintillators
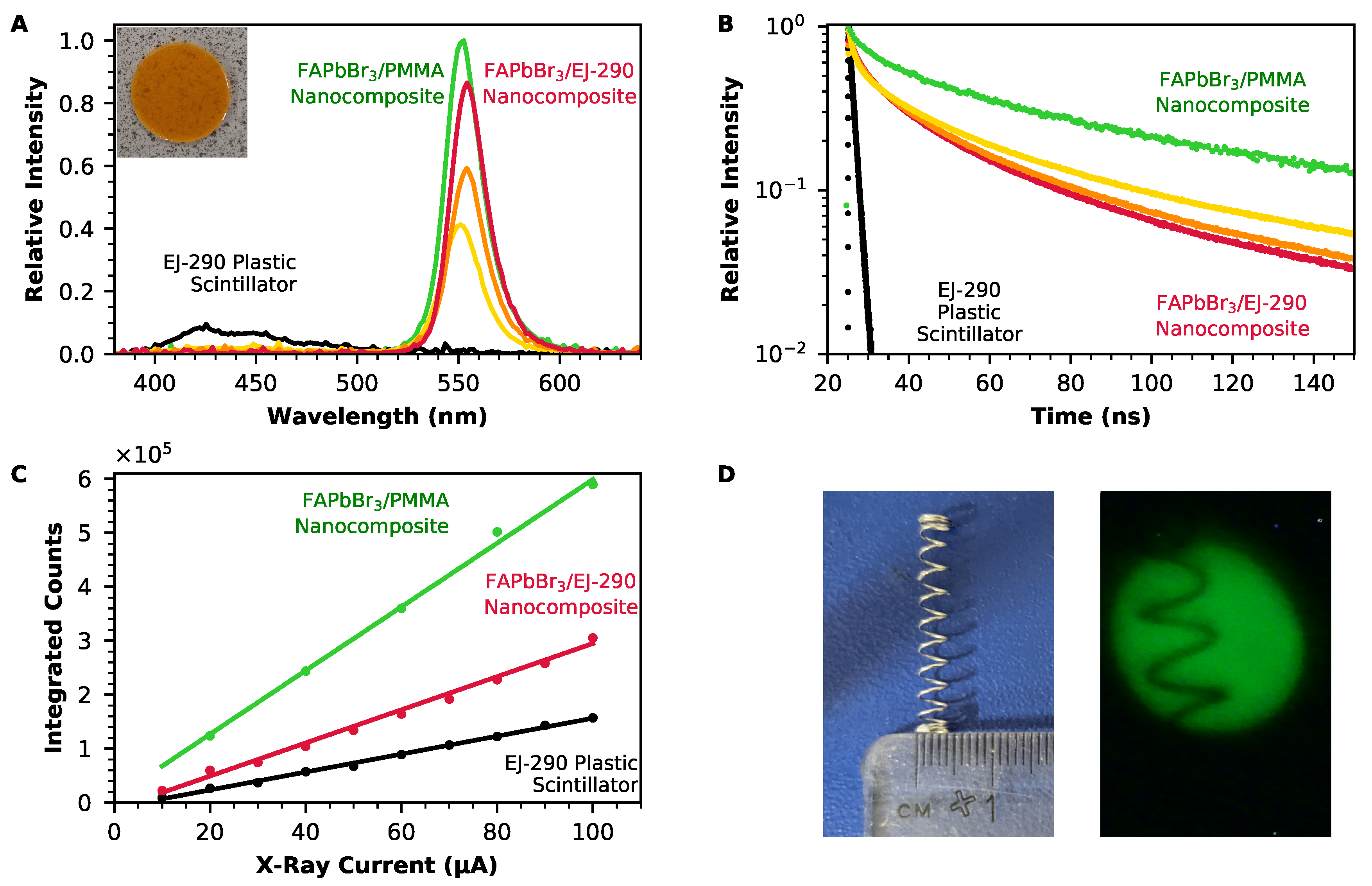
2.3. Performance of Nanocomposite Scintillators
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Perovskite Nanocrystal Synthesis
4.3. Surface Modification with BMEP
4.4. Fabrication of EJ-290 Nanocomposites
4.5. Fabrication of PMMA Nanocomposites
4.6. Calculations
4.7. Characterisation Methods
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maddalena, F.; Tjahjana, L.; Xie, A.; Arramel; Zeng, S.; Wang, H.; Coquet, P.; Drozdowski, W.; Dujardin, C.; Dang, C.; et al. Inorganic, Organic, and Perovskite Halides with Nanotechnology for High-Light Yield X- and γ-ray Scintillators. Crystals 2019, 9, 88. [Google Scholar] [CrossRef] [Green Version]
- Knoll, G.F. Radiation Detection and Measurement, 4th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2010. [Google Scholar]
- Hajagos, T.J.; Liu, C.; Cherepy, N.J.; Pei, Q. High-Z Sensitized Plastic Scintillators: A Review. Adv. Mater. 2018, 30, 1706956. [Google Scholar] [CrossRef]
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal Halide Perovskites as Visible-Light Sensitizers for Photovoltaic Cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef]
- Jena, A.K.; Kulkarni, A.; Miyasaka, T. Halide Perovskite Photovoltaics: Background, Status, and Future Prospects. Chem. Rev. 2019, 119, 3036–3103. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, B.R.; Sargent, E.H. Perovskite Photonic Sources. Nat. Photonics 2016, 10, 295–302. [Google Scholar] [CrossRef]
- Shirasaki, Y.; Supran, G.J.; Bawendi, M.G.; Bulović, V. Emergence of Colloidal Quantum-Dot Light-Emitting Technologies. Nat. Photonics 2013, 7, 13–23. [Google Scholar] [CrossRef]
- Birowosuto, M.D.; Cortecchia, D.; Drozdowski, W.; Brylew, K.; Lachmanski, W.; Bruno, A.; Soci, C. X-ray Scintillation in Lead Halide Perovskite Crystals. Sci. Rep. 2016, 6, 37254. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Hei, D.; Xu, Q.; Tan, X.; Ruan, J.; Ouyang, X.; Nie, J.; Wei, K.; Xu, Q.; Sun, B. Low Temperature Scintillation Performance of a Br-doped CH3NH3PbCl3 Single-Crystalline Perovskite. RSC Adv. 2021, 11, 2020–2024. [Google Scholar] [CrossRef]
- Schmidt, L.C.; Pertegás, A.; González-Carrero, S.; Malinkiewicz, O.; Agouram, S.; Mínguez Espallargas, G.; Bolink, H.J.; Galian, R.E.; Pérez-Prieto, J. Nontemplate Synthesis of CH3NH3PbBr3 Perovskite Nanoparticles. J. Am. Chem. Soc. 2014, 136, 850–853. [Google Scholar] [CrossRef]
- Chen, W.; Liu, Y.; Yuan, Z.; Xu, Z.; Zhang, Z.; Liu, K.; Jin, Z.; Tang, X. X-ray Radioluminescence Effect of All-Inorganic Halide Perovskite CsPbBr3 Quantum Dots. J. Radioanal. Nucl. Chem. 2017, 314, 2327–2337. [Google Scholar] [CrossRef]
- Graham, E.; Gooding, D.; Gruszko, J.; Grant, C.; Naranjo, B.; Winslow, L. Light Yield of Perovskite Nanocrystal-Doped Liquid Scintillator. J. Instrum. 2019, 14, P11024. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Sun, R.; Ou, X.; Fu, K.; Chen, Q.; Ding, Y.; Xu, L.J.; Liu, L.; Han, Y.; Malko, A.V.; et al. Metal Halide Perovskite Nanosheet for X-ray High-Resolution Scintillation Imaging Screens. ACS Nano 2019, 13, 2520–2525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCall, K.M.; Sakhatskyi, K.; Lehmann, E.; Walfort, B.; Losko, A.S.; Montanarella, F.; Bodnarchuk, M.I.; Krieg, F.; Kelestemur, Y.; Mannes, D.; et al. Fast Neutron Imaging with Semiconductor Nanocrystal Scintillators. ACS Nano 2020, 14, 14686–14697. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Wu, J.; Ou, X.; Huang, B.; Almutlaq, J.; Zhumekenov, A.A.; Guan, X.; Han, S.; Liang, L.; Yi, Z.; et al. All-Inorganic Perovskite Nanocrystal Scintillators. Nature 2018, 561, 88–93. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, H.; Zhou, M.; Zhao, L.; Chen, W.; Wang, T.; Yu, X.; Zhou, D.; Qiu, J.; Xu, X. High-Stable X-ray Imaging from All-Inorganic Perovskite Nanocrystals under a High Dose Radiation. J. Phys. Chem. Lett. 2020, 11, 9203–9209. [Google Scholar] [CrossRef]
- Yang, H.; Li, H.; Yuan, R.; Chen, J.; Zhao, J.; Wang, S.; Liu, Y.; Li, Q.; Zhang, Z. A Novel Scintillation Screen for Achieving High-Energy Ray Detection with Fast and Full-Color Emission. J. Mater. Chem. C 2021, 9, 7905–7909. [Google Scholar] [CrossRef]
- Heo, J.H.; Shin, D.H.; Park, J.K.; Kim, D.H.; Lee, S.J.; Im, S.H. High-Performance Next-Generation Perovskite Nanocrystal Scintillator for Nondestructive X-Ray Imaging. Adv. Mater. 2018, 30, 1801743. [Google Scholar] [CrossRef]
- Gandini, M.; Villa, I.; Beretta, M.; Gotti, C.; Imran, M.; Carulli, F.; Fantuzzi, E.; Sassi, M.; Zaffalon, M.; Brofferio, C.; et al. Efficient, fast and reabsorption-free perovskite nanocrystal-based sensitized plastic scintillators. Nat. Nanotechnol. 2020, 15, 462–468. [Google Scholar] [CrossRef]
- Protesescu, L.; Yakunin, S.; Bodnarchuk, M.I.; Krieg, F.; Caputo, R.; Hendon, C.H.; Yang, R.X.; Walsh, A.; Kovalenko, M.V. Nanocrystals of Cesium Lead Halide Perovskites (CsPbX3, X = Cl, Br, and I): Novel Optoelectronic Materials Showing Bright Emission with Wide Color Gamut. Nano Lett. 2015, 15, 3692–3696. [Google Scholar] [CrossRef] [Green Version]
- Trinh, C.T.; Minh, D.N.; Ahn, K.J.; Kang, Y.; Lee, K.G. Organic–Inorganic FAPbBr3 Perovskite Quantum Dots as a Quantum Light Source: Single-Photon Emission and Blinking Behaviors. ACS Photonics 2018, 5, acsphotonics.8b01130. [Google Scholar] [CrossRef]
- Akkerman, Q.A.; Rainò, G.; Kovalenko, M.V.; Manna, L. Genesis, challenges and opportunities for colloidal lead halide perovskite nanocrystals. Nat. Mater. 2018, 17, 394–405. [Google Scholar] [CrossRef] [PubMed]
- Tamarat, P.; Bodnarchuk, M.I.; Trebbia, J.b.; Erni, R.; Kovalenko, M.V.; Even, J.; Lounis, B. The ground exciton state of formamidinium lead bromide perovskite nanocrystals is a singlet dark state. Nat. Mater. 2019, 18, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Becker, M.A.; Vaxenburg, R.; Nedelcu, G.; Sercel, P.C.; Shabaev, A.; Mehl, M.J.; Michopoulos, J.G.; Lambrakos, S.G.; Bernstein, N.; Lyons, J.L.; et al. Bright triplet excitons in caesium lead halide perovskites. Nature 2018, 553, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Krentz, T.M.; Wang, L.; Benicewicz, B.C.; Schadler, L.S. Ligand Engineering of Polymer Nanocomposites: From the Simple to the Complex. ACS Appl. Mater. Interfaces 2014, 6, 6005–6021. [Google Scholar] [CrossRef]
- Caseri, W.R. Nanocomposites of polymers and inorganic particles: Preparation, structure and properties. Mater. Sci. Technol. 2006, 22, 807–817. [Google Scholar] [CrossRef]
- Balazs, A.C.; Emrick, T.; Russell, T.P. Nanoparticle Polymer Composites: Where Two Small Worlds Meet. Science 2006, 314, 1107–1110. [Google Scholar] [CrossRef]
- Hiemenz, P.C.; Rajagopalan, R. Principles of Colloid and Surface Chemistry; Marcel Dekker Inc.: New York, NY, USA, 1997. [Google Scholar]
- Cai, W.; Chen, Q.; Cherepy, N.; Dooraghi, A.; Kishpaugh, D.; Chatziioannou, A.; Payne, S.; Xiang, W.; Pei, Q. Synthesis of bulk-size transparent gadolinium oxide–polymer nanocomposites for gamma ray spectroscopy. J. Mater. Chem. C 2013, 1, 1970. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Hajagos, T.J.; Kishpaugh, D.; Jin, Y.; Hu, W.; Chen, Q.; Pei, Q. Facile Single-Precursor Synthesis and Surface Modification of Hafnium Oxide Nanoparticles for Nanocomposite γ-Ray Scintillators. Adv. Funct. Mater. 2015, 25, 4607–4616. [Google Scholar] [CrossRef]
- Jin, Y.; Kishpaugh, D.; Liu, C.; Hajagos, T.J.; Chen, Q.; Li, L.; Chen, Y.; Pei, Q. Partial ligand exchange as a critical approach to the synthesis of transparent ytterbium fluoride–polymer nanocomposite monoliths for gamma ray scintillation. J. Mater. Chem. C 2016, 4, 3654–3660. [Google Scholar] [CrossRef]
- Liu, C.; Li, Z.; Hajagos, T.J.; Kishpaugh, D.; Chen, D.Y.; Pei, Q. Transparent Ultra-High-Loading Quantum Dot/Polymer Nanocomposite Monolith for Gamma Scintillation. ACS Nano 2017, 11, 6422–6430. [Google Scholar] [CrossRef] [Green Version]
- Nie, J.; Li, C.; Zhou, S.; Huang, J.; Ouyang, X.; Xu, Q. High Photoluminescence Quantum Yield Perovskite/Polymer Nanocomposites for High Contrast X-ray Imaging. ACS Appl. Mater. Interfaces 2021, 13, 54348–54353. [Google Scholar] [CrossRef] [PubMed]
- Maddalena, F.; Xie, A.; Chin, X.Y.; Begum, R.; Witkowski, M.E.; Makowski, M.; Mahler, B.; Drozdowski, W.; Springham, S.V.; Rawat, R.S.; et al. Deterministic Light Yield, Fast Scintillation, and Microcolumn Structures in Lead Halide Perovskite Nanocrystals. J. Phys. Chem. C 2021, 125, 14082–14088. [Google Scholar] [CrossRef]
- Zhang, F.; Zhong, H.; Chen, C.; Wu, X.G.; Hu, X.; Huang, H.; Han, J.; Zou, B.; Dong, Y. Brightly luminescent and color-tunable colloidal CH3NH3PbX3 (X = Br, I, Cl) quantum dots: Potential alternatives for display technology. ACS Nano 2015, 9, 4533–4542. [Google Scholar] [CrossRef]
- Perumal, A.; Shendre, S.; Li, M.; Tay, Y.K.E.; Sharma, V.K.; Chen, S.; Wei, Z.; Liu, Q.; Gao, Y.; Buenconsejo, P.J.S.; et al. High brightness formamidinium lead bromide perovskite nanocrystal light emitting devices. Sci. Rep. 2016, 6, 36733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ravi, V.K.; Markad, G.B.; Nag, A. Band Edge Energies and Excitonic Transition Probabilities of Colloidal CsPbX3 (X = Cl, Br, I) Perovskite Nanocrystals. ACS Energy Lett. 2016, 1, 665–671. [Google Scholar] [CrossRef]
- Umebayashi, T.; Asai, K.; Kondo, T.; Nakao, A. Electronic structures of lead iodide based low-dimensional crystals. Phys. Rev. B Condens. Matter Mater. Phys. 2003, 67, 2–7. [Google Scholar] [CrossRef]
- Dou, Y.; Wang, S.; Zhang, C.; Luo, H.; Li, X.; Wang, H.; Cao, F.; Shen, P.; Zhang, J.; Yang, X. Ten-Gram-Scale Synthesis of FAPbX3 Perovskite Nanocrystals by a High-Power Room-Temperature Ultrasonic-Assisted Strategy and Their Electroluminescence. Adv. Mater. Technol. 2020, 5, 1901089. [Google Scholar] [CrossRef]
- Levchuk, I.; Osvet, A.; Tang, X.; Brandl, M.; Perea, J.D.; Hoegl, F.; Matt, G.J.; Hock, R.; Batentschuk, M.; Brabec, C.J. Brightly Luminescent and Color-Tunable Formamidinium Lead Halide Perovskite FAPbX3 (X = Cl, Br, I) Colloidal Nanocrystals. Nano Lett. 2017, 17, 2765–2770. [Google Scholar] [CrossRef]
- Imran, M.; Caligiuri, V.; Wang, M.; Goldoni, L.; Prato, M.; Krahne, R.; De Trizio, L.; Manna, L. Benzoyl Halides as Alternative Precursors for the Colloidal Synthesis of Lead-Based Halide Perovskite Nanocrystals. J. Am. Chem. Soc. 2018, 140, 2656–2664. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Li, H.; Yang, P.; Wei, J.; Wang, F.; Shen, H.; Li, D.; Li, F. Room-temperature synthesized formamidinium lead halide perovskite quantum dots with bright luminescence and color-tunability for efficient light emitting. Org. Electron. 2019, 68, 76–84. [Google Scholar] [CrossRef]
- Forster, T.H. Transfer Mechanisms of Electronic Excitation. Discuss. Faraday Soc. 1959, 27, 7–17. [Google Scholar] [CrossRef]
- Singldinger, A.; Gramlich, M.; Gruber, C.; Lampe, C.; Urban, A.S. Nonradiative Energy Transfer between Thickness-Controlled Halide Perovskite Nanoplatelets. ACS Energy Lett. 2020, 5, 1380–1385. [Google Scholar] [CrossRef]
- Ida, T.; Ando, M.; Toraya, H. Extended pseudo-Voigt function for approximating the Voigt profile. J. Appl. Crystallogr. 2000, 33, 1311–1316. [Google Scholar] [CrossRef]
- Prahl, S. MiePython v1.3.1. 2020. Available online: https://github.com/scottprahl/miepython (accessed on 26 May 2022).
- Ndione, P.F.; Li, Z.; Zhu, K. Effects of alloying on the optical properties of organic–inorganic lead halide perovskite thin films. J. Mater. Chem. C 2016, 4, 7775–7782. [Google Scholar] [CrossRef]
- Protesescu, L.; Yakunin, S.; Bodnarchuk, M.I.; Bertolotti, F.; Masciocchi, N.; Guagliardi, A.; Kovalenko, M.V. Monodisperse Formamidinium Lead Bromide Nanocrystals with Bright and Stable Green Photoluminescence. J. Am. Chem. Soc. 2016, 43, 14202–14205. [Google Scholar] [CrossRef] [Green Version]
- Fan, L.; Ding, K.; Chen, H.; Xiang, S.; Zhang, R.; Guo, R.; Liu, Z.; Wang, L. Efficient pure green light-emitting diodes based on formamidinium lead bromide perovskite nanocrystals. Org. Electron. 2018, 60, 64–70. [Google Scholar] [CrossRef]
- Kim, Y.H.; Lee, G.H.; Kim, Y.T.; Wolf, C.; Yun, H.J.; Kwon, W.; Park, C.G.; Lee, T.-W. High efficiency perovskite light-emitting diodes of ligand-engineered colloidal formamidinium lead bromide nanoparticles. Nano Energy 2017, 38, 51–58. [Google Scholar] [CrossRef]
- Minh, D.N.; Kim, J.; Hyon, J.; Sim, J.H.; Sowlih, H.H.; Seo, C.; Nam, J.; Eom, S.; Suk, S.; Lee, S.; et al. Room-Temperature Synthesis of Widely Tunable Formamidinium Lead Halide Perovskite Nanocrystals. Chem. Mater. 2017, 29, 5713–5719. [Google Scholar] [CrossRef]
- Aygüler, M.F.; Weber, M.D.; Puscher, B.M.D.; Medina, D.D.; Docampo, P.; Costa, R.D. Light-emitting electrochemical cells based on hybrid lead halide perovskite nanoparticles. J. Phys. Chem. C 2015, 119, 12047. [Google Scholar] [CrossRef] [Green Version]
- Jana, A.; Mittal, M.; Singla, A.; Sapra, S. Solvent-free, mechanochemical syntheses of bulk trihalide perovskites and their nanoparticles. Chem. Commun. 2017, 53, 3046–3049. [Google Scholar] [CrossRef]
- Eperon, G.; Stranks, S.D.; Menelaou, C.; Johnston, M.B.; Herz, L.M.; Snaith, H.J. Formamidinium lead trihalide: A broadly tunable perovskite for efficient planar heterojunction solar cells. Energy Environ. Sci. 2014, 7, 982–988. [Google Scholar] [CrossRef]
| : Component from EJ-290 | : Component from FAPbBr | : Component from FAPbBr | ||
|---|---|---|---|---|
| EJ-290 | 1.2 ns (100%) | - | - | (1.2 ± 0.1) ns |
| FAPbBr/EJ-290, 0.4% Loading | 1.5 ns (44%) | 17.5 ns (36%) | 90.3 ns (20%) | (25.0 ± 0.1) ns |
| FAPbBr/EJ-290, 0.9% Loading | 1.8 ns (38%) | 15.6 ns (43%) | 75.1 ns (19%) | (21.8 ± 0.1) ns |
| FAPbBr/EJ-290, 1.8% Loading | 1.9 ns (36%) | 14.9 ns (46%) | 71.0 ns (18%) | (20.3 ± 0.1) ns |
| FAPbBr/PMMA, 1.8% Loading | - | 16.9 ns (56%) | 119.7 ns (44%) | (62.0 ± 0.4) ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Braddock, I.H.B.; Al Sid Cheikh, M.; Ghosh, J.; Mulholland, R.E.; O’Neill, J.G.; Stolojan, V.; Crean, C.; Sweeney, S.J.; Sellin, P.J. Formamidinium Lead Halide Perovskite Nanocomposite Scintillators. Nanomaterials 2022, 12, 2141. https://doi.org/10.3390/nano12132141
Braddock IHB, Al Sid Cheikh M, Ghosh J, Mulholland RE, O’Neill JG, Stolojan V, Crean C, Sweeney SJ, Sellin PJ. Formamidinium Lead Halide Perovskite Nanocomposite Scintillators. Nanomaterials. 2022; 12(13):2141. https://doi.org/10.3390/nano12132141
Chicago/Turabian StyleBraddock, Isabel H. B., Maya Al Sid Cheikh, Joydip Ghosh, Roma E. Mulholland, Joseph G. O’Neill, Vlad Stolojan, Carol Crean, Stephen J. Sweeney, and Paul J. Sellin. 2022. "Formamidinium Lead Halide Perovskite Nanocomposite Scintillators" Nanomaterials 12, no. 13: 2141. https://doi.org/10.3390/nano12132141
APA StyleBraddock, I. H. B., Al Sid Cheikh, M., Ghosh, J., Mulholland, R. E., O’Neill, J. G., Stolojan, V., Crean, C., Sweeney, S. J., & Sellin, P. J. (2022). Formamidinium Lead Halide Perovskite Nanocomposite Scintillators. Nanomaterials, 12(13), 2141. https://doi.org/10.3390/nano12132141







