Effect of Li+ Doping on Photoelectric Properties of Double Perovskite Cs2SnI6: First Principles Calculation and Experimental Investigation
Abstract
:1. Introduction
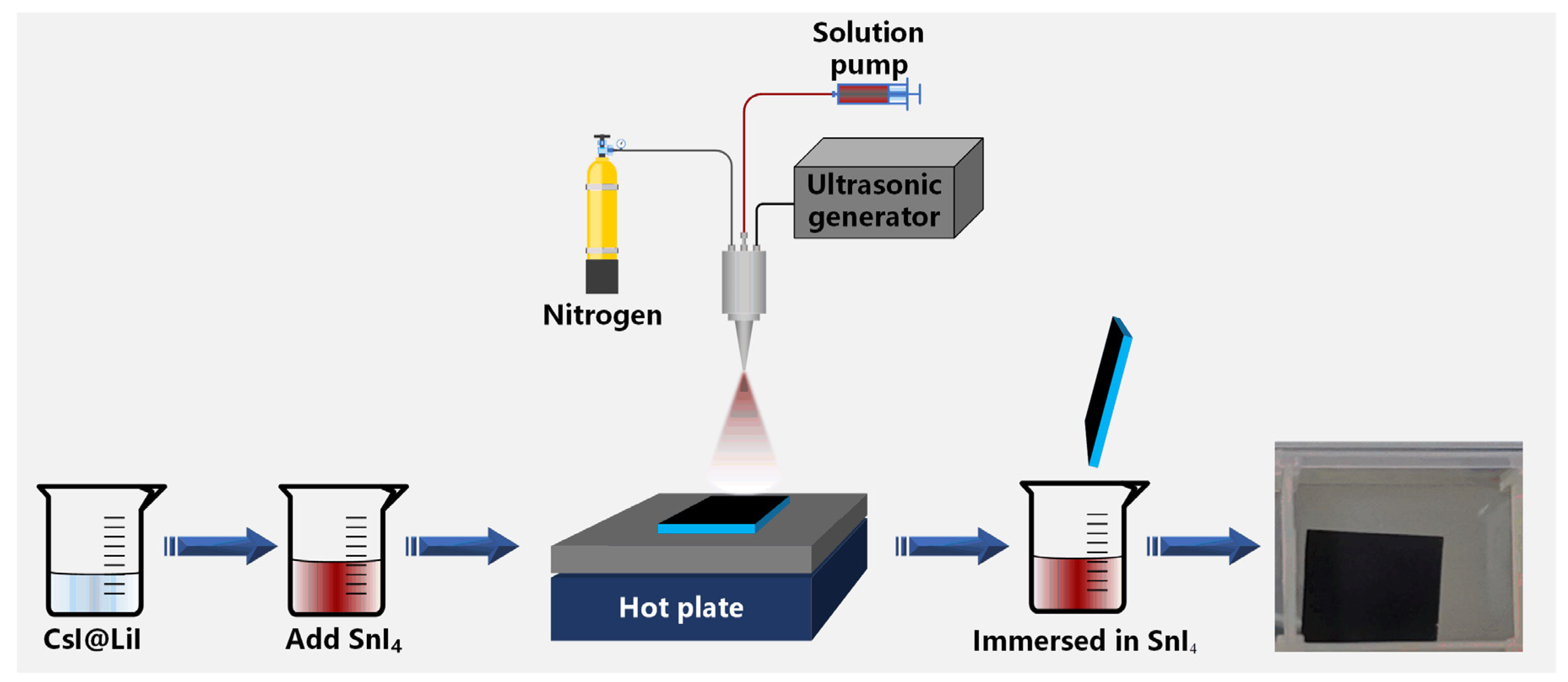
2. Materials and Methods
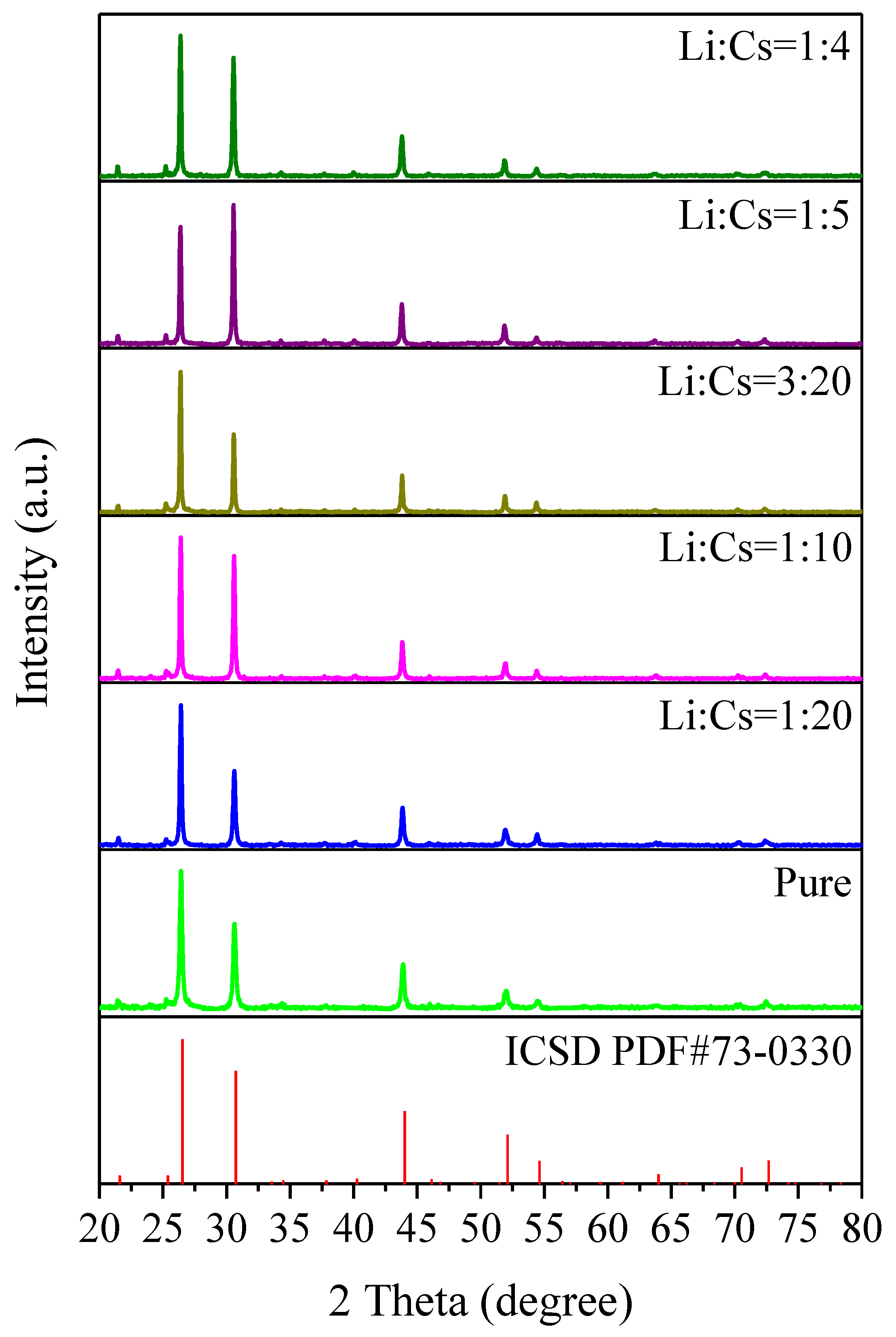
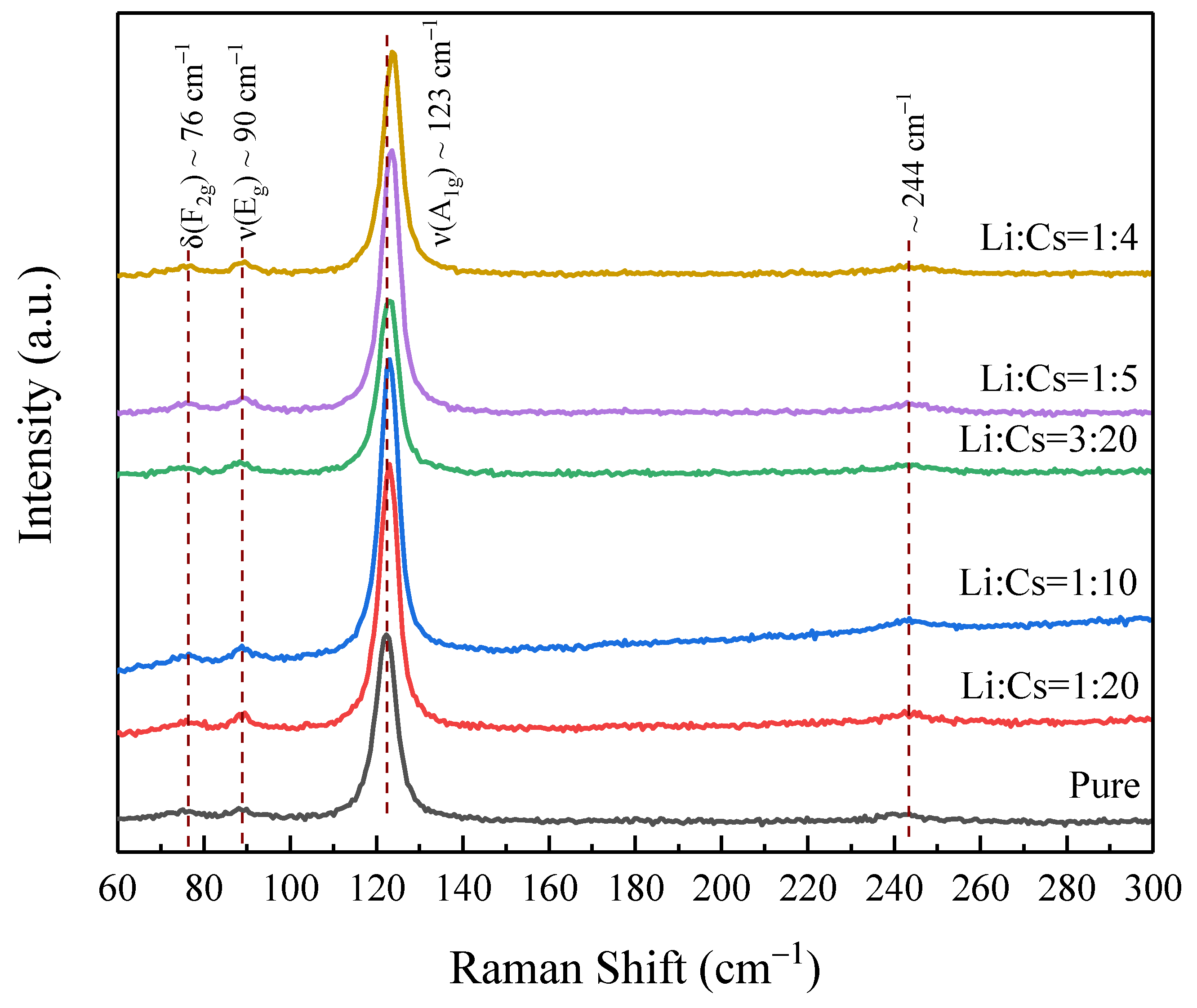
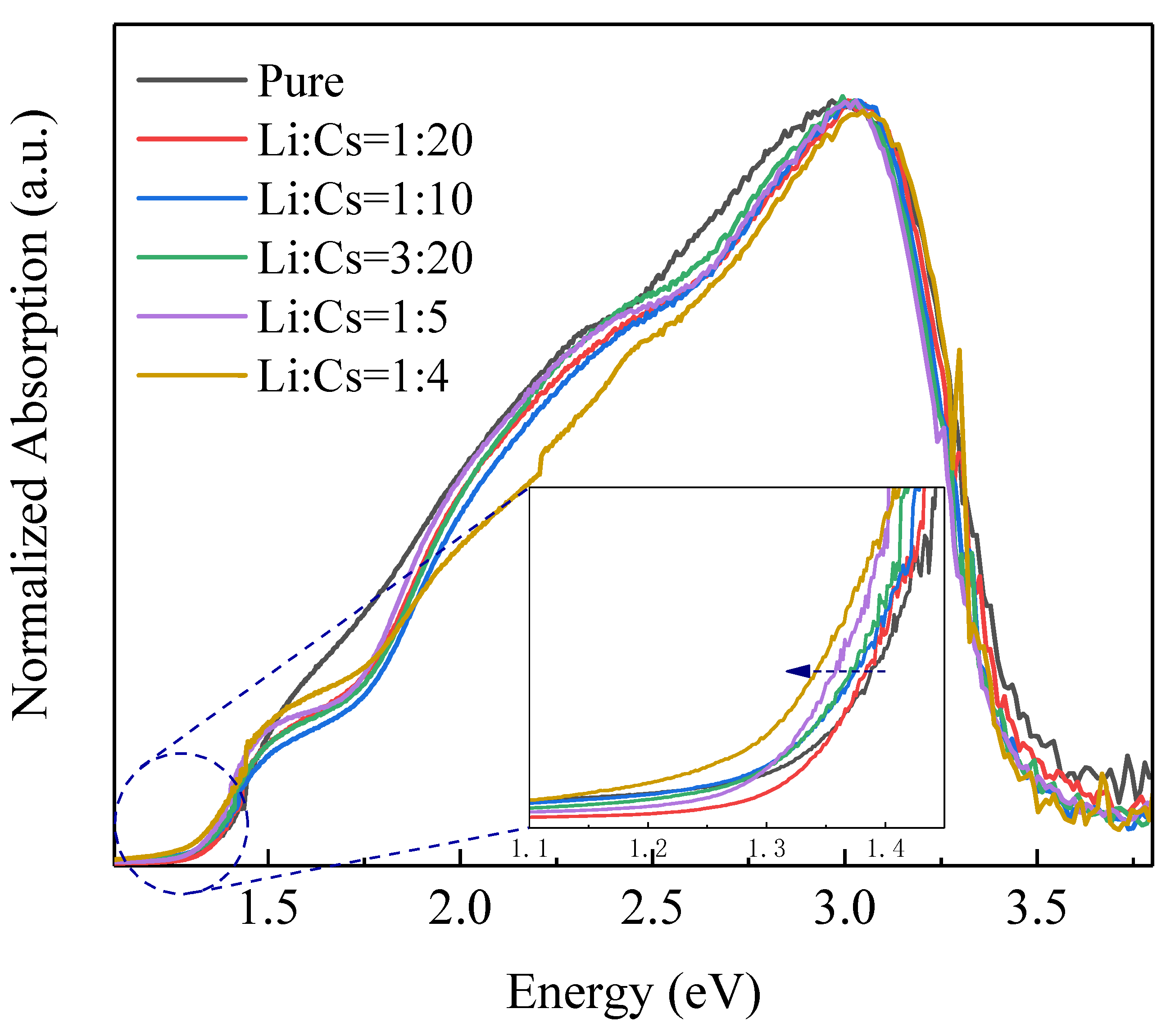
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Grätzel, M. The light and shade of perovskite solar cells. Nat. Mater. 2014, 13, 838–842. [Google Scholar] [CrossRef] [PubMed]
- Jena, A.K.; Kulkarni, A.; Miyasaka, T. Halide perovskite photovoltaics: Background, status, and future prospects. Chem. Rev. 2019, 119, 3036–3103. [Google Scholar] [CrossRef]
- Snaith, H.J. Perovskites: The emergence of a new era for low–cost, high–efficiency solar cells. J. Phys. Chem. Lett. 2013, 4, 3623–3630. [Google Scholar] [CrossRef]
- Kazim, S.; Nazeeruddin, M.K.; Grätzel, M.; Ahmad, S. Perovskite as light harvester: A game changer in photovoltaics. Angew. Chem. Int. Ed. 2014, 53, 2812–2824. [Google Scholar] [CrossRef] [PubMed]
- Panigrahi, S.; Jana, S.; Calmeiro, T.; Nunes, D.; Deuermeier, J.; Martins, R.; Fortunato, E. Mapping the space charge carrier dynamics in plasmon-based perovskite solar cells. J. Mater. Chem. A 2019, 7, 19811–19819. [Google Scholar] [CrossRef]
- Liang, P.-W.; Chueh, C.-C.; Xin, X.-K.; Zuo, F.; Williams, S.T.; Liao, C.-Y.; Jen, A.K.-Y. High-performance planar-heterojunction solar cells based on ternary halide large-band-gap perovskites. Adv. Energy Mater. 2015, 5, 1400960. [Google Scholar] [CrossRef]
- Li, M.; Wang, Z.-K.; Zhou, M.-P.; Hu, Y.; Hu, K.-H.; Ye, Q.-Q.; Jain, S.M.; Yang., Y.-G.; Gao, X.-Y.; Liao, L.-S. Pb–Sn–Cu ternary organometallic halide perovskite solar cells. Adv. Mater. 2018, 30, 1800258. [Google Scholar] [CrossRef]
- Jiang, J.; Wang, Q.; Jin, Z.; Zhang, X.; Lei, J.; Bin, H.; Zhang, Z.-G.; Li, Y.; Liu, S. Polymer doping for high-efficiency perovskite solar cells with improved moisture stability. Adv. Energy Mater. 2018, 8, 1701757. [Google Scholar] [CrossRef]
- Atwater, H.A.; Polman, A. Plasmonics for improved photovoltaic devices. Nat. Mater. 2010, 9, 205–213. [Google Scholar] [CrossRef]
- NREL. Best Research—Cell Efficiencies. Available online: https://www.nrel.gov/pv/cell–efficiency.html (accessed on 20 May 2022).
- Tian, J.; Xue, Q.; Tang, X.; Chen, Y.; Li, N.; Hu, Z.; Shi, T.; Wang, X.; Huang, F.; Brabec, C.J.; et al. Dual interfacial design for efficient CsPbI2Br perovskite solar cells with improved photostability. Adv. Mater. 2019, 31, 1901152. [Google Scholar] [CrossRef]
- Saliba, M.; Matsui, T.; Seo, J.-Y.; Domanski, K.; Correa–Baena, J.-P.; Nazeeruddin, M.K.; Zakeeruddin, S.M.; Tress, W.; Abate, A.; Hagfeldtd, A.; et al. Cesium–containing triple cation perovskite solar cells: Improved stability, reproducibility and high efficiency. Energy Environ. Sci. 2016, 9, 1989–1997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Correa-Baena, J.-P.; Saliba, M.; Buonassisi, T.; Grätzel, M.; Abate, A.; Tress, W.; Hagfeldt, A. Promises and challenges of perovskite solar cells. Science 2017, 358, 739–744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, I.; Lee, B.; He, J.; Chang, R.P.H.; Kanatzidis, M.G. All–solid–state dye–sensitized solar cells with high efficiency. Nature 2012, 485, 486–489. [Google Scholar] [CrossRef] [PubMed]
- Marshall, K.P.; Walker, M.; Walton, R.I.; Hatton, R.A. Enhanced stability and efficiency in hole-transport-layer-free CsSnI3 perovskite photovoltaics. Nat. Energy 2016, 1, 16178. [Google Scholar] [CrossRef] [Green Version]
- Lee, B.; Stoumpos, C.C.; Zhou, N.; Hao, F.; Malliakas, C.; Yeh, C.-Y.; Marks, T.J.; Kanatzidis, M.G.; Chang, R.P.H. Air-stable molecular semiconducting iodosalts for solar cell applications: Cs2SnI6 as a hole conductor. J. Am. Chem. Soc. 2014, 136, 15379–15385. [Google Scholar] [CrossRef]
- Shin, H.; Kim, B.-M.; Jang, T.; Kim, K.M.; Roh, D.-H.; Nam, J.S.; Kim, J.S.; Kim, U.-Y.; Lee, B.; Pang, Y.; et al. Surface state–mediated charge transfer of Cs2SnI6 and its application in dye–sensitized solar cells. Adv. Energy Mater. 2019, 9, 1803243. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, C.; Wang, L.; Li, Y.; Ren, Y.; Shum, K. Energy barrier at the N719–dye/CsSnI3 interface for photogenerated holes in dye–sensitized solar cells. Sci. Rep. 2014, 4, 6954. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, C.; Liao, Y.; Li, S.; Yang, P.; Xi, Y.; Cai, C.; Liu, W. Investigating optical adsorption properties of lead–free double perovskite semiconductors Cs2SnI6–xBrx (x = 0–6) via first principles calculation. Microw. Opt. Technol. Lett. 2021, 1–7. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, J.; Liao, Y.; Pan, Z.; Rao, H.; Zhong, X. Cs2SnI6 nanocrystals enhancing hole extraction for efficient carbon—Based CsPbI2Br perovskite solar cells. Chem. Eng. J. 2022, 440, 135710. [Google Scholar] [CrossRef]
- Lien, T.T.D.; Dai, N.T.; Thanh, N.T.; Van Phuc, P.; Oanh, N.T.T.; Long, P.D.; Hoi, P.V.; Chi, L.H. Tin fluoride assisted growth of air stable perovskite derivative Cs2SnI6 thin film as a hole transport layer. Mater. Res. Express 2019, 6, 116442. [Google Scholar] [CrossRef]
- Saparov, B.; Sun, J.-P.; Meng, W.; Xiao, Z.; Duan, H.-S.; Gunawan, O.; Shin, D.; Hill, I.G.; Yan, Y.; Mitzi, D.B. Thin-film deposition and characterization of a Sn–deficient perovskite derivative Cs2SnI6. Chem. Mater. 2016, 28, 2315–2322. [Google Scholar] [CrossRef]
- Zhang, J.; Li, S.; Yang, P.; Liu, W.; Liao, Y. Enhanced stability of lead-free perovskite heterojunction for photovoltaic applications. J. Mater. Sci. 2018, 53, 378–4386. [Google Scholar] [CrossRef]
- Kapil, G.; Ohta, T.; Koyanagi, T.; Vigneshwaran, M.; Zhang, Y.; Ogomi, Y.; Pandey, S.S.; Yoshino, K.; Shen, Q.; Toyoda, T.; et al. Investigation of interfacial charge transfer in solution processed Cs2SnI6 thin films. J. Phys. Chem. C 2017, 121, 13092–13100. [Google Scholar] [CrossRef]
- Lee, B.; Ezhumalai, Y.; Lee, W.; Chen, M.-C.; Yeh, C.-Y.; Marks, T.J.; Chang, R.P.H. Cs2SnI6—Encapsulated multidye-sensitized all-solid-state solar cells. ACS Appl. Mater. Interfaces 2019, 11, 21424–21434. [Google Scholar] [CrossRef]
- Kaltzoglou, A.; Antoniadou, M.; Kontos, A.G.; Stoumpos, C.C.; Perganti, D.; Siranidi, E.; Raptis, V.; Trohidou, K.; Psycharis, V.; Kanatzidis, M.G.; et al. Optical-vibrational properties of the Cs2SnX6 (X = Cl, Br, I) defect perovskites and hole-transport efficiency in dye-sensitized solar cells. J. Phys. Chem. C 2016, 120, 11777–11785. [Google Scholar] [CrossRef]
- Xiao, Z.; Zhou, Y.; Hosono, H.; Kamiya, T. Undoped defects in photovoltaic perovskite variant Cs2SnI6. Phys. Chem. Chem. Phys. 2015, 17, 18900–18903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konstantakoua, M.; Stergiopoulos, T. A critical review on tin halide perovskite solar cells. J. Mater. Chem. A 2017, 5, 11518–11549. [Google Scholar] [CrossRef]
- Yuan, G.; Huang, S.; Qin, S.; Wu, X.; Ding, H.; Lu, A. Structural, optical, and thermal properties of Cs2SnI6–xBrx mixed perovskite solid solutions. Eur. J. Inorg. Chem. 2019, 2019, 2524–2529. [Google Scholar] [CrossRef]





| Doping Position | Energy Difference |
|---|---|
| Substitution site | 0.0416 eV/atom |
| Interstitial site 1 | −0.01898 eV/atom |
| Interstitial site 2 | −0.01813 eV/atom |
| Li+:Cs+ | Conductivity Type | Carrier Concentration (1/cm3) | Hall Mobility (cm2/Vs) |
|---|---|---|---|
| 1:20 | p | 9.392 × 1013 | 89.51 |
| 1:10 | p | 1.996 × 1014 | 91.89 |
| 3:20 | p | 2.071 × 1014 | 104.5 |
| 1:5 | p | 2.5 × 1014 | 110.45 |
| 1:4 | p | 3.18 × 1014 | 356.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Yang, C.; Liao, Y.; Li, S.; Yang, P.; Xi, Y.; Liu, W.; Golosov, D.A.; Zavadski, S.M.; Melnikov, S.N. Effect of Li+ Doping on Photoelectric Properties of Double Perovskite Cs2SnI6: First Principles Calculation and Experimental Investigation. Nanomaterials 2022, 12, 2279. https://doi.org/10.3390/nano12132279
Zhang J, Yang C, Liao Y, Li S, Yang P, Xi Y, Liu W, Golosov DA, Zavadski SM, Melnikov SN. Effect of Li+ Doping on Photoelectric Properties of Double Perovskite Cs2SnI6: First Principles Calculation and Experimental Investigation. Nanomaterials. 2022; 12(13):2279. https://doi.org/10.3390/nano12132279
Chicago/Turabian StyleZhang, Jin, Chen Yang, Yulong Liao, Shijie Li, Pengfei Yang, Yingxue Xi, Weiguo Liu, Dmitriy A. Golosov, Sergey M. Zavadski, and Sergei N. Melnikov. 2022. "Effect of Li+ Doping on Photoelectric Properties of Double Perovskite Cs2SnI6: First Principles Calculation and Experimental Investigation" Nanomaterials 12, no. 13: 2279. https://doi.org/10.3390/nano12132279







