Microstructure and Electrical Conductivity of Electrospun Titanium Oxynitride Carbon Composite Nanofibers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of TiON/C-CNF Fabrics
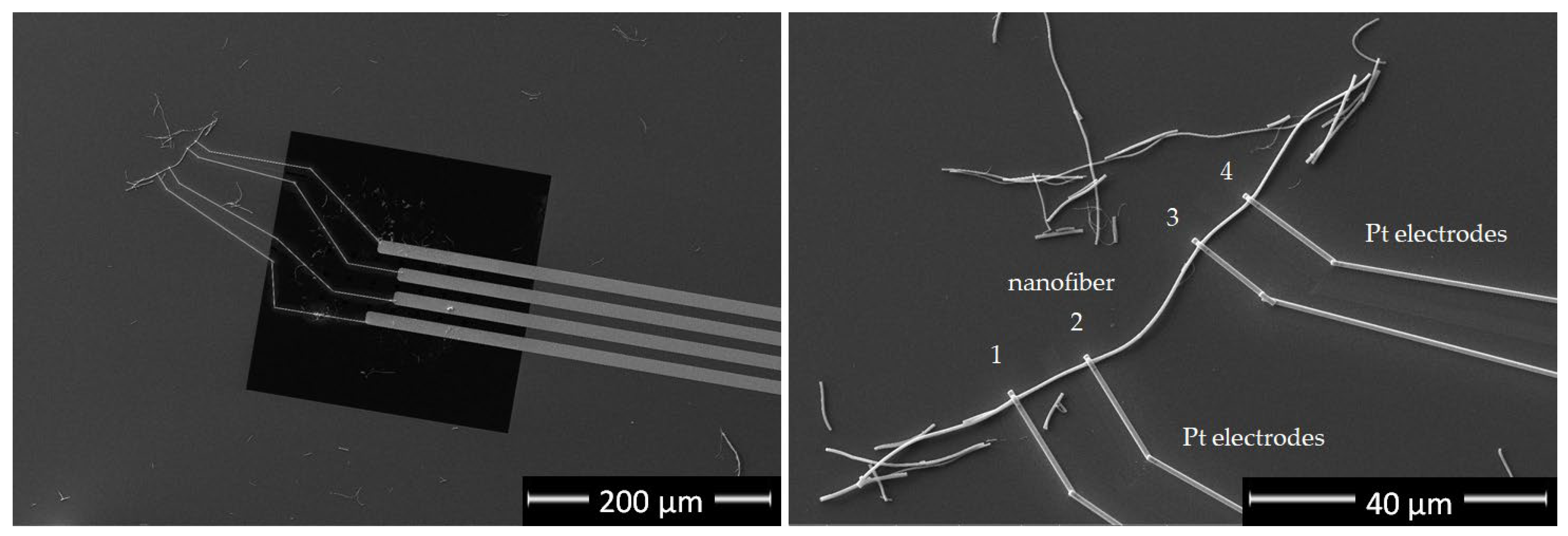
2.2. Preparation of Single TiON/C-CNF2 Samples for Electrical Measurements
2.3. Electrical Measurements of TiON/C-CNF1 Fabric
2.4. Electrochemical Measurements of TiON/C-CNF1 Fabric
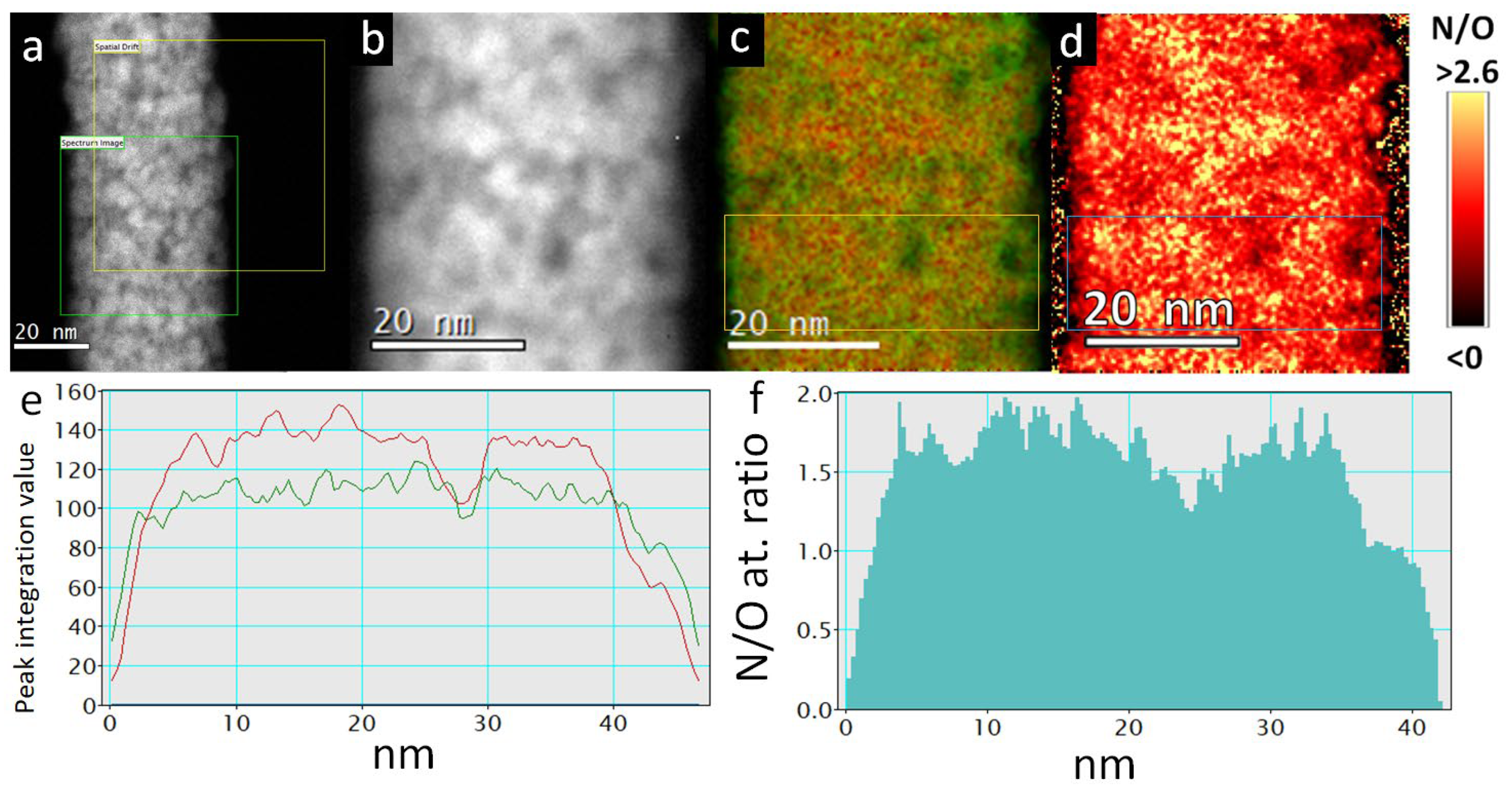
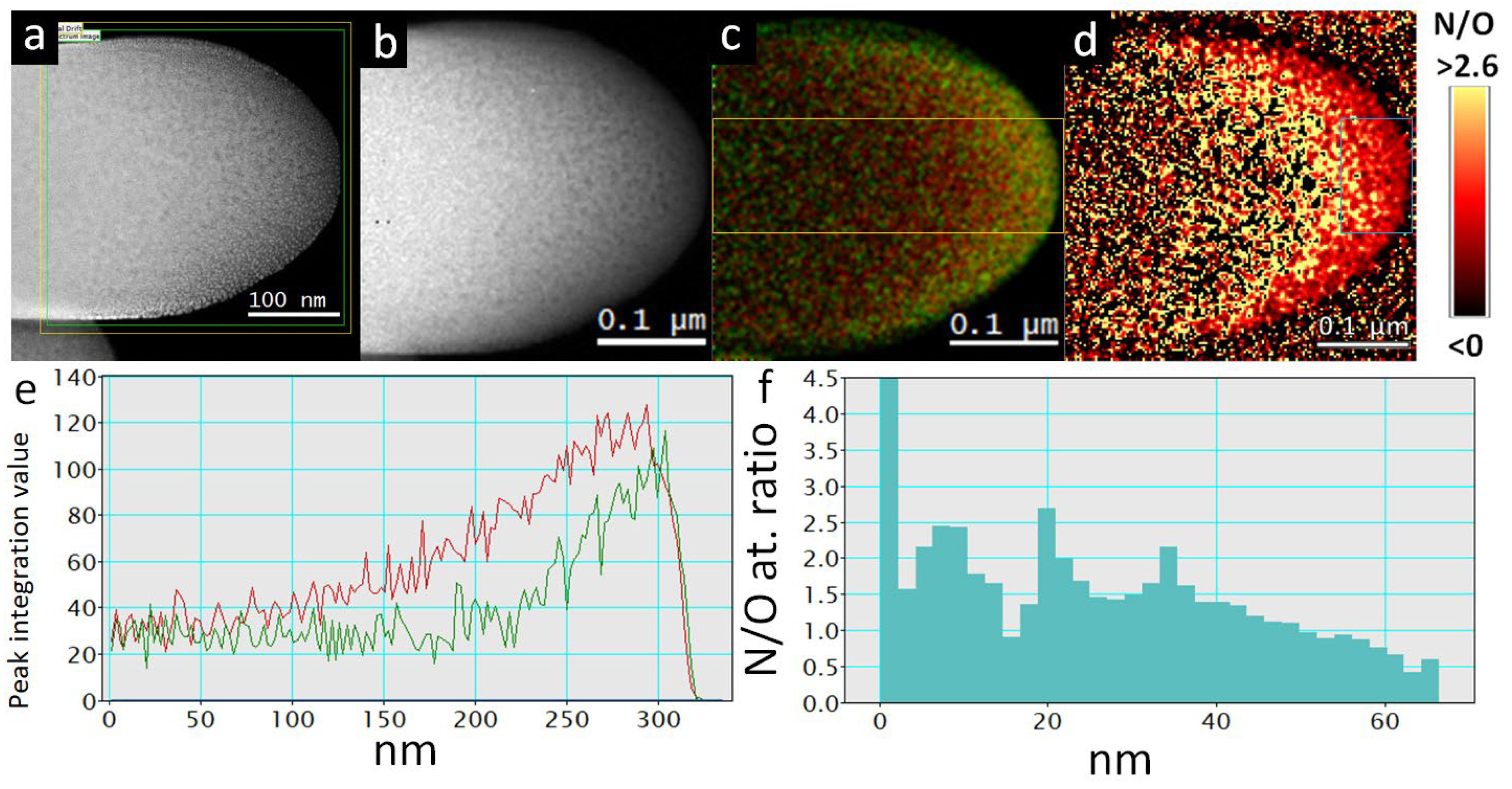
2.5. STEM, EELS and STEM EDXS Analysis
2.6. Sample Preparation of TiON/C-CNF2s for STEM, EELS and STEM EDXS Analysis
2.7. Raman Spectroscopy
2.8. SEM and SEM EDXS Analysis
2.9. XRD
2.10. TGA
2.11. CHNS Analysis
2.12. Programs Used
3. Results and Discussion
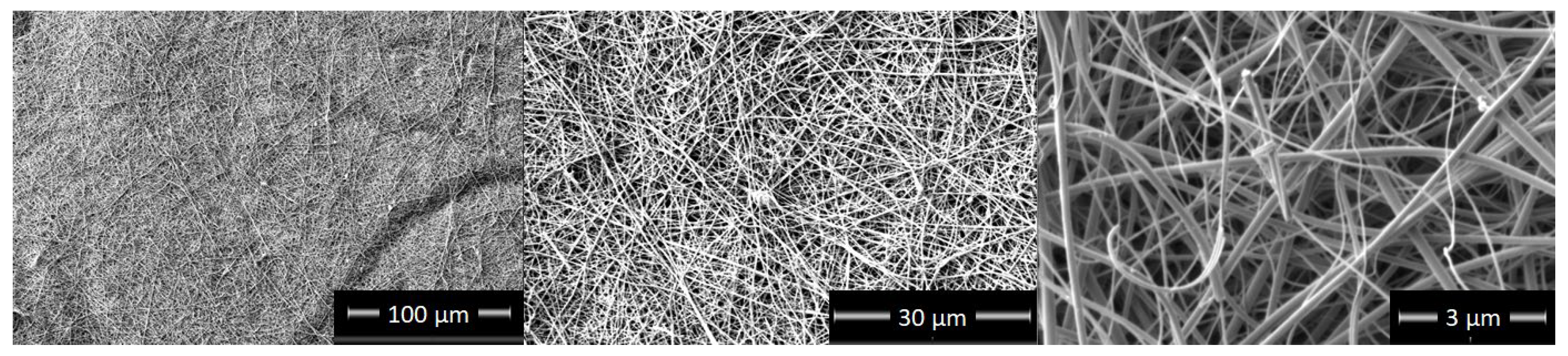
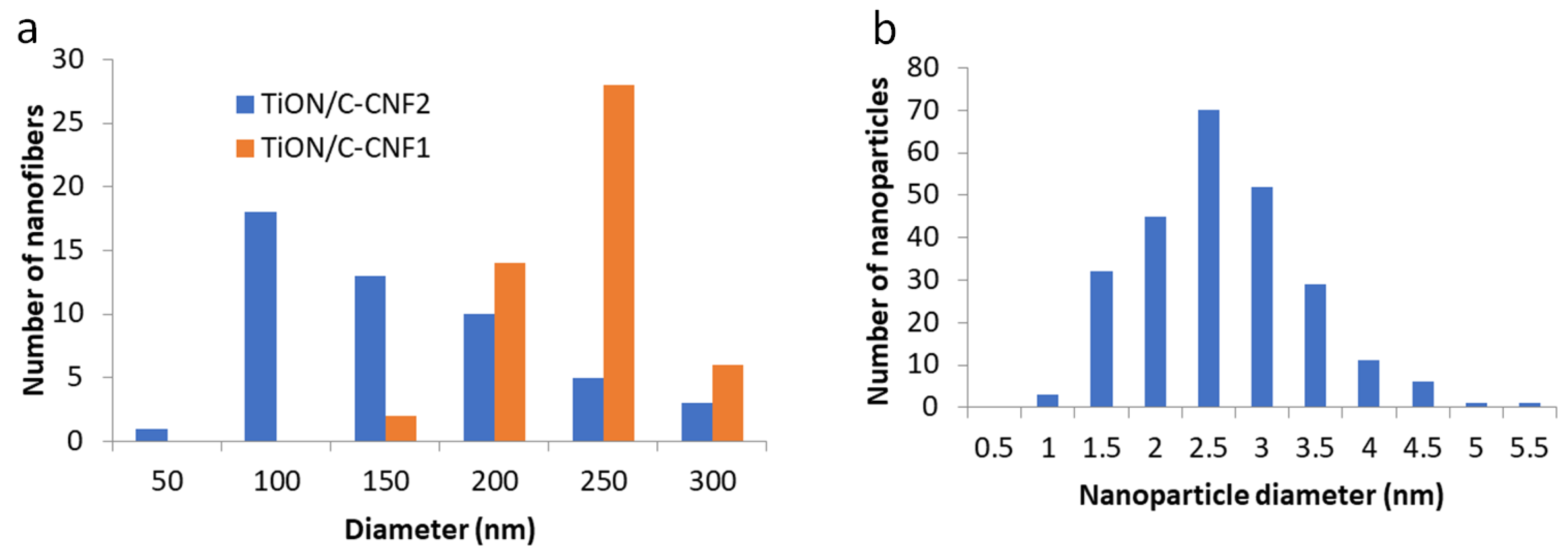
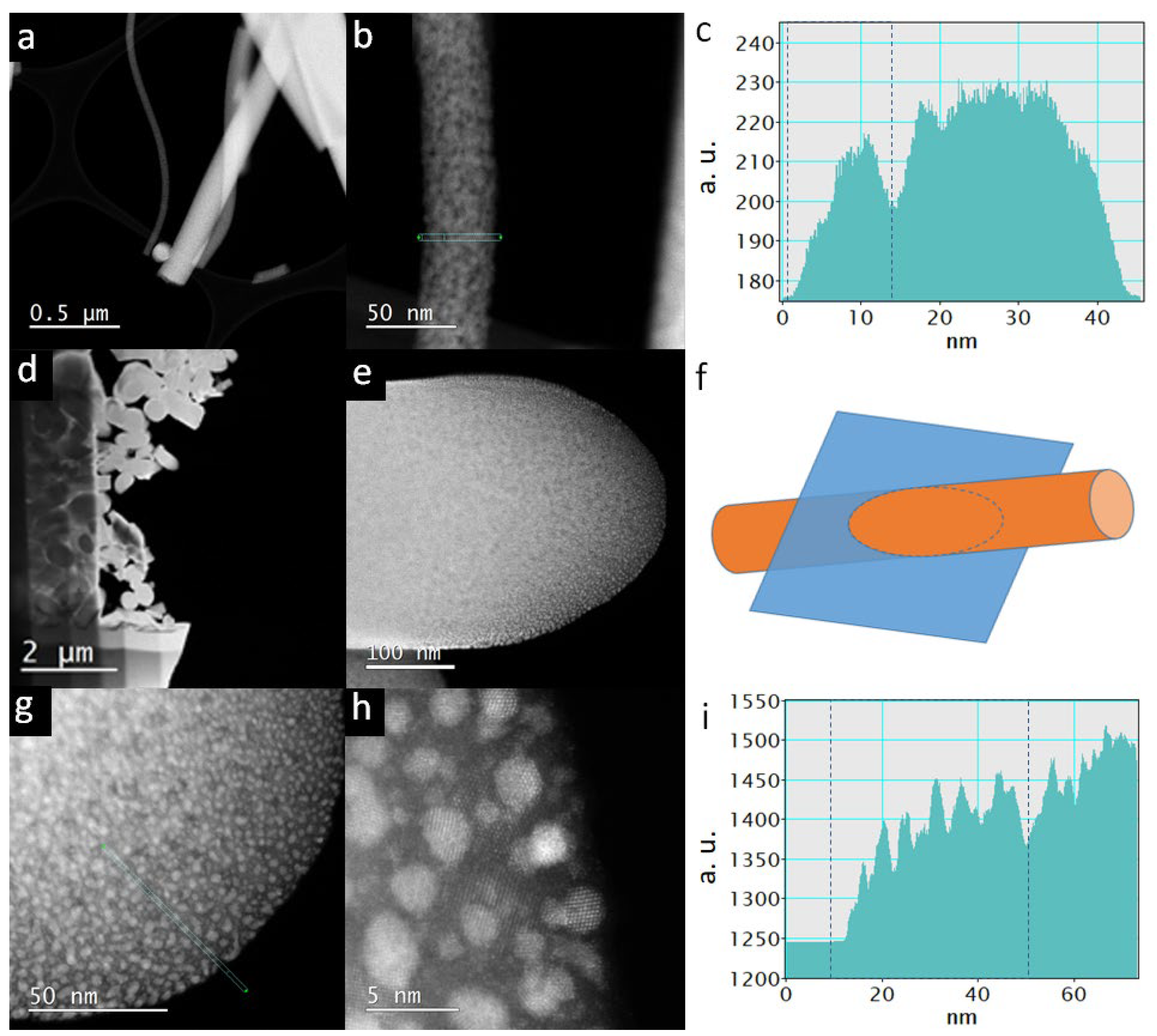
3.1. Micro- and Nanostructure of TiON/C-CNFs
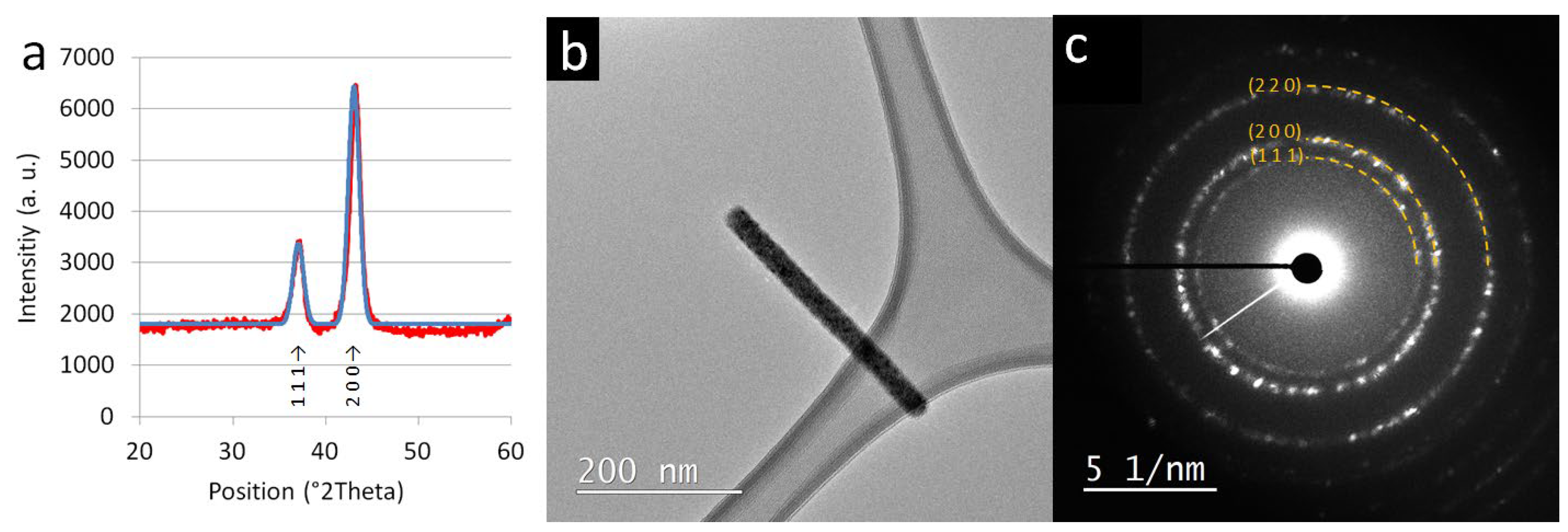
3.2. Chemical Composition and Crystal Structure of TiON/C-CNFs
3.3. Electrical Conductivity and Electrochemical Measurements
3.4. Estimation of Electrical Conductivity of TiON/C-CNF2s
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Enríquez, E.; Mariscal, A.; Serna, R.; Fernández, J.F. Transparent High Conductive Titanium Oxynitride Nanofilms Obtained by Nucleation Control for Sustainable Optolectronics. Appl. Surf. Sci. 2022, 574, 151631. [Google Scholar] [CrossRef]
- Reddy Mucha, N.; Som, J.; Choi, J.; Shaji, S.; Gupta, R.K.; Meyer, H.M.; Cramer, C.L.; Elliott, A.M.; Kumar, D.; Mucha, N.R.; et al. High-Performance Titanium Oxynitride Thin Films for Electrocatalytic Water Oxidation. ACS Appl. Energy Mater. 2020, 3, 8366–8374. [Google Scholar] [CrossRef]
- Chin, T.K.; Liao, M.W.; Perng, T.P. Enabling Higher Electrochemical Activity of Pt Nanoparticles Uniformly Coated on Cubic Titanium Oxynitride by Vertical Forced-Flow Atomic Layer Deposition. J. Power Sources 2019, 434, 226716. [Google Scholar] [CrossRef]
- Lee, E.J.; Lee, L.; Abbas, M.A.; Bang, J.H. The Influence of Surface Area, Porous Structure, and Surface State on the Supercapacitor Performance of Titanium Oxynitride: Implications for a Nanostructuring Strategy. Phys. Chem. Chem. Phys. 2017, 19, 21140–21151. [Google Scholar] [CrossRef]
- Shashikant Samdani, J.; Kang, T.-H.; Lee, B.-J.; Hee Jang, Y.; Yu, J.-S.; Shanmugam, S. Heterostructured Titanium Oxynitride-Manganese Cobalt Oxide Nanorods as High-Performance Electrode Materials for Supercapacitor Devices. ACS Appl. Mater. Interfaces 2020, 12, 54524–54536. [Google Scholar] [CrossRef]
- Han, J.H.; Bang, J.H. A Hollow Titanium Oxynitride Nanorod Array as an Electrode Substrate Prepared by the Hot Ammonia-Induced Kirkendall Effect. J. Mater. Chem. A 2014, 2, 10568–10576. [Google Scholar] [CrossRef]
- Chen, T.T.; Liu, H.P.; Wei, Y.J.; Chang, I.C.; Yang, M.H.; Lin, Y.S.; Chan, K.L.; Chiu, H.T.; Lee, C.Y. Porous Titanium Oxynitride Sheets as Electrochemical Electrodes for Energy Storage. Nanoscale 2014, 6, 5106–5109. [Google Scholar] [CrossRef]
- Dong, J.; Jiang, Y.; Li, Q.; Wei, Q.; Yang, W.; Tan, S.; Xu, X.; An, Q.; Mai, L. Pseudocapacitive Titanium Oxynitride Mesoporous Nanowires with Iso-Oriented Nanocrystals for Ultrahigh-Rate Sodium Ion Hybrid Capacitors. J. Mater. Chem. A 2017, 5, 10827–10835. [Google Scholar] [CrossRef]
- Noh, Y.; Kim, Y.; Han, H.; Park, S.; Yoon, W.; Lee, S.; Kim, J.G.; Kim, Y.; Kim, H.J.; Kim, W.B. Heat Treatment–Controlled Morphology Modification of Electrospun Titanium Oxynitride Nanowires for Capacitive Energy Storage and Electrocatalytic Reactions. Energy Technol. 2020, 8, 2000184. [Google Scholar] [CrossRef]
- Niu, W.; Guan, Y.; Luo, Y.; Liu, G.; Li, J. A Macroporous Titanium Oxynitride-Supported Bifunctional Oxygen Electrocatalyst for Zinc–Air Batteries. Catal. Sci. Technol. 2021. [Google Scholar] [CrossRef]
- Do, H.; Wu, Y.H.; Dai, V.T.; Peng, C.Y.; Yen, T.C.; Chang, L. Structure and Property of Epitaxial Titanium Oxynitride Grown on MgO(001) Substrate by Pulsed Laser Deposition. Surf. Coatings Technol. 2013, 214, 91–96. [Google Scholar] [CrossRef]
- Bele, M.; Stojanovski, K.; Jovanovič, P.; Moriau, L.; Podboršek, G.K.; Moškon, J.; Umek, P.; Sluban, M.; Dražič, G.; Hodnik, N.; et al. Towards Stable and Conductive Titanium Oxynitride High-Surface-Area Support for Iridium Nanoparticles as Oxygen Evolution Reaction Electrocatalyst. ChemCatChem 2019, 11, 5038–5044. [Google Scholar] [CrossRef]
- Moriau, L.; Bele, M.; Marinko, Ž.; Ruiz-Zepeda, F.; Koderman Podboršek, G.; Šala, M.; Šurca, A.K.; Kovač, J.; Arčon, I.; Jovanovič, P.; et al. Effect of the Morphology of the High-Surface-Area Support on the Performance of the Oxygen-Evolution Reaction for Iridium Nanoparticles. ACS Catal. 2021, 11, 670–681. [Google Scholar] [CrossRef]
- Bele, M.; Jovanovič, P.; Marinko, Ž.; Drev, S.; Šelih, V.S.; Kovač, J.; Gaberšček, M.; Koderman Podboršek, G.; Dražić, G.; Hodnik, N.; et al. Increasing the Oxygen-Evolution Reaction Performance of Nanotubular Titanium Oxynitride-Supported Ir Nanoparticles by a Strong Metal-Support Interaction. ACS Catal. 2020, 10, 13688–13700. [Google Scholar] [CrossRef]
- Thavasi, V.; Singh, G.; Ramakrishna, S. Electrospun Nanofibers in Energy and Environmental Applications. Energy Environ. Sci. 2008, 1, 205–221. [Google Scholar] [CrossRef]
- Jiang, H.; Yang, L.; Li, C.; Yan, C.; Lee, P.S.; Ma, J. High–Rate Electrochemical Capacitors from Highly Graphitic Carbon–Tipped Manganese Oxide/Mesoporous Carbon/Manganese Oxide Hybrid Nanowires. Energy Environ. Sci. 2011, 4, 1813–1819. [Google Scholar] [CrossRef]
- Ghosh, S.; Jeong, S.M.; Polaki, S.R. A Review on Metal Nitrides/Oxynitrides as an Emerging Supercapacitor Electrode beyond Oxide. Korean J. Chem. Eng. 2018, 35, 1389–1408. [Google Scholar] [CrossRef]
- Long, Y.; Duvail, J.; Li, M.; Gu, C.; Liu, Z.; Ringer, S.P. Electrical Conductivity Studies on Individual Conjugated Polymer Nanowires: Two-Probe and Four-Probe Results. Nanoscale Res. Lett. 2010, 5, 237–242. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.L.; Kim, C.; Kim, I.D. Ultrasmall Titanium Oxide/Titanium Oxynitride Composite Nanoparticle-Embedded Carbon Nanofiber Mats as High-Capacity and Free-Standing Electrodes for Lithium Sulfur Batteries. RSC Adv. 2017, 7, 44804–44808. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.G.; Shi, D.; Kong, K.J.; Heo, Y.U.; Kim, J.H.; Jo, M.R.; Lee, Y.C.; Kang, Y.M.; Dou, S.X. Structurally and Electronically Designed TiO2Nx Nanofibers for Lithium Rechargeable Batteries. ACS Appl. Mater. Interfaces 2013, 5, 691–696. [Google Scholar] [CrossRef]
- Sharma, S.; Pollet, B.G. Support Materials for PEMFC and DMFC Electrocatalysts—A Review. J. Power Sources 2012, 208, 96–119. [Google Scholar] [CrossRef]
- Kaur, A.; Chauhan, R.P. Modifications Induced by Silicon and Nickel Ion Beams in the Electrical Conductivity of Zinc Nanowires. J. Mater. Sci. Mater. Electron. 2013, 24, 4302–4310. [Google Scholar] [CrossRef]
- Haroun, A.A.; Youssef, A.M. Synthesis and Electrical Conductivity Evaluation of Novel Hybrid Poly (Methyl Methacrylate)/Titanium Dioxide Nanowires. Synth. Met. 2011, 161, 2063–2069. [Google Scholar] [CrossRef]
- Othman, M.A.; Amat, N.F.; Ahmad, B.H.; Rajan, J. Electrical Conductivity Characteristic of TiO2 Nanowires From Hydrothermal Method. J. Phys. Conf. Ser. 2014, 495, 012027. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Pan, W.; Zhang, W.; Huang, S.; Wu, H. TiN Nanofibers: A New Material with High Conductivity and Transmittance for Transparent Conductive Electrodes. Adv. Funct. Mater. 2013, 23, 209–214. [Google Scholar] [CrossRef]
- Chen, P.; Li, H.; Hu, S.; Zhou, T.; Yan, Y.; Pan, W. Copper-Coated TiN Nanofibers with High Electrical Conductivity: A New Advance in Conductive One-Dimensional Nanostructures. J. Mater. Chem. C 2015, 3, 7272–7276. [Google Scholar] [CrossRef]
- Sharma, C.S.; Katepalli, H.; Sharma, A.; Madou, M. Fabrication and Electrical Conductivity of Suspended Carbon Nanofiber Arrays. Carbon N. Y. 2011, 49, 1727–1732. [Google Scholar] [CrossRef]
- Podyacheva, O.Y.; Cherepanova, S.V.; Romanenko, A.I.; Kibis, L.S.; Svintsitskiy, D.A.; Boronin, A.I.; Stonkus, O.A.; Suboch, A.N.; Puzynin, A.V.; Ismagilov, Z.R. Nitrogen Doped Carbon Nanotubes and Nanofibers: Composition, Structure, Electrical Conductivity and Capacity Properties. Carbon N. Y. 2017, 122, 475–483. [Google Scholar] [CrossRef]
- Zhou, G.; Xiong, T.; Jiang, S.; Jian, S.; Zhou, Z.; Hou, H. Flexible Titanium Carbide–Carbon Nanofibers with High Modulus and High Conductivity by Electrospinning. Mater. Lett. 2016, 165, 91–94. [Google Scholar] [CrossRef]
- Horkstra, J.; van der Pauw, L.J.; Philips, N.V. Measurement of the Resistivity Constants of Anistotropic Conductors by Means of Plane-Parallel Discs of Arbitrary Shape. J. Electron. Control 2007, 7. [Google Scholar] [CrossRef]
- Van der Pauw, L.J. A Method of Measuring Specific Resistivity and Hall Effect of Discs of Arbitrary Shape. Philips Res. Rep. 1958, 13, 1–9. [Google Scholar]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [Green Version]
- Ritchie, N.W.M.; Davis, J.; Newbury, D.E. DTSA-II: A New Tool for Simulating and Quantifying EDS Spectra—Application to Difficult Overlaps. Microsc. Microanal. 2008, 14, 1176–1177. [Google Scholar] [CrossRef] [Green Version]
- Patterson, A.L. The Scherrer Formula for X-Ray Particle Size Determination. Phys. Rev. 1939, 56, 978. [Google Scholar] [CrossRef]
- Seo, S.G.; Park, C.H.; Kim, H.Y.; Nam, W.H.; Jeong, M.; Choi, Y.N.; Lim, Y.S.; Seo, W.S.; Kim, S.J.; Lee, J.Y.; et al. Preparation and Visible-Light Photocatalysis of Hollow Rock-Salt TiO1−xNx Nanoparticles. J. Mater. Chem. A 2013, 1, 3639–3644. [Google Scholar] [CrossRef]
- Im, J.S.; Kim, S.J.; Kang, P.H.; Lee, Y.S. The Improved Electrical Conductivity of Carbon Nanofibers by Fluorinated MWCNTs. J. Ind. Eng. Chem. 2009, 15, 699–702. [Google Scholar] [CrossRef]
- Ge, J.J.; Hou, H.; Li, Q.; Graham, M.J.; Greiner, A.; Reneker, D.H.; Harris, F.W.; Cheng, S.Z.D. Assembly of Well-Aligned Multiwalled Carbon Nanotubes in Confined Polyacrylonitrile Environments: Electrospun Composite Nanofiber Sheets. J. Am. Chem. Soc. 2004, 126, 15754–15761. [Google Scholar] [CrossRef]
- Almora, O.; Aranda, C.; Mas-Marzá, E.; Garcia-Belmonte, G. On Mott-Schottky Analysis Interpretation of Capacitance Measurements in Organometal Perovskite Solar Cells. Appl. Phys. Lett. 2016, 109, 173903. [Google Scholar] [CrossRef] [Green Version]
- Wen, J.; Zeng, Z.; Yang, L.; Zeng, Q.; Lou, H.; Sheng, H.; Miller, D.J.; Yang, W.; Mao, H.-K. TEM Study of Amorphous Carbon with Fully Sp3-Bonded Structure. Microsc. Microanal 2022, 23, 2017. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Wang, C.; Pan, L.; Fu, X.; Yin, P.; Zou, H. Electrical Conductivity of Single Polycrystalline-Amorphous Carbon Nanocoils. Carbon N. Y. 2016, 98, 285–290. [Google Scholar] [CrossRef]
- Chappé, J.M.; Martin, N.; Lintymer, J.; Sthal, F.; Terwagne, G.; Takadoum, J. Titanium Oxynitride Thin Films Sputter Deposited by the Reactive Gas Pulsing Process. Appl. Surf. Sci. 2007, 253, 5312–5316. [Google Scholar] [CrossRef]
- Chappé, J.M.; Martin, N.; Pierson, J.F.; Terwagne, G.; Lintymer, J.; Gavoille, J.; Takadoum, J. Influence of Substrate Temperature on Titanium Oxynitride Thin Films Prepared by Reactive Sputtering. Appl. Surf. Sci. 2004, 225, 29–38. [Google Scholar] [CrossRef]
- Fabreguette, F.; Imhoff, L.; Maglione, M.; Domenichini, B.; Marco De Lucas, M.C.; Sibillot, P.; Bourgeois, S.; Sacilotti, M. Correlation Between the Electrical Properties and Morphology of Low-Pressure MOCVD Titanium Oxynitride Thin Films Grown at Various Temperatures. Chem. Vap. Depos. 2000, 6, 109–114. [Google Scholar] [CrossRef]
- Bruggeman, D.A.G. Berechnung Verschiedener Physikalischer Konstanten von Heterogenen Substanzen. I. Dielektrizitätskonstanten Und Leitfähigkeiten Der Mischkörper Aus Isotropen Substanzen. Ann. Phys. 1935, 416, 636–664. [Google Scholar] [CrossRef]
- Begun, G.M.; Bamberger, C.E. Raman Spectroscopic Observation of Laser-Induced Oxidation of Transition-Metal Borides, Carbides, and Nitrides. Appl. Spectrosc. 2016, 43, 134–138. [Google Scholar] [CrossRef]
- Doiron, B.; Li, Y.; Mihai, A.; Bower, R.; Alford, N.M.N.; Petrov, P.K.; Maier, S.A.; Oulton, R.F. Plasmon-Enhanced Electron Harvesting in Robust Titanium Nitride Nanostructures. J. Phys. Chem. C 2019, 123, 18521–18527. [Google Scholar] [CrossRef]
- Ohsaka, T.; Izumi, F.; Fujiki, Y. Raman Spectrum of Anatase, TiO2. J. Raman Spectrosc. 1978, 7, 321–324. [Google Scholar] [CrossRef]
- Spengler, W.; Kaiser, R. First and Second Order Raman Scattering in Transition Metal Compounds. Solid State Commun. 1976, 18, 881–884. [Google Scholar] [CrossRef]
- Bernard, M.; Deneuville, A.; Thomas, O.; Gergaud, P.; Sandstrom, P.; Birch, J. Raman Spectra of TiN/AlN Superlattices. Thin Solid Films 2000, 380, 252–255. [Google Scholar] [CrossRef]
- Ding, Z.H.; Yao, B.; Qiu, L.X.; Lv, T.Q. Raman Scattering Investigation of Nanocrystalline δ-TiNx Synthesized by Solid-State Reaction. J. Alloys Compd. 2006, 421, 247–251. [Google Scholar] [CrossRef]



| Sample | At.% | Wt.% | N/O at. Ratio | n(Ti):n(C) | m(TiON):m(C) | ||
|---|---|---|---|---|---|---|---|
| TiON/C-CNF1 | Ti | 36.8 | Ti | 65.6 | 1.13 | 4.84 | 28.4 |
| O | 26.1 | O | 15.6 | ||||
| N | 29.5 | N | 15.4 | ||||
| C | 7.6 | C | 3.4 | ||||
| TiON/C-CNF2 | Ti | 33.8 | Ti | 63.2 | 1.67 | 2.66 | 6.87 |
| O | 20.0 | O | 12.5 | ||||
| N | 33.4 | N | 18.3 | ||||
| C | 12.7 | C | 6.0 | ||||
| Nanofiber Sample No. | Resistance R (kΩ) | Average Nanofiber Radius r (nm) | Nanofiber Length L (µm) | Resistivity Ro (mΩm) | Conductivity (kS/m) |
|---|---|---|---|---|---|
| 1 | 100 | 207 | 22.3 | 0.6 | 1.7 |
| 2 | 120 | 230 | 35.2 | 0.6 | 1.8 |
| 3 | 872 | 170 | 44.5 | 1.8 | 0.6 |
| 4 | 130 | 230 | 18.0 | 1.2 | 0.8 |
| Average | 1.0 ± 0.5 | 1.2 ± 0.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koderman Podboršek, G.; Zupančič, Š.; Kaufman, R.; Surca, A.K.; Marsel, A.; Pavlišič, A.; Hodnik, N.; Dražić, G.; Bele, M. Microstructure and Electrical Conductivity of Electrospun Titanium Oxynitride Carbon Composite Nanofibers. Nanomaterials 2022, 12, 2177. https://doi.org/10.3390/nano12132177
Koderman Podboršek G, Zupančič Š, Kaufman R, Surca AK, Marsel A, Pavlišič A, Hodnik N, Dražić G, Bele M. Microstructure and Electrical Conductivity of Electrospun Titanium Oxynitride Carbon Composite Nanofibers. Nanomaterials. 2022; 12(13):2177. https://doi.org/10.3390/nano12132177
Chicago/Turabian StyleKoderman Podboršek, Gorazd, Špela Zupančič, Rok Kaufman, Angelja Kjara Surca, Aleš Marsel, Andraž Pavlišič, Nejc Hodnik, Goran Dražić, and Marjan Bele. 2022. "Microstructure and Electrical Conductivity of Electrospun Titanium Oxynitride Carbon Composite Nanofibers" Nanomaterials 12, no. 13: 2177. https://doi.org/10.3390/nano12132177






