Bio-Inspired Surface Modification of Magnetite Nanoparticles with Dopamine Conjugates
Abstract
:1. Introduction
2. Results
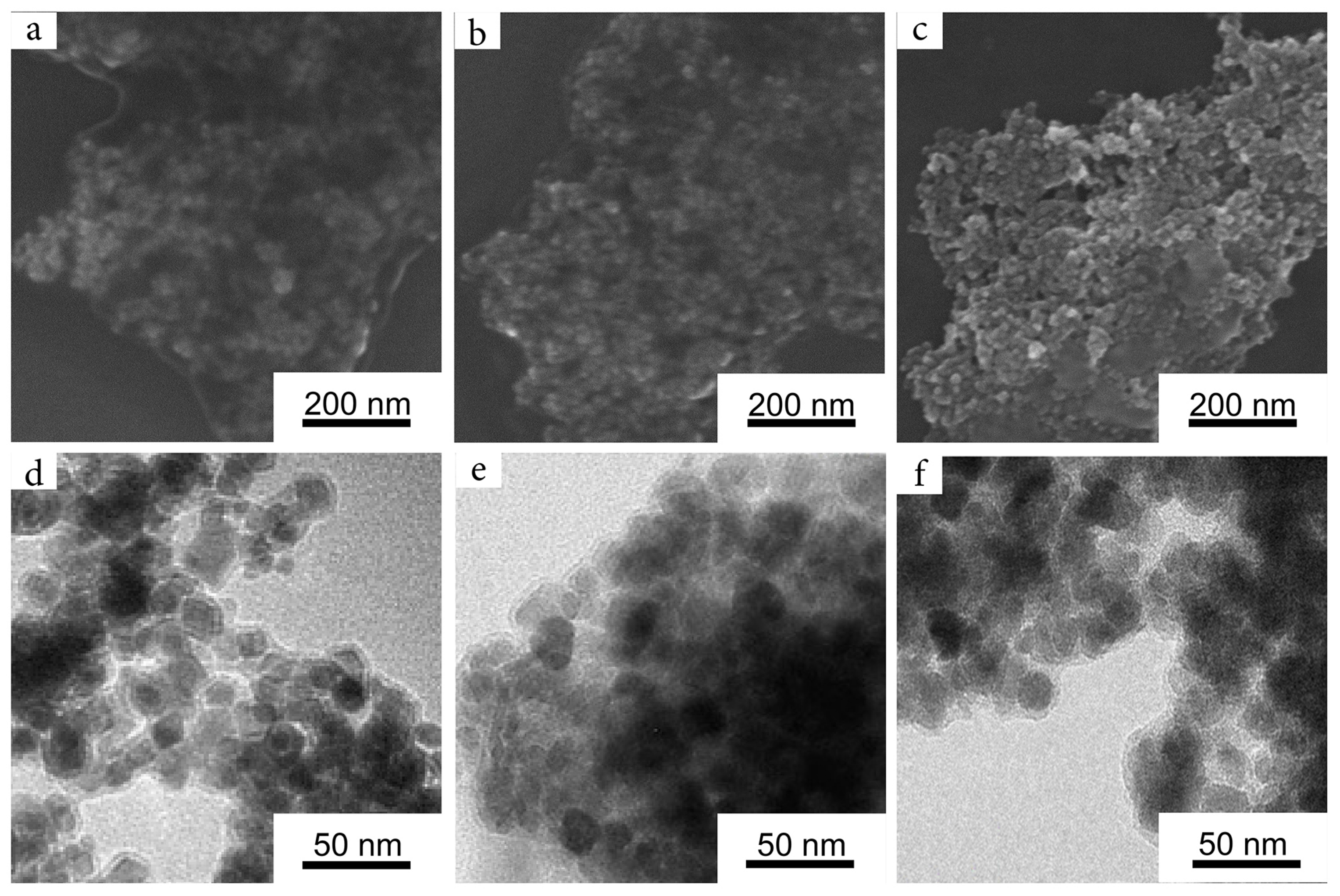
2.1. Magnetic Nanoparticles
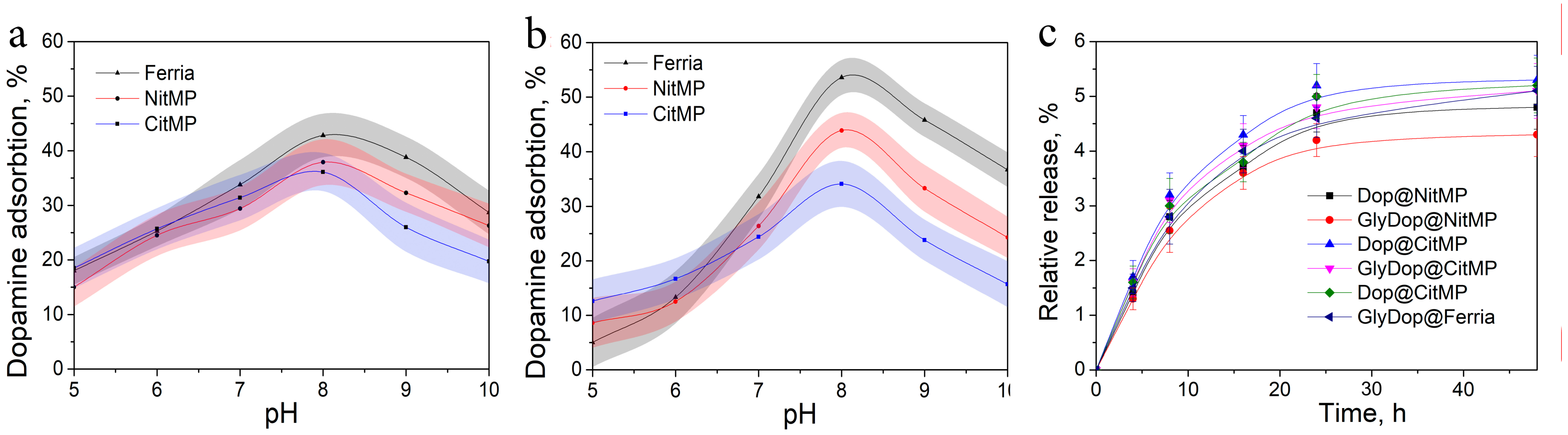
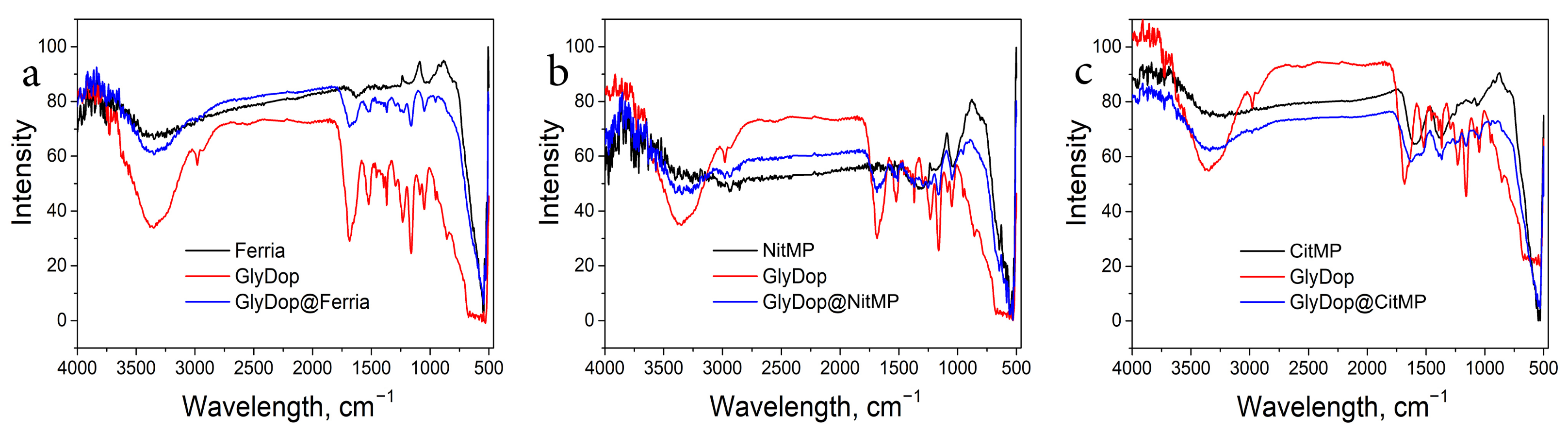
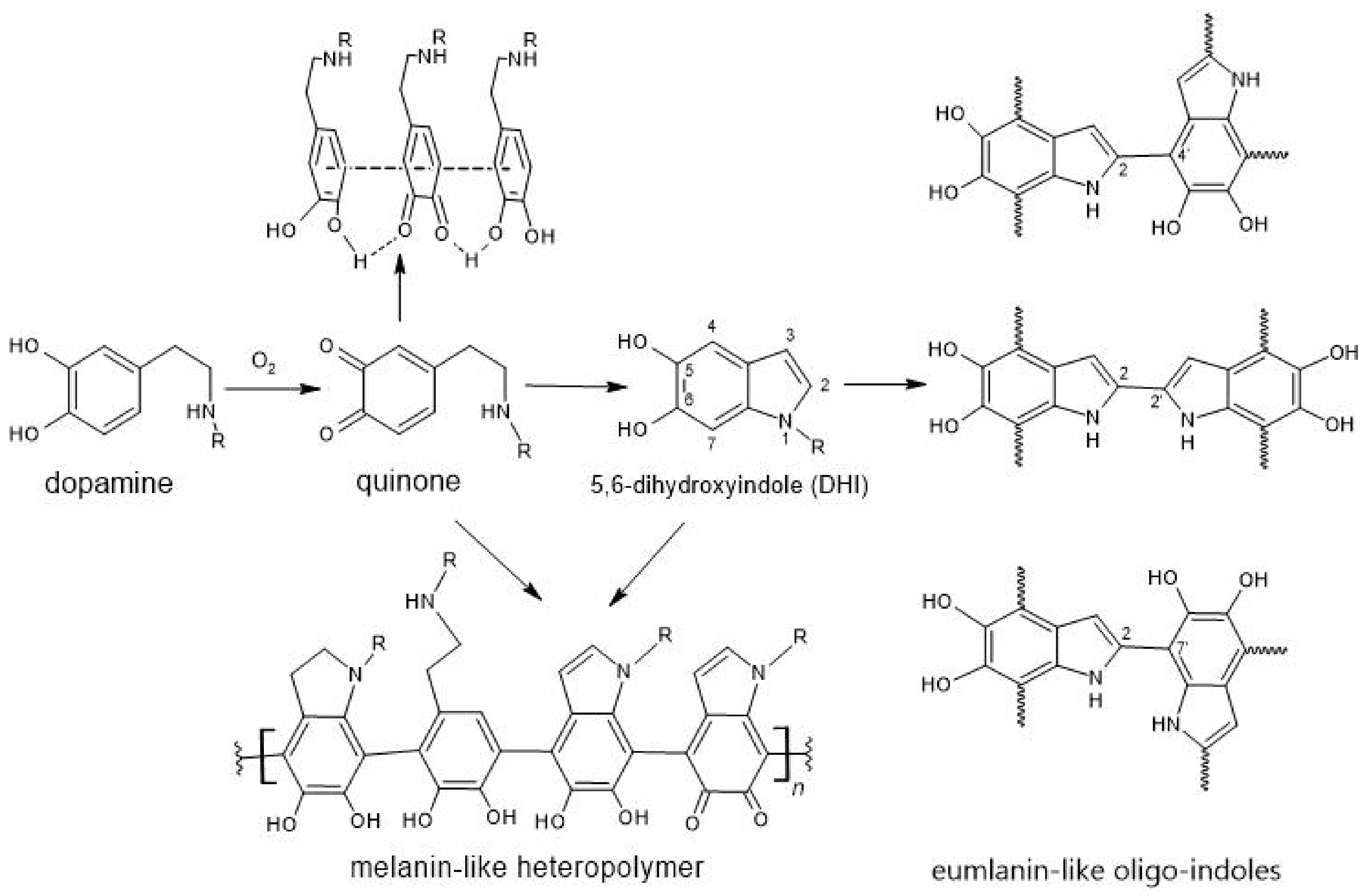
2.2. Biomimetic Coating of the Nanoparticles
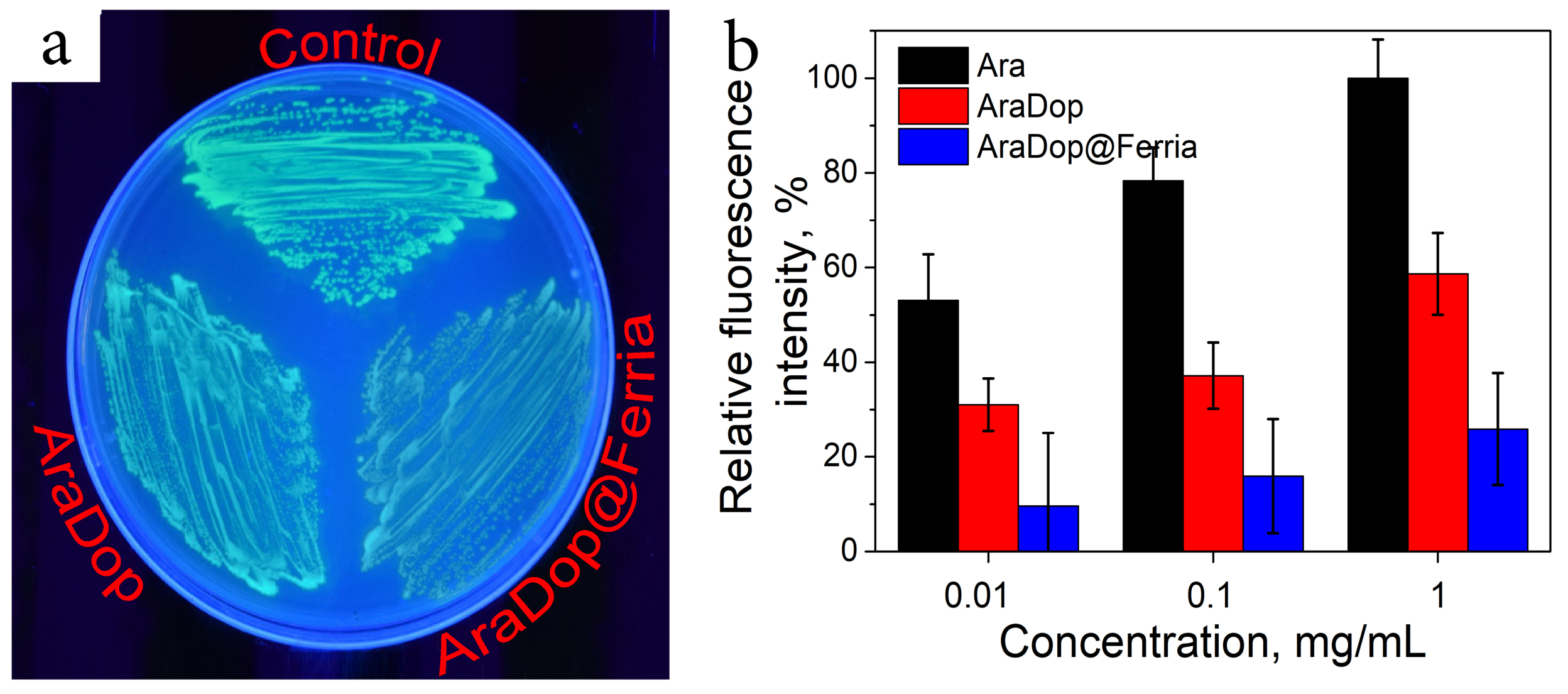
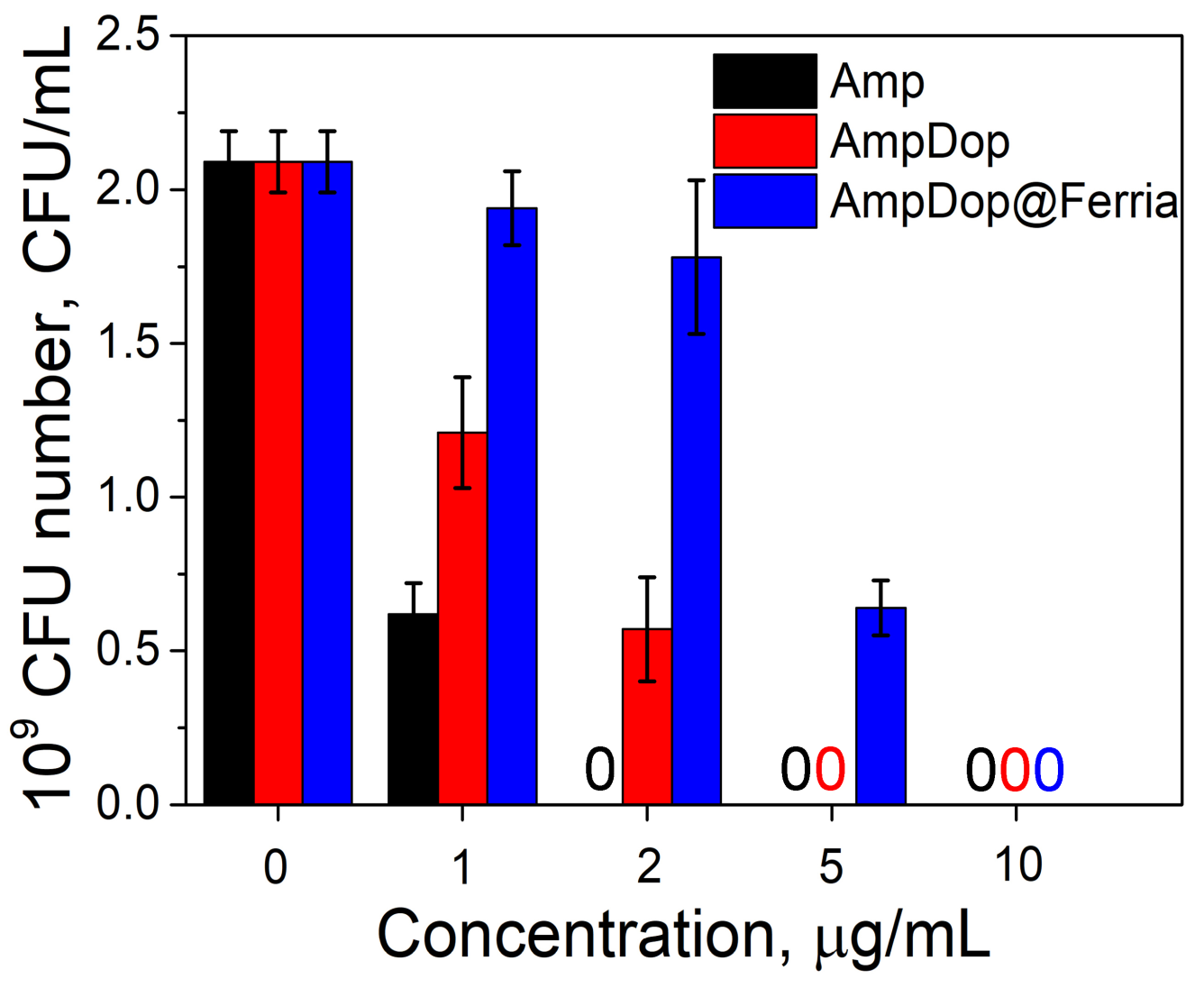
2.3. Bioactivity of the Dopamine-Coated NPs
3. Discussion
4. Conclusions
5. Materials and Methods
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhu, N.; Ji, H.; Yu, P.; Niu, J.; Farooq, M.; Akram, M.W.; Udego, I.; Li, H.; Niu, X. Surface modification of magnetic iron oxide nanoparticles. Nanomaterials 2018, 8, 810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neděla, O.; Slepička, P.; Švorčík, V. Surface modification of polymer substrates for biomedical applications. Materials 2017, 10, 1115. [Google Scholar] [CrossRef]
- Barui, A.K.; Oh, J.Y.; Jana, B.; Kim, C.; Ryu, J.H. Cancer-targeted nanomedicine: Overcoming the barrier of the protein corona. Adv. Ther. 2020, 3, 1900124. [Google Scholar] [CrossRef]
- Drozdov, A.S.; Nikitin, P.I.; Rozenberg, J.M. Systematic Review of Cancer Targeting by Nanoparticles Revealed a Global Association between Accumulation in Tumors and Spleen. Int. J. Mol. Sci. 2021, 22, 13011. [Google Scholar] [CrossRef] [PubMed]
- Kango, S.; Kalia, S.; Celli, A.; Njuguna, J.; Habibi, Y.; Kumar, R. Surface modification of inorganic nanoparticles for development of organic–inorganic nanocomposites—A review. Prog. Polym. Sci. 2013, 38, 1232–1261. [Google Scholar] [CrossRef]
- Kaseem, M.; Fatimah, S.; Nashrah, N.; Ko, Y.G. Recent progress in surface modification of metals coated by plasma electrolytic oxidation: Principle, structure, and performance. Prog. Mater. Sci. 2021, 117, 100735. [Google Scholar] [CrossRef]
- Lam, M.; Migonney, V.; Falentin-Daudre, C. Review of silicone surface modification techniques and coatings for antibacterial/antimicrobial applications to improve breast implant surfaces. Acta Biomater. 2021, 121, 68–88. [Google Scholar] [CrossRef]
- Zhang, J.; Mou, L.; Jiang, X. Surface chemistry of gold nanoparticles for health-related applications. Chem. Sci. 2020, 11, 923–936. [Google Scholar] [CrossRef] [Green Version]
- Nemani, S.K.; Annavarapu, R.K.; Mohammadian, B.; Raiyan, A.; Heil, J.; Haque, M.A.; Abdelaal, A.; Sojoudi, H. Surface modification of polymers: Methods and applications. Adv. Mater. Interfaces 2018, 5, 1801247. [Google Scholar] [CrossRef]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-inspired surface chemistry for multifunctional coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef] [Green Version]
- Qiu, W.Z.; Yang, H.C.; Xu, Z.K. Dopamine-assisted co-deposition: An emerging and promising strategy for surface modification. Adv. Colloid Interface Sci. 2018, 256, 111–125. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.H.; Messersmith, P.B.; Lee, H. Polydopamine surface chemistry: A decade of discovery. ACS Appl. Mater. Interfaces 2018, 10, 7523–7540. [Google Scholar] [CrossRef] [PubMed]
- Klosterman, L.; Riley, J.K.; Bettinger, C.J. Control of heterogeneous nucleation and growth kinetics of dopamine-melanin by altering substrate chemistry. Langmuir 2015, 31, 3451–3458. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.A.; Park, E.; Lee, H. Polydopamine and its derivative surface chemistry in material science: A focused review for studies at KAIST. Adv. Mater. 2020, 32, 1907505. [Google Scholar] [CrossRef]
- Liebscher, J.; Mrówczyński, R.; Scheidt, H.A.; Filip, C.; Hadade, N.D.; Turcu, R.; Bende, A.; Beck, S. Structure of polydopamine: A never-ending story? Langmuir 2013, 29, 10539–10548. [Google Scholar] [CrossRef]
- Ball, V. Polydopamine nanomaterials: Recent advances in synthesis methods and applications. Front. Bioeng. Biotechnol. 2018, 6, 109. [Google Scholar] [CrossRef] [Green Version]
- Palladino, P.; Bettazzi, F.; Scarano, S. Polydopamine: Surface coating, molecular imprinting, and electrochemistry—Successful applications and future perspectives in (bio) analysis. Anal. Bioanal. Chem. 2019, 411, 4327–4338. [Google Scholar] [CrossRef]
- Ding, Y.; Floren, M.; Tan, W. Mussel-inspired polydopamine for bio-surface functionalization. Biosurface Biotribol. 2016, 2, 121–136. [Google Scholar] [CrossRef]
- Ye, Q.; Zhou, F.; Liu, W. Bioinspired catecholic chemistry for surface modification. Chem. Soc. Rev. 2011, 40, 4244–4258. [Google Scholar] [CrossRef]
- Liu, C.Y.; Huang, C.J. Functionalization of polydopamine via the aza-michael reaction for antimicrobial interfaces. Langmuir 2016, 32, 5019–5028. [Google Scholar] [CrossRef]
- Kung, M.L.; Lin, P.Y.; Peng, S.W.; Wu, D.C.; Wu, W.J.; Yeh, B.W.; Tai, M.H.; Hung, H.S.; Hsieh, S. Biomimetic polymer-based Ag nanocomposites as a antimicrobial platform. Appl. Mater. Today 2016, 4, 31–39. [Google Scholar] [CrossRef]
- Huang, Z.H.; Peng, S.W.; Hsieh, S.L.; Kirankumar, R.; Huang, P.F.; Chang, T.M.; Dwivedi, A.K.; Chen, N.F.; Wu, H.M.; Hsieh, S. Polydopamine ultrathin film growth on mica via in-situ polymerization of dopamine with applications for silver-based antimicrobial coatings. Materials 2021, 14, 671. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.F.; Liao, Y.H.; Lin, P.Y.; Chen, W.F.; Wen, Z.H.; Hsieh, S. Investigation of the Characteristics and Antibacterial Activity of Polymer-Modified Copper Oxide Nanoparticles. Int. J. Mol. Sci. 2021, 22, 12913. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ai, K.; Lu, L. Polydopamine and its derivative materials: Synthesis and promising applications in energy, environmental, and biomedical fields. Chem. Rev. 2014, 114, 5057–5115. [Google Scholar] [CrossRef]
- Lee, H.A.; Ma, Y.; Zhou, F.; Hong, S.; Lee, H. Material-independent surface chemistry beyond polydopamine coating. Accounts Chem. Res. 2019, 52, 704–713. [Google Scholar] [CrossRef]
- Singh, I.; Dhawan, G.; Gupta, S.; Kumar, P. Recent advances in a polydopamine-mediated antimicrobial adhesion system. Front. Microbiol. 2021, 11, 3326. [Google Scholar] [CrossRef]
- Li, H.; Yin, D.; Li, W.; Tang, Q.; Zou, L.; Peng, Q. Polydopamine-based nanomaterials and their potentials in advanced drug delivery and therapy. Colloids Surf. B Biointerfaces 2021, 199, 111502. [Google Scholar] [CrossRef]
- Jaramillo, A.M.; Barrera-Gutiérrez, R.; Cortes, M.T. Synthesis, Follow-Up, and Characterization of Polydopamine-like Coatings Departing from Micromolar Dopamine-o-Quinone Precursor Concentrations. ACS Omega 2020, 5, 15016–15027. [Google Scholar] [CrossRef]
- Suarez-Garcia, S.; Esposito, T.V.; Neufeld-Peters, J.; Bergamo, M.; Yang, H.; Saatchi, K.; Schaffer, P.; Hafeli, U.O.; Ruiz-Molina, D.; Rodriguez-Rodriguez, C.; et al. Hybrid Metal–Phenol Nanoparticles with Polydopamine-like Coating for PET/SPECT/CT Imaging. ACS Appl. Mater. Interfaces 2021, 13, 10705–10718. [Google Scholar] [CrossRef]
- Massaro, M.; Campisciano, V.; Viseras Iborra, C.; Liotta, L.F.; Sánchez-Polo, M.; Riela, S.; Gruttadauria, M. New Mussel Inspired Polydopamine-Like Silica-Based Material for Dye Adsorption. Nanomaterials 2020, 10, 1416. [Google Scholar] [CrossRef]
- Shin, M.; Ryu, J.H.; Kim, K.; Kim, M.J.; Jo, S.; Lee, M.S.; Lee, D.Y.; Lee, H. Hemostatic swabs containing polydopamine-like catecholamine chitosan-catechol for normal and coagulopathic animal models. ACS Biomater. Sci. Eng. 2018, 4, 2314–2318. [Google Scholar] [CrossRef] [PubMed]
- Barclay, T.G.; Hegab, H.M.; Clarke, S.R.; Ginic-Markovic, M. Versatile surface modification using polydopamine and related polycatecholamines: Chemistry, structure, and applications. Adv. Mater. Interfaces 2017, 4, 1601192. [Google Scholar] [CrossRef]
- Mazur, M.; Barras, A.; Kuncser, V.; Galatanu, A.; Zaitzev, V.; Turcheniuk, K.V.; Woisel, P.; Lyskawa, J.; Laure, W.; Siriwardena, A.; et al. Iron oxide magnetic nanoparticles with versatile surface functions based on dopamine anchors. Nanoscale 2013, 5, 2692–2702. [Google Scholar] [CrossRef] [PubMed]
- Shapovalova, O.E.; Drozdov, A.S.; Bryushkova, E.A.; Morozov, M.I.; Vinogradov, V.V. Room-temperature fabrication of magnetite-boehmite sol-gel composites for heavy metal ions removal. Arab. J. Chem. 2020, 13, 1933–1944. [Google Scholar] [CrossRef]
- Drozdov, A.S.; Ivanovski, V.; Avnir, D.; Vinogradov, V.V. A universal magnetic ferrofluid: Nanomagnetite stable hydrosol with no added dispersants and at neutral pH. J. Colloid Interface Sci. 2016, 468, 307–312. [Google Scholar] [CrossRef]
- Kosmulski, M. The pH dependent surface charging and points of zero charge. VII. Update. Adv. Colloid Interface Sci. 2018, 251, 115–138. [Google Scholar] [CrossRef]
- Anastasova, E.I.; Ivanovski, V.; Fakhardo, A.F.; Lepeshkin, A.I.; Omar, S.; Drozdov, A.S.; Vinogradov, V.V. A pure magnetite hydrogel: Synthesis, properties and possible applications. Soft Matter 2017, 13, 8651–8660. [Google Scholar] [CrossRef] [Green Version]
- Qureashi, A.; Pandith, A.H.; Bashir, A.; Manzoor, T.; Malik, L.A.; Sheikh, F.A. Citrate coated magnetite: A complete magneto dielectric, electrochemical and DFT study for detection and removal of heavy metal ions. Surf. Interfaces 2021, 23, 101004. [Google Scholar] [CrossRef]
- Liu, J.; Sun, Z.; Deng, Y.; Zou, Y.; Li, C.; Guo, X.; Xiong, L.; Gao, Y.; Li, F.; Zhao, D. Highly water-dispersible biocompatible magnetite particles with low cytotoxicity stabilized by citrate groups. Angew. Chem. Int. Ed. 2009, 48, 5875–5879. [Google Scholar] [CrossRef]
- Andreeva, Y.I.; Drozdov, A.S.; Fakhardo, A.F.; Cheplagin, N.A.; Shtil, A.A.; Vinogradov, V.V. The controllable destabilization route for synthesis of low cytotoxic magnetic nanospheres with photonic response. Sci. Rep. 2017, 7, 11343. [Google Scholar] [CrossRef] [Green Version]
- RuizMoreno, R.; Martinez, A.; Castro-Rodriguez, R.; Bartolo, P. Synthesis and characterization of citrate coated magnetite nanoparticles. J. Supercond. Nov. Magn. 2013, 26, 709–712. [Google Scholar] [CrossRef]
- Mérida, F.; Chiu-Lam, A.; Bohórquez, A.C.; Maldonado-Camargo, L.; Pérez, M.E.; Pericchi, L.; Torres-Lugo, M.; Rinaldi, C. Optimization of synthesis and peptization steps to obtain iron oxide nanoparticles with high energy dissipation rates. J. Magn. Magn. Mater. 2015, 394, 361–371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mandel, K.; Szczerba, W.; Thünemann, A.F.; Riesemeier, H.; Girod, M.; Sextl, G. Nitric acid-stabilized superparamagnetic iron oxide nanoparticles studied with X-rays. J. Nanopart. Res. 2012, 14, 1066. [Google Scholar] [CrossRef]
- Salomäki, M.; Marttila, L.; Kivelä, H.; Ouvinen, T.; Lukkari, J. Effects of pH and oxidants on the first steps of polydopamine formation: A thermodynamic approach. J. Phys. Chem. B 2018, 122, 6314–6327. [Google Scholar] [CrossRef] [PubMed]
- Alfieri, M.L.; Panzella, L.; Oscurato, S.L.; Salvatore, M.; Avolio, R.; Errico, M.E.; Maddalena, P.; Napolitano, A.; d’Ischia, M. The chemistry of polydopamine film formation: The amine-quinone interplay. Biomimetics 2018, 3, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ivanovski, V.; Shapovalova, O.E.; Drozdov, A.S. Structural Rearrangements of Carbonic Anhydrase Entrapped in Sol-Gel Magnetite Determined by ATR–FTIR Spectroscopy. Int. J. Mol. Sci. 2022, 23, 5975. [Google Scholar] [CrossRef] [PubMed]
- Kuznowicz, M.; Jędrzak, A.; Rębiś, T.; Jesionowski, T. Biomimetic magnetite/polydopamine/β-cyclodextrins nanocomposite for long-term glucose measurements. Biochem. Eng. J. 2021, 174, 108127. [Google Scholar] [CrossRef]
- Ding, Y.; Weng, L.T.; Yang, M.; Yang, Z.; Lu, X.; Huang, N.; Leng, Y. Insights into the aggregation/deposition and structure of a polydopamine film. Langmuir 2014, 30, 12258–12269. [Google Scholar] [CrossRef]
- Yang, J.; Stuart, M.A.C.; Kamperman, M. Jack of all trades: Versatile catechol crosslinking mechanisms. Chem. Soc. Rev. 2014, 43, 8271–8298. [Google Scholar] [CrossRef]
- Bernsmann, F.; Ersen, O.; Voegel, J.C.; Jan, E.; Kotov, N.A.; Ball, V. Melanin-containing films: Growth from dopamine solutions versus layer-by-layer deposition. ChemPhysChem 2010, 11, 3299–3305. [Google Scholar] [CrossRef] [Green Version]
- Tan, J.; Cho, T.J.; Tsai, D.H.; Liu, J.; Pettibone, J.M.; You, R.; Hackley, V.A.; Zachariah, M.R. Surface modification of cisplatin-complexed gold nanoparticles and its influence on colloidal stability, drug loading, and drug release. Langmuir 2018, 34, 154–163. [Google Scholar] [CrossRef]
- Cinier, M.; Petit, M.; Williams, M.N.; Fabre, R.M.; Pecorari, F.; Talham, D.R.; Bujoli, B.; Tellier, C. Bisphosphonate adaptors for specific protein binding on zirconium phosphonate-based microarrays. Bioconjug. Chem. 2009, 20, 2270–2277. [Google Scholar] [CrossRef]
- Queffelec, C.; Petit, M.; Janvier, P.; Knight, D.A.; Bujoli, B. Surface modification using phosphonic acids and esters. Chem. Rev. 2012, 112, 3777–3807. [Google Scholar] [CrossRef] [PubMed]
- Soisson, S.M.; MacDougall-Shackleton, B.; Schleif, R.; Wolberger, C. Structural basis for ligand-regulated oligomerization of AraC. Science 1997, 276, 421–425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zapun, A.; Contreras-Martel, C.; Vernet, T. Penicillin-binding proteins and β-lactam resistance. FEMS Microbiol. Rev. 2008, 32, 361–385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, A.; Pandey, A.K.; Singh, S.S.; Shanker, R.; Dhawan, A. Cellular uptake and mutagenic potential of metal oxide nanoparticles in bacterial cells. Chemosphere 2011, 83, 1124–1132. [Google Scholar] [CrossRef]
- Jayawardena, H.S.N.; Jayawardana, K.W.; Chen, X.; Yan, M. Maltoheptaose promotes nanoparticle internalization by Escherichia coli. Chem. Commun. 2013, 49, 3034–3036. [Google Scholar] [CrossRef] [Green Version]
- Joo, S.H.; Aggarwal, S. Factors impacting the interactions of engineered nanoparticles with bacterial cells and biofilms: Mechanistic insights and state of knowledge. J. Environ. Manag. 2018, 225, 62–74. [Google Scholar] [CrossRef]
- Rumyantceva, V.; Rumyantceva, V.; Andreeva, Y.; Tsvetikova, S.; Radaev, A.; Vishnevskaya, M.; Vinogradov, V.; Drozdov, A.S.; Koshel, E. Magnetically controlled carbonate nanocomposite with ciprofloxacin for biofilm eradication. Int. J. Mol. Sci. 2021, 22, 6187. [Google Scholar] [CrossRef]
- Kolchanov, D.S.; Slabov, V.; Keller, K.; Sergeeva, E.; Zhukov, M.V.; Drozdov, A.S.; Vinogradov, A.V. Sol–gel magnetite inks for inkjet printing. J. Mater. Chem. C 2019, 7, 6426–6432. [Google Scholar] [CrossRef]
- Nikitin, M.P.; Shipunova, V.O.; Deyev, S.M.; Nikitin, P.I. Biocomputing based on particle disassembly. Nat. Nanotechnol. 2014, 9, 716–722. [Google Scholar] [CrossRef] [PubMed]
- Dheyab, M.A.; Aziz, A.A.; Jameel, M.S.; Noqta, O.A.; Khaniabadi, P.M.; Mehrdel, B. Simple rapid stabilization method through citric acid modification for magnetite nanoparticles. Sci. Rep. 2020, 10, 10793. [Google Scholar] [CrossRef] [PubMed]



| Name | pH of the Colloid | Surface Groups | Crystallite Diameter, nm | Hydrodynamic Radius, nm | Isoelectric Point, pH | Potential at pH 7, mV |
|---|---|---|---|---|---|---|
| CitMP | 4.6 | –COOH | 10.47 | 40 | 6.3 | −7 |
| NitMP | 2.3 | Fe–OH | 10.74 | 38 | 7.3 | +8 |
| Ferria | 6.8 | Fe(II)–OH | 10.31 | 33 | 8.2 | +32 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Volov, A.; Shkodenko, L.; Koshel, E.; Drozdov, A.S. Bio-Inspired Surface Modification of Magnetite Nanoparticles with Dopamine Conjugates. Nanomaterials 2022, 12, 2230. https://doi.org/10.3390/nano12132230
Volov A, Shkodenko L, Koshel E, Drozdov AS. Bio-Inspired Surface Modification of Magnetite Nanoparticles with Dopamine Conjugates. Nanomaterials. 2022; 12(13):2230. https://doi.org/10.3390/nano12132230
Chicago/Turabian StyleVolov, Alexander, Liubov Shkodenko, Elena Koshel, and Andrey S. Drozdov. 2022. "Bio-Inspired Surface Modification of Magnetite Nanoparticles with Dopamine Conjugates" Nanomaterials 12, no. 13: 2230. https://doi.org/10.3390/nano12132230






