Electrochemically Promoted Benzylation of [60]Fullerooxazolidinone
Abstract
:1. Introduction
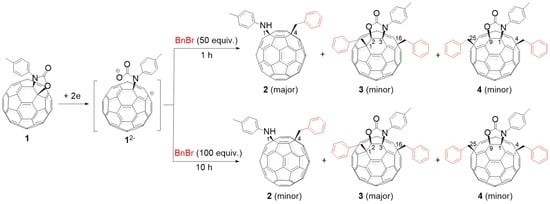
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.2. Generation of Dianion 12−
3.3. Synthesis of Compounds 2–4
3.4. Single-Crystal Growth and Characterization of 3
3.5. CVs and DPVs of Compounds 1–4 along with PCBM
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Anilkumar, P.; Lu, F.; Cao, L.; Luo, P.G.; Liu, J.-H.; Sahu, S.; Tackett, K.N., II; Wang, Y.; Sun, Y.-P. Fullerenes for applications in biology and medicine. Curr. Med. Chem. 2011, 18, 2045–2059. [Google Scholar] [CrossRef] [PubMed]
- Castro, E.; Hernandez Garcia, A.; Zavala, G.; Echegoyen, L. Fullerenes in biology and medicine. J. Mater. Chem. B 2017, 5, 6523–6535. [Google Scholar] [CrossRef] [PubMed]
- Nierengarten, J.-F. Fullerene hexa-adduct scaffolding for the construction of giant molecules. Chem. Commun. 2017, 53, 11855–11868. [Google Scholar] [CrossRef] [PubMed]
- Guldi, D.M.; Illescas, B.M.; Atienza, C.M.; Wielopolski, M.; Martín, N. Fullerene for organic electronics. Chem. Soc. Rev. 2009, 38, 1587–1597. [Google Scholar] [CrossRef]
- Zieleniewska, A.; Lodermeyer, F.; Roth, A.; Guldi, D.M. Fullerenes - how 25 years of charge transfer chemistry have shaped our understanding of (interfacial) interactions. Chem. Soc. Rev. 2018, 47, 702–714. [Google Scholar] [CrossRef]
- Megiatto, J.D., Jr.; Guldi, D.M.; Schuster, D.I. Design, synthesis and photoinduced processes in molecular interlocked photosynthetic [60]fullerene systems. Chem. Soc. Rev. 2020, 49, 8–20. [Google Scholar] [CrossRef]
- Cui, C.; Li, Y.; Li, Y. Fullerene derivatives for the applications as acceptor and cathode buffer layer materials for organic and perovskite solar cells. Adv. Energy Mater. 2017, 7, 1601251. [Google Scholar] [CrossRef]
- Gatti, T.; Menna, E.; Meneghetti, M.; Maggini, M.; Petrozza, A.; Lamberti, F. The Renaissance of fullerenes with perovskite solar cells. Nano Energy 2017, 41, 84–100. [Google Scholar] [CrossRef]
- Castro, E.; Murillo, J.; Fernandez-Delgado, O.; Echegoyen, L. Progress in fullerene-based hybrid perovskite solar cells. J. Mater. Chem. C 2018, 6, 2635–2651. [Google Scholar] [CrossRef]
- Umeyama, T.; Imahori, H. Isomer effects of fullerene derivatives on organic photovoltaics and perovskite solar cells. Acc. Chem. Res. 2019, 52, 2046–2055. [Google Scholar] [CrossRef]
- Jia, L.; Chen, M.; Yang, S. Functionalization of fullerene materials toward applications in perovskite solar cells. Mater. Chem. Front. 2020, 4, 2256–2282. [Google Scholar] [CrossRef]
- Murata, M.; Murata, Y.; Komatsu, K. Surgery of fullerenes. Chem. Commun. 2008, 6083–6094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vougioukalakis, G.C.; Roubelakis, M.M.; Orfanopoulos, M. Open-cage fullerenes: Towards the construction of nanosized molecular containers. Chem. Soc. Rev. 2010, 39, 817–844. [Google Scholar] [CrossRef] [PubMed]
- Itami, K. Molecular catalysis for fullerene functionalization. Chem. Rec. 2011, 11, 226–235. [Google Scholar] [CrossRef]
- Zhu, S.-E.; Li, F.; Wang, G.-W. Mechanochemistry of fullerenes and related materials. Chem. Soc. Rev. 2013, 42, 7535–7570. [Google Scholar] [CrossRef]
- Wang, G.-W. Fullerene mechanochemistry: Serendipitous discovery of dumb-bell-shaped C120 and beyond. Chin. J. Chem. 2021, 39, 1797–1803. [Google Scholar] [CrossRef]
- Maroto, E.E.; Izquierdo, M.; Reboredo, S.; Marco-Martínez, J.; Filippone, S.; Martín, N. Chiral fullerenes from asymmetric catalysis. Acc. Chem. Res. 2014, 47, 2660–2670. [Google Scholar] [CrossRef]
- Gan, L. Peroxide-mediated selective cleavage of [60]fullerene skeleton bonds: Towards the synthesis of open-cage fulleroid C55O5. Chem. Rec. 2015, 15, 189–198. [Google Scholar] [CrossRef]
- Niu, C.; Wang, G.-W. Progress in electrochemical reactions of [60]fullerene-fused heterocycles. Chin. J. Org. Chem. 2020, 40, 3633–3645. [Google Scholar] [CrossRef]
- Caron, C.; Subramanian, R.; D’Souza, F.; Kim, J.; Kutner, W.; Jones, M.T.; Kadish, K.M. Selective electrosynthesis of dimethylfullerene [(CH3)2C60]: A novel method for the controlled functionalization of fullerenes. J. Am. Chem. Soc. 1993, 115, 8505–8506. [Google Scholar] [CrossRef]
- Kadish, K.M.; Gao, X.; Van Caemelbecke, E.; Suenobu, T.; Fukuzumi, S. Effect of addition pattern on the electrochemical and spectroscopic properties of neutral and reduced 1,2- and 1,4-(C6H5CH2)2C60 isomers. J. Phys. Chem. A 2000, 104, 3878–3883. [Google Scholar] [CrossRef]
- Lukoyanova, O.; Cardona, C.M.; Altable, M.; Filippone, S.; Domenech, Á.M.; Martín, N.; Echegoyen, L. Selective electrochemical retro-cycloaddition reaction of pyrrolidinofullerenes. Angew. Chem. Int. Ed. 2006, 45, 7430–7433. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Li, F.-F.; Ni, L.; Yang, W.-W.; Gao, X. Synthesis and identification of heterocyclic derivatives of fullerene C60: Unexpected reaction of anionic C60 with benzonitrile. J. Org. Chem. 2008, 73, 3159–3168. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.-W.; Li, Z.-J.; Gao, X. Reaction of C602− with organic halides revisited in DMF: Proton transfer from water to RC60− and unexpected formation of 1,2-dihydro[60]fullerenes. J. Org. Chem. 2010, 75, 4086–4094. [Google Scholar] [CrossRef] [PubMed]
- Li, F.-F.; Rodríguez-Fortea, A.; Peng, P.; Campos Chavez, G.A.; Poblet, J.M.; Echegoyen, L. Electrosynthesis of a Sc3N@Ih-C80 methano derivative from trianionic Sc3N@Ih-C80. J. Am. Chem. Soc. 2012, 134, 7480–7487. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhu, S.-E.; Liu, D.-J.; Suzuki, M.; Lu, X.; Wang, G.-W. Regioselective electrosynthesis of rare 1,2,3,16-functionalized [60]fullerene derivatives. Angew. Chem. Int. Ed. 2014, 53, 3006–3010. [Google Scholar] [CrossRef]
- Lin, H.-S.; Matsuo, Y.; Wang, J.-J.; Wang, G.-W. Regioselective acylation and carboxylation of [60]fulleroindoline via electrochemical synthesis. Org. Chem. Front. 2017, 4, 603–607. [Google Scholar] [CrossRef]
- Liu, K.-Q.; Wang, J.-J.; Yan, X.-X.; Niu, C.; Wang, G.-W. Regioselective electrosynthesis of tetra- and hexa-functionalized [60]fullerene derivatives with unprecedented addition patterns. Chem. Sci. 2020, 11, 384–388. [Google Scholar] [CrossRef] [Green Version]
- Yan, X.-X.; Li, B.; Lin, H.-S.; Jin, F.; Niu, C.; Liu, K.-Q.; Wang, G.-W.; Yang, S. Successively regioselective electrosynthesis and electron transport property of stable multiply functionalized [60]fullerene derivatives. Research 2020, 2020, 2059190. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Niu, C.; Chen, M.; Yang, S.; Wang, G.-W. Electrochemical regioselective alkylations of a [60]fulleroindoline with bulky alkyl bromides. Org. Biomol. Chem. 2020, 18, 4783–4787. [Google Scholar] [CrossRef]
- Li, F.; Wang, J.-J.; Wang, G.-W. Palladium-catalyzed synthesis of [60]fullerene-fused benzofurans via heteroannulation of phenols. Chem. Commun. 2017, 53, 1852–1855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hussain, M.; Chen, M.; Yang, S.; Wang, G.-W. Palladium-catalyzed heteroannulation of indole-1-carboxamides with [60]fullerene and subsequent electrochemical transformations. Org. Lett. 2019, 21, 8568–8571. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liu, Z.; Yin, Z.-C.; Wang, G.-W. Synthesis of [60]fullerene-fused dihydrobenzooxazepines via the palladium-catalyzed oxime-directed C–H bond activation and subsequent electrochemical functionalization. Org. Chem. Front. 2020, 7, 2518–2525. [Google Scholar] [CrossRef]
- Hussain, M.; Niu, C.; Wang, G.-W. Palladium-catalyzed synthesis of [60]fullerene-fused furochromenones and further electrochemical functionalization. Org. Chem. Front. 2020, 7, 1249–1254. [Google Scholar] [CrossRef]
- Liu, Z.; Yin, Z.-C.; Lu, W.-Q.; Niu, C.; Chen, M.; Yang, S.; Wang, G.-W. Cu(I)-catalyzed synthesis of [60]fullerene-fused lactams and further electrochemical functionalization. Org. Lett. 2021, 23, 4051–4056. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.-W.; Li, Z.-J.; Li, F.-F.; Gao, X. Electrochemical and H/D-labeling study of oxazolino[60]fullerene rearrangement. J. Org. Chem. 2011, 76, 1384–1389. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.-L.; Li, Z.-J.; Gao, X. Reductive benzylation of C60 imidazoline with a bulky addend. Org. Lett. 2014, 16, 712–715. [Google Scholar] [CrossRef]
- Li, Z.-J.; Li, S.-H.; Sun, T.; Hou, H.-L.; Gao, X. Reductive benzylation of singly bonded 1,2,4,15-C60 dimers with an oxazoline or imidazoline heterocycle: Unexpected formation of 1,2,3,16-C60 adducts and insights into the reactivity of singly bonded C60 dimers. J. Org. Chem. 2015, 80, 3566–3571. [Google Scholar] [CrossRef]
- You, X.; Wang, G.-W. Ferric chloride-catalyzed reaction of [60]fullerene with tert-butyl N-substituted carbamates: Synthesis of oxazolidino[4,5:1,2][60]fullerenes. J. Org. Chem. 2014, 79, 117–121. [Google Scholar] [CrossRef]
- Niu, C.; Li, B.; Yin, Z.-C.; Yang, S.; Wang, G.-W. Electrochemical benzylation of [60]fullerene-fused lactones: Unexpected formation of ring-opened adducts and their photovoltaic performance. Org. Lett. 2019, 21, 7346–7350. [Google Scholar] [CrossRef]
- He, F.-G.; Li, Z.-J.; Gao, X. Methoxylation of singly bonded 1,4–1′,4′-BnC60–C60Bn dimer: Preferential formation of 1,4-C60 adduct with sterically less demanding addends and stability difference between 1,2-and 1,4-OMe(Bn)C60. J. Org. Chem. 2016, 81, 6838–6842. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Song, M.; Li, X.; Huang, Y.; Zhao, T.; Wei, Z.; Lan, Y.; Tan, H. Synthesis of heterobiaryl 4-aryl furans through a base-promoted decarboxylative propargylation/cycloisomerization annulation. Org. Lett. 2020, 22, 8752–8757. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Meng, X.; Yang, S. Interface engineering for highly efficient and stable planar p-i-n perovskite solar cells. Adv. Energy Mater. 2018, 8, 1701883. [Google Scholar] [CrossRef]
- Li, B.; Zhen, J.; Wan, Y.; Lei, X.; Liu, Q.; Liu, Y.; Jia, L.; Wu, X.; Zeng, H.; Zhang, W.; et al. Anchoring fullerene onto perovskite film via grafting pyridine toward enhanced electron transport in high-efficiency solar cells. ACS Appl. Mater. Interfaces 2018, 10, 32471–32482. [Google Scholar] [CrossRef]
- Wong, W.-Y.; Wang, X.-Z.; He, Z.; Djurišić, A.B.; Yip, C.-T.; Cheung, K.-Y.; Wang, H.; Mak, C.S.K.; Chan, W.-K. Metallated conjugated polymers as a new avenue towards high-efficiency polymer solar cells. Nat. Mater. 2007, 6, 521–527. [Google Scholar] [CrossRef] [Green Version]
- Li, N.; Brabec, C.J. Air-processed polymer tandem solar cells with power conversion efficiency exceeding 10%. Energy Environ. Sci. 2015, 8, 2902–2909. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Hu, X.; Liu, P.; Li, W.; Gong, X.; Huang, F.; Cao, Y. Donor–acceptor conjugated polymer based on naphtho[1,2-c:5,6-c]bis[1,2,5]thiadiazole for high-performance polymer solar cells. J. Am. Chem. Soc. 2011, 133, 9638–9641. [Google Scholar] [CrossRef]





| Compd | E1a (V) | λonset b (nm) | Eg,optc (eV) | LUMO Level d (eV) | HOMO Level e (eV) |
|---|---|---|---|---|---|
| 1 | −1.028 | 679 | 1.826 | −3.772 | −5.598 |
| 2 | −1.096 | 687 | 1.805 | −3.704 | −5.509 |
| 3 | −1.148 | 690 | 1.797 | −3.652 | −5.449 |
| 4 | −1.051 | 672 | 1.845 | −3.749 | −5.594 |
| PCBM | −1.164 | 695 | 1.784 | −3.636 | −5.420 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, X.-X.; Niu, C.; Ye, S.-Q.; Zhao, B.-C.; Wang, G.-W. Electrochemically Promoted Benzylation of [60]Fullerooxazolidinone. Nanomaterials 2022, 12, 2281. https://doi.org/10.3390/nano12132281
Yan X-X, Niu C, Ye S-Q, Zhao B-C, Wang G-W. Electrochemically Promoted Benzylation of [60]Fullerooxazolidinone. Nanomaterials. 2022; 12(13):2281. https://doi.org/10.3390/nano12132281
Chicago/Turabian StyleYan, Xing-Xing, Chuang Niu, Shi-Qi Ye, Bo-Chen Zhao, and Guan-Wu Wang. 2022. "Electrochemically Promoted Benzylation of [60]Fullerooxazolidinone" Nanomaterials 12, no. 13: 2281. https://doi.org/10.3390/nano12132281
APA StyleYan, X.-X., Niu, C., Ye, S.-Q., Zhao, B.-C., & Wang, G.-W. (2022). Electrochemically Promoted Benzylation of [60]Fullerooxazolidinone. Nanomaterials, 12(13), 2281. https://doi.org/10.3390/nano12132281