Antimony Nanoparticles Encapsulated in Self-Supported Organic Carbon with a Polymer Network for High-Performance Lithium-Ion Batteries Anode
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis of the3DPNS-Sb/C Composites
2.3. Materials Characterization
2.4. Electrochemical Characterization
3. Results and Discussion
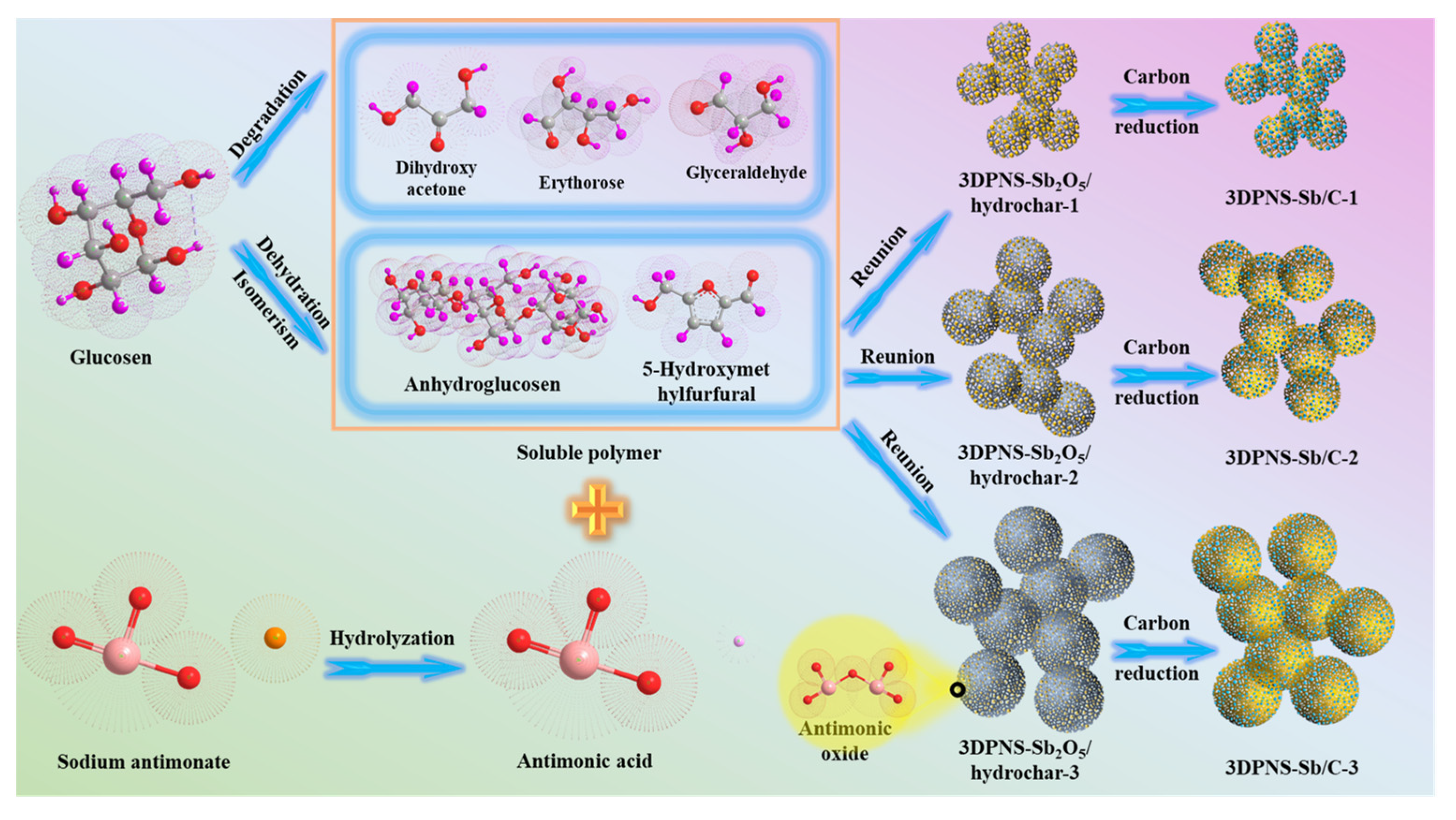
3.1. Experimental Synthesis Mechanism
3.2. Morphology Analysis
3.3. Microstructure and Component Analysis
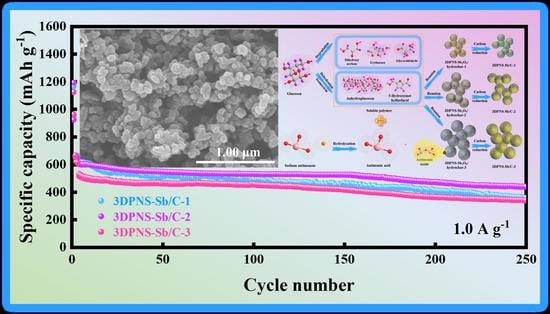
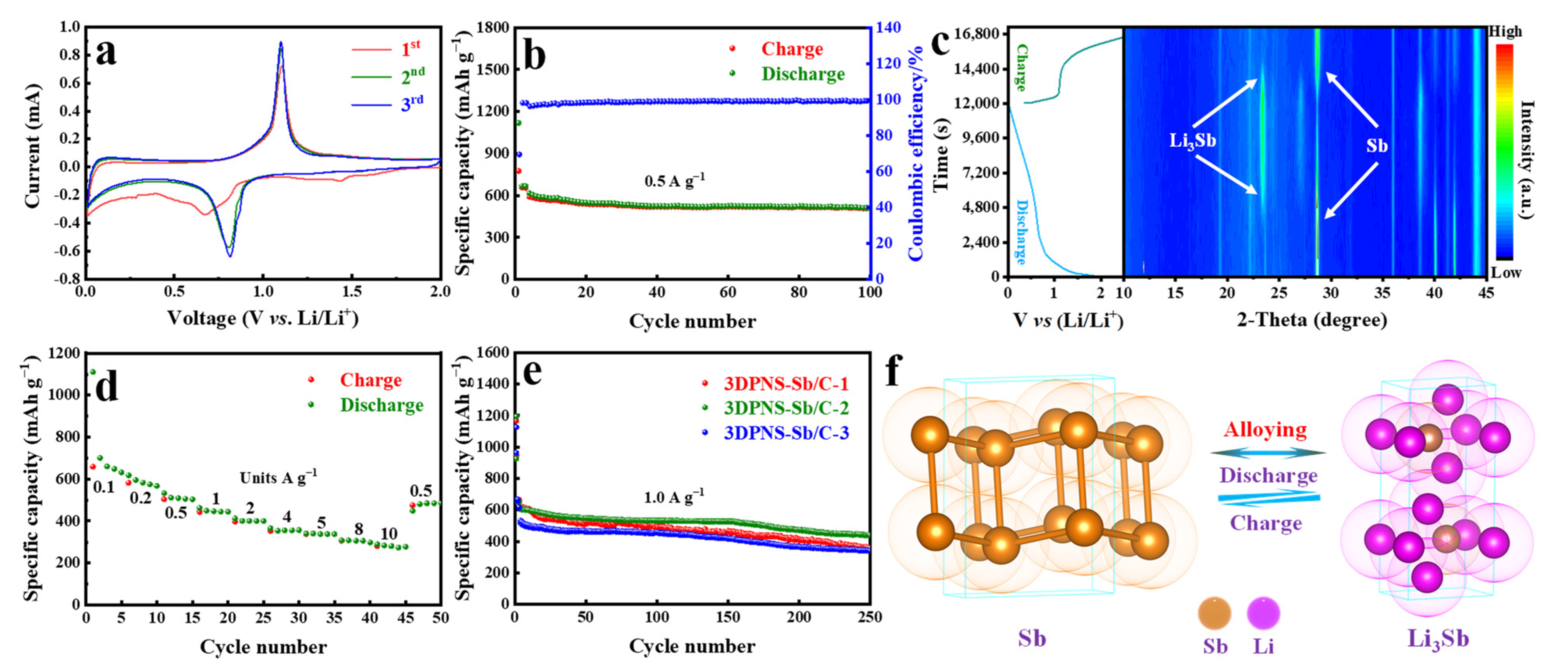
4. Electrochemical Evaluation in LIBs
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Demirocak, D.E.; Srinivasan, S.S.; Stefanakos, E.K. A Review on Nanocomposite Materials for Rechargeable Li-ion Batteries. Appl. Sci. 2017, 7, 731. [Google Scholar] [CrossRef] [Green Version]
- Deng, D. Li-ion batteries: Basics, progress, and challenges. Energy Sci. Eng. 2015, 3, 385–418. [Google Scholar] [CrossRef]
- Shahjalal, M.; Roy, P.K.; Shams, T.; Fly, A.; Chowdhury, J.I.; Ahmed, R.; Liu, K. A review on second-life of Li-ion batteries: Prospects, challenges, and issues. Energy 2021, 241, 122881. [Google Scholar] [CrossRef]
- Tian, Y.; Zeng, G.; Rutt, A.; Shi, T.; Kim, H.; Wang, J.; Koettgen, J.; Sun, Y.; Ouyang, B.; Chen, T.; et al. Promises and Challenges of Next-Generation “Beyond Li-ion” Batteries for Electric Vehicles and Grid Decarbonization. Chem. Rev. 2020, 121, 1623–1669. [Google Scholar] [CrossRef]
- Nie, P.; Le, Z.; Chen, G.; Liu, D.; Liu, X.; Bin Wu, H.; Xu, P.; Li, X.; Liu, F.; Chang, L.; et al. Graphene Caging Silicon Particles for High-Performance Lithium-Ion Batteries. Small 2018, 14, e1800635. [Google Scholar] [CrossRef] [PubMed]
- Ong, S.P.; Chevrier, V.L.; Hautier, G.; Jain, A.; Moore, C.; Kim, S.; Ma, X.; Ceder, G. Voltage, stability and diffusion barrier differences between sodium-ion and lithium-ion intercalation materials. Energy Environ. Sci. 2011, 4, 3680–3688. [Google Scholar] [CrossRef] [Green Version]
- Roselin, L.S.; Juang, R.-S.; Hsieh, C.-T.; Sagadevan, S.; Umar, A.; Selvin, R.; Hegazy, H.H. Recent Advances and Perspectives of Carbon-Based Nanostructures as Anode Materials for Li-ion Batteries. Materials 2019, 12, 1229. [Google Scholar] [CrossRef] [Green Version]
- Roy, P.; Srivastava, S.K. Nanostructured anode materials for lithium ion batteries. J. Mater. Chem. A 2014, 3, 2454–2484. [Google Scholar] [CrossRef]
- Saritha, D. A concise review on the advancement of anode materials for Li-ion batteries. Mater. Today Proc. 2019, 19, 726–730. [Google Scholar] [CrossRef]
- Fan, L.; Zhang, J.; Cui, J.; Zhu, Y.; Liang, J.; Wang, L.; Qian, Y. Electrochemical performance of rod-like Sb-C composite as anodes for Li-ion and Na-ion batteries. J. Mater. Chem. A 2015, 3, 3276–3280. [Google Scholar] [CrossRef]
- He, M.; Kravchyk, K.; Walter, M.; Kovalenko, M.V. Monodisperse Antimony Nanocrystals for High-Rate Li-ion and Na-ion Battery Anodes: Nano versus Bulk. Nano Lett. 2014, 14, 1255–1262. [Google Scholar] [CrossRef]
- Hou, H.; Jing, M.; Yang, Y.; Zhu, Y.; Fang, L.; Song, W.; Pan, C.; Yang, X.; Ji, X. Sodium/Lithium Storage Behavior of Antimony Hollow Nanospheres for Rechargeable Batteries. ACS Appl. Mater. Interfaces 2014, 6, 16189–16196. [Google Scholar] [CrossRef]
- Liu, J.; Yu, L.; Wu, C.; Wen, Y.; Yin, K.; Chiang, F.-K.; Hu, R.; Liu, J.; Sun, L.; Gu, L.; et al. New Nanoconfined Galvanic Replacement Synthesis of Hollow Sb@C Yolk–Shell Spheres Constituting a Stable Anode for High-Rate Li/Na-Ion Batteries. Nano Lett. 2017, 17, 2034–2042. [Google Scholar] [CrossRef]
- Yang, Q.; Zhou, J.; Zhang, G.; Guo, C.; Li, M.; Zhu, Y.; Qian, Y. Sb nanoparticles uniformly dispersed in 1-D N-doped porous carbon as anodes for Li-ion and Na-ion batteries. J. Mater. Chem. A 2017, 5, 12144–12148. [Google Scholar] [CrossRef]
- Yuan, Y.; Jan, S.; Wang, Z.; Jin, X. A simple synthesis of nanoporous Sb/C with high Sb content and dispersity as an advanced anode for sodium ion batteries. J. Mater. Chem. A 2018, 6, 5555–5559. [Google Scholar] [CrossRef]
- Wang, B.; Deng, Z.; Xia, Y.; Hu, J.; Li, H.; Wu, H.; Zhang, Q.; Zhang, Y.; Liu, H.; Dou, S. Realizing Reversible Conversion-Alloying of Sb(V) in Polyantimonic Acid for Fast and Durable Lithium- and Potassium-Ion Storage. Adv. Energy Mater. 2019, 10, 1903119. [Google Scholar] [CrossRef]
- Darwiche, A.; Marino, C.; Sougrati, M.T.; Fraisse, B.; Stievano, L.; Monconduit, L. Better Cycling Performances of Bulk Sb in Na-Ion Batteries Compared to Li-Ion Systems: An Unexpected Electrochemical Mechanism. J. Am. Chem. Soc. 2012, 134, 20805–20811. [Google Scholar] [CrossRef]
- Ding, Y.-L.; Wu, C.; Kopold, P.; van Aken, P.A.; Maier, J.; Yu, Y. Graphene-Protected 3D Sb-based Anodes Fabricated via Electrostatic Assembly and Confinement Replacement for Enhanced Lithium and Sodium Storage. Small 2015, 11, 6026–6035. [Google Scholar] [CrossRef]
- Ko, Y.N.; Kang, Y.C. Electrochemical properties of ultrafine Sb nanocrystals embedded in carbon microspheres for use as Na-ion battery anode materials. Chem. Commun. 2014, 50, 12322–12324. [Google Scholar] [CrossRef]
- Luo, W.; Li, F.; Gaumet, J.-J.; Magri, P.; Diliberto, S.; Zhou, L.; Mai, L. Bottom-Up Confined Synthesis of Nanorod-in-Nanotube Structured Sb@N-C for Durable Lithium and Sodium Storage. Adv. Energy Mater. 2018, 8, 1703237. [Google Scholar] [CrossRef]
- Han, Q.; Zhang, X.; Li, X.; Li, Y.; Zhang, W.; Sheng, Y. Temperature-Dependent Nanopolyhedron Carbon-Decorated Sb for High-Performance Lithium-Ion Batteries. ChemElectroChem 2021, 8, 1486–1492. [Google Scholar] [CrossRef]
- Kim, D.; Kim, H.; Lim, H.; Kim, K.J.; Jung, H.G.; Byun, D.; Kim, C.; Choi, W. A facile control in free-carbon domain with divinylbenzene for the high-rate-performing Sb/SiOC composite anode material in sodium-ion batteries. Int. J. Energy Res. 2020, 44, 11473–11486. [Google Scholar] [CrossRef]
- Le, H.T.T.; Pham, X.-M.; Park, C.-J. Facile citrate gel synthesis of an antimony–carbon nanosponge with enhanced lithium storage. New J. Chem. 2019, 43, 10716–10725. [Google Scholar] [CrossRef]
- Li, Q.; Liang, Z.; Zhang, W.; Lin, D.; Wang, G.; Wang, J.; Guang, C.; Huang, S. Constructing a hierarchical Sb@C nanoarchitectures as free-standing anode for high-performance lithium-ion batteries. Mater. Lett. 2021, 303, 130563. [Google Scholar] [CrossRef]
- Liang, S.; Cheng, Y.-J.; Wang, X.; Xu, Z.; Ma, L.; Xu, H.; Ji, Q.; Zuo, X.; Müller-Buschbaum, P.; Xia, Y. Impact of CO2 activation on the structure, composition, and performance of Sb/C nanohybrid lithium/sodium-ion battery anodes. Nanoscale Adv. 2021, 3, 1942–1953. [Google Scholar] [CrossRef]
- Liang, S.-Z.; Wang, X.-Y.; Xia, Y.-G.; Xia, S.-L.; Metwalli, E.; Qiu, B.; Ji, Q.; Yin, S.-S.; Xie, S.; Fang, K.; et al. Scalable Synthesis of Hierarchical Antimony/Carbon Micro-/Nanohybrid Lithium/Sodium-Ion Battery Anodes Based on Dimethacrylate Monomer. Acta Metall. Sin. 2018, 31, 910–922. [Google Scholar] [CrossRef] [Green Version]
- Ma, Z.; Chen, X.; Wu, H.; Xiao, Y.; Feng, C. Synthesis and electrochemical properties of Pb/Sb@C composite for lithium-ion battery application. Ionics 2020, 26, 5343–5348. [Google Scholar] [CrossRef]
- Mao, Y.; Chen, R.; You, H.; Liu, Y.; Luan, S.; Hou, L.; Gao, F. Advanced performance of S and N co-doped Sb@CNFs with a 3D conductive network as superior lithium-ion battery anodes. J. Alloy. Compd. 2022, 904, 164000. [Google Scholar] [CrossRef]
- Song, J.; Xiao, D.; Jia, H.; Zhu, G.; Engelhard, M.; Xiao, B.; Feng, S.; Li, D.; Reed, D.; Sprenkle, V.L.; et al. A comparative study of pomegranate Sb@C yolk–shell microspheres as Li and Na-ion battery anodes. Nanoscale 2019, 11, 348–355. [Google Scholar] [CrossRef]
- Tian, J.; Yang, H.; Fu, C.; Sun, M.; Wang, L.; Liu, T. In-situ synthesis of microspherical Sb@C composite anode with high tap density for lithium/sodium-ion batteries. Compos. Commun. 2020, 17, 177–181. [Google Scholar] [CrossRef]
- Wang, L.; Zhu, L.; Zhang, W.; Ding, G.; Yang, G.; Xie, L.; Cao, X. Revealing the unique process of alloying reaction in Ni-Co-Sb/C nanosphere anode for high-performance lithium storage. J. Colloid Interface Sci. 2020, 586, 730–740. [Google Scholar] [CrossRef]
- Wang, Z.; Qu, J.; Hao, S.; Zhang, Y.; Kong, F.; Yang, D.; Yu, Z. Sb Nanoparticles Embedded in a Nitrogen-Doped Carbon Matrix with Tuned Voids and Interfacial Bonds for High-Rate Lithium Storage. ChemElectroChem 2018, 5, 2653–2659. [Google Scholar] [CrossRef]
- Wen, J.; Pei, Y.; Liu, L.; Su, D.; Yang, M.; Wang, Q.; Zhang, W.; Dai, J.; Feng, Y.; Wu, T.; et al. Fully encapsulated Sb2Se3/Sb/C nanofibers: Towards high-rate, ultralong-lifespan lithium-ion batteries. J. Alloy. Compd. 2021, 874, 159961. [Google Scholar] [CrossRef]
- Wu, Y.; Pan, Q.; Zheng, F.; Ou, X.; Yang, C.; Xiong, X.; Liu, M.; Hu, D.; Huang, C. Sb@C/expanded graphite as high-performance anode material for lithium ion batteries. J. Alloy. Compd. 2018, 744, 481–486. [Google Scholar] [CrossRef]
- Zeng, T.; Hu, X.; Ji, P.; Peng, Q.; Shang, B.; Gong, S. General synthesis of nano-M embedded Li4Ti5O12/C composites (M = Sn, Sb and Bi) with high capacity and good cycle stability. Electrochim. Acta 2016, 217, 299–309. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, G.; Bin Wu, H.; Yu, L.; Lou, X.W. Hierarchical Tubular Structures Constructed by Carbon-Coated SnO2 Nanoplates for Highly Reversible Lithium Storage. Adv. Mater. 2013, 25, 2589–2593. [Google Scholar] [CrossRef]
- Xu, Y.; Zhu, Y.; Liu, Y.; Wang, C. Electrochemical Performance of Porous Carbon/Tin Composite Anodes for Sodium-Ion and Lithium-Ion Batteries. Adv. Energy Mater. 2012, 3, 128–133. [Google Scholar] [CrossRef]
- Li, H.; Zhou, H. Enhancing the performances of Li-ion batteries by carbon-coating: Present and future. Chem. Commun. 2011, 48, 1201–1217. [Google Scholar] [CrossRef]
- Luo, W.; Lorger, S.; Wang, B.; Bommier, C.; Ji, X. Facile synthesis of one-dimensional peapod-like Sb@C submicron-structures. Chem. Commun. 2014, 50, 5435–5437. [Google Scholar] [CrossRef]
- Fan, L.; Zhu, Y.; Zhang, J.; Liang, J.; Wang, L.; Wei, D.; Li, X.; Qian, Y. Uniformly dispersed Sn-MnO@C nanocomposite derived from MnSn(OH)6 precursor as anode material for lithium-ion batteries. Electrochim. Acta 2014, 121, 21–26. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Z.; Liu, W.; Xiao, W.; Lou, X.W. Amorphous CoSnO3@C nanoboxes with superior lithium storage capability. Energy Environ. Sci. 2013, 6, 87–91. [Google Scholar] [CrossRef]
- Li, L.; Seng, K.H.; Li, D.; Xia, Y.Y.; Liu, H.K.; Guo, Z.P. SnSb@carbon nanocable anchored on graphene sheets for sodium ion batteries. Nano Res. 2014, 7, 1466–1476. [Google Scholar] [CrossRef]
- Sougrati, M.T.; Fullenwarth, J.; Debenedetti, A.; Fraisse, B.; Jumas, J.C.; Monconduit, L. TiSnSb a new efficient negative electrode for Li-ion batteries: Mechanism investigations by operando-XRD and Mössbauer techniques. J. Mater. Chem. 2011, 21, 10069–10076. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Wang, C.; Zhang, N.; Li, F.; Cheng, F.; Chen, J. High Anode Performance of in Situ Formed Cu2Sb Nanoparticles Integrated on Cu Foil via Replacement Reaction for Sodium-Ion Batteries. ACS Energy Lett. 2016, 2, 256–262. [Google Scholar] [CrossRef]
- Xu, J.; Wu, H.; Wang, F.; Xia, Y.; Zheng, G. Zn4Sb3 Nanotubes as Lithium Ion Battery Anodes with High Capacity and Cycling Stability. Adv. Energy Mater. 2013, 3, 286–289. [Google Scholar] [CrossRef]
- Yang, R.; Huang, J.; Zhao, W.; Lai, W.; Zhang, X.; Zheng, J.; Li, X. Bubble assisted synthesis of Sn–Sb–Cu alloy hollow nanostructures and their improved lithium storage properties. J. Power Sources 2010, 195, 6811–6816. [Google Scholar] [CrossRef]
- Sevilla, M.; Fuertes, A.B. Chemical and Structural Properties of Carbonaceous Products Obtained by Hydrothermal Carbonization of Saccharides. Chem.–A Eur. J. 2009, 15, 4195–4203. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Yu, L.; Ji, S.; Xu, X.; Liu, Z.; Liu, J.; Liu, J. Facile synthesis of three-dimensional porous interconnected carbon matrix embedded with Sb nanoparticles as superior anode for Na-ion batteries. Chem. Eng. J. 2019, 374, 502–510. [Google Scholar] [CrossRef]
- Liu, X.; Tian, Y.; Cao, X.; Li, X.; Le, Z.; Zhang, D.; Li, X.; Nie, P.; Li, H. Aerosol-Assisted Synthesis of Spherical Sb/C Composites as Advanced Anodes for Lithium Ion and Sodium Ion Batteries. ACS Appl. Energy Mater. 2018, 1, 6381–6387. [Google Scholar] [CrossRef]
- Lannin, J.S.; Calleja, J.M.; Cardona, M. Second-order Raman scattering in the group-Vbsemimetals: Bi, Sb, and As. Phys. Rev. B 1975, 12, 585–593. [Google Scholar] [CrossRef]
- Ramireddy, T.; Rahman, M.; Xing, T.; Chen, Y.; Glushenkov, A.M. Stable anode performance of an Sb–carbon nanocomposite in lithium-ion batteries and the effect of ball milling mode in the course of its preparation. J. Mater. Chem. A 2014, 2, 4282–4291. [Google Scholar] [CrossRef] [Green Version]
- Ramireddy, T.; Sharma, N.; Xing, T.; Chen, Y.; Leforestier, J.; Glushenkov, A.M. Size and Composition Effects in Sb-Carbon Nanocomposites for Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2016, 8, 30152–30164. [Google Scholar] [CrossRef]
- Wang, X.; Kunc, K.; Loa, I.; Schwarz, U.; Syassen, K. Effect of pressure on the Raman modes of antimony. Phys. Rev. B 2006, 74, 134305. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez, J.R.; Hamann, H.J.; Mitchell, G.M.; Ortalan, V.; Pol, V.G.; Ramachandran, P.V. Three-Dimensional Antimony Nanochains for Lithium-Ion Storage. ACS Appl. Nano Mater. 2019, 2, 5351–5355. [Google Scholar] [CrossRef]
- Bodenes, L.; Darwiche, A.; Monconduit, L.; Martinez, H. The Solid Electrolyte Interphase a key parameter of the high performance of Sb in sodium-ion batteries: Comparative X-ray Photoelectron Spectroscopy study of Sb/Na-ion and Sb/Li-ion batteries. J. Power Sources 2015, 273, 14–24. [Google Scholar] [CrossRef]
- Marino, C.; Darwiche, A.; Dupre, N.; Wilhelm, H.A.; Lestriez, B.; Martinez, H.; Dedryvère, R.; Zhang, W.; Ghamouss, F.; Lemordant, D.; et al. Study of the Electrode/Electrolyte Interface on Cycling of a Conversion Type Electrode Material in Li Batteries. J. Phys. Chem. C 2013, 117, 19302–19313. [Google Scholar] [CrossRef]
- Pham, X.-M.; Ngo, D.T.; Le, H.T.T.; Didwal, P.N.; Verma, R.; Min, C.-W.; Park, C.-N.; Park, C.-J. A self-encapsulated porous Sb–C nanocomposite anode with excellent Na-ion storage performance. Nanoscale 2018, 10, 19399–19408. [Google Scholar] [CrossRef]
- Dailly, A.; Ghanbaja, J.; Willmann, P.; Billaud, D. Lithium insertion into new graphite–antimony composites. Electrochim. Acta 2003, 48, 977–984. [Google Scholar] [CrossRef]
- Sethuraman, V.; Srinivasan, V.; Newman, J. Analysis of Electrochemical Lithiation and Delithiation Kinetics in Silicon. J. Electrochem. Soc. 2012, 160, A394–A403. [Google Scholar] [CrossRef]
- Sethuraman, V.A.; Srinivasan, V.; Bower, A.F.; Guduru, P.R. In Situ Measurements of Stress-Potential Coupling in Lithiated Silicon. J. Electrochem. Soc. 2010, 157, A1253–A1261. [Google Scholar] [CrossRef]
- Kim, H.; Cho, J. Template Synthesis of Hollow Sb Nanoparticles as a High-Performance Lithium Battery Anode Material. Chem. Mater. 2008, 20, 1679–1681. [Google Scholar] [CrossRef]
- Zhu, J.; Sun, T.; Chen, J.; Shi, W.; Zhang, X.; Lou, X.; Mhaisalkar, S.; Hng, H.H.; Boey, F.; Ma, J.; et al. Controlled Synthesis of Sb Nanostructures and Their Conversion to CoSb3 Nanoparticle Chains for Li-Ion Battery Electrodes. Chem. Mater. 2010, 22, 5333–5339. [Google Scholar] [CrossRef]
- Lv, H.; Qiu, S.; Lu, G.; Fu, Y.; Li, X.; Hu, C.; Liu, J. Nanostructured Antimony/carbon Composite Fibers as Anode Material for Lithium-ion Battery. Electrochim. Acta 2015, 151, 214–221. [Google Scholar] [CrossRef]


| Material | Reversible Capacity/mAh g−1 | Current Density (mA g−1) | Areal Mass Loading (mg cm−2) | Batteries | Ref. |
|---|---|---|---|---|---|
| Hollow Sb Nanoparticles | 615/100th cycles | 120 | Li-ion | [61] | |
| Sb nanoparticles | 120/70th cycles | 120 | Li-ion | [62] | |
| Sb-carbon nanocomposite | 550/250th cycles | 230 | 1.07–1.11 | Li-ion | [51] |
| Sb/C composite fibers | 315.9/100th cycles | 100 | Li-ion | [63] | |
| Sb HNSs | 627.3/50th cycles | 100 | Li-ion | [12] | |
| Sb nanocrystals | 600/100th cycles | 660 | Li-ion | [11] | |
| Spherical Sb/C Composites | 590/80th cycles | 100 | 1 | Li-ion | [49] |
| Sb@C nanosponges | 447.1/500th cycles | 660 | 1.5 | Li-ion | [23] |
| Sb/C micro-/nanohybrid | 793/100th cycles | 66 | Li-ion | [26] | |
| Sb@C composites | 598.6/100th cycles | 100 | 1.132 | Li-ion | [21] |
| Sb/C/G nanocomposites | 413/700th cycles | 1000 | 1.0 | Li-ion | [32] |
| Sb/NPC | 556/100th cycles | 200 | 1.00 | Li-ion | [14] |
| Sb@C composites | 280/500th cycles | 100 | 1.35 | Li-ion | [30] |
| Sb@CNFs | 394.5/2000th cycles | 2000 | 0.8 | Li-ion | [28] |
| Sb2Se3/Sb/C nanofibers | 764/300th cycles | 100 | Li-ion | [33] | |
| Sb@C/EG | 486/600th cycles | 1000 | 0.5 | Li-ion | [34] |
| Ni-Co-Sb/C Nanosphere | 354/100th cycles | 100 | ~0.55 | Li-ion | [31] |
| Sb@C | 525/400th cycles | 500 | 1.2–1.5 | Li-ion | [24] |
| 3DPNS-Sb/C composites | 511.5/100th cycles | 500 | 0.93–1.12 | Li-ion | this work (586 Wh L−1) (ICE: 69.35%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Zeng, F.; Zhang, D.; Shen, Y.; Wang, S.; Cheng, Y.; Li, C.; Wang, L. Antimony Nanoparticles Encapsulated in Self-Supported Organic Carbon with a Polymer Network for High-Performance Lithium-Ion Batteries Anode. Nanomaterials 2022, 12, 2322. https://doi.org/10.3390/nano12142322
Wang Z, Zeng F, Zhang D, Shen Y, Wang S, Cheng Y, Li C, Wang L. Antimony Nanoparticles Encapsulated in Self-Supported Organic Carbon with a Polymer Network for High-Performance Lithium-Ion Batteries Anode. Nanomaterials. 2022; 12(14):2322. https://doi.org/10.3390/nano12142322
Chicago/Turabian StyleWang, Zhaomin, Fanming Zeng, Dongyu Zhang, Yabin Shen, Shaohua Wang, Yong Cheng, Chun Li, and Limin Wang. 2022. "Antimony Nanoparticles Encapsulated in Self-Supported Organic Carbon with a Polymer Network for High-Performance Lithium-Ion Batteries Anode" Nanomaterials 12, no. 14: 2322. https://doi.org/10.3390/nano12142322