A First Assessment of Carbon Nanotubes Grown on Oil-Well Cement via Chemical Vapor Deposition
Abstract
:1. Introduction
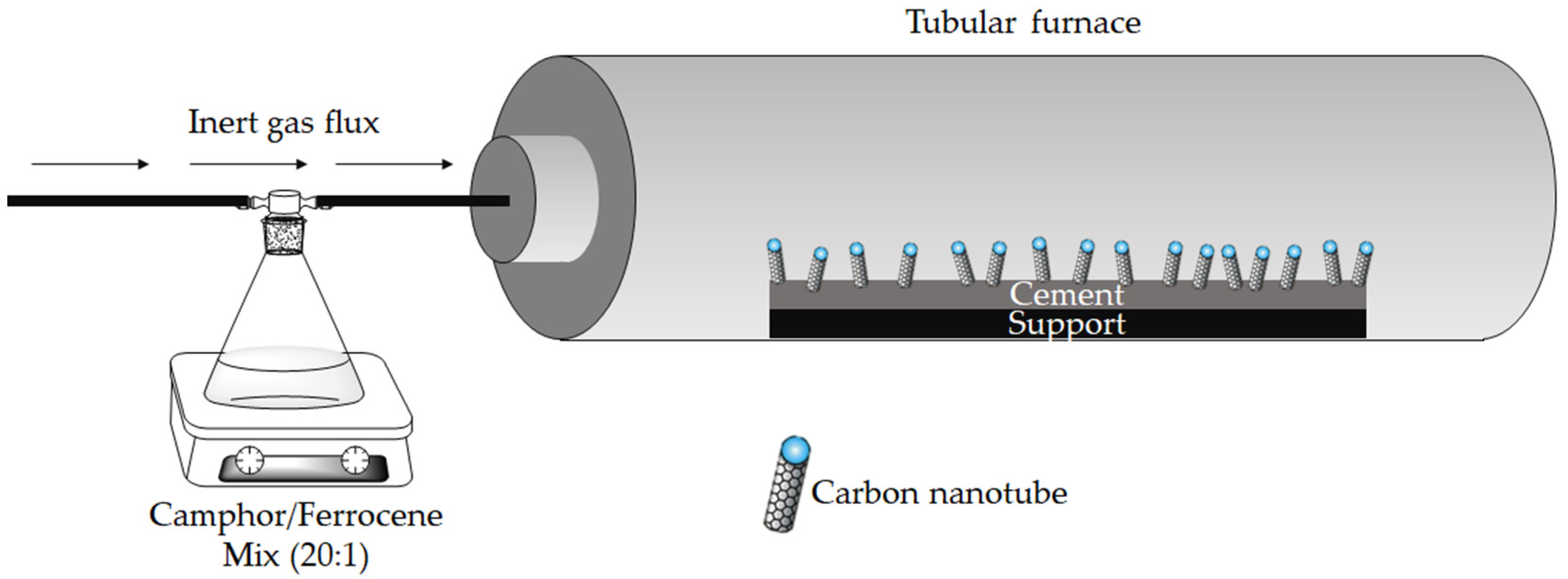
2. Materials and Methods
3. Results and Discussion
3.1. Assessment Analysis of CNTs Based Cement Composites
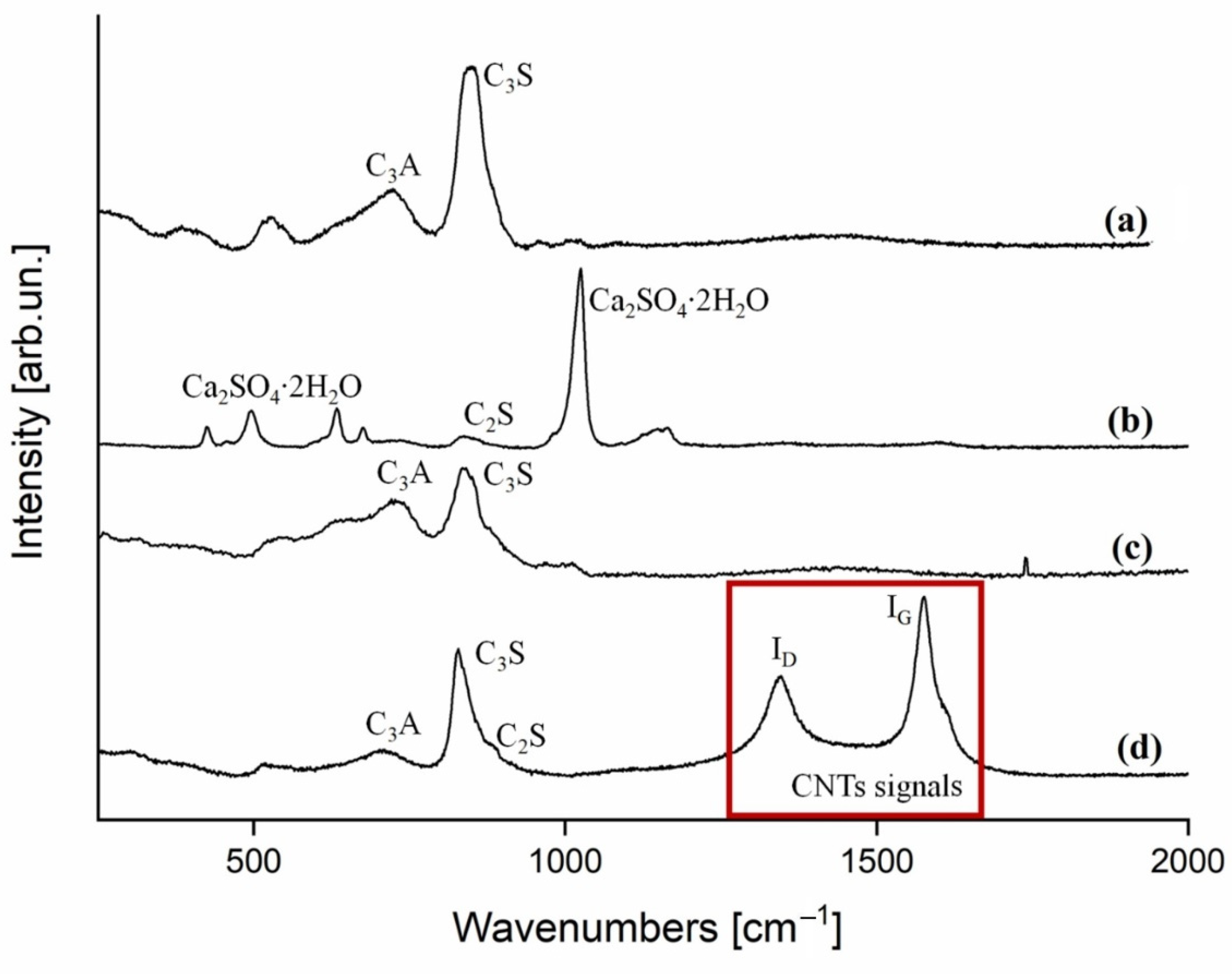
3.2. X-ray Diffraction Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Radushkevich, L.; Lukyanovich, V.Á. O strukture ugleroda, obrazujucegosja pri termiceskom razlozenii okisi ugleroda na zeleznom kontakte. Zurn Fis. Chim. 1952, 26, 88–95. [Google Scholar]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Iijima, S.; Ichihashi, T. Single-shell carbon nanotubes of 1-nm diameter. Nature 1993, 363, 603–605. [Google Scholar] [CrossRef]
- Grobert, N. Carbon nanotubes–becoming clean. Mater. Today 2007, 10, 28–35. [Google Scholar] [CrossRef]
- Lavagna, L.; Massella, D.; Pantano, M.F.; Bosia, F.; Pugno, N.M.; Pavese, M. Grafting carbon nanotubes onto carbon fibres doubles their effective strength and the toughness of the composite. Compos. Sci. Technol. 2018, 166, 140–149. [Google Scholar] [CrossRef]
- Lavagna, L.; Marchisio, S.; Merlo, A.; Nisticò, R.; Pavese, M. Polyvinyl butyral-based composites with carbon nanotubes: Efficient dispersion as a key to high mechanical properties. Polym. Compos. 2020, 41, 3627–3637. [Google Scholar] [CrossRef]
- Kunwar, P.; Soman, P. Direct Laser Writing of Fluorescent Silver Nanoclusters: A Review of Methods and Applications. ACS Appl. Nano Mater. 2020, 3, 7325–7342. [Google Scholar] [CrossRef]
- Vinante, M.; Digregorio, G.; Lunelli, L.; Forti, S.; Musso, S.; Vanzetti, L.; Lui, A.; Pasquardini, L.; Giorcelli, M.; Tagliaferro, A. Human plasma protein adsorption on carbon-based materials. J. Nanosci. Nanotechnol. 2009, 9, 3785–3791. [Google Scholar] [CrossRef]
- Kumar, M.; Ando, Y. Chemical vapor deposition of carbon nanotubes: A review on growth mechanism and mass production. J. Nanosci. Nanotechnol. 2010, 10, 3739–3758. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Li, J. Carbon nanotube in different shapes. Mater. Today 2009, 12, 12–18. [Google Scholar] [CrossRef]
- Öncel, Ç.; Yürüm, Y. Carbon nanotube synthesis via the catalytic CVD method: A review on the effect of reaction parameters. Fuller. Nanotub. Carbon Nonstruct. 2006, 14, 17–37. [Google Scholar] [CrossRef] [Green Version]
- De Greef, N.; Zhang, L.; Magrez, A.; Forró, L.; Locquet, J.-P.; Verpoest, I.; Seo, J.W. Direct growth of carbon nanotubes on carbon fibers: Effect of the CVD parameters on the degradation of mechanical properties of carbon fibers. Diam. Relat. Mater. 2015, 51, 39–48. [Google Scholar] [CrossRef]
- Dong, X.; Li, B.; Wei, A.; Cao, X.; Chan-Park, M.B.; Zhang, H.; Li, L.-J.; Huang, W.; Chen, P. One-step growth of graphene–carbon nanotube hybrid materials by chemical vapor deposition. Carbon 2011, 49, 2944–2949. [Google Scholar] [CrossRef]
- Reales, O.A.M.; Toledo Filho, R.D. A review on the chemical, mechanical and microstructural characterization of carbon nanotubes-cement based composites. Constr. Build. Mater. 2017, 154, 697–710. [Google Scholar] [CrossRef]
- Azhari, F.; Banthia, N. Cement-based sensors with carbon fibers and carbon nanotubes for piezoresistive sensing. Cem. Concr. Compos. 2012, 34, 866–873. [Google Scholar] [CrossRef]
- Fu, X.; Lu, W.; Chung, D. Improving the strain-sensing ability of carbon fiber-reinforced cement by ozone treatment of the fibers. Cem. Concr. Res. 1998, 28, 183–187. [Google Scholar] [CrossRef]
- Materazzi, A.L.; Ubertini, F.; D’Alessandro, A. Carbon nanotube cement-based transducers for dynamic sensing of strain. Cem. Concr. Compos. 2013, 37, 2–11. [Google Scholar] [CrossRef]
- Tafesse, M.; Kim, H.-K. The role of carbon nanotube on hydration kinetics and shrinkage of cement composite. Compos. Part B Eng. 2019, 169, 55–64. [Google Scholar] [CrossRef]
- Yang, K.; Yi, Z.; Jing, Q.; Yue, R.; Jiang, W.; Lin, D. Sonication-assisted dispersion of carbon nanotubes in aqueous solutions of the anionic surfactant SDBS: The role of sonication energy. Chin. Sci. Bull. 2013, 58, 2082–2090. [Google Scholar] [CrossRef] [Green Version]
- Arrigo, R.; Teresi, R.; Gambarotti, C.; Parisi, F.; Lazzara, G.; Dintcheva, N.T. Sonication-induced modification of carbon nanotubes: Effect on the rheological and thermo-oxidative behaviour of polymer-based nanocomposites. Materials 2018, 11, 383. [Google Scholar] [CrossRef] [Green Version]
- Rausch, J.; Zhuang, R.-C.; Mäder, E. Surfactant assisted dispersion of functionalized multi-walled carbon nanotubes in aqueous media. Compos. Part A Appl. Sci. Manuf. 2010, 41, 1038–1046. [Google Scholar] [CrossRef]
- Lavagna, L.; Nisticò, R.; Musso, S.; Pavese, M. Functionalization as a way to enhance dispersion of carbon nanotubes in matrices: A review. Mater. Today Chem. 2021, 20, 100477. [Google Scholar] [CrossRef]
- Sinnott, S.B. Chemical functionalization of carbon nanotubes. J. Nanosci. Nanotechnol. 2002, 2, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Lavagna, L.; Bartoli, M.; Suarez-Riera, D.; Cagliero, D.; Musso, S.; Pavese, M. Oxidation of Carbon Nanotubes for Improving the Mechanical and Electrical Properties of Oil-Well Cement-Based Composites. ACS Appl. Nano Mater. 2022, 5, 6671–6678. [Google Scholar] [CrossRef]
- Huang, Y.Y.; Terentjev, E.M. Dispersion of carbon nanotubes: Mixing, sonication, stabilization, and composite properties. Polymers 2012, 4, 275–295. [Google Scholar] [CrossRef] [Green Version]
- Adhikary, S.K.; Rudžionis, Ž.; Rajapriya, R. The effect of carbon nanotubes on the flowability, mechanical, microstructural and durability properties of cementitious composite: An overview. Sustainability 2020, 12, 8362. [Google Scholar] [CrossRef]
- Lavagna, L.; Musso, S.; Pavese, M. A facile method to oxidize carbon nanotubes in controlled flow of oxygen at 350° C. Mater. Lett. 2021, 283, 128816. [Google Scholar] [CrossRef]
- Wepasnick, K.A.; Smith, B.A.; Schrote, K.E.; Wilson, H.K.; Diegelmann, S.R.; Fairbrother, D.H. Surface and structural characterization of multi-walled carbon nanotubes following different oxidative treatments. Carbon 2011, 49, 24–36. [Google Scholar] [CrossRef]
- Ludvig, P.; Calixto, J.M.; Ladeira, L.O.; Gaspar, I.C. Using converter dust to produce low cost cementitious composites by in situ carbon nanotube and nanofiber synthesis. Materials 2011, 4, 575–584. [Google Scholar] [CrossRef] [Green Version]
- de Paula, J.N.; Calixto, J.M.; Ladeira, L.O.; Ludvig, P.; Souza, T.C.C.; Rocha, J.M.; de Melo, A.A.V. Mechanical and rheological behavior of oil-well cement slurries produced with clinker containing carbon nanotubes. J. Pet. Sci. Eng. 2014, 122, 274–279. [Google Scholar] [CrossRef]
- Musso, S.; Porro, S.; Giorcelli, M.; Chiodoni, A.; Ricciardi, C.; Tagliaferro, A. Macroscopic growth of carbon nanotube mats and their mechanical properties. Carbon 2007, 45, 1133–1136. [Google Scholar] [CrossRef]
- Orlando, A.; Franceschini, F.; Muscas, C.; Pidkova, S.; Bartoli, M.; Rovere, M.; Tagliaferro, A. A Comprehensive Review on Raman Spectroscopy Applications. Chemosensors 2021, 9, 262. [Google Scholar] [CrossRef]
- Deng, C.-S.; Breen, C.; Yarwood, J.; Habesch, S.; Phipps, J.; Craster, B.; Maitland, G. Ageing of oilfield cement at high humidity: A combined FEG-ESEM and Raman microscopic investigation. J. Mater. Chem. 2002, 12, 3105–3112. [Google Scholar] [CrossRef]
- Tagliaferro, A.; Rovere, M.; Padovano, E.; Bartoli, M.; Giorcelli, M. Introducing the Novel Mixed Gaussian-Lorentzian Lineshape in the Analysis of the Raman Signal of Biochar. Nanomaterials 2020, 10, 1748. [Google Scholar] [CrossRef]
- West, R.R.; Sutton, W.J. Thermography of gypsum. J. Am. Ceram. Soc. 1954, 37, 221–224. [Google Scholar] [CrossRef]
- Ghaharpour, F.; Bahari, A.; Abbasi, M.; Ashkarran, A.A. Parametric investigation of CNT deposition on cement by CVD process. Constr. Build. Mater. 2016, 113, 523–535. [Google Scholar] [CrossRef]
- Oh, J.S.; Wheelock, T.D. Reductive decomposition of calcium sulfate with carbon monoxide: Reaction mechanism. Ind. Eng. Chem. Res. 1990, 29, 544–550. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Shen, X.; Tang, M.; Li, X. Stability of tricalcium silicate and other primary phases in portland cement clinker. Ind. Eng. Chem. Res. 2014, 53, 1954–1964. [Google Scholar] [CrossRef]
- Tenório, J.A.S.; Pereira, S.S.R.; Ferreira, A.V.; Espinosa, D.C.R.; da Silva Araújo, F.G. CCT diagrams of tricalcium silicate: Part I. Influence of the Fe2O3 content. Mater. Res. Bull. 2005, 40, 433–438. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lavagna, L.; Bartoli, M.; Musso, S.; Suarez-Riera, D.; Tagliaferro, A.; Pavese, M. A First Assessment of Carbon Nanotubes Grown on Oil-Well Cement via Chemical Vapor Deposition. Nanomaterials 2022, 12, 2346. https://doi.org/10.3390/nano12142346
Lavagna L, Bartoli M, Musso S, Suarez-Riera D, Tagliaferro A, Pavese M. A First Assessment of Carbon Nanotubes Grown on Oil-Well Cement via Chemical Vapor Deposition. Nanomaterials. 2022; 12(14):2346. https://doi.org/10.3390/nano12142346
Chicago/Turabian StyleLavagna, Luca, Mattia Bartoli, Simone Musso, Daniel Suarez-Riera, Alberto Tagliaferro, and Matteo Pavese. 2022. "A First Assessment of Carbon Nanotubes Grown on Oil-Well Cement via Chemical Vapor Deposition" Nanomaterials 12, no. 14: 2346. https://doi.org/10.3390/nano12142346
APA StyleLavagna, L., Bartoli, M., Musso, S., Suarez-Riera, D., Tagliaferro, A., & Pavese, M. (2022). A First Assessment of Carbon Nanotubes Grown on Oil-Well Cement via Chemical Vapor Deposition. Nanomaterials, 12(14), 2346. https://doi.org/10.3390/nano12142346










