A Preparation Method of Nano-Pesticide Improves the Selective Toxicity toward Natural Enemies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical Reagents
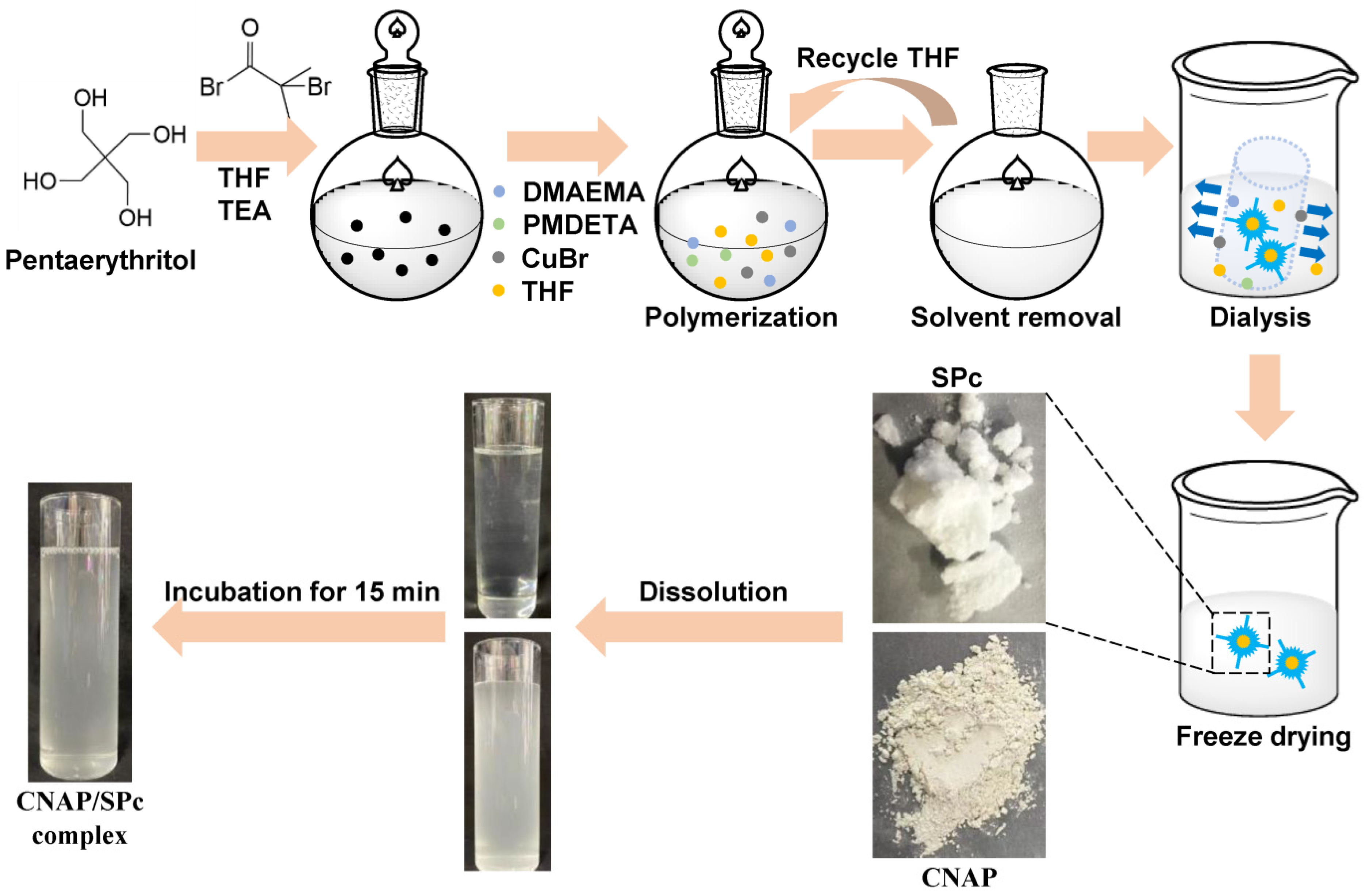
2.2. SPc Synthesis and Preparation of CNAP/SPc Complex
2.3. Loading Capacity Measurement
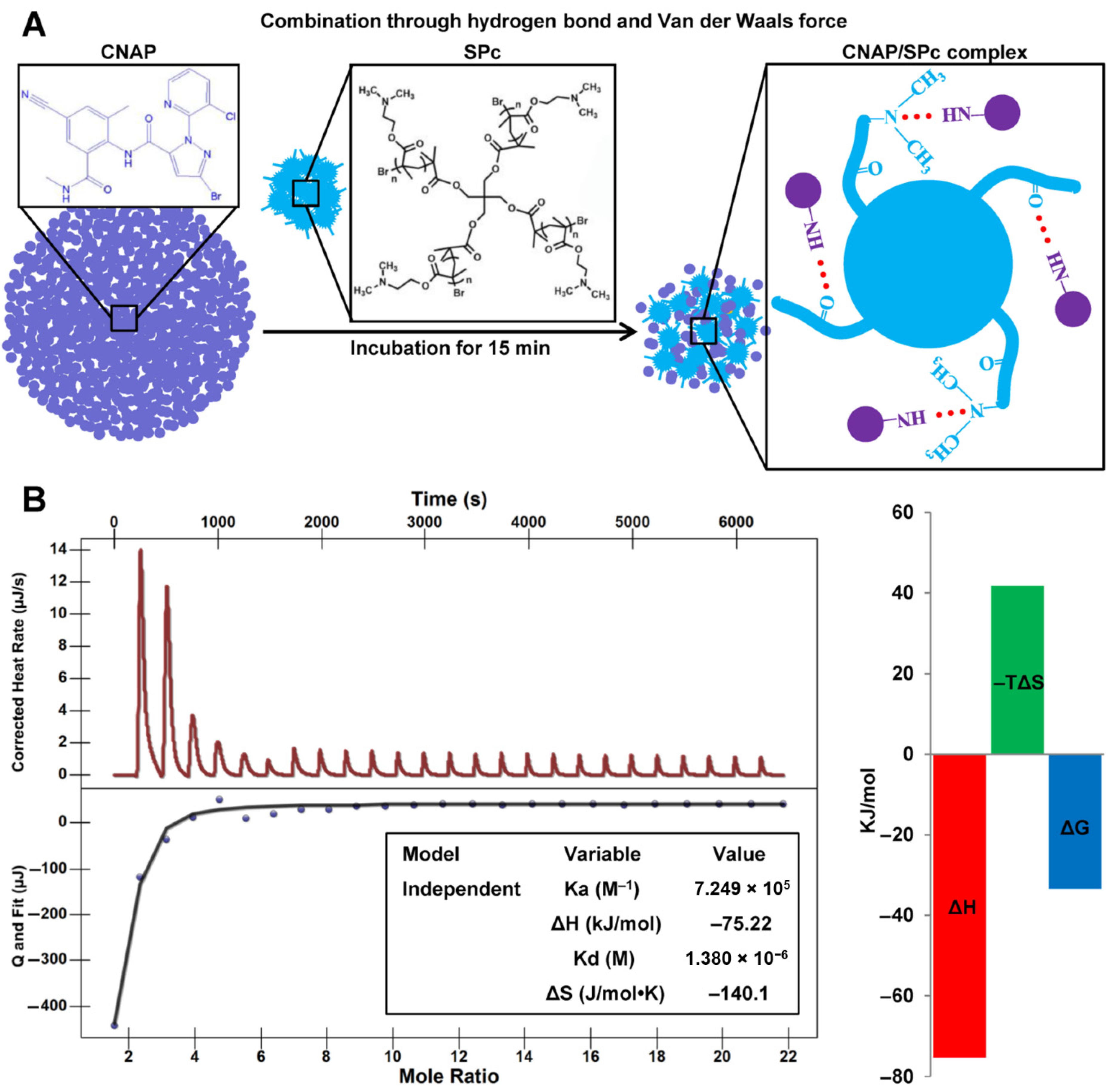
2.4. Isothermal Titration Calorimetry (ITC) Assay
2.5. Particle Size Measurement and Complex Morphology Characterization
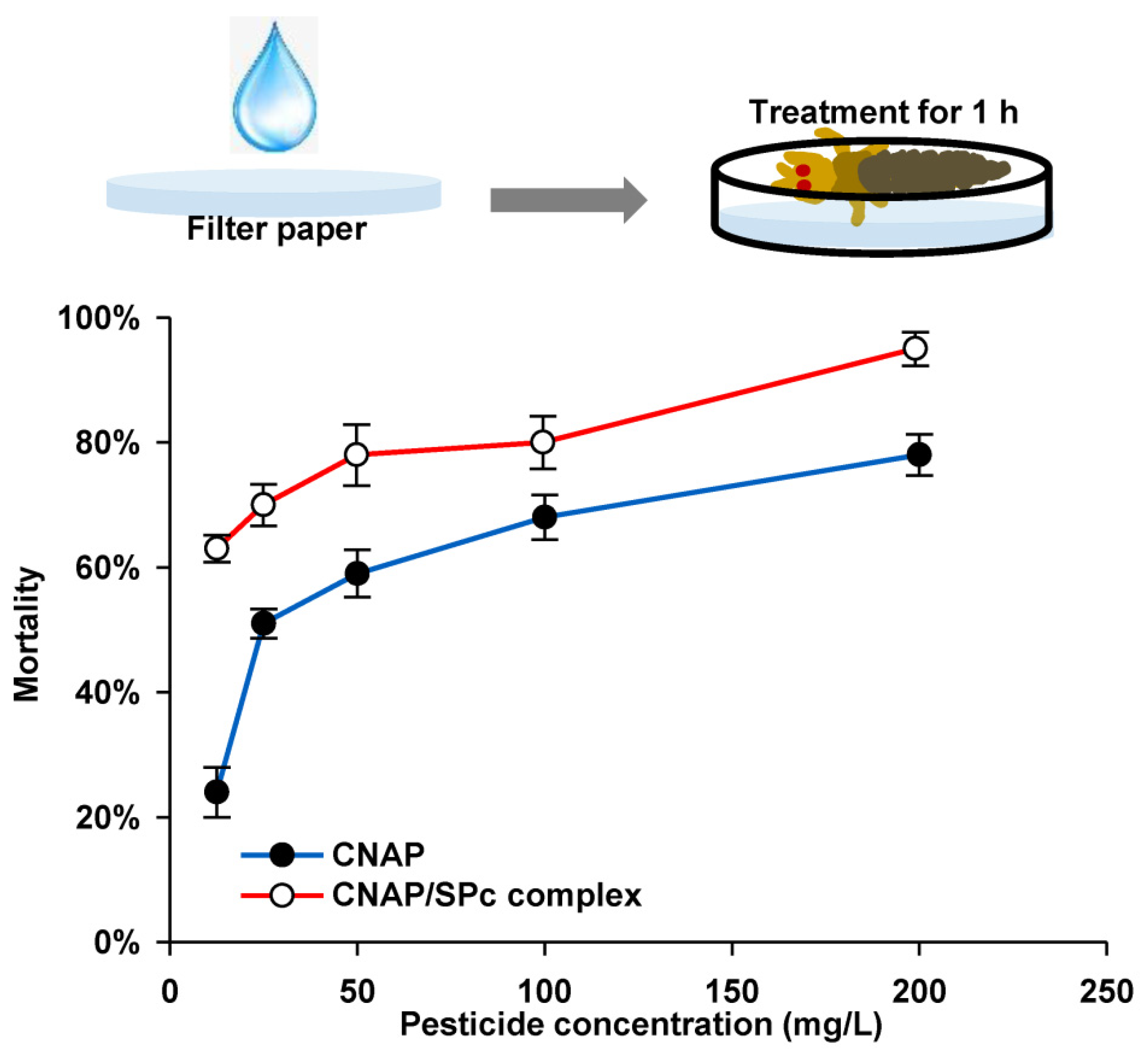
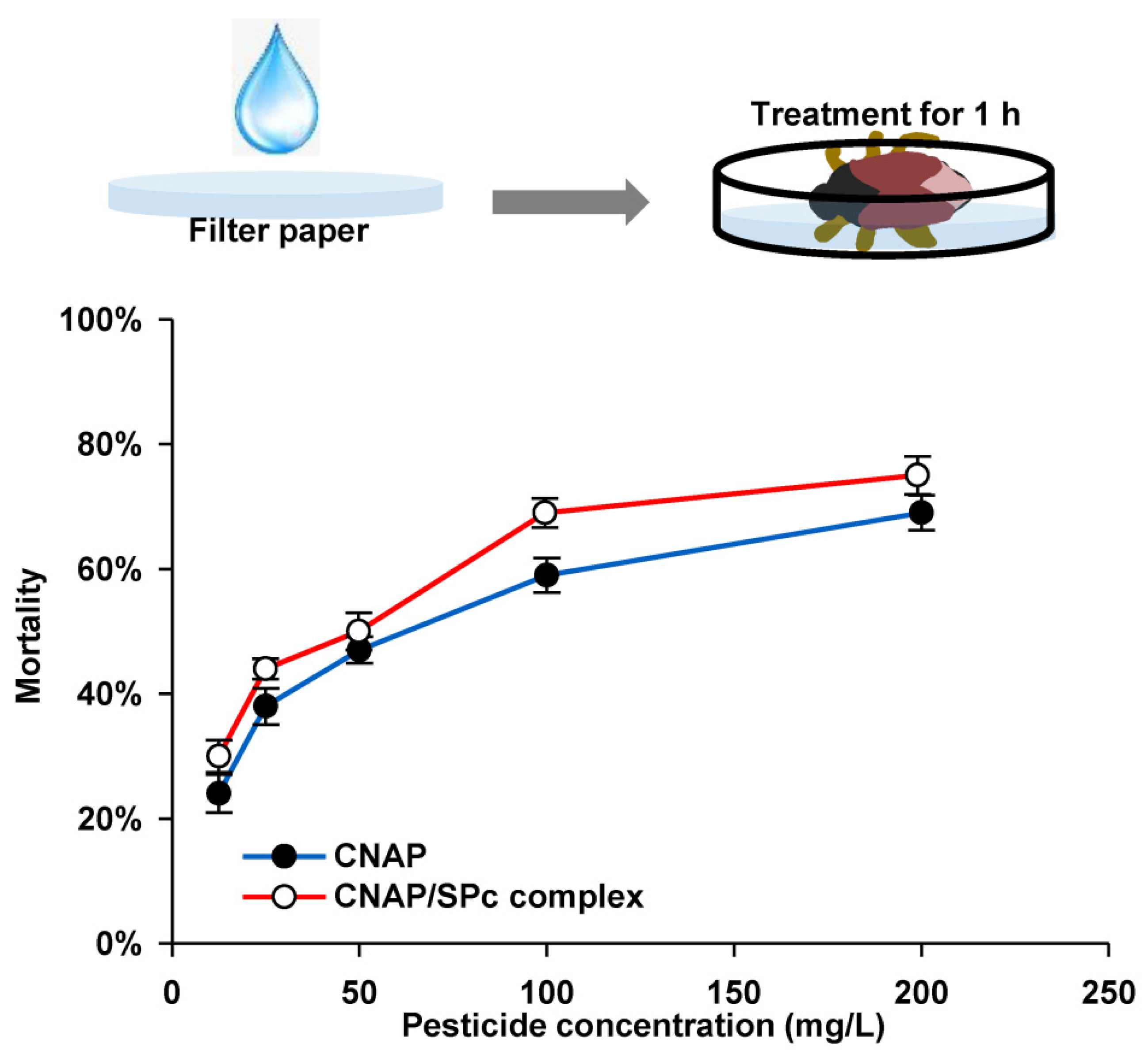
2.6. Bioassay of SPc-Loaded CNAP toward WFTs
2.7. Bioassy of SPc-Loaded CNAP toward Orius sauteri
2.8. Data Analysis
3. Results
3.1. SPc Synthesis and Its Loading Capacity
3.2. Interaction of CNAP with SPc
3.3. Characterization of CNAP/SPc Complex
3.4. Toxicity of CNAP/SPc Complex against WFTs
3.5. Selective Toxicity of CNAP/SPc Complex against O. sauteri
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kumari, P.; Ghosh, B.; Biswas, S. Nanocarriers for cancer-targeted drug delivery. J. Drug. Target. 2016, 24, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Hoosen, Y.; Pradeep, P.; Kumar, P.; Du Toit, L.C.; Choonara, Y.E.; Pillay, V. Nanotechnology and glycosaminoglycans: Paving the way forward for ovarian cancer intervention. Int. J. Mol. Sci. 2018, 19, 731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruiz-Gatón, L.; Espuelas, S.; Larrañeta, E.; Reviakine, I.; Yate, L.A.; Irache, J.M. Pegylated poly (anhydride) nanoparticles for oral delivery of docetaxel. Eur. J. Pharm. Sci. 2018, 118, 165–175. [Google Scholar] [CrossRef] [Green Version]
- Kang, E.J.; Baek, Y.M.; Hahm, E.; Lee, S.H.; Pham, X.H.; Noh, M.S.; Lim, D.E.; Jun, B.H. Functionalized β-cyclodextrin immobilized on Ag-embedded silica nanoparticles as a drug carrier. Int. J. Mol. Sci. 2019, 20, 315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhatia, R.; Sharma, A.; Narang, R.K.; Rawal, R.K. Recent nanocarrier approaches for targeted drug delivery in cancer therapy. Curr. Mol. Pharmacol. 2021, 14, 350–366. [Google Scholar] [CrossRef]
- Prasad, S.R.; Kumar, T.S.S.; Jayakrishnan, A. Nanocarrier-based drug delivery systems for bone cancer therapy: A review. Biomed. Mater. 2021, 16, 044107. [Google Scholar]
- Barahuie, F.; Hussein, M.Z.; Fakurazi, S.; Zainal, Z. Development of drug delivery systems based on layered hydroxides for nanomedicine. Int. J. Mol. Sci. 2014, 15, 7750–7786. [Google Scholar] [CrossRef] [Green Version]
- Gogos, A.; Knauer, K.; Bucheli, T.D. Nanomaterials in plant protection and fertilization: Current state, foreseen applications, and research priorities. J. Agric. Food Chem. 2012, 60, 9781–9792. [Google Scholar] [CrossRef]
- De Oliveira, J.L.; Campos, E.V.R.; Bakshi, M.; Abhilash, P.C.; Fraceto, L.F. Application of nanotechnology for the encapsulation of botanical insecticides for sustainable agriculture: Prospects and promises. Biotechnol. Adv. 2014, 32, 1550–1561. [Google Scholar] [CrossRef]
- Hou, Q.; Zhang, H.; Bao, L.; Song, Z.; Liu, C.; Jiang, Z.; Zheng, Y. NCs-delivered pesticides: A promising candidate in smart agriculture. Int. J. Mol. Sci. 2021, 22, 13043. [Google Scholar] [CrossRef]
- Zhang, Y.; Fu, L.; Li, S.; Yan, J.; Sun, M.; Giraldo, J.P.; Matyjaszewski, K.; Tilton, R.D.; Lowry, G.V. Star polymer size, charge content, and hydrophobicity affect their leaf uptake and translocation in plants. Environ. Sci. Technol. 2021, 55, 10758–10768. [Google Scholar] [CrossRef] [PubMed]
- Stejskal, V.; Vendl, T.; Aulicky, R.; Athanassiou, C. Synthetic and natural insecticides: Gas, liquid, gel and solid formulations for stored-product and food-industry. Insects 2021, 12, 590. [Google Scholar] [CrossRef]
- Zheng, Y.; You, S.; Ji, C.; Yin, M.; Yang, W.; Shen, J. Development of an amino acid-functionalized fluorescent nanocarrier to deliver a toxin to kill insect pests. Adv. Mater. 2016, 28, 1375–1380. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Hu, Y.; Yan, S.; Zhou, H.; Song, D.; Yin, M.; Shen, J. A polymer/detergent formulation improves dsRNA penetration through the body wall and RNAi-induced mortality in the soybean aphid Aphis glycines. Pest. Manag. Sci. 2019, 75, 1993–1999. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Cui, H.; Wang, Y.; Sun, C.; Cui, B.; Zeng, Z. Development strategies and prospects of nano-based smart pesticide formulation. J. Agric. Food Chem. 2017, 66, 6504–6512. [Google Scholar] [CrossRef]
- Cao, L.; Ma, D.; Zhou, Z.; Xu, C.; Cao, C.; Zhao, P.; Huang, Q. Efficient photocatalytic degradation of herbicide glyphosate in water by magnetically separable and recyclable BiOBr/Fe3O4 nanocomposites under visible light irradiation. Chem. Eng. J. 2019, 368, 212–222. [Google Scholar] [CrossRef]
- Yan, S.; Ren, B.; Zeng, B.; Shen, J. Improving RNAi efficiency for pest control in crop species. BioTechniques 2020, 68, 283–290. [Google Scholar] [CrossRef] [Green Version]
- Yan, S.; Ren, B.; Shen, J. Nanoparticle-mediated double-stranded RNA delivery system: A promising approach for sustainable pest management. Insect Sci. 2021, 28, 21–34. [Google Scholar] [CrossRef]
- Kumar, S.; Nehra, M.; Dilbaghi, N.; Marrazza, G.; Hassan, A.A.; Kim, K.H. Nano-based smart pesticide formulations: Emerging opportunities for agriculture. J. Control Release 2019, 294, 131–153. [Google Scholar] [CrossRef]
- Hao, L.; Gong, L.; Chen, L.; Guan, M.; Zhou, H.; Qiu, S.; Wen, H.; Chen, H.; Zhou, X.; Akbulut, M. Composite pesticide nanocarriers involving functionalized boron nitride nanoplatelets for pH-responsive release and enhanced UV stability. Chem. Eng. J. 2020, 396, 125233. [Google Scholar] [CrossRef]
- Xu, C.; Shan, Y.; Bilal, M.; Xu, B.; Cao, L.; Huang, Q. Copper ions chelated mesoporous silica nanoparticles via dopamine chemistry for controlled pesticide release regulated by coordination bonding. Chem. Eng. J. 2020, 395, 125093. [Google Scholar] [CrossRef]
- Chen, L.; Lin, Y.; Zhou, H.; Hao, L.; Chen, H.; Zhou, X. A stable polyamine-modified zein-based nanoformulation with high foliar affinity and lowered toxicity for sustained avermectin release. Pest. Manag. Sci. 2021, 77, 3300–3312. [Google Scholar] [CrossRef]
- Li, J.; Qian, J.; Xu, Y.; Yan, S.; Shen, J.; Yin, M. A facile-synthesized star polycation constructed as a highly efficient gene vector in pest management. ACS Sustain. Chem. Eng. 2019, 7, 6316–6322. [Google Scholar] [CrossRef]
- Yan, S.; Qian, J.; Cai, C.; Ma, Z.; Li, J.; Yin, M.; Ren, B.; Shen, J. Spray method application of transdermal dsRNA delivery system for efficient gene silencing and pest control on soybean aphid Aphis glycines. J. Pest. Sci. 2020, 93, 449–459. [Google Scholar] [CrossRef]
- Wang, X.; Zheng, K.; Cheng, W.; Li, J.; Liang, X.; Shen, J.; Dou, D.; Yin, M.; Yan, S. Field application of star polymer-delivered chitosan to amplify plant defense against potato late blight. Chem. Eng. J. 2021, 417, 129327. [Google Scholar] [CrossRef]
- Ma, Z.; Zheng, Y.; Chao, Z.; Chen, H.; Zhang, Y.; Yin, M.; Shen, J.; Yan, S. Visualization of the process of a nanocarrier-mediated gene delivery: Stabilization, endocytosis and endosomal escape of genes for intracellular spreading. J. Nanobiotechnol. 2022, 20, 124. [Google Scholar] [CrossRef]
- Ma, Z.; Zhang, Y.; Li, M.; Chao, Z.; Du, X.; Yan, S.; Shen, J. A first greenhouse application of bacteria-expressed and nanocarrier-delivered RNA pesticide for Myzus persicae control. J. Pest. Sci. 2022, 1–13. [Google Scholar] [CrossRef]
- Yang, J.; Yan, S.; Xie, S.; Yin, M.; Shen, J.; Li, Z.; Zhou, Y.; Duan, L. Construction and application of star polycation nanocarrier-based microRNA delivery system in Arabidopsis and maize. J. Nanobiotechnol. 2022, 20, 219. [Google Scholar] [CrossRef]
- Yan, S.; Hu, Q.; Li, J.; Cao, Z.; Cai, C.; Yin, M.; Du, X.; Shen, J. A star polycation acts a drug nanocarrier to improve the toxicity and persistence of botanical pesticides. ACS Sustain. Chem. Eng. 2019, 7, 17406–17413. [Google Scholar] [CrossRef]
- Yan, S.; Hu, Q.; Jiang, Q.; Chen, H.; Wei, J.; Yin, M.; Du, X.; Shen, J. Simple osthole/nanocarrier pesticide efficiently controls both pests and diseases fulfilling the need of green production of strawberry. ACS Appl. Mater. Inter. 2021, 13, 36350–36360. [Google Scholar] [CrossRef]
- Yan, S.; Cheng, W.Y.; Han, Z.H.; Wang, D.; Yin, M.Z.; Du, X.G.; Shen, J. Nanometerization of thiamethoxam by a cationic star polymer nanocarrier efficiently enhances the contact and plant-uptake dependent stomach toxicity against green peach aphids. Pest. Manag. Sci. 2021, 77, 1954–1962. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Xie, Y.; Peng, M.; Wang, Z.; Li, T.; Yin, M.; Shen, J.; Yan, S. A nanocarrier pesticide delivery system with promising benefits in the case of dinotefuran: Strikingly enhanced bioactivity and reduced pesticide residue. Environ. Sci. Nano 2022, 9, 988–999. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, Y.H.; Jiang, Q.H.; Chen, H.T.; Ma, R.H.; Wang, Z.J.; Yin, M.Z.; Shen, J.; Yan, S. Efficient polymer-mediated delivery system for thiocyclam: Nanometerization remarkably improves the bioactivity toward green peach aphids. Insect Sci. 2022. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.; Chen, D.; Che, L.; Gu, N.; Yin, M.; Du, X.; Shen, J.; Yan, S. Biotoxicity evaluation of a cationic star polymer on a predatory ladybird and cooperative pest control by polymer-delivered pesticides and ladybird. ACS Appl. Mater. Inter. 2022, 14, 6083–6092. [Google Scholar] [CrossRef]
- Kirk, W.D.J.; de Kogel, W.J.; Koschier, E.H.; Teulon, D.A.J. Semiochemicals for thrips and their use in pest management. Annu. Rev. Entomol. 2021, 66, 101–119. [Google Scholar] [CrossRef]
- Xu, X.; Enkegaard, A. Prey preference of Orius sauteri between western flower thrips and spider mites. Entomol. Exp. Appl. 2009, 132, 93–98. [Google Scholar] [CrossRef]
- Jacobson, A.L.; Kennedy, G.G. Effect of cyantraniliprole on feeding behavior and virus transmission of Frankliniella fusca and Frankliniella occidentalis (Thysanoptera: Thripidae) on Capsicum annuum. Crop. Prot. 2013, 54, 251–258. [Google Scholar] [CrossRef]
- Bielza, P.; Guillén, J. Cyantraniliprole: A valuable tool for Frankliniella occidentalis (Pergande) management. Pest. Manag. Sci. 2015, 71, 1068–1074. [Google Scholar] [CrossRef]
- Lin, Q.C.; Chen, H.; Babendreier, D.; Zhang, J.P.; Zhang, F.; Dai, X.Y.; Sun, Z.W.; Shi, Z.P.; Dong, X.L.; Wu, G.A.; et al. Improved control of Frankliniella occidentalis on greenhouse pepper through the integration of Orius sauteri and neonicotinoid insecticides. J. Pest. Sci. 2021, 94, 101–109. [Google Scholar] [CrossRef]
- Zhang, R.; Jiang, E.B.; He, S.; Chen, J. Lethal and sublethal effects of cyantraniliprole on Bactrocera dorsalis (Hendel) (Diptera: Tephritidae). Pest. Manag. Sci. 2015, 71, 250–256. [Google Scholar] [CrossRef]
- Dong, J.; Wang, K.; Li, Y.; Wang, S. Lethal and sublethal effects of cyantraniliprole on Helicoverpa assulta (Lepidoptera: Noctuidae). Pestic. Biochem. Phys. 2017, 136, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Moreno, I.; Belando, A.; Grávalos, C.; Bielza, P. Baseline susceptibility of Mediterranean strains of Trialeurodes vaporariorum (Westwood) to cyantraniliprole. Pest. Manag. Sci. 2018, 74, 1552–1557. [Google Scholar] [CrossRef] [PubMed]
- Qian, Z.; Zhang, F.; Yao, X.; Yu, H.; Sun, S.; Li, X.; Zhang, J.; Jiang, X. Growth, DNA damage and biochemical toxicity of cyantraniliprole in earthworms (Eisenia fetida). Chemosphere 2019, 236, 124328. [Google Scholar]
- Costa, N.C.R.; Picelli, E.C.M.; Silva, F.M.A.; Gonring, A.H.R.; Guedes, R.N.C.; Durigan, M.R.; Fernandes, F.L. Cyantraniliprole susceptibility baseline, resistance survey and control failure likelihood in the coffee berry borer Hypothenemus hampei. Ecotoxicol. Environ. Saf. 2020, 203, 110947. [Google Scholar] [CrossRef]
- Cordova, D.; Benner, E.A.; Sacher, M.D.; Rauh, J.J.; Sopa, J.S.; Lahm, G.P.; Selby, T.P.; Stevenson, T.M.; Flexner, L.; Gutteridge, S.; et al. Anthranilic diamides: A new class of insecticides with a novel mode of action, ryanodine receptor activation. Pestic. Biochem. Physiol. 2006, 84, 196–214. [Google Scholar] [CrossRef]
- Sattelle, D.B.; Cordova, D.; Cheek, T.R. Insect ryanodine receptors: Molecular targets for novel pest control chemicals. Invertebr. Neurosci. 2008, 8, 107–119. [Google Scholar] [CrossRef]
- Jeanguenat, A. The story of a new insecticidal chemistry class: The diamides. Pest. Manag. Sci. 2013, 69, 7–14. [Google Scholar] [CrossRef]
- Sparks, T.C.; Nauen, R. IRAC: Mode of action classification and insecticide resistance management. Pestic. Biochem. Physiol. 2015, 121, 122–128. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Li, D.; Li, D.; Xu, P.; Mao, K.; Zhang, Y.; Qin, X.; Tang, T.; Wan, H.; Li, J.; et al. Efficacy of an adhesive nanopesticide on insect pests of rice in field trials. J. Asia-Pac. Entomol. 2020, 23, 1222–1227. [Google Scholar]
- Li, X.; Shi, H.; Gao, X.; Liang, P. Characterization of UDP-Glucuronosyltransferase Genes and their Possible Roles in Multi-Insecticide Resistance in Plutella Xylostella (L.). Pest. Manag. Sci. 2018, 74, 695–704. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Z.; Liu, H. Evaluation of the selective toxicity of six acaricides to Tetranychus cinnabarinus and Neoseiulus cucumeris. Plant. Prot. 2014, 40, 209–212. [Google Scholar]
- Shen, X.; Huang, C.; Yu, X.; Shang, S.; Wang, X.; Wang, X.; Liu, M.; Yang, M. Safety evaluation and toxicity determination of 5 biopesticides to Myzus persicae (Sulzer) and Aphidoletes aphidimyza (Rondani). Chin. Tob. Sci. 2020, 41, 56–61, 66. [Google Scholar]
- Zhou, F.; Zhang, X.; Pan, Y.; Wang, R.; Li, X.; Zhang, J.; Yang, X.; Lei, F. Safety assessment of five pesticides to thrips-predatory natural enemy mites. Guizhou Agric. Sci. 2020, 48, 69–72. [Google Scholar]
- Doyle, M.L. Characterization of binding interactions by isothermal titration calorimetry. Curr. Opin. Biotechnol. 1997, 8, 31–35. [Google Scholar] [CrossRef]
- Grolier, J.P.E.; Del Rio, J.M. Isothermal titration calorimetry: A thermodynamic interpretation of measurements. J. Chem. Thermodyn. 2012, 55, 193–202. [Google Scholar] [CrossRef]
- Ross, P.D.; Subramanian, S. Thermodynamics of protein association reactions: Forces contributing to stability. Biochemistry 1981, 20, 3096–3102. [Google Scholar] [CrossRef]
- Yang, Y.; Jiang, Q.; Peng, M.; Zhou, Z.; Du, X.; Yin, M.; Shen, J.; Yan, S. A star polyamine-based nanocarrier delivery system for enhanced avermectin contact and stomach toxicity against green peach aphids. Nanomaterials 2022, 12, 1445. [Google Scholar] [CrossRef]
- Xiao, D.; Guo, X.J.; Wang, S.; Zhang, J.M.; Zhang, F. The toxicity of three insecticides to natural enemy. J. Environ. Entomol. 2014, 36, 951–958. [Google Scholar]
- Gao, Y.; Reitz, S.R.; Wang, J.; Tamez-Guerra, P.; Wang, E.; Xu, X.; Lei, Z. Potential use of the fungus Beauveria bassiana against the western flower thrips Frankliniella occidentalis without reducing the effectiveness of its natural predator Orius sauteri (Hemiptera: Anthocoridae). Biocontrol Sci. Techn. 2012, 22, 803–812. [Google Scholar] [CrossRef]

| Sample Number | Weight of Applied CNAP (mg) | Weight of Applied SPc (mg) | Weight of CNAP-Loaded Complex (mg) | Weight of CNAP Loaded in Complex (mg) | Pesticide Loading Content (%) | Average Pesticide Loading Content (%) |
|---|---|---|---|---|---|---|
| 1 | 10.0 | 10.0 | 13.5 | 3.5 | 25.93 | 24.79 ± 0.87 |
| 2 | 10.0 | 10.0 | 13.0 | 3.0 | 23.08 | |
| 3 | 10.0 | 10.0 | 13.4 | 3.4 | 25.37 |
| Formulation | Sample Number | Mass Ratio | Polydispersity | Size (nm) | Average Size (nm) |
|---|---|---|---|---|---|
| CNAP | 1 | - | 0.211 | 789.71 | 807.86 ± 49.34 |
| 2 | 0.230 | 900.94 | |||
| 3 | 0.286 | 732.94 | |||
| CNAP/SPc complex | 1 | 1:1 | 0.290 | 335.94 | 298.92 ± 50.79 |
| 2 | 0.153 | 198.49 | |||
| 3 | 0.238 | 362.34 | |||
| 1 | 1:3.03 | 0.251 | 251.45 | 289.01 ± 24.40 | |
| 2 | 0.242 | 280.83 | |||
| 3 | 0.235 | 334.76 | |||
| F2,6 = 47,094, p < 0.001 | |||||
| Formulation | LC50 (mg/L) (95% Confidence Limits) | Slope ± SE | χ2(df) a | Efficiency Ratio |
|---|---|---|---|---|
| CNAP | 98.695 (74.040–146.350) | 0.968 ± 0.141 | 15.480 (48) | 1.837 |
| CNAP/SPc complex | 53.714 (42.517–68.443) | 1.146 ± 0.142 | 19.357 (48) |
| Formulation | LC50 (mg/L) (95% Confidence Limits) | Slope ± SE | χ2(df) a | Efficiency Ratio |
|---|---|---|---|---|
| CNAP | 229.662 (155.278–437.831) | 1.012 ± 0.153 | 13.736 (48) | 1.324 |
| CNAP/SPc complex | 173.437 (119.378–319.255) | 0.918 ± 0.145 | 15.978 (48) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, S.; Gu, N.; Peng, M.; Jiang, Q.; Liu, E.; Li, Z.; Yin, M.; Shen, J.; Du, X.; Dong, M. A Preparation Method of Nano-Pesticide Improves the Selective Toxicity toward Natural Enemies. Nanomaterials 2022, 12, 2419. https://doi.org/10.3390/nano12142419
Yan S, Gu N, Peng M, Jiang Q, Liu E, Li Z, Yin M, Shen J, Du X, Dong M. A Preparation Method of Nano-Pesticide Improves the Selective Toxicity toward Natural Enemies. Nanomaterials. 2022; 12(14):2419. https://doi.org/10.3390/nano12142419
Chicago/Turabian StyleYan, Shuo, Na Gu, Min Peng, Qinhong Jiang, Enliang Liu, Zhiqiang Li, Meizhen Yin, Jie Shen, Xiangge Du, and Min Dong. 2022. "A Preparation Method of Nano-Pesticide Improves the Selective Toxicity toward Natural Enemies" Nanomaterials 12, no. 14: 2419. https://doi.org/10.3390/nano12142419
APA StyleYan, S., Gu, N., Peng, M., Jiang, Q., Liu, E., Li, Z., Yin, M., Shen, J., Du, X., & Dong, M. (2022). A Preparation Method of Nano-Pesticide Improves the Selective Toxicity toward Natural Enemies. Nanomaterials, 12(14), 2419. https://doi.org/10.3390/nano12142419






