Optical and Photocatalytic Properties of Br-Doped BiOCl Nanosheets with Rich Oxygen Vacancies and Dominating {001} Facets
Abstract
:1. Introduction
2. Materials and Methods
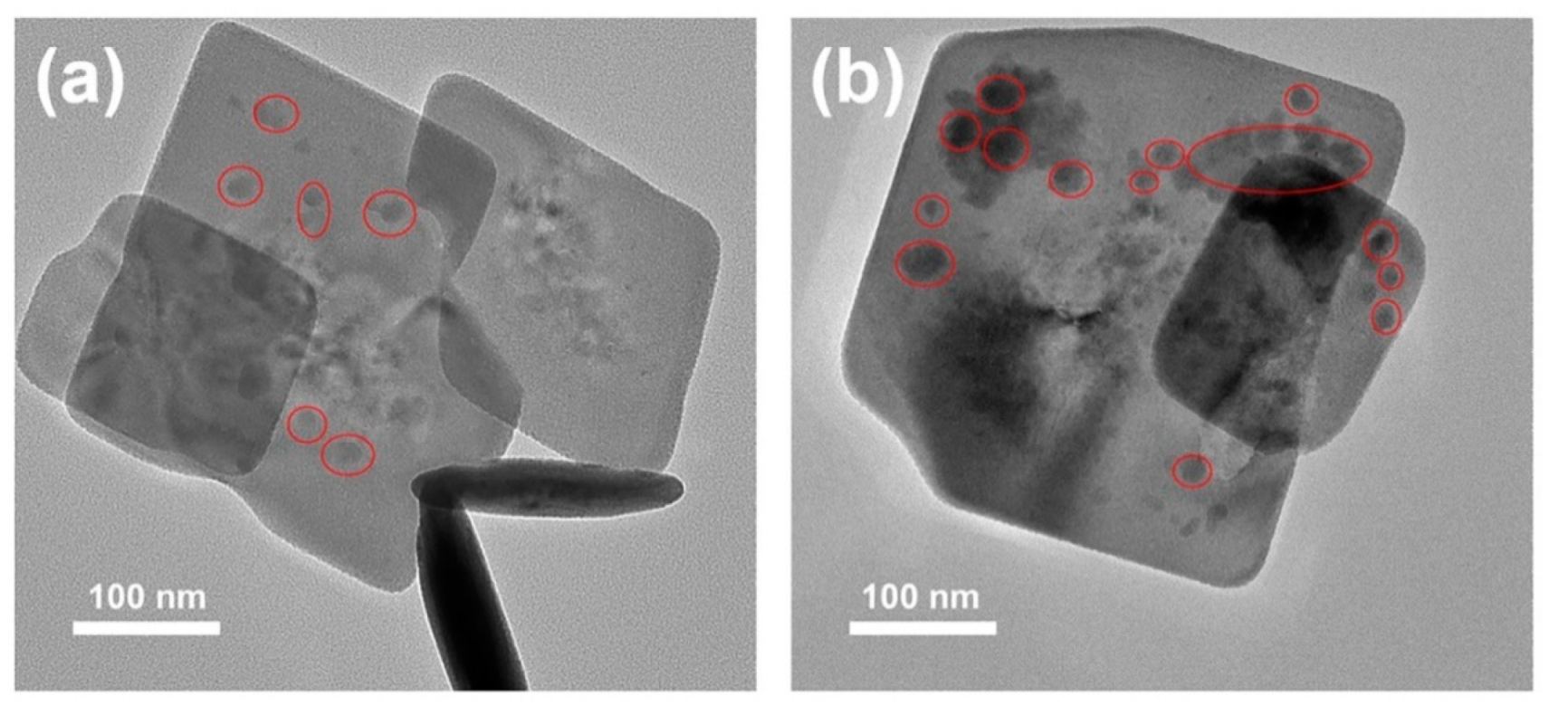
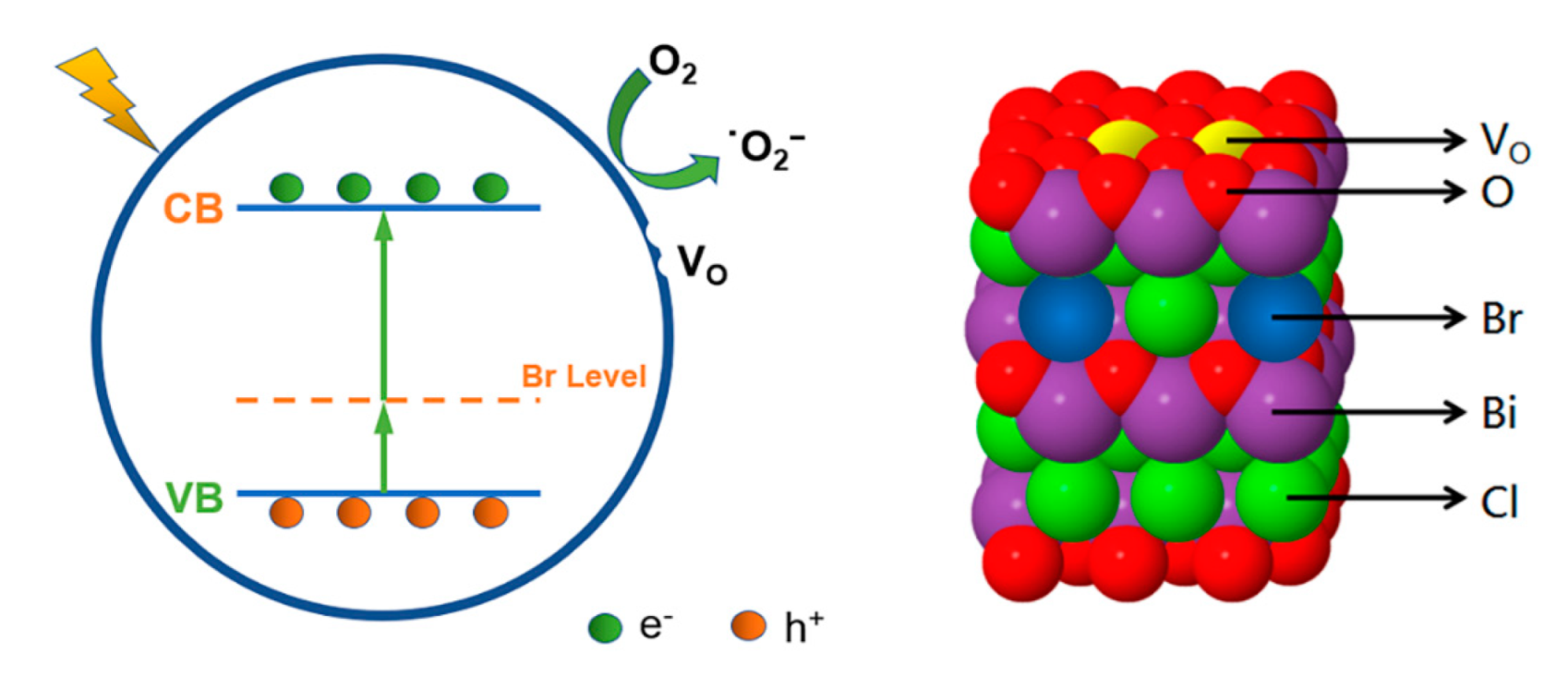
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zaman, F.; Xie, B.; Zhang, J.; Gong, T.; Cui, K.; Hou, L.; Xu, J.; Zhai, Z.; Yuan, C. MOFs derived hetero-ZnO/Fe2O3 nanoflowers with enhanced photocatalytic performance towards efficient degradation of organic dyes. Nanomaterials 2021, 11, 3239. [Google Scholar] [CrossRef]
- Rameshbabu, R.; Sandhiya, M.; Pecchi, G.; Sathish, M. Effective coupling of Cu (II) with BiOCl nanosheets for high performance electrochemical supercapacitor and enhanced photocatalytic applications. Appl. Surf. Sci. 2020, 521, 146362. [Google Scholar] [CrossRef]
- Kanagaraj, T.; Thiripuranthagan, S.; Paskalis, S.M.K.; Abe, H. Visible light photocatalytic activities of template free porous graphitic carbon nitride-BiOBr composite catalysts towards the mineralization of reactive dyes. Appl. Surf. Sci. 2017, 426, 1030–1045. [Google Scholar] [CrossRef]
- Li, H.; Luo, X.; Long, Z.; Huang, G.; Zhu, L. Plasmonic Ag Nanoparticle-Loaded np Bi2O2CO3/α-Bi2O3 Heterojunction Microtubes with Enhanced Visible-Light-Driven Photocatalytic Activity. Nanomaterials 2022, 12, 1608. [Google Scholar] [CrossRef]
- Pascariu, P.; Cojocaru, C.; Homocianu, M.; Samoila, P.; Dascalu, A.; Suchea, M. New La3+ doped TiO2 nanofibers for photocatalytic degradation of organic pollutants: Effects of thermal treatment and doping loadings. Ceram. Int. 2022, 48, 4953–4964. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhao, X.; Duan, L.; Shen, H.; Liu, R. Controlling oxygen vacancies and enhanced visible light photocatalysis of CeO2/ZnO nanocomposites. J. Photochem. Photobiol. A Chem. 2020, 392, 112156. [Google Scholar] [CrossRef]
- Cao, D.; Wang, Q.; Zhu, S.; Zhang, X.; Li, Y.; Cui, Y.; Xue, Z.; Gao, S. Hydrothermal construction of flower-like MoS2 on TiO2 NTs for highly efficient environmental remediation and photocatalytic hydrogen evolution. Sep. Purif. Technol. 2021, 265, 118463. [Google Scholar] [CrossRef]
- Xu, T.; Yang, M.; Chen, C.; Duan, R.; Shen, Q.; Sun, C. Photocatalytic activation of C-Br bond on facet-dependent BiOCl with oxygen vacancies. Appl. Surf. Sci. 2021, 548, 149243. [Google Scholar] [CrossRef]
- Yang, Z.; Shang, Z.; Liu, F.; Chen, Y.; Wang, X.; Zhang, B.; Liu, G. Hollow porous BiOCl microspheres assembled with single layer of nanocrystals: Spray solution combustion synthesis and the enhanced photocatalytic properties. Nanotechnology 2021, 32, 205602. [Google Scholar] [CrossRef]
- Wang, L.; Lv, D.; Yue, Z.; Zhu, H.; Wang, L.; Wang, D.; Xu, X.; Hao, W.; Dou, S.; Du, Y. Promoting photoreduction properties via synergetic utilization between plasmonic effect and highly active facet of BiOCl. Nano Energy 2019, 57, 398–404. [Google Scholar] [CrossRef]
- Liu, W.; Qiao, L.; Zhu, A.; Liu, Y.; Pan, J. Constructing 2D BiOCl/C3N4 layered composite with large contact surface for visible-light-driven photocatalytic degradation. Appl. Surf. Sci. 2017, 426, 897–905. [Google Scholar] [CrossRef]
- Gao, X.; Tang, G.; Peng, W.; Guo, Q.; Luo, Y. Surprise in the phosphate modification of BiOCl with oxygen vacancy: In situ construction of hierarchical Z-scheme BiOCl-OV-BiPO4 photocatalyst for the degradation of carbamazepine. Chem. Eng. J. 2019, 360, 1320–1329. [Google Scholar] [CrossRef]
- Han, X.; Dong, S.; Yu, C.; Wang, Y.; Yang, K.; Sun, J. Controllable synthesis of Sn-doped BiOCl for efficient photocatalytic degradation of mixed-dye wastewater under natural sunlight irradiation. J. Alloys Compd. 2016, 685, 997–1007. [Google Scholar] [CrossRef]
- Wu, D.; Wang, R.; Yang, C.; An, Y.; Lu, H.; Wang, H.; Cao, K.; Gao, Z.; Zhang, W.; Xu, F.; et al. Br doped porous bismuth oxychloride micro-sheets with rich oxygen vacancies and dominating {001} facets for enhanced nitrogen photo-fixation performances. J. Colloid Interface Sci. 2019, 556, 111–119. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, W.; Zhong, L.; Liu, D.; Cao, X.; Cui, F. Oxygen vacancy-rich 2D/2D BiOCl-g-C3N4 ultrathin heterostructure nanosheets for enhanced visible-light-driven photocatalytic activity in environmental remediation. Appl. Catal. B Environ. 2018, 220, 290–302. [Google Scholar] [CrossRef]
- Li, Z.; Qu, Y.; Hu, K.; Humayun, M.; Chen, S.; Jing, L. Improved photoelectrocatalytic activities of BiOCl with high stability for water oxidation and MO degradation by coupling RGO and modifying phosphate groups to prolong carrier lifetime. Appl. Catal. B Environ. 2017, 203, 355–362. [Google Scholar] [CrossRef]
- Xu, Y.; Xu, S.; Wang, S.; Zhang, Y.; Li, G. Citric acid modulated electrochemical synthesis and photocatalytic behavior of BiOCl nanoplates with exposed {001} facets. Dalton Trans. 2014, 43, 479–485. [Google Scholar] [CrossRef]
- Wang, J.; Liu, W.; Zhong, D.; Ma, Y.; Ma, Q.; Wang, Z.; Pan, J. Fabrication of bismuth titanate nanosheets with tunable crystal facets for photocatalytic degradation of antibiotic. J. Mater. Sci. 2019, 54, 13740–13752. [Google Scholar] [CrossRef]
- Bai, S.; Li, X.; Kong, Q.; Long, R.; Wang, C.; Jiang, J.; Xiong, Y. Toward enhanced photocatalytic oxygen evolution: Synergetic utilization of plasmonic effect and schottky junction via interfacing facet selection. Adv. Mater. 2015, 27, 3444–3452. [Google Scholar] [CrossRef]
- Jiang, J.; Zhao, K.; Xiao, X.; Zhang, L. Synthesis and facet-dependent photoreactivity of BiOCl single-crystalline nanosheets. J. Am. Chem. Soc. 2012, 134, 4473–4476. [Google Scholar] [CrossRef]
- Liu, W.; Zhong, D.; Dai, Z.; Liu, Y.; Wang, J.; Wang, Z.; Pan, J. Synergetic utilization of photoabsorption and surface facet in crystalline/amorphous contacted BiOCl-Bi2S3 composite for photocatalytic degradation. J. Alloys Compd. 2019, 780, 907–916. [Google Scholar] [CrossRef]
- Zhong, S.; Wang, X.; Wang, Y.; Zhou, F.; Li, J.; Liang, S.; Li, C. Preparation of Y3+-doped BiOCl photocatalyst and its enhancing effect on degradation of tetracycline hydrochloride wastewater. J. Alloys Compd. 2020, 843, 155598. [Google Scholar] [CrossRef]
- Yu, H.; Ge, D.; Liu, Y.; Lu, Y.; Wang, X.; Huo, M.; Qin, W. One-pot synthesis of BiOCl microflowers co-modified with Mn and oxygen vacancies for enhanced photocatalytic degradation of tetracycline under visible light. Sep. Purif. Technol. 2020, 251, 117414. [Google Scholar] [CrossRef]
- Liu, W.; Shang, Y.; Zhu, A.; Tan, P.; Liu, Y.; Qiao, L.; Chu, D.; Xiong, X.; Pan, J. Enhanced performance of doped BiOCl nanoplates for photocatalysis: Understanding from doping insight into improved spatial carrier separation. J. Mater. Chem. A 2017, 5, 12542–12549. [Google Scholar] [CrossRef]
- Verma, R.; Samdarshi, S.K. Correlating oxygen vacancies and phase ratio/interface with efficient photocatalytic activity in mixed phase TiO2. J. Alloys Compd. 2015, 629, 105–112. [Google Scholar] [CrossRef]
- Wang, L.; Lv, D.; Dong, F.; Wu, X.; Cheng, N.; Scott, J.; Xu, X.; Hao, W.; Du, Y. Boosting visible-light-driven photo-oxidation of BiOCl by promoted charge separation via vacancy engineering. ACS Sustain. Chem. Eng. 2019, 7, 3010–3017. [Google Scholar] [CrossRef]
- Cai, Y.; Li, D.; Sun, J.; Chen, M.; Li, Y.; Zou, Z.; Zhang, H.; Xu, H.; Xia, D. Synthesis of BiOCl nanosheets with oxygen vacancies for the improved photocatalytic properties. Appl. Surf. Sci. 2018, 439, 697–704. [Google Scholar] [CrossRef]
- Wang, X.; Liu, X.; Liu, G.; Zhang, C.; Liu, G.; Xu, S.; Cui, P.; Li, D. Rapid synthesis of BiOCl graded microspheres with highly exposed (110) facets and oxygen vacancies at room temperature to enhance visible light photocatalytic activity. Catal. Commun. 2019, 130, 105769. [Google Scholar] [CrossRef]
- Wang, J.; Chen, R.; Xiang, L.; Komarneni, S. Synthesis, properties and applications of ZnO nanomaterials with oxygen vacancies: A review. Ceram. Int. 2018, 44, 7357–7377. [Google Scholar] [CrossRef]
- Yu, H.; Ge, D.; Wang, Y.; Zhu, S.; Wang, X.; Huo, M.; Lu, Y. Facile synthesis of Bi-modified Nb-doped oxygen defective BiOCl microflowers with enhanced visible-light-driven photocatalytic performance. J. Alloys Compd. 2019, 786, 155–162. [Google Scholar] [CrossRef]
- Song, Z.; Dong, X.; Fang, J.; Xiong, C.; Wang, N.; Tang, X. Improved photocatalytic degradation of perfluorooctanoic acid on oxygen vacancies-tunable bismuth oxychloride nanosheets prepared by a facile hydrolysis. J. Hazard. Mater. 2019, 377, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Wang, X.; Wang, H.; Wang, F.; Wang, D.; Gao, Z.; Wang, X.; Xu, F.; Jiang, K. Ultrasonic-assisted synthesis of two dimensional BiOCl/MoS2 with tunable band gap and fast charge separation for enhanced photocatalytic performance under visible light. J. Colloid Interface Sci. 2019, 533, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, F.; Cao, Y.; Zhang, F.; Zou, Y.; Huang, Z.; Ye, L.; Zhou, Y. Interfacial Oxygen Vacancy Engineered Two-Dimensional g-C3N4/BiOCl Heterostructures with Boosted Photocatalytic Conversion of CO2. ACS Appl. Energy Mater. 2020, 3, 4610–4618. [Google Scholar] [CrossRef]
- Zhang, Q.; Hou, T.; Shen, H.; Guan, C.; Duan, L.; Zhao, X. Optical and photocatalytic properties of S doped BiOCl nanosheets with tunable exposed {001} facets and band gap. Appl. Surface Sci. 2022, 600, 154020. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, W.; Sun, S.; Jiang, D.; Gao, E. Selective transport of electron and hole among {001} and {110} facets of BiOCl for pure water splitting. Appl. Catal. B Environ. 2015, 162, 470–474. [Google Scholar] [CrossRef]
- Zhang, N.; Li, L.; Shao, Q.; Zhu, T.; Huang, X.; Xiao, X. Fe-doped BiOCl nanosheets with light-switchable oxygen vacancies for photocatalytic nitrogen fixation. ACS Appl. Energy Mater. 2019, 2, 8394–8398. [Google Scholar] [CrossRef]
- Li, H.; Qin, F.; Yang, Z.; Cui, X.; Wang, J.; Zhang, L. New reaction pathway induced by plasmon for selective benzyl alcohol oxidation on BiOCl possessing oxygen vacancies. J. Am. Chem. Soc. 2017, 139, 3513–3521. [Google Scholar] [CrossRef]
- Liu, R.; Li, H.; Duan, L.; Shen, H.; Zhang, Q.; Zhao, X. The synergistic effect of graphene oxide and silver vacancy in Ag3PO4-based photocatalysts for rhodamine B degradation under visible light. Appl. Surf. Sci. 2018, 462, 263–269. [Google Scholar] [CrossRef]
- Shen, H.; Zhao, X.; Duan, L.; Liu, R.; Li, H. Enhanced visible light photocatalytic activity in SnO2@g-C3N4 core-shell structures. Mater. Sci. Eng. B 2017, 218, 23–30. [Google Scholar] [CrossRef]
- Shen, H.; Zhao, X.; Duan, L.; Liu, R.; Wu, H.; Hou, T.; Jiang, X.; Gao, H. Influence of interface combination of RGO-photosensitized SnO2@RGO core-shell structures on their photocatalytic performance. Appl. Surf. Sci. 2017, 391, 627–634. [Google Scholar] [CrossRef]
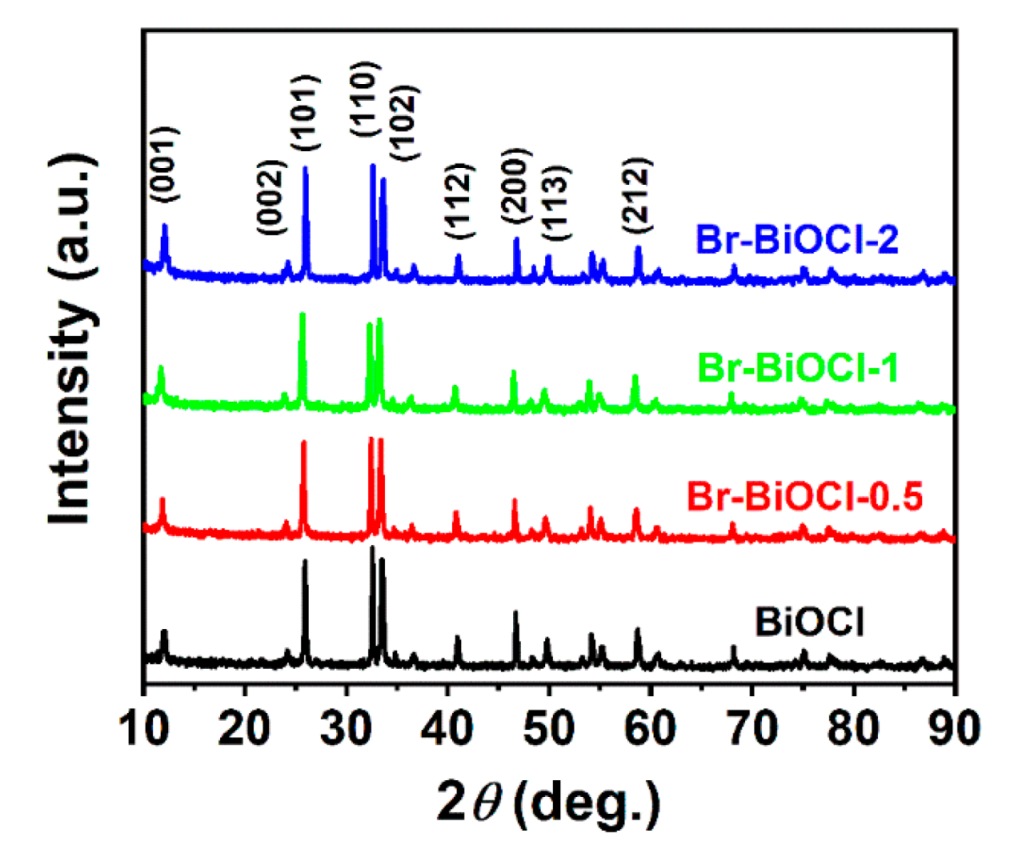
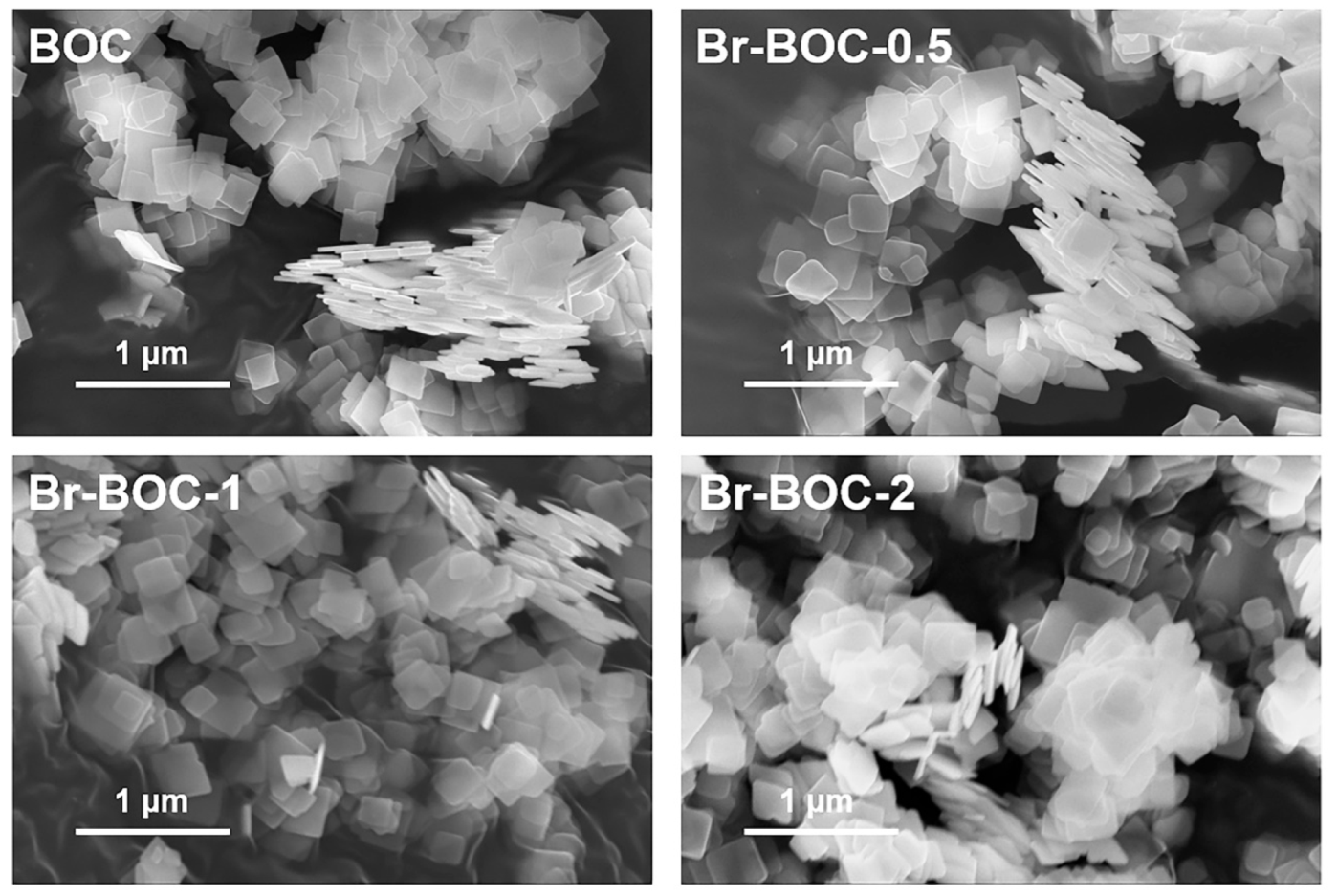
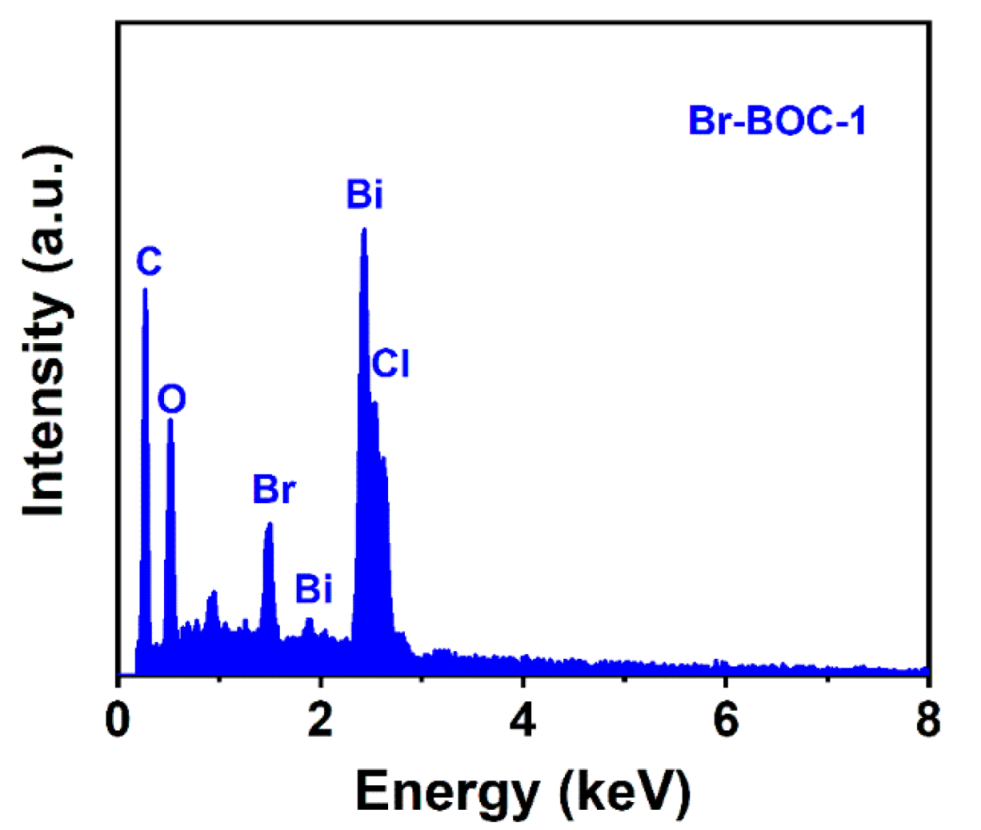

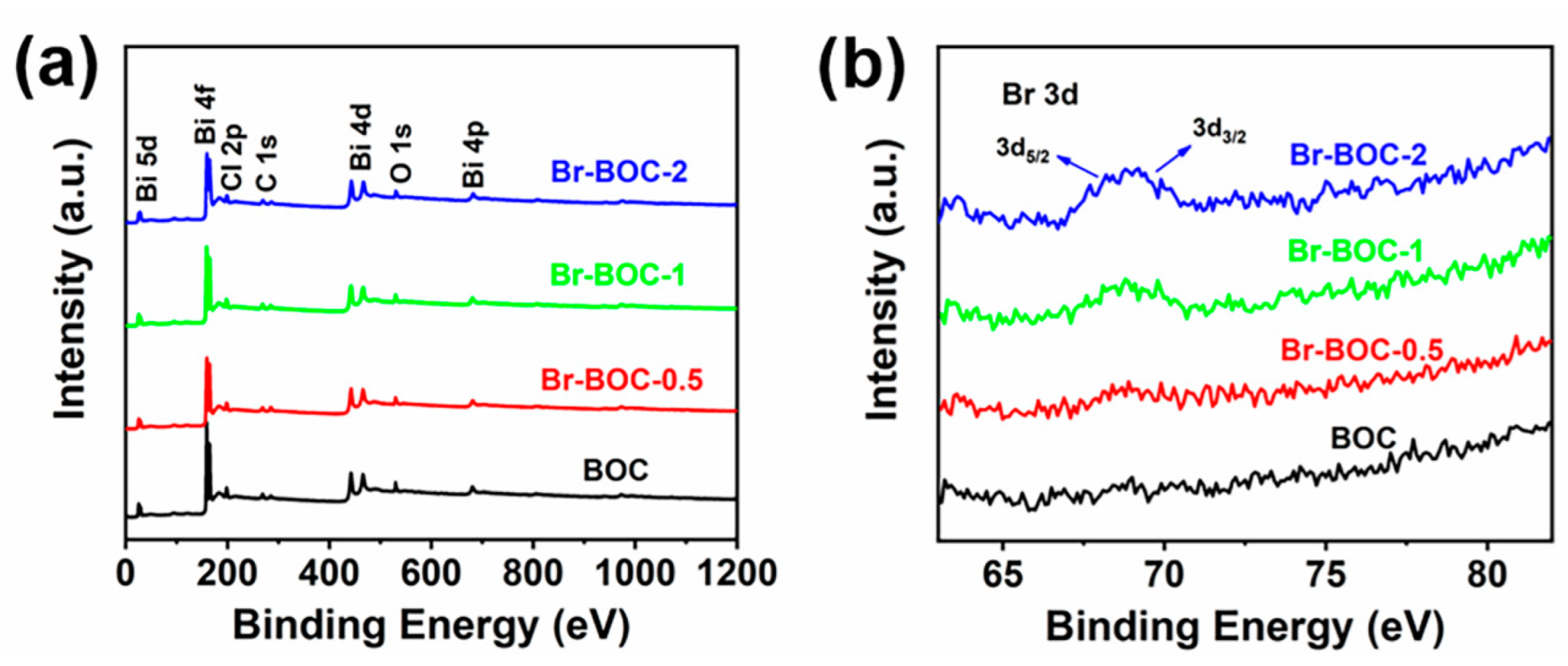
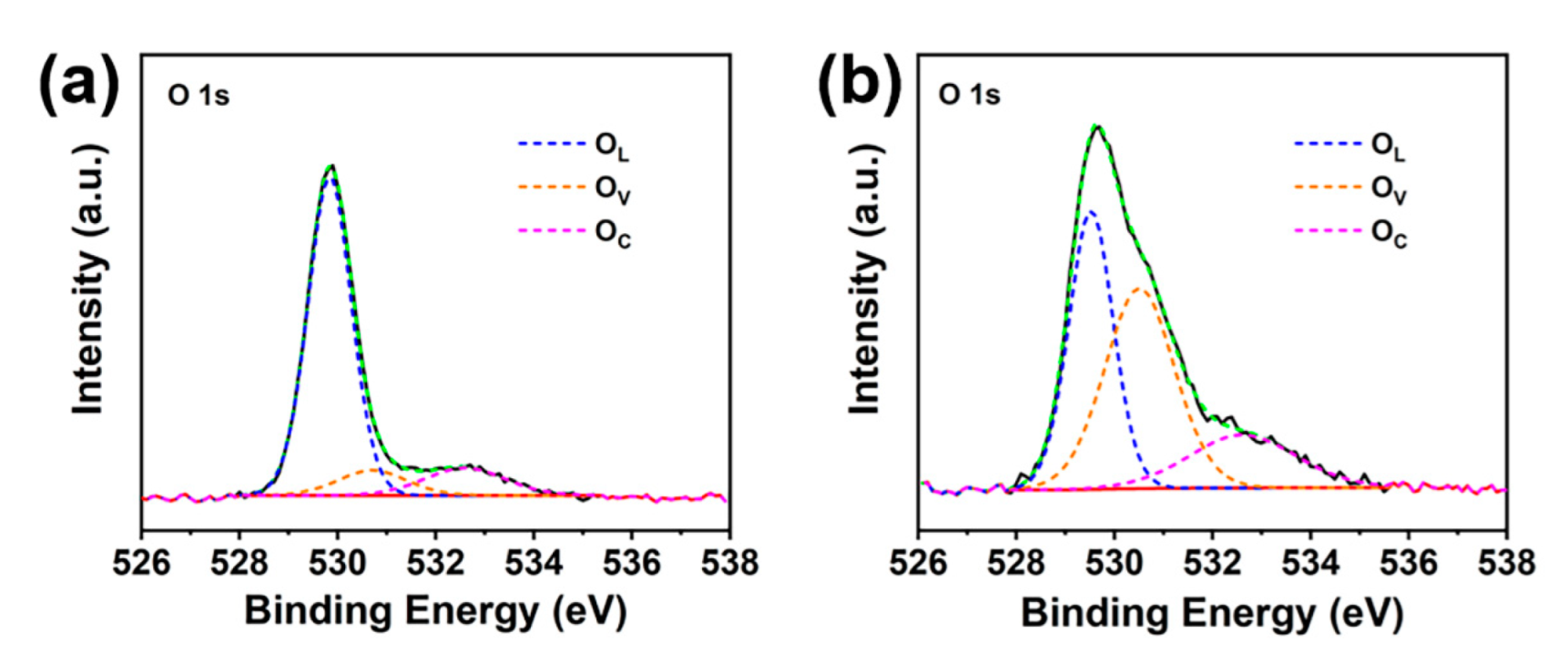
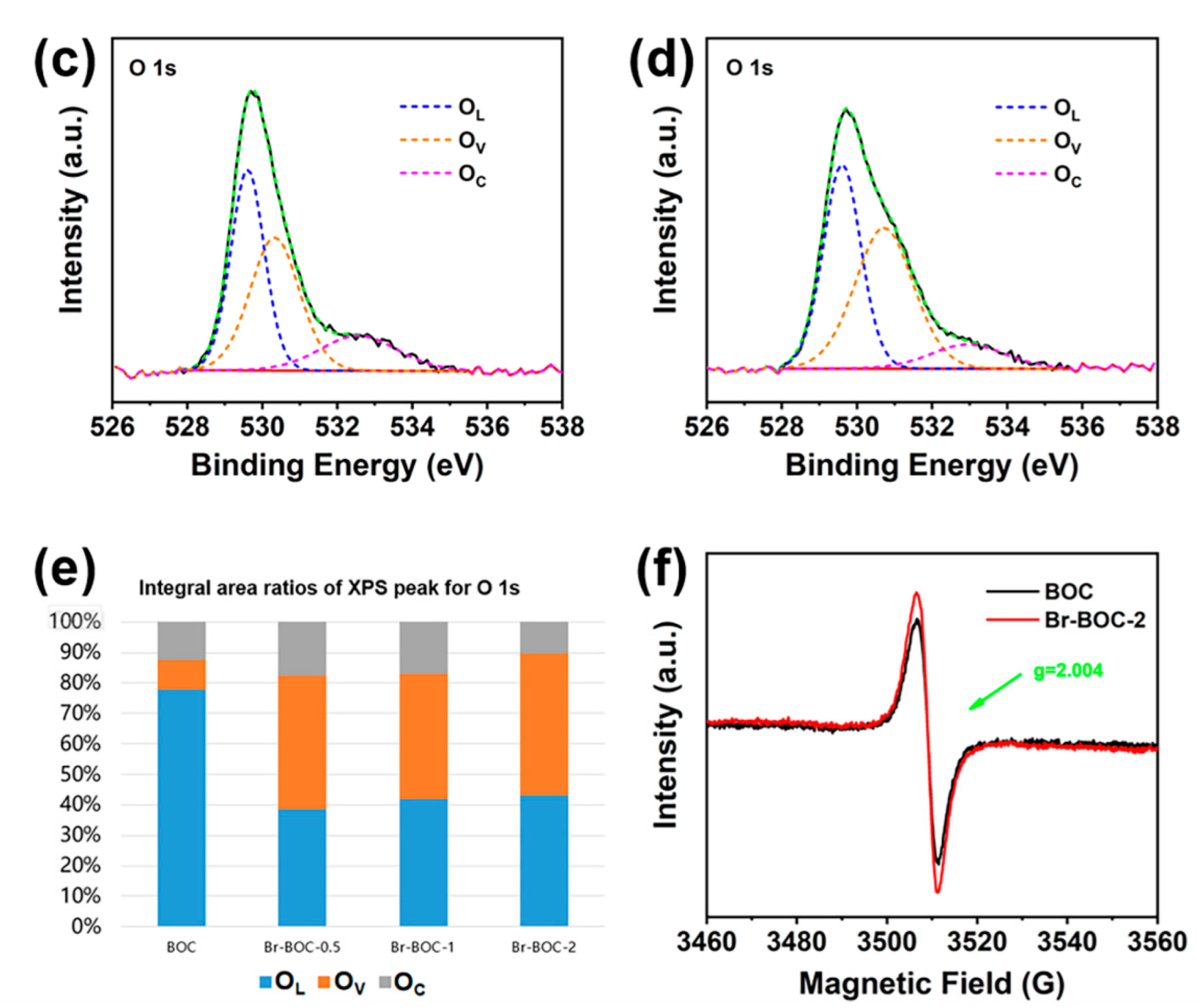


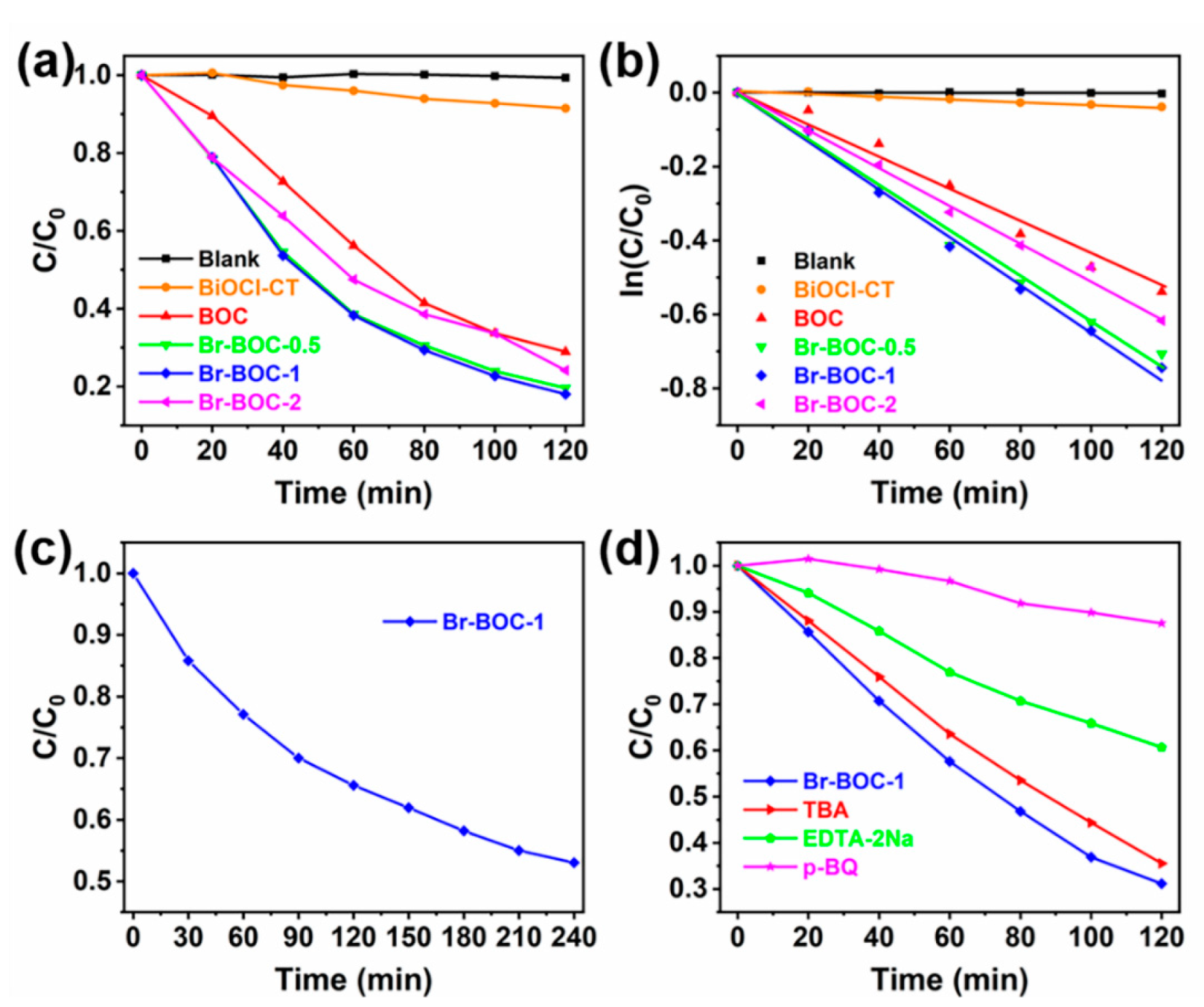
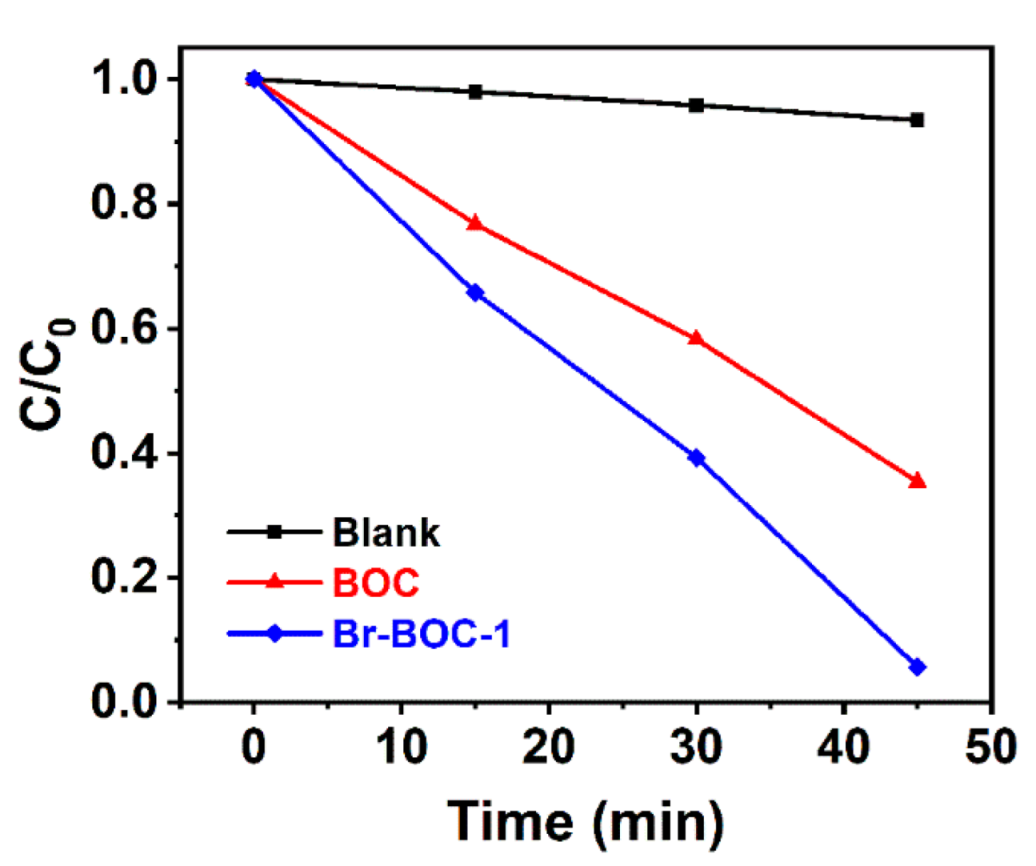
| BOC | Br−BOC−0.5 | Br−BOC−1 | Br−BOC−2 | |
|---|---|---|---|---|
| Eg (eV) | 3.21 | 3.06 | 3.02 | 2.96 |
| SBET (m2g−1) | 11.18 | 12.45 | 12.85 | 12.67 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.; Nie, W.; Hou, T.; Shen, H.; Li, Q.; Guan, C.; Duan, L.; Zhao, X. Optical and Photocatalytic Properties of Br-Doped BiOCl Nanosheets with Rich Oxygen Vacancies and Dominating {001} Facets. Nanomaterials 2022, 12, 2423. https://doi.org/10.3390/nano12142423
Zhang Q, Nie W, Hou T, Shen H, Li Q, Guan C, Duan L, Zhao X. Optical and Photocatalytic Properties of Br-Doped BiOCl Nanosheets with Rich Oxygen Vacancies and Dominating {001} Facets. Nanomaterials. 2022; 12(14):2423. https://doi.org/10.3390/nano12142423
Chicago/Turabian StyleZhang, Qian, Wuyang Nie, Tian Hou, Hao Shen, Qiang Li, Chongshang Guan, Libing Duan, and Xiaoru Zhao. 2022. "Optical and Photocatalytic Properties of Br-Doped BiOCl Nanosheets with Rich Oxygen Vacancies and Dominating {001} Facets" Nanomaterials 12, no. 14: 2423. https://doi.org/10.3390/nano12142423
APA StyleZhang, Q., Nie, W., Hou, T., Shen, H., Li, Q., Guan, C., Duan, L., & Zhao, X. (2022). Optical and Photocatalytic Properties of Br-Doped BiOCl Nanosheets with Rich Oxygen Vacancies and Dominating {001} Facets. Nanomaterials, 12(14), 2423. https://doi.org/10.3390/nano12142423






