Synthesis and Characterization of Monodispersed Spherical Calcium Oxide and Calcium Carbonate Nanoparticles via Simple Pyrolysis
Abstract
:1. Introduction
2. Experimental Conditions
2.1. Required Materials
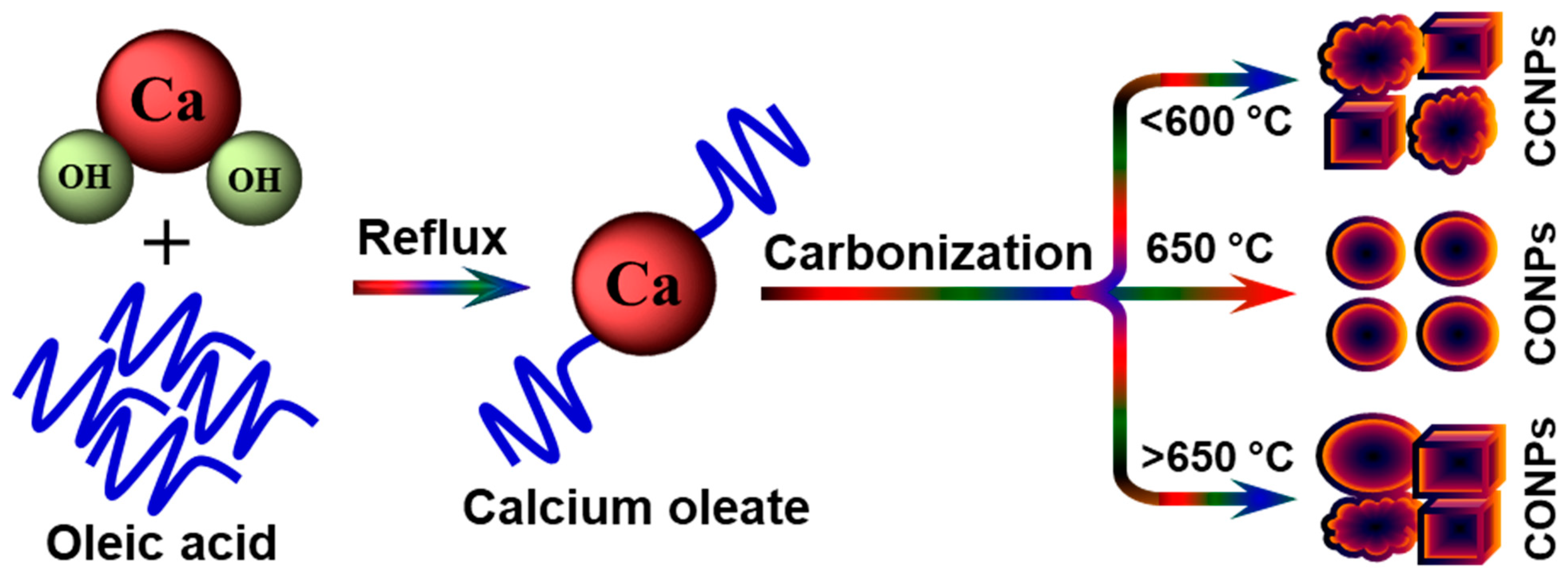
2.2. Preparation of Calcium Oleate, CCNPs, and CONPs
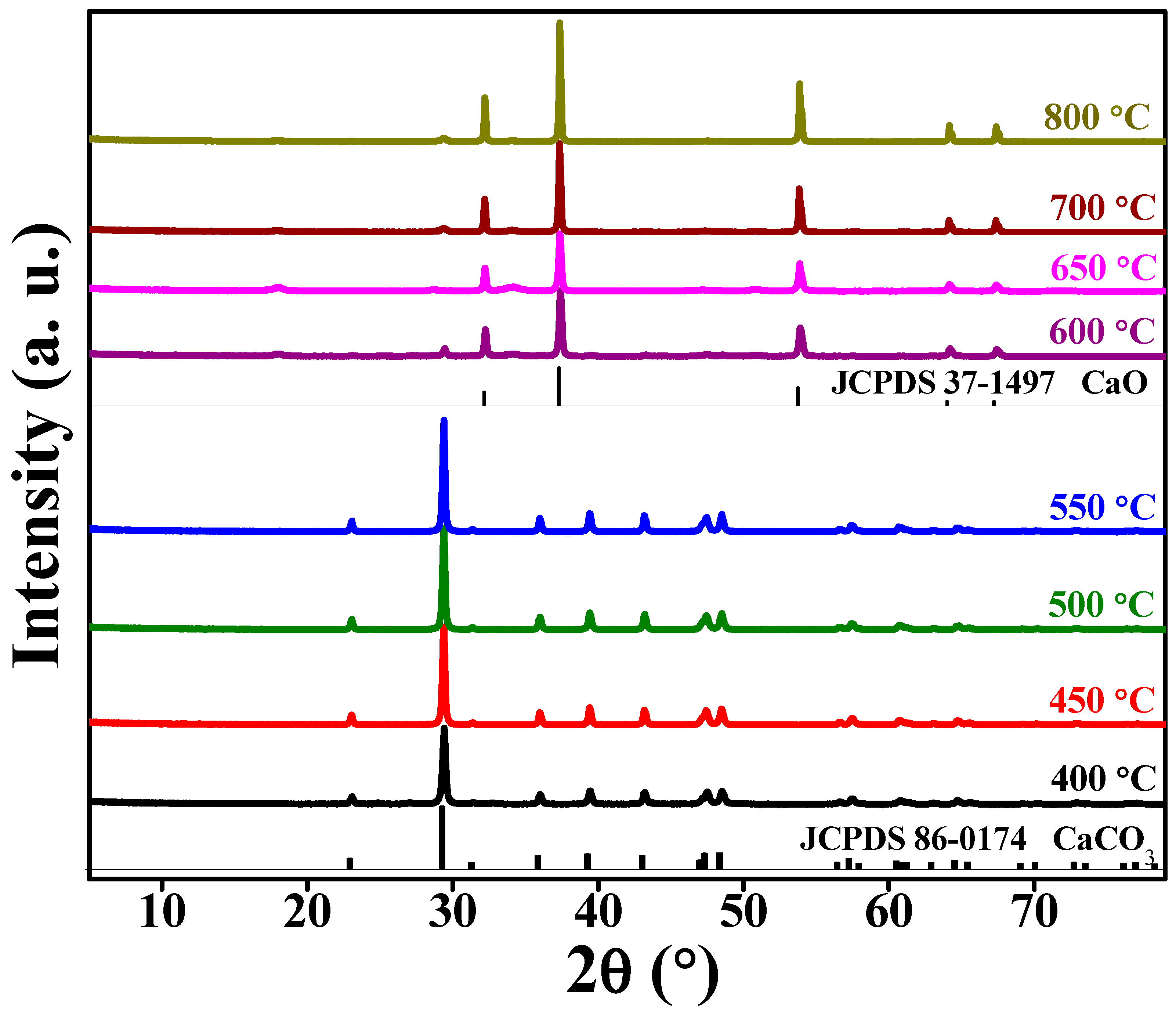
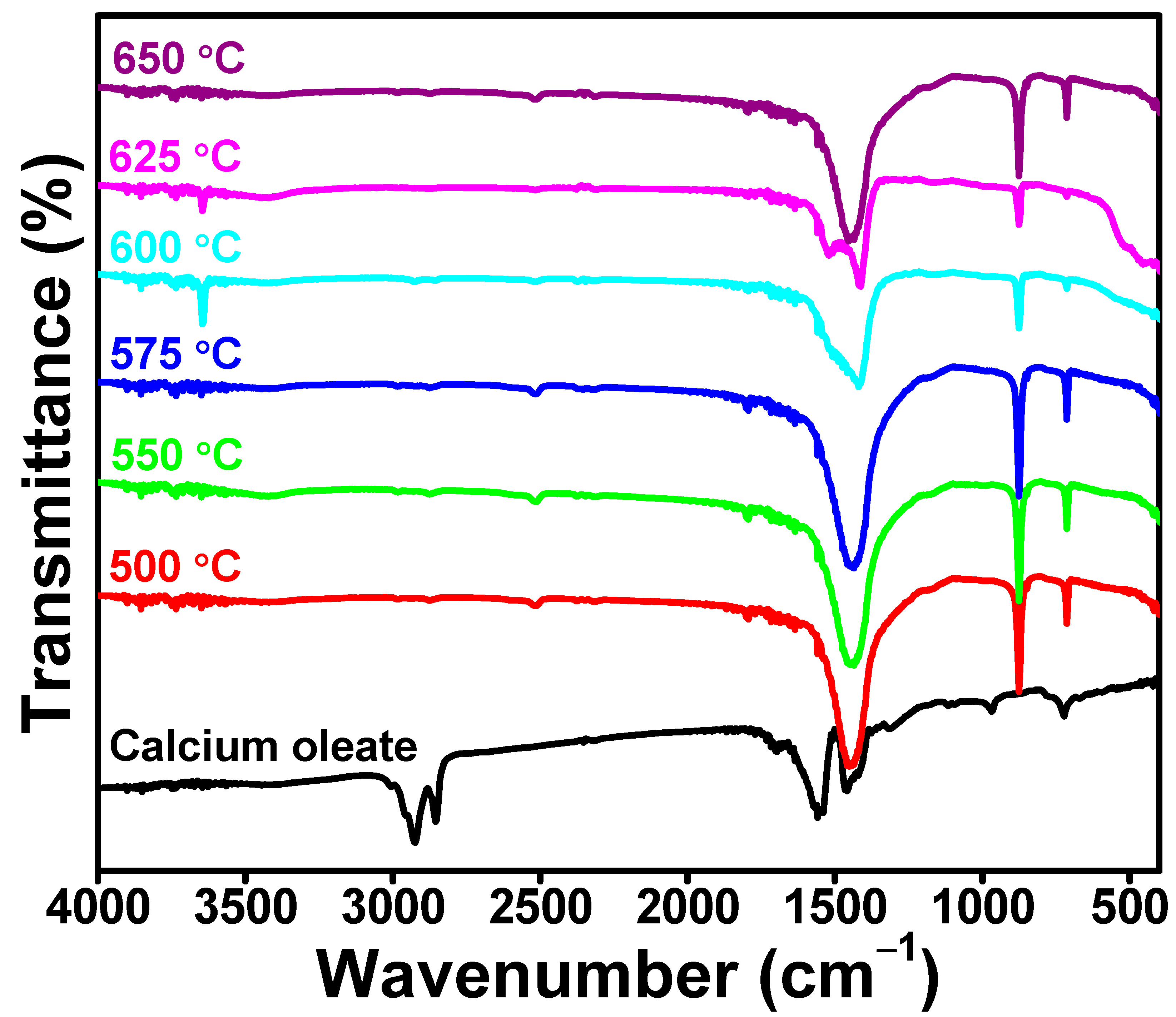
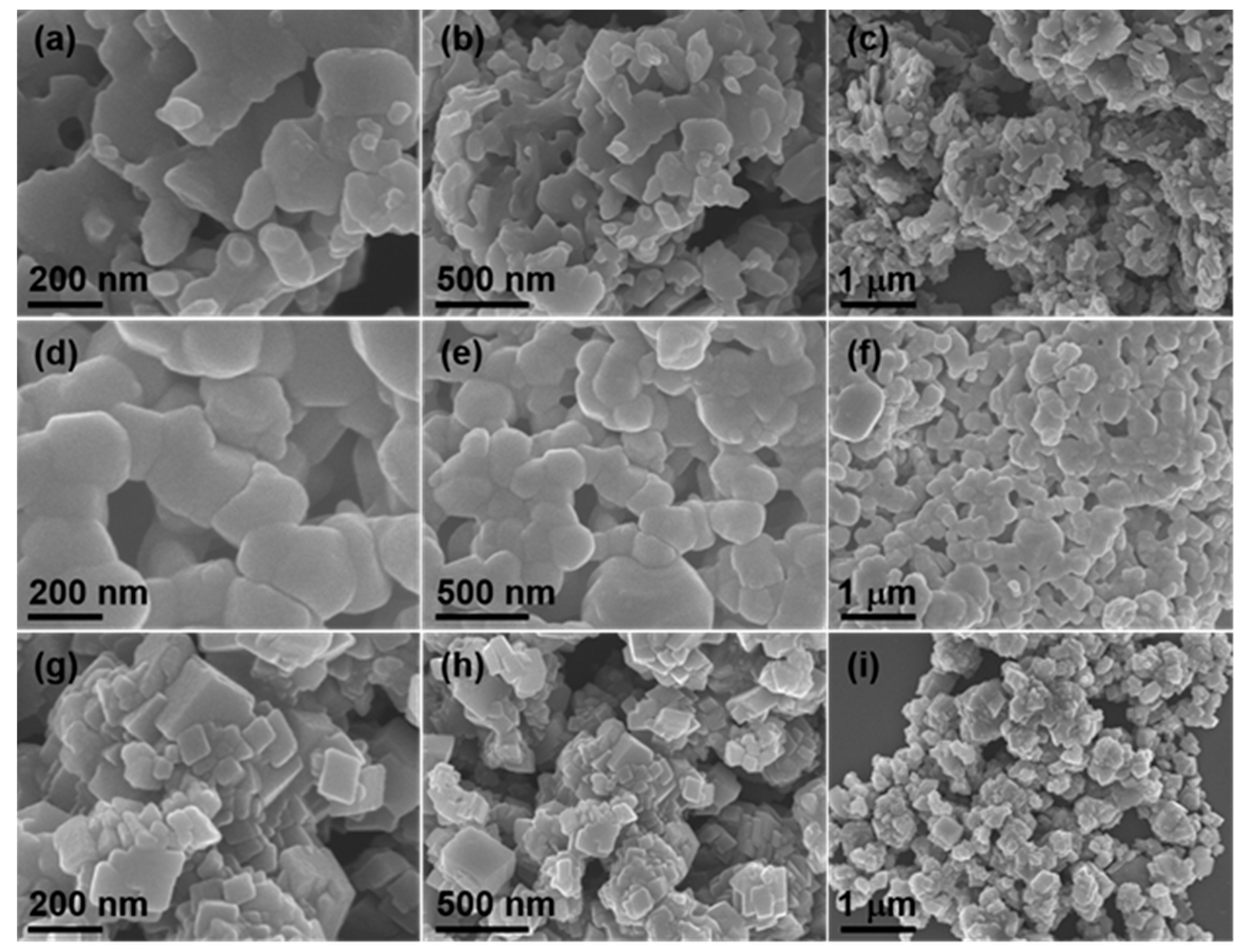
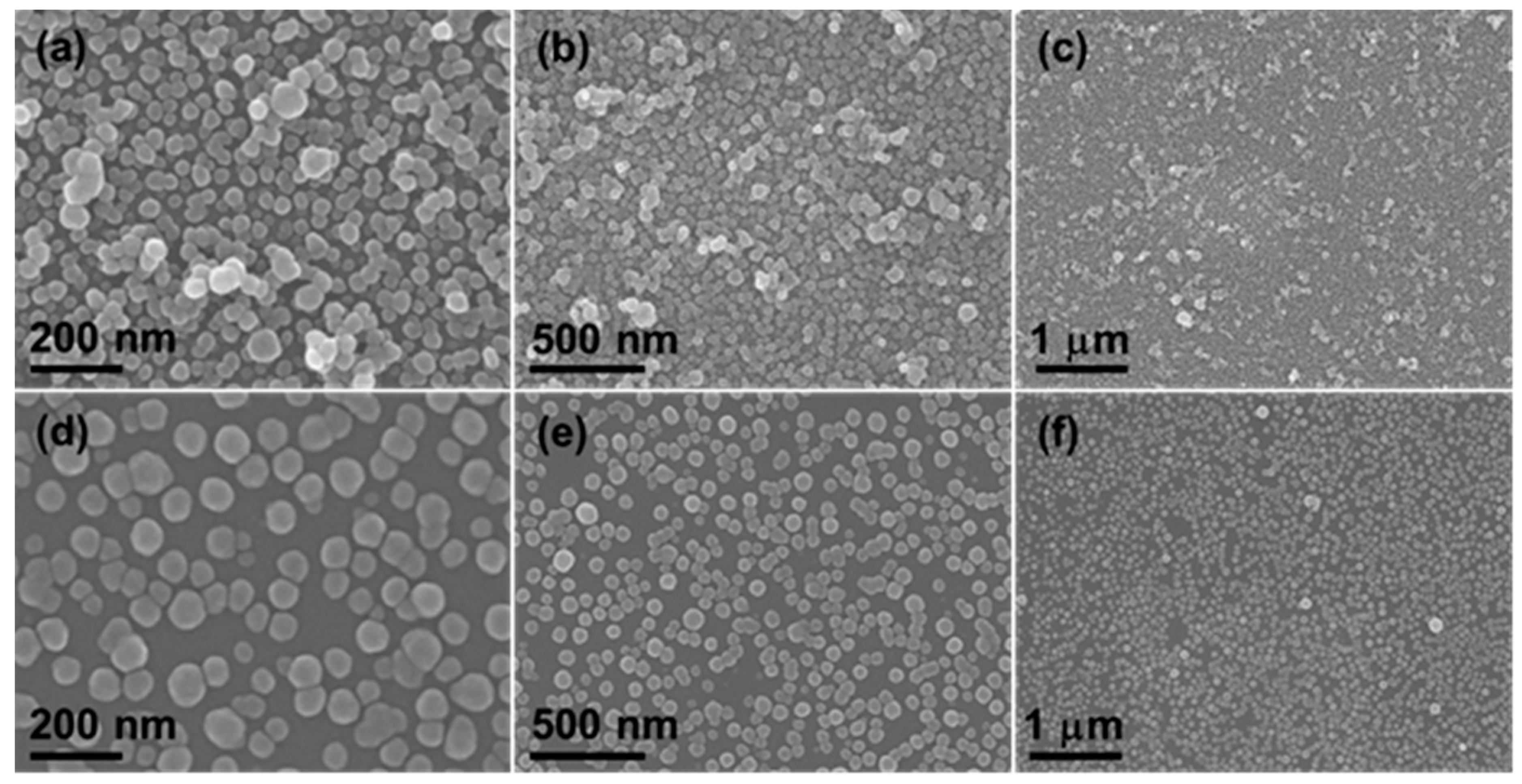
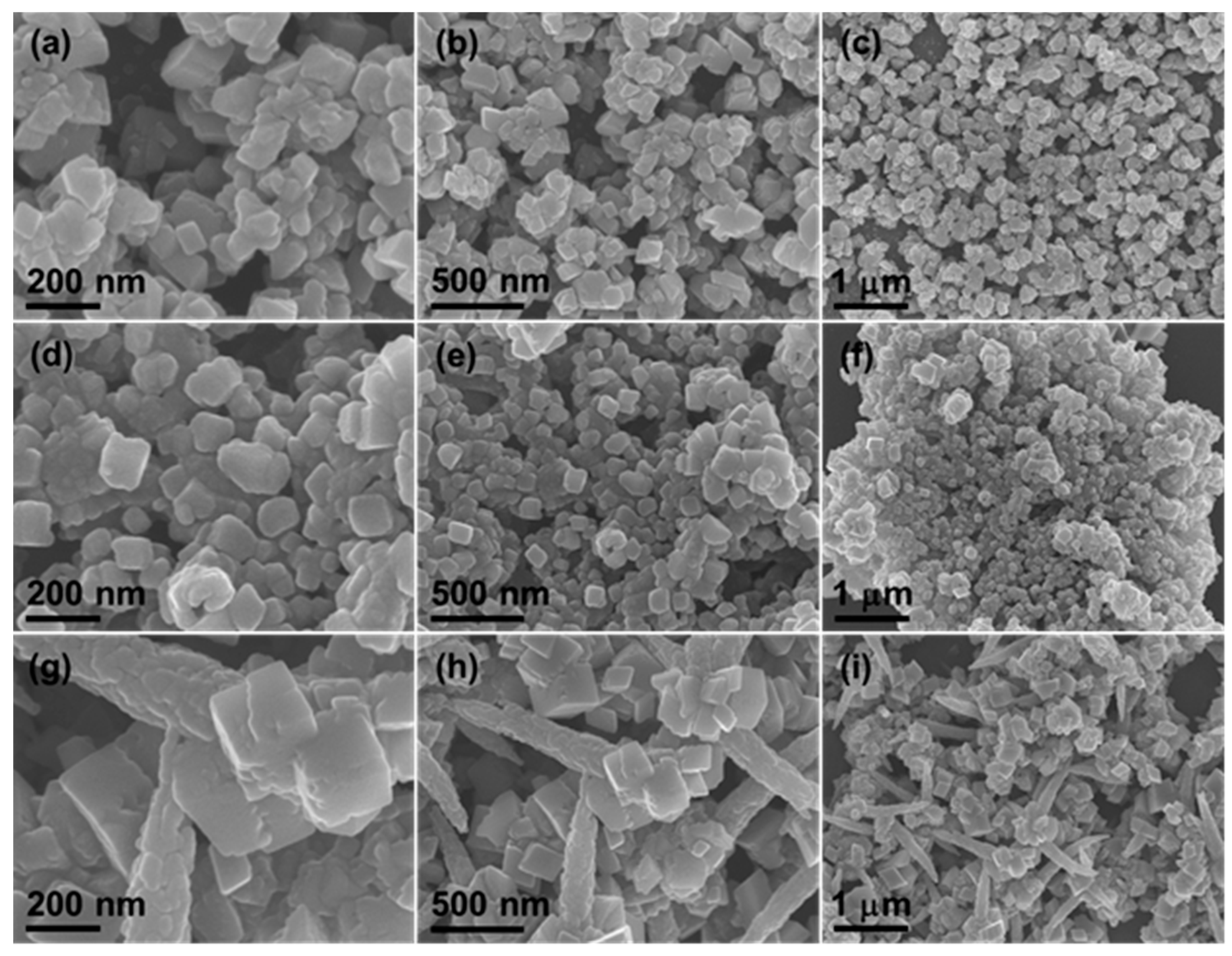
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jiao, J.; Liu, X.; Gao, W.; Wang, C.; Feng, H.; Zhao, X.; Chen, L. Two-step synthesis flowerlike calcium carbonate/biopolymer composite materials. CrystEngComm 2009, 11, 1886–1891. [Google Scholar] [CrossRef]
- Sugih, A.K.; Shukla, D.; Heeres, H.J.; Mehra, A. CaCO3 nanoparticle synthesis by carbonation of lime solution in microemulsion systems. Nanotechnology 2007, 18, 035607. [Google Scholar] [CrossRef]
- Atchudan, R.; Na, H.B.; Cheong, I.W.; Jool, J. Facile Synthesis of Monodispersed Cubic and Spherical Calcite Nanoparticles in the Presence of Cetyltrimethylammonium Bromide. J. Nanosci. Nanotechnol. 2015, 15, 2702–2714. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Li, L.; Lan, J.; Chen, L.; You, J. Biomimetic crystallization of calcium carbonate spherules controlled by hyperbranched polyglycerols. J. Mater. Chem. 2008, 18, 2789–2797. [Google Scholar] [CrossRef]
- Zhao, L.; Feng, J.; Wang, Z. In situ synthesis and modification of calcium carbonate nanoparticles via a bobbling method. Sci. China Ser. B Chem. 2009, 52, 924–929. [Google Scholar] [CrossRef]
- Zhuravlev, Y.N.; Atuchin, V.V. Comprehensive Density Functional Theory Studies of Vibrational Spectra of Carbonates. Nanomaterials 2020, 10, 2275. [Google Scholar] [CrossRef]
- Wójcik, J.; Jones, A. Particle disruption of precipitated CaCO3 crystal agglomerates in turbulently agitated suspensions. Chem. Eng. Sci. 1998, 53, 1097–1101. [Google Scholar] [CrossRef]
- Vučak, M.; Perić, J.; Krstulović, R. Precipitation of calcium carbonate in a calcium nitrate and monoethanolamine solution. Powder Technol. 1997, 91, 69–74. [Google Scholar] [CrossRef]
- Franke, J.; Mersmann, A. The influence of the operational conditions on the precipitation process. Chem. Eng. Sci. 1995, 50, 1737–1753. [Google Scholar] [CrossRef]
- Du, L.; Wang, Y.; Luo, G. In situ preparation of hydrophobic CaCO3 nanoparticles in a gas–liquid microdispersion process. Particuology 2013, 11, 421–427. [Google Scholar] [CrossRef]
- Safaei-Ghomi, J.; Ghasemzadeh, M.A.; Mehrabi, M. Calcium oxide nanoparticles catalyzed one-step multicomponent synthesis of highly substituted pyridines in aqueous ethanol media. Sci. Iran. 2013, 20, 549–554. [Google Scholar] [CrossRef]
- Mirghiasi, Z.; Bakhtiari, F.; Darezereshki, E.; Esmaeilzadeh, E. Preparation and characterization of CaO nanoparticles from Ca(OH)2 by direct thermal decomposition method. J. Ind. Eng. Chem. 2014, 20, 113–117. [Google Scholar] [CrossRef]
- Zhu, Y.; Wu, S.; Wang, X. Nano CaO grain characteristics and growth model under calcination. Chem. Eng. J. 2011, 175, 512–518. [Google Scholar] [CrossRef]
- Feng, B.; An, H.; Tan, E. Screening of CO2 Adsorbing Materials for Zero Emission Power Generation Systems. Energy Fuels 2007, 21, 426–434. [Google Scholar] [CrossRef]
- Luo, C.; Zheng, Y.; Guo, J.; Feng, B. Effect of sulfation on CO2 capture of CaO-based sorbents during calcium looping cycle. Fuel 2014, 127, 124–130. [Google Scholar] [CrossRef]
- Atchudan, R.; Lone, N.; Joo, J. Preparation of CaCO₃ and CaO Nanoparticles via Solid-State Conversion of Calcium Oleate Precursor. J. Nanosci. Nanotechnol. 2018, 18, 1958–1964. [Google Scholar] [CrossRef]
- Samudrala, R.K.; Azeem, P.A. Preliminary biological evaluation of tantalum containing soda lime borosilicate bioactive glasses. J. Alloys Compd. 2019, 810, 151853. [Google Scholar] [CrossRef]
- Guo, X.; Liu, L.; Wang, W.; Zhang, J.; Wang, Y.; Yu, S.-H. Controlled crystallization of hierarchical and porous calcium carbonate crystals using polypeptide type block copolymer as crystal growth modifier in a mixed solution. CrystEngComm 2011, 13, 2054–2061. [Google Scholar] [CrossRef]
- Zhang, Y.; Cho, Y.; Yamaguchi, A.; Peng, X.; Miyauchi, M.; Abe, H.; Fujita, T. CO2 oxidative coupling of methane using an earth-abundant CaO-based catalyst. Sci. Rep. 2019, 9, 15454. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Pan, F.; Wu, S. The grain growth mechanism of nano-CaO regenerated by nano-CaCO3 in calcium looping. RSC Adv. 2019, 9, 26949–26955. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Zhu, Y.; Zhou, B.; Guo, Y.; Gao, W.; Ma, Y.; Guan, S.; Wang, L.; Wang, Z. Hydrophilic CaCO3 nanoparticles designed for poly (ethylene terephthalate). Powder Technol. 2010, 204, 21–26. [Google Scholar] [CrossRef]
- Wu, G.; Wang, Y.; Zhu, S.; Wang, J. Preparation of ultrafine calcium carbonate particles with micropore dispersion method. Powder Technol. 2007, 172, 82–88. [Google Scholar] [CrossRef]
- Golovnev, N.N.; Molokeev, M.S.; Vereshchagin, S.N.; Atuchin, V.V. Calcium and strontium thiobarbiturates with discrete and polymeric structures. J. Coord. Chem. 2013, 66, 4119–4130. [Google Scholar] [CrossRef]
- Golovnev, N.N.; Solovyov, L.A.; Lesnikov, M.K.; Vereshchagin, S.N.; Atuchin, V.V. Hydrated and anhydrous cobalt (II) barbiturates: Crystal structures, spectroscopic and thermal properties. Inorg. Chim. Acta 2017, 467, 39–45. [Google Scholar] [CrossRef]
- Denisenko, Y.G.; Molokeev, M.S.; Oreshonkov, A.S.; Krylov, A.S.; Aleksandrovsky, A.S.; Azarapin, N.O.; Andreev, O.V.; Razumkova, I.A.; Atuchin, V.V. Crystal Structure, Vibrational, Spectroscopic and Thermochemical Properties of Double Sulfate Crystalline Hydrate [CsEu(H2O)3(SO4)2]·H2O and Its Thermal Dehydration Product CsEu(SO4)2. Crystals 2021, 11, 1027. [Google Scholar] [CrossRef]
- Chen, X.; Zhu, Y.; Guo, Y.; Zhou, B.; Zhao, X.; Du, Y.; Lei, H.; Li, M.; Wang, Z. Carbonization synthesis of hydrophobic CaCO3 at room temperature. Colloids Surf. A Physicochem. Eng. Asp. 2010, 353, 97–103. [Google Scholar] [CrossRef]
- Gönen, M.; Öztürk, S.; Balköse, D.; Okur, S.; Ülkü, S. Preparation and Characterization of Calcium Stearate Powders and Films Prepared by Precipitation and Langmuir−Blodgett Techniques. Ind. Eng. Chem. Res. 2010, 49, 1732–1736. [Google Scholar] [CrossRef] [Green Version]
- Uçurum, M.; Bayram, Ö.; Toraman, Ö.Y.; Kılıç, H.; Yalçın, Ş. Changes of surface properties of calcite particles with calcium stearate using conventional experimental design and properties of coated calcite. Physicochem. Probl. Miner. Process. 2018, 54, 688–700. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atchudan, R.; Perumal, S.; Joo, J.; Lee, Y.R. Synthesis and Characterization of Monodispersed Spherical Calcium Oxide and Calcium Carbonate Nanoparticles via Simple Pyrolysis. Nanomaterials 2022, 12, 2424. https://doi.org/10.3390/nano12142424
Atchudan R, Perumal S, Joo J, Lee YR. Synthesis and Characterization of Monodispersed Spherical Calcium Oxide and Calcium Carbonate Nanoparticles via Simple Pyrolysis. Nanomaterials. 2022; 12(14):2424. https://doi.org/10.3390/nano12142424
Chicago/Turabian StyleAtchudan, Raji, Suguna Perumal, Jin Joo, and Yong Rok Lee. 2022. "Synthesis and Characterization of Monodispersed Spherical Calcium Oxide and Calcium Carbonate Nanoparticles via Simple Pyrolysis" Nanomaterials 12, no. 14: 2424. https://doi.org/10.3390/nano12142424








