Crystalline Biomimetic Calcium Phosphate Coating on Mini-Pin Implants to Accelerate Osseointegration and Extend Drug Release Duration for an Orthodontic Application
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
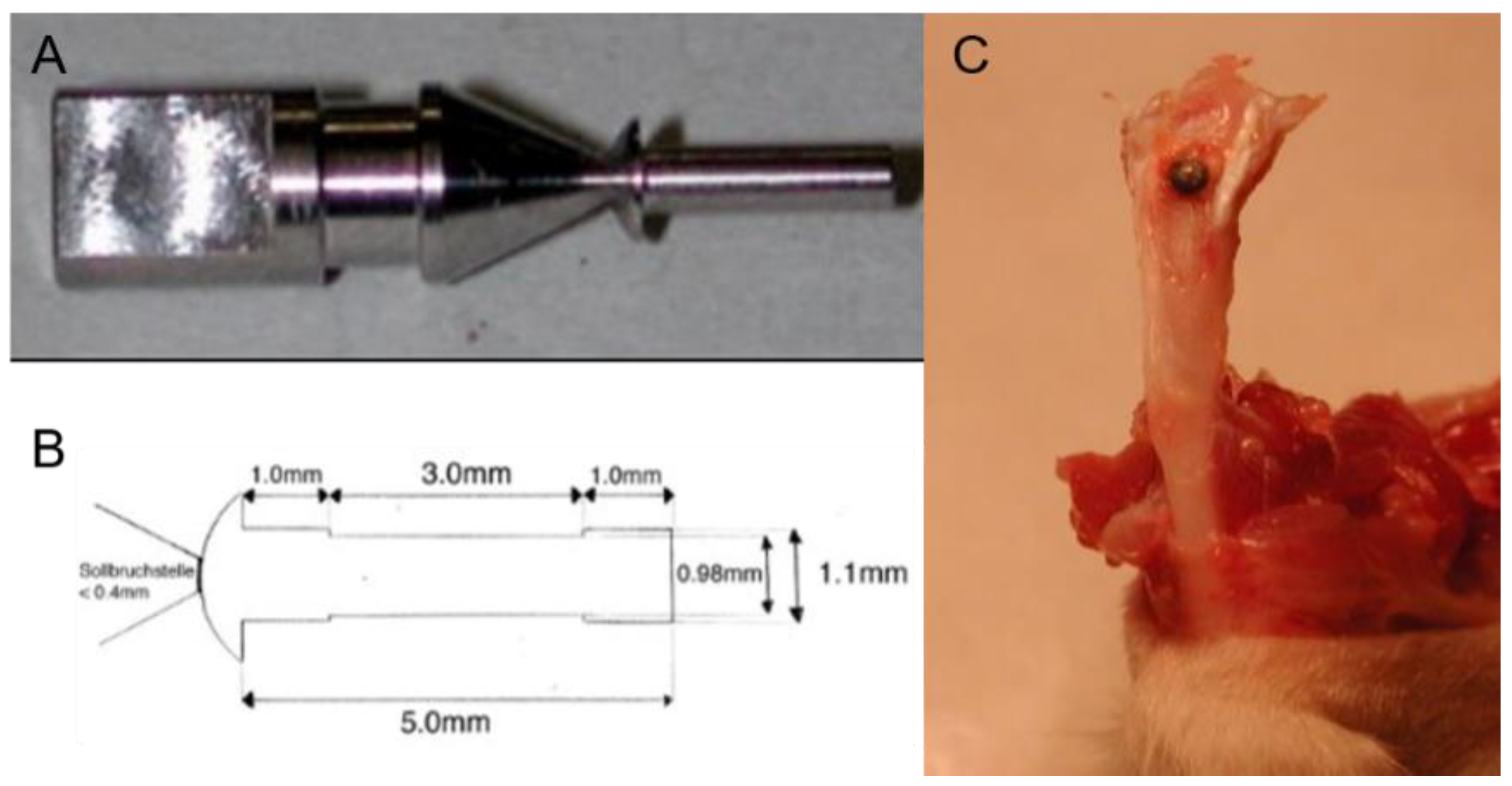
2.1.1. Design of the Mini-Pin Implant
2.1.2. Coating Procedure on Titanium Pin
2.2. In Vitro Study
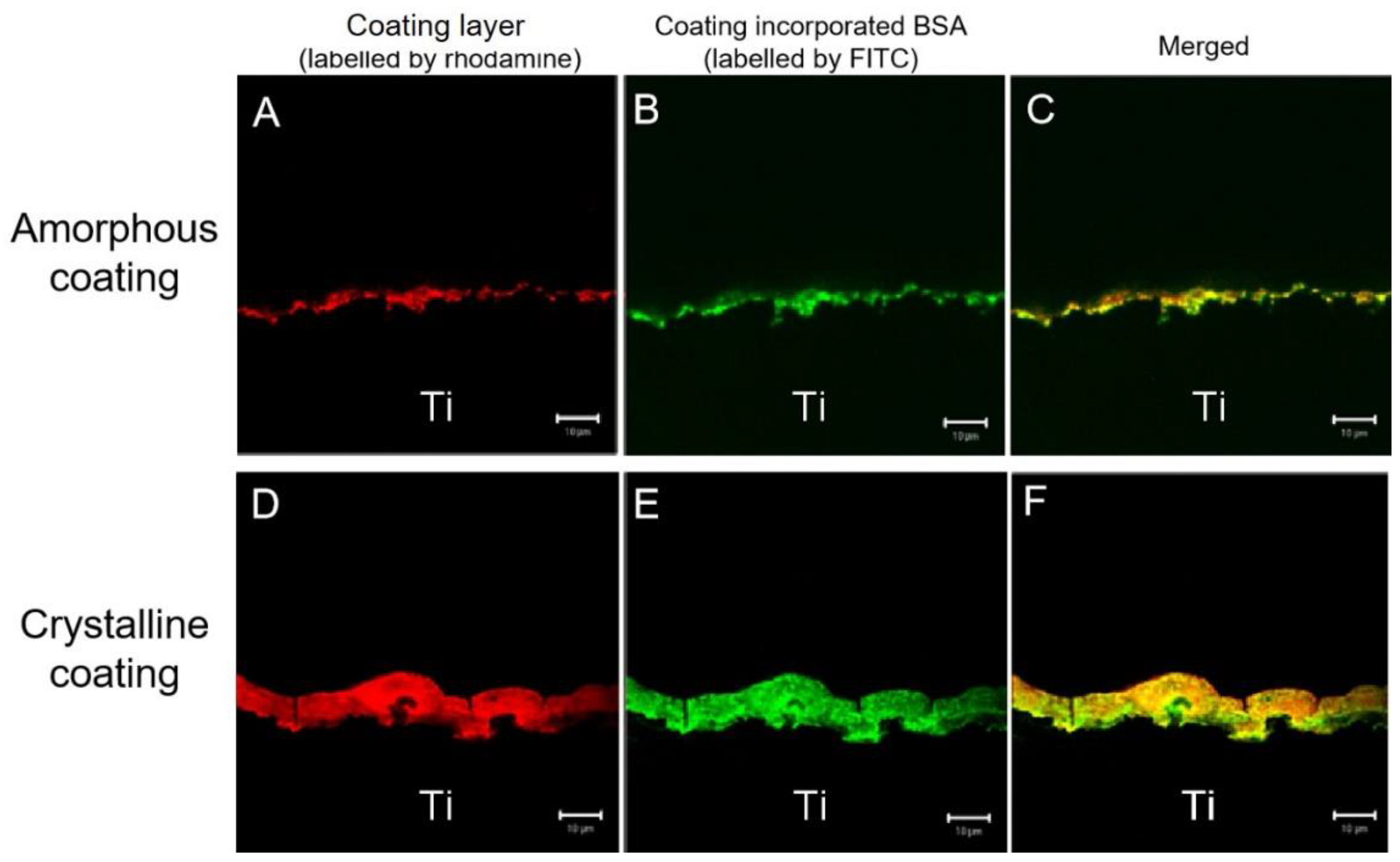
2.2.1. Characterization of Coatings
2.2.2. Loading and Release Kinetics of BSA In Vitro
2.2.3. Alkaline Phosphatase (ALP) Activity of Primary Osteoblasts
2.3. In Vivo Study
2.3.1. Experiment Grouping
2.3.2. Surgical Procedure
2.3.3. Histological Process
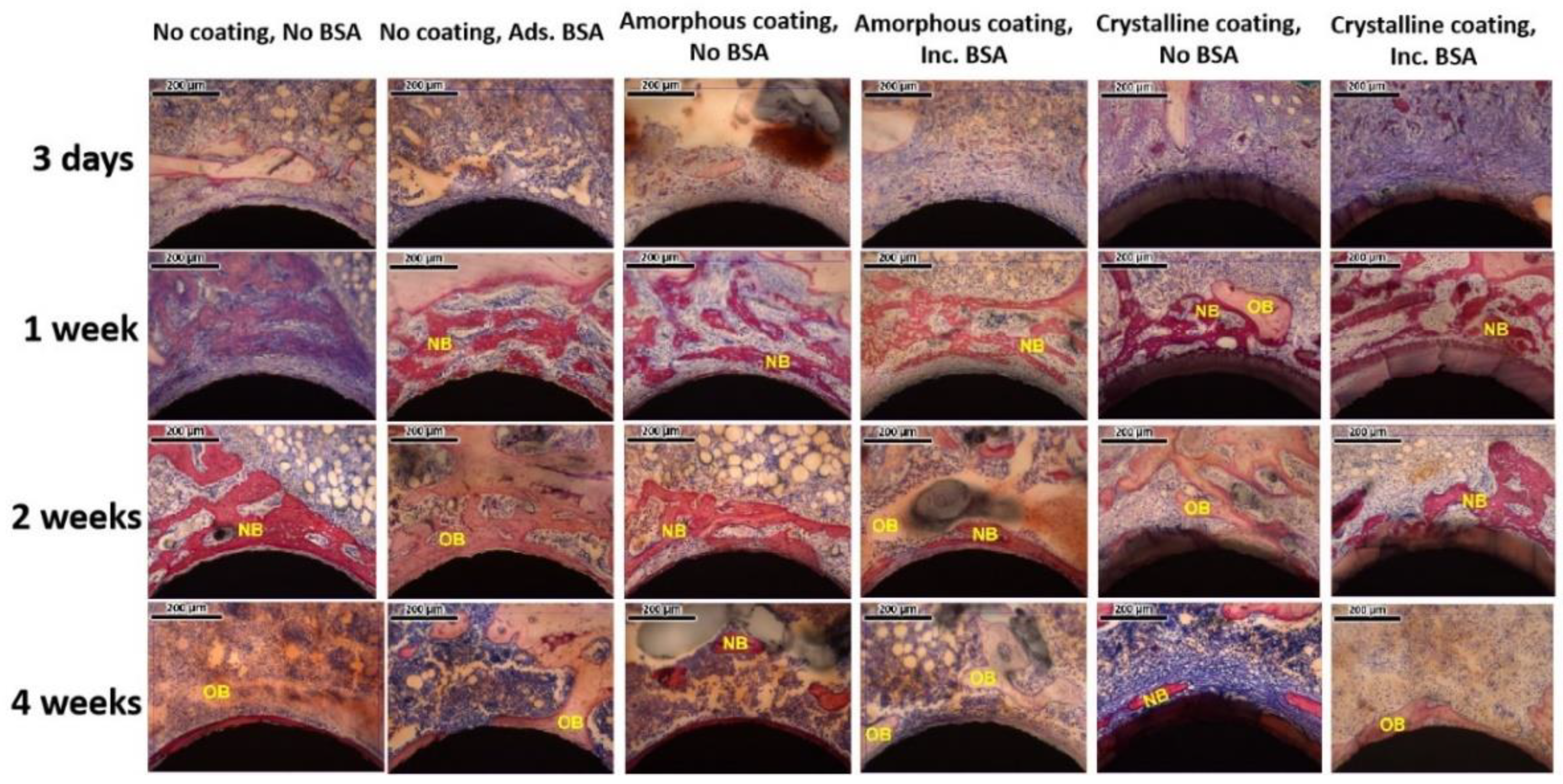
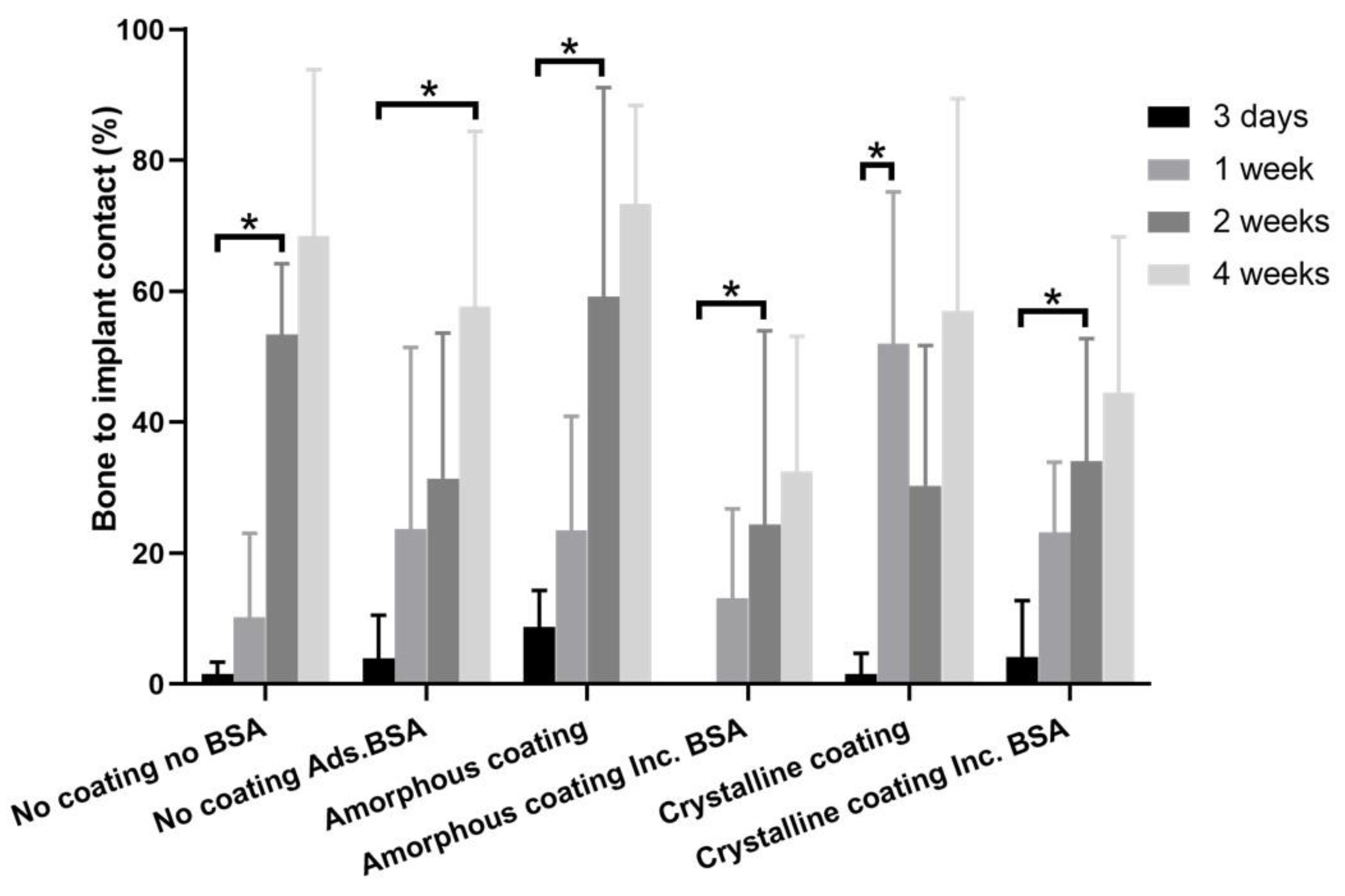
2.3.4. Histomorphometric Analysis
2.4. Statistical Analysis
3. Results
3.1. Coating Characterization
3.2. Loading and Release Kinetics of BSA In Vitro
3.3. In Vitro Cellular Experiments
3.4. In Vivo Study
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Scribante, A.; Montasser, M.A.; Radwan, E.S.; Bernardinelli, L.; Alcozer, R.; Gandini, P.; Sfondrini, M.F. Reliability of Orthodontic Miniscrews: Bending and Maximum Load of Different Ti-6Al-4V Titanium and Stainless Steel Temporary Anchorage Devices (TADs). Materials 2018, 11, 1138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bollero, P.; Di Fazio, V.; Pavoni, C.; Cordaro, M.; Cozza, P.; Lione, R. Titanium alloy vs. stainless steel miniscrews: An in vivo split-mouth study. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 2191–2198. [Google Scholar] [PubMed]
- Papadopoulos, M.A.; Tarawneh, F. The use of miniscrew implants for temporary skeletal anchorage in orthodontics: A comprehensive review. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2007, 103, e6–e15. [Google Scholar] [CrossRef] [PubMed]
- Iijima, M.; Takano, M.; Yasuda, Y.; Muguruma, T.; Nakagaki, S.; Sakakura, Y.; Ochi, M.; Mizoguchi, I. Effect of the quantity and quality of cortical bone on the failure force of a miniscrew implant. Eur. J. Orthod. 2012, 35, 583–589. [Google Scholar] [CrossRef] [Green Version]
- Jing, Z.; Wu, Y.; Jiang, W.; Zhao, L.; Jing, D.; Zhang, N.; Cao, X.; Xu, Z.; Zhao, Z. Factors Affecting the Clinical Success Rate of Miniscrew Implants for Orthodontic Treatment. Int. J. Oral Maxillofac. Implant. 2016, 31, 835–841. [Google Scholar] [CrossRef] [Green Version]
- Lai, T.-T.; Chen, M.-H. Factors affecting the clinical success of orthodontic anchorage: Experience with 266 temporary anchorage devices. J. Dent. Sci. 2014, 9, 49–55. [Google Scholar] [CrossRef] [Green Version]
- Hyde, J.D.; King, G.J.; Greenlee, G.M.; Spiekerman, C.; Huang, G.J. Survey of orthodontists’ attitudes and experiences regarding miniscrew implants. J. Clin. Orthod. JCO 2010, 44, 481–486. [Google Scholar]
- Mohammed, H.; Wafaie, K.; Rizk, M.Z.; Almuzian, M.; Sosly, R.; Bearn, D.R. Role of anatomical sites and correlated risk factors on the survival of orthodontic miniscrew implants: A systematic review and meta-analysis. Prog. Orthod. 2018, 19, 36. [Google Scholar] [CrossRef] [Green Version]
- Ramírez-Ossa, D.M.; Escobar-Correa, N.; Ramírez-Bustamante, M.A.; Agudelo-Suárez, A.A. An Umbrella Review of the Effectiveness of Temporary Anchorage Devices and the Factors That Contribute to Their Success or Failure. J. Évid. Based Dent. Pract. 2020, 20, 101402. [Google Scholar] [CrossRef]
- Melo, A.C.M.; Andrighetto, A.R.; Hirt, S.D.; Bongiolo, A.L.M.; Silva, S.U.; Da Silva, M.A.D. Risk factors associated with the failure of miniscrews—A ten-year cross sectional study. Braz. Oral Res. 2016, 30, e124. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Zhao, L.; Wu, Y.; Wang, H.; Zhao, Z.; Xu, Z.; Wei, X.; Tang, T. The effect of varying healing times on orthodontic mini-implant stability: A microscopic computerized tomographic and biomechanical analysis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2011, 112, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Trisi, P.; Berardini, M.; Falco, A.; Vulpiani, M.P. Effect of Implant Thread Geometry on Secondary Stability, Bone Density, and Bone-to-Implant Contact. Implant Dent. 2015, 24, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Bueno, R.D.B.E.L.; Ponce, K.; Dias, A.; Bello, D.G.; Brunski, J.; Nanci, A. Influence of Nanotopography on Early Bone Healing during Controlled Implant Loading. Nanomaterials 2020, 10, 2191. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhao, Z.; Li, Y.; Wu, J.; Zheng, L.; Tang, T. Osseointegration of Orthodontic Micro-screws After Immediate and Early Loading. Angle Orthod. 2010, 80, 354–360. [Google Scholar] [CrossRef] [Green Version]
- Fleischmann, L.; Crismani, A.; Falkensammer, F.; Bantleon, H.-P.; Rausch-Fan, X.; Andrukhov, O. Behavior of osteoblasts on TI surface with two different coating designed for orthodontic devices. J. Mater. Sci. Mater. Electron. 2015, 26, 1–9. [Google Scholar] [CrossRef]
- Pellegrini, G.; Francetti, L.; Barbaro, B.; Del Fabbro, M. Novel surfaces and osseointegration in implant dentistry. J. Investig. Clin. Dent. 2018, 9, e12349. [Google Scholar] [CrossRef]
- Shah, F.A.; Thomsen, P.; Palmquist, A. Osseointegration and current interpretations of the bone-implant interface. Acta Biomater. 2018, 84, 1–15. [Google Scholar] [CrossRef]
- Shadanbaz, S.; Dias, G.J. Calcium phosphate coatings on magnesium alloys for biomedical applications: A review. Acta Biomater. 2012, 8, 20–30. [Google Scholar] [CrossRef]
- Bandyopadhyay, A.; Shivaram, A.; Mitra, I.; Bose, S. Electrically polarized TiO2 nanotubes on Ti implants to enhance early-stage osseointegration. Acta Biomater. 2019, 96, 686–693. [Google Scholar] [CrossRef]
- Qadir, M.; Li, Y.; Wen, C. Ion-substituted calcium phosphate coatings by physical vapor deposition magnetron sputtering for biomedical applications: A review. Acta Biomater. 2019, 89, 14–32. [Google Scholar] [CrossRef]
- Sut, T.N.; Tan, S.W.; Jeon, W.-Y.; Yoon, B.K.; Cho, N.-J.; Jackman, J.A. Streamlined Fabrication of Hybrid Lipid Bilayer Membranes on Titanium Oxide Surfaces: A Comparison of One- and Two-Tail SAM Molecules. Nanomaterials 2022, 12, 1153. [Google Scholar] [CrossRef] [PubMed]
- Kokubo, T.; Ito, S.; Huang, Z.T.; Hayashi, T.; Sakka, S.; Kitsugi, T.; Yamamuro, T. Ca, P-rich layer formed on high-strength bioactive glass-ceramic A-W. J. Biomed. Mater. Res. 1990, 24, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Hunziker, E.; Randall, N.; de Groot, K.; Layrolle, P. Proteins incorporated into biomimetically prepared calcium phosphate coatings modulate their mechanical strength and dissolution rate. Biomaterials 2002, 24, 65–70. [Google Scholar] [CrossRef]
- Wang, J.; Guo, J.; Liu, J.; Wei, L.; Wu, G. BMP-Functionalised Coatings to Promote Osteogenesis for Orthopaedic Implants. Int. J. Mol. Sci. 2014, 15, 10150–10168. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; De Groot, K.; Hunziker, E.B. BMP-2 liberated from biomimetic implant coatings induces and sustains direct ossification in an ectopic rat model. Bone 2005, 36, 745–757. [Google Scholar] [CrossRef]
- Wu, G.; Liu, Y.; Iizuka, T.; Hunziker, E.B. Biomimetic Coating of Organic Polymers with a Protein-Functionalized Layer of Calcium Phosphate: The Surface Properties of the Carrier Influence Neither the Coating Characteristics Nor the Incorporation Mechanism or Release Kinetics of the Protein. Tissue Eng. Part C Methods 2010, 16, 1255–1265. [Google Scholar] [CrossRef]
- Lin, X.; Chen, J.; Liao, Y.; Pathak, J.L.; Li, H.; Liu, Y. Biomimetic Calcium Phosphate Coating as a Drug Delivery Vehicle for Bone Tissue Engineering: A Mini-Review. Coatings 2020, 10, 1118. [Google Scholar] [CrossRef]
- Liu, Y.; Enggist, L.; Kuffer, A.F.; Buser, D.; Hunziker, E.B. The influence of BMP-2 and its mode of delivery on the osteoconductivity of implant surfaces during the early phase of osseointegration. Biomaterials 2007, 28, 2677–2686. [Google Scholar] [CrossRef]
- Uskoković, V.; Janković-Častvan, I.; Wu, V.M. Bone Mineral Crystallinity Governs the Orchestration of Ossification and Resorption during Bone Remodeling. ACS Biomater. Sci. Eng. 2019, 5, 3483–3498. [Google Scholar] [CrossRef]
- Hägi, T.T.; Enggist, L.; Michel, D.; Ferguson, S.J.; Liu, Y.; Hunziker, E.B. Mechanical insertion properties of calcium-phosphate implant coatings. Clin. Oral Implant. Res. 2010, 21, 1214–1222. [Google Scholar] [CrossRef]
- Fan, H.; Ikoma, T.; Tanaka, J.; Zhang, X. Surface Structural Biomimetics and the Osteoinduction of Calcium Phosphate Biomaterials. J. Nanosci. Nanotechnol. 2007, 7, 808–813. [Google Scholar] [CrossRef] [PubMed]
- Florkiewicz, W.; Słota, D.; Placek, A.; Pluta, K.; Tyliszczak, B.; Douglas, T.; Sobczak-Kupiec, A. Synthesis and Characterization of Polymer-Based Coatings Modified with Bioactive Ceramic and Bovine Serum Albumin. J. Funct. Biomater. 2021, 12, 21. [Google Scholar] [CrossRef] [PubMed]
- Barrere, F.; van Blitterswijk, C.; de Groot, K.; Layrolle, P. Influence of ionic strength and carbonate on the Ca-P coating formation from SBF×5 solution. Biomaterials 2002, 23, 1921–1930. [Google Scholar] [CrossRef]
- Liu, T.-Y.; Chen, S.-Y.; Liu, D.-M.; Liou, S.-C. On the study of BSA-loaded calcium-deficient hydroxyapatite nano-carriers for controlled drug delivery. J. Control. Release 2005, 107, 112–121. [Google Scholar] [CrossRef]
- Wang, J.; Layrolle, P.; Stigter, M.; de Groot, K. Biomimetic and electrolytic calcium phosphate coatings on titanium alloy: Physicochemical characteristics and cell attachment. Biomaterials 2003, 25, 583–592. [Google Scholar] [CrossRef]
- Dekker, R.J.; de Bruijn, J.D.; Stigter, M.; Barrere, F.; Layrolle, P.; van Blitterswijk, C.A. Bone tissue engineering on amorphous carbonated apatite and crystalline octacalcium phosphate-coated titanium discs. Biomaterials 2005, 26, 5231–5239. [Google Scholar] [CrossRef]
- Wang, H.; Lin, C.-J.; Hu, R.; Zhang, F.; Lin, L.-W. A novel nano-micro structured octacalcium phosphate/protein composite coating on titanium by using an electrochemically induced deposition. J. Biomed. Mater. Res. Part A 2008, 87A, 698–705. [Google Scholar] [CrossRef]
- Cross, K.J.; Huq, N.L.; Reynolds, E.C. Casein Phosphopeptide–Amorphous Calcium Phosphate Nanocomplexes: A Structural Model. Biochemistry 2016, 55, 4316–4325. [Google Scholar] [CrossRef]
- Lin, X.; Hunziker, E.B.; Liu, T.; Hu, Q.; Liu, Y. Enhanced biocompatibility and improved osteogenesis of coralline hydroxyapatite modified by bone morphogenetic protein 2 incorporated into a biomimetic coating. Mater. Sci. Eng. C 2018, 96, 329–336. [Google Scholar] [CrossRef]
- Miyawaki, S.; Koyama, I.; Inoue, M.; Mishima, K.; Sugahara, T.; Takano-Yamamoto, T. Factors associated with the stability of titanium screws placed in the posterior region for orthodontic anchorage. Am. J. Orthod. Dentofac. Orthop. 2003, 124, 373–378. [Google Scholar] [CrossRef]
- Duboisset, J.; Ferrand, P.; Baroni, A.; Grünewald, T.A.; Dicko, H.; Grauby, O.; Vidal-Dupiol, J.; Saulnier, D.; Gilles, L.M.; Rosenthal, M.; et al. Amorphous-to-crystal transition in the layer-by-layer growth of bivalve shell prisms. Acta Biomater. 2022, 142, 194–207. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhou, P.; Liu, S.; Attarilar, S.; Ma, R.L.-W.; Zhong, Y.; Wang, L. Multi-Scale Surface Treatments of Titanium Implants for Rapid Osseointegration: A Review. Nanomaterials 2020, 10, 1244. [Google Scholar] [CrossRef] [PubMed]
- Bucci, R.; Montanaro, D.; Rongo, R.; Valletta, R.; Michelotti, A.; D’Antò, V. Effects of maxillary expansion on the upper airways: Evidence from systematic reviews and meta-analyses. J. Oral Rehabil. 2019, 46, 377–387. [Google Scholar] [CrossRef]
- Choi, S.; Hwang, C. Factors Affecting the Failure of TADs and Efforts to Improve the Biomechanical Stability of TADs. In Temporary Anchorage Devices in Clinical Orthodontics; Wiley: Hoboken, NJ, USA, 2020; pp. 61–67. [Google Scholar]
- Lee, S.-J.; Ahn, S.-J.; Lee, J.W.; Kim, S.-H.; Kim, T.-W. Survival analysis of orthodontic mini-implants. Am. J. Orthod. Dentofac. Orthop. 2010, 137, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Al-Thomali, Y.; Basha, S.; Mohamed, R.N. Effect of surface treatment on the mechanical stability of orthodontic miniscrews: A systematic review with meta-analysis. Angle Orthod. 2021, 92, 127–136. [Google Scholar] [CrossRef]
- Moghaddam, S.F.; Mohammadi, A.; Behroozian, A. The effect of sandblasting and acid etching on survival rate of orthodontic miniscrews: A split-mouth randomized controlled trial. Prog. Orthod. 2021, 22, 1–7. [Google Scholar] [CrossRef]
- Seker, E.D.; Yavuz, I.; Yucesoy, T.; Cenkci, E.; Yay, A. Comparison of the Stability of Sandblasted, Large-Grit, and Acid-Etched Treated Mini-Screws with Two Different Surface Roughness Values: A Histomorphometric Study. J. Craniofacial Surg. 2021, 33, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Oh, N.-H.; Kim, S.-H.; Kook, Y.-A.; Lee, K.-H.; Kang, Y.-G.; Mo, S.-S. Removal torque of sandblasted large grit, acid etched treated mini-implant. Korean J. Orthod. 2006, 36, 324–330. [Google Scholar]
- Kim, S.-H.; Cho, J.-H.; Chung, K.-R.; Kook, Y.-A.; Nelson, G. Removal torque values of surface-treated mini-implants after loading. Am. J. Orthod. Dentofac. Orthop. 2008, 134, 36–43. [Google Scholar] [CrossRef]
- Cho, I.-S.; Kim, S.-K.; Chang, Y.-I.; Baek, S.-H. In vitro and in vivo mechanical stability of orthodontic mini-implants. Angle Orthod. 2012, 82, 611–617. [Google Scholar] [CrossRef] [Green Version]
- Gharbavi, M.; Danafar, H.; Sharafi, A. Microemulsion and bovine serum albumin nanoparticles as a novel hybrid nanocarrier system for efficient multifunctional drug delivery. J. Biomed. Mater. Res. Part A 2020, 108, 1688–1702. [Google Scholar] [CrossRef] [PubMed]
- Khodashenas, B.; Ardjmand, M.; Baei, M.S.; Rad, A.S.; Khiyavi, A.A. Bovine serum albumin/gold nanoparticles as a drug delivery system for Curcumin: Experimental and computational studies. J. Biomol. Struct. Dyn. 2019, 38, 4644–4654. [Google Scholar] [CrossRef] [PubMed]
- Ferrado, J.B.; Perez, A.A.; Ruiz, M.C.; León, I.E.; Santiago, L.G. Chrysin-loaded bovine serum albumin particles as bioactive nanosupplements. Food Funct. 2020, 11, 6007–6019. [Google Scholar] [CrossRef] [PubMed]
- Yedomon, B.; Fessi, H.; Charcosset, C. Preparation of Bovine Serum Albumin (BSA) nanoparticles by desolvation using a membrane contactor: A new tool for large scale production. Eur. J. Pharm. Biopharm. 2013, 85, 398–405. [Google Scholar] [CrossRef]
- Liu, Y.; Layrolle, P.; de Bruijn, J.; van Blitterswijk, C.; de Groot, K. Biomimetic coprecipitation of calcium phosphate and bovine serum albumin on titanium alloy. J. Biomed. Mater. Res. 2001, 57, 327–335. [Google Scholar] [CrossRef]
- Wen, H.B.; De Wijn, J.R.; van Blitterswijk, C.; De Groot, K. Incorporation of bovine serum albumin in calcium phosphate coating on titanium. J. Biomed. Mater. Res. 1999, 46, 245–252. [Google Scholar] [CrossRef]
- Feng, B.; Weng, J.; Qu, S.X.; Zhang, X.D. The Influence of Calcium at the Titanium Surface on Co-Precipitation of Ca-P and Bovine Serum Albumin. Mater. Sci. Forum 2005, 479, 2375–2378. [Google Scholar] [CrossRef]
- Dong, G.; He, L.; Pang, D.; Wei, L.; Deng, C. An in situ study of the deposition of a calcium phosphate mineralized layer on a silicon-substituted hydroxyapatite sensor modulated by bovine serum albumin using QCM-D technology. Ceram. Int. 2016, 42, 18648–18656. [Google Scholar] [CrossRef]
- Combes, C.; Rey, C. Adsorption of proteins and calcium phosphate materials bioactivity. Biomaterials 2002, 23, 2817–2823. [Google Scholar] [CrossRef]
- Exposto, C.R.; Oz, U.; Westgate, P.M.; Huja, S.S. Influence of mini-screw diameter and loading conditions on static and dynamic assessments of bone-implant contact: An animal study. Orthod. Craniofacial Res. 2019, 22, 96–100. [Google Scholar] [CrossRef]
- Lian, Z.; Guan, H.; Ivanovski, S.; Loo, Y.-C.; Johnson, N.; Zhang, H. Effect of bone to implant contact percentage on bone remodelling surrounding a dental implant. Int. J. Oral Maxillofac. Surg. 2010, 39, 690–698. [Google Scholar] [CrossRef] [Green Version]
- Yu, W.-P.; Tsai, M.-T.; Yu, J.-H.; Huang, H.-L.; Hsu, J.-T. Bone quality affects stability of orthodontic miniscrews. Sci. Rep. 2022, 12, 2849. [Google Scholar] [CrossRef] [PubMed]
- Offermanns, V.; Andersen, O.Z.; Riede, G.; Sillassen, M.; Jeppesen, C.S.; Almtoft, K.P.; Talasz, H.; Öhman-Mägi, C.; Lethaus, B.; Tolba, R.; et al. Effect of strontium surface-functionalized implants on early and late osseointegration: A histological, spectrometric and tomographic evaluation. Acta Biomater. 2018, 69, 385–394. [Google Scholar] [CrossRef]
- Roberts, W. Bone tissue interface. J. Dent. Educ. 1988, 52, 804–809. [Google Scholar] [CrossRef]
- Bang, S.-M.; Moon, H.-J.; Kwon, Y.-D.; Yoo, J.-Y.; Pae, A.; Kwon, I.K. Osteoblastic and osteoclastic differentiation on SLA and hydrophilic modified SLA titanium surfaces. Clin. Oral Implant. Res. 2013, 25, 831–837. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Fan, H.; Xiao, Y.; Li, D.; Zhang, H.; Luxbacher, T.; Zhang, X. Effect of surface structure on protein adsorption to biphasic calcium-phosphate ceramics in vitro and in vivo. Acta Biomater. 2009, 5, 1311–1318. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhao, S.; Liu, B.; Chen, M.; Mao, J.; He, H.; Zhao, Y.; Huang, N.; Wan, G. Corrosion-Controlling and Osteo-Compatible Mg Ion-Integrated Phytic Acid (Mg-PA) Coating on Magnesium Substrate for Biodegradable Implants Application. ACS Appl. Mater. Interfaces 2014, 6, 19531–19543. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Chen, Y.; Zhang, W.; Mo, X.; Zou, D.; Soliman, H.; Zhou, C.; Huang, N.; Sang, H.; Zeng, H.; et al. Micro/Nano-Structured Metal–Organic/Inorganic Hybrid Coatings on Biodegradable Zn for Osteogenic and Biocompatible Improvement. Adv. Mater. Interfaces 2022, 9, 2101852. [Google Scholar] [CrossRef]
- Jimbo, R.; Coelho, P.G.; Vandeweghe, S.; Schwartz-Filho, H.O.; Hayashi, M.; Ono, D.; Andersson, M.; Wennerberg, A. Histological and three-dimensional evaluation of osseointegration to nanostructured calcium phosphate-coated implants. Acta Biomater. 2011, 7, 4229–4234. [Google Scholar] [CrossRef]
- Bouler, J.; Pilet, P.; Gauthier, O.; Verron, E. Biphasic calcium phosphate ceramics for bone reconstruction: A review of biological response. Acta Biomater. 2017, 53, 1–12. [Google Scholar] [CrossRef]
- Lin, X.; De Groot, K.; Wang, D.; Hu, Q.; Wismeijer, D.; Liu, Y. A Review Paper on Biomimetic Calcium Phosphate Coatings. Open Biomed. Eng. J. 2015, 9, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Wang, T.; Xu, Y.; Guo, Y.; Li, G.; Lian, J. A polydopamine-based calcium phosphate/graphene oxide composite coating on magnesium alloy to improve corrosion resistance and biocompatibility for biomedical applications. Materialia 2022, 21, 101315. [Google Scholar] [CrossRef]
- Yoshinari, M.; Oda, Y.; Inoue, T.; Matsuzaka, K.; Shimono, M. Bone response to calcium phosphate-coated and bisphosphonate-immobilized titanium implants. Biomaterials 2002, 23, 2879–2885. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J.; Jiang, M.; Li, X.; Zhang, Q.; He, H. Osteoinductive hybrid hydrogel membranes for in situ bone regeneration in hyperglycemia. Colloids Surf. B Biointerfaces 2022, 214, 112450. [Google Scholar] [CrossRef]
- Rustom, L.E.; Poellmann, M.J.; Johnson, A.J.W. Mineralization in micropores of calcium phosphate scaffolds. Acta Biomater. 2018, 83, 435–455. [Google Scholar] [CrossRef]
- Xiao, D.; Zhang, J.; Zhang, C.; Barbieri, D.; Yuan, H.; Moroni, L.; Feng, G. The role of calcium phosphate surface structure in osteogenesis and the mechanisms involved. Acta Biomater. 2020, 106, 22–33. [Google Scholar] [CrossRef]
- Kim, H.; Choi, A.; Gong, M.-K.; Park, H.; Kim, Y.-I. Effect of Remineralized Collagen on Dentin Bond Strength Through Calcium Phosphate Ion Clusters or Metastable Calcium Phosphate Solution. Nanomaterials 2020, 10, 2203. [Google Scholar] [CrossRef]
- Stadelmann, V.A.; Thompson, K.; Zeiter, S.; Camenisch, K.; Styger, U.; Patrick, S.; McDowell, A.; Nehrbass, D.; Richards, R.G.; Moriarty, T.F. Longitudinal time-lapse in vivo micro-CT reveals differential patterns of peri-implant bone changes after subclinical bacterial infection in a rat model. Sci. Rep. 2020, 10, 20901. [Google Scholar] [CrossRef] [PubMed]
- Wheelis, S.E.; Biguetti, C.C.; Natarajan, S.; Arteaga, A.; El Allami, J.; Chandrashekar, B.L.; Garlet, G.P.; Rodrigues, D.C. Cellular and Molecular Dynamics during Early Oral Osseointegration: A Comprehensive Characterization in the Lewis Rat. ACS Biomater. Sci. Eng. 2021, 7, 2392–2407. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Wu, G.; Wang, M.; Hunziker, E.B.; Liu, Y. Crystalline Biomimetic Calcium Phosphate Coating on Mini-Pin Implants to Accelerate Osseointegration and Extend Drug Release Duration for an Orthodontic Application. Nanomaterials 2022, 12, 2439. https://doi.org/10.3390/nano12142439
Li M, Wu G, Wang M, Hunziker EB, Liu Y. Crystalline Biomimetic Calcium Phosphate Coating on Mini-Pin Implants to Accelerate Osseointegration and Extend Drug Release Duration for an Orthodontic Application. Nanomaterials. 2022; 12(14):2439. https://doi.org/10.3390/nano12142439
Chicago/Turabian StyleLi, Menghong, Gang Wu, Mingjie Wang, Ernst B. Hunziker, and Yuelian Liu. 2022. "Crystalline Biomimetic Calcium Phosphate Coating on Mini-Pin Implants to Accelerate Osseointegration and Extend Drug Release Duration for an Orthodontic Application" Nanomaterials 12, no. 14: 2439. https://doi.org/10.3390/nano12142439
APA StyleLi, M., Wu, G., Wang, M., Hunziker, E. B., & Liu, Y. (2022). Crystalline Biomimetic Calcium Phosphate Coating on Mini-Pin Implants to Accelerate Osseointegration and Extend Drug Release Duration for an Orthodontic Application. Nanomaterials, 12(14), 2439. https://doi.org/10.3390/nano12142439







