Microbial Electrosynthesis Inoculated with Anaerobic Granular Sludge and Carbon Cloth Electrodes Functionalized with Copper Nanoparticles for Conversion of CO2 to CH4
Abstract
:1. Introduction
2. Materials and Methods
2.1. Inoculum
2.2. Electrodes Synthesis and Characterization
2.3. MES System Set-Up
2.4. Analytical Methods
2.5. High-Throughput 16S rRNA Gene Sequencing
3. Results and Discussion
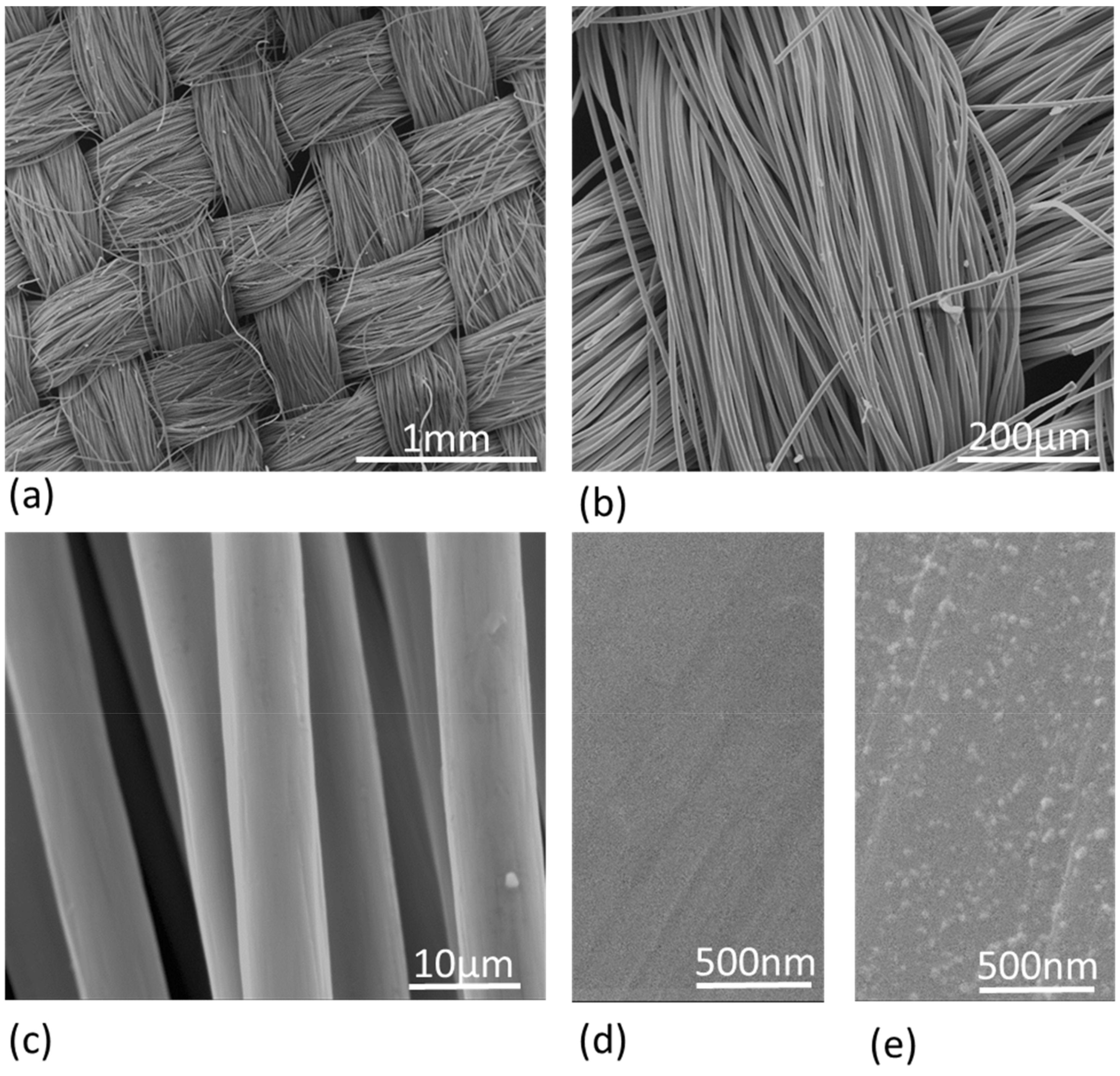
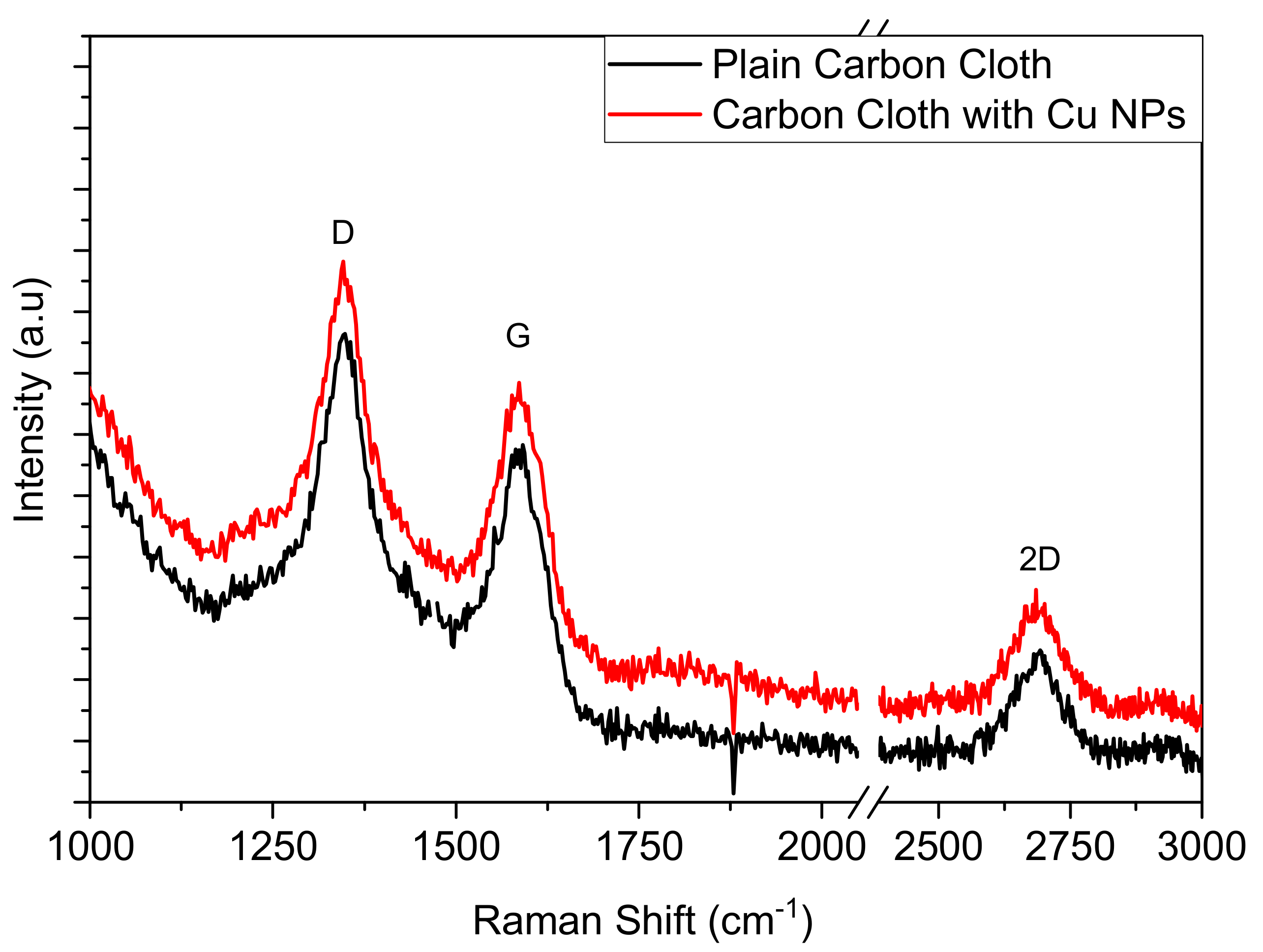
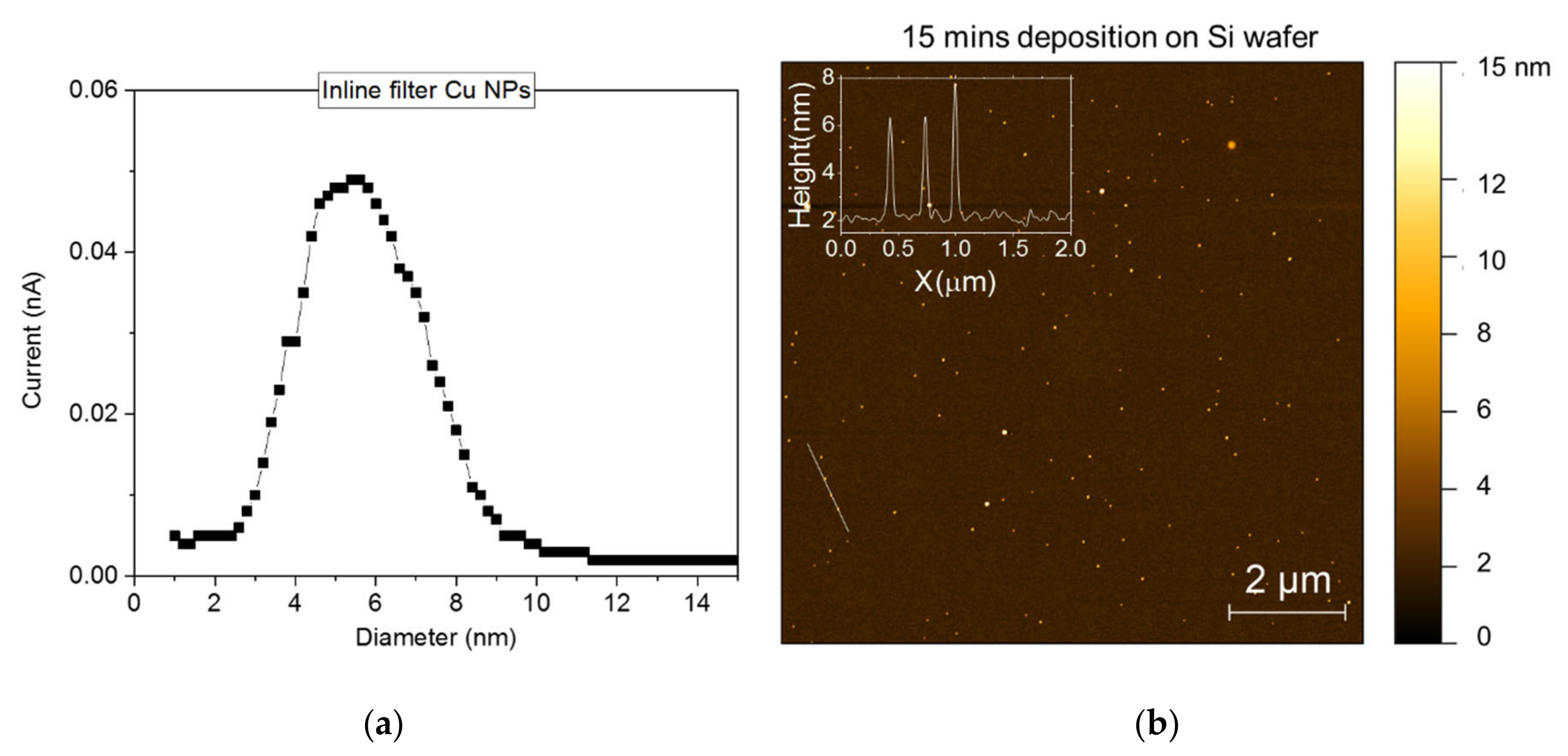
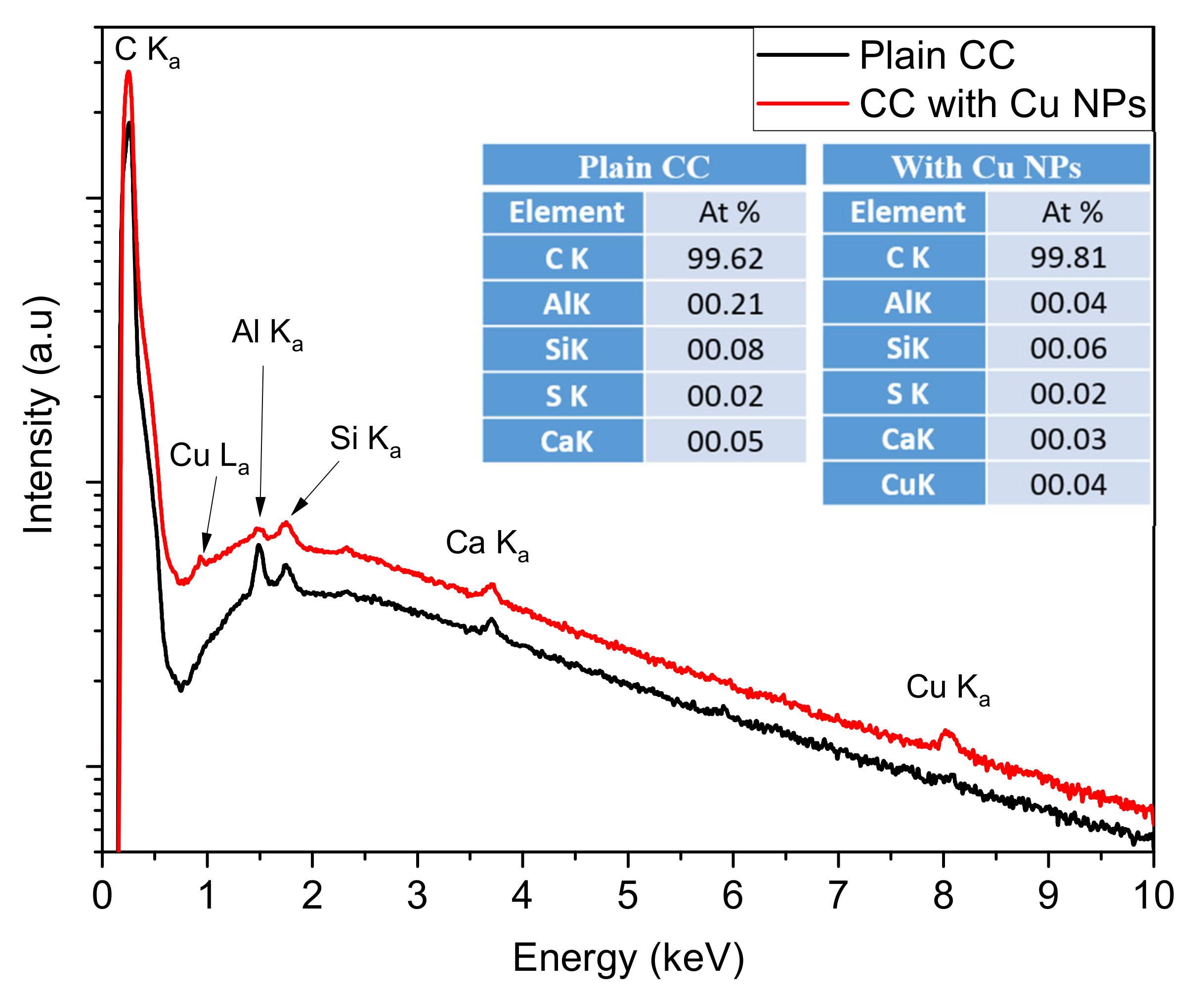
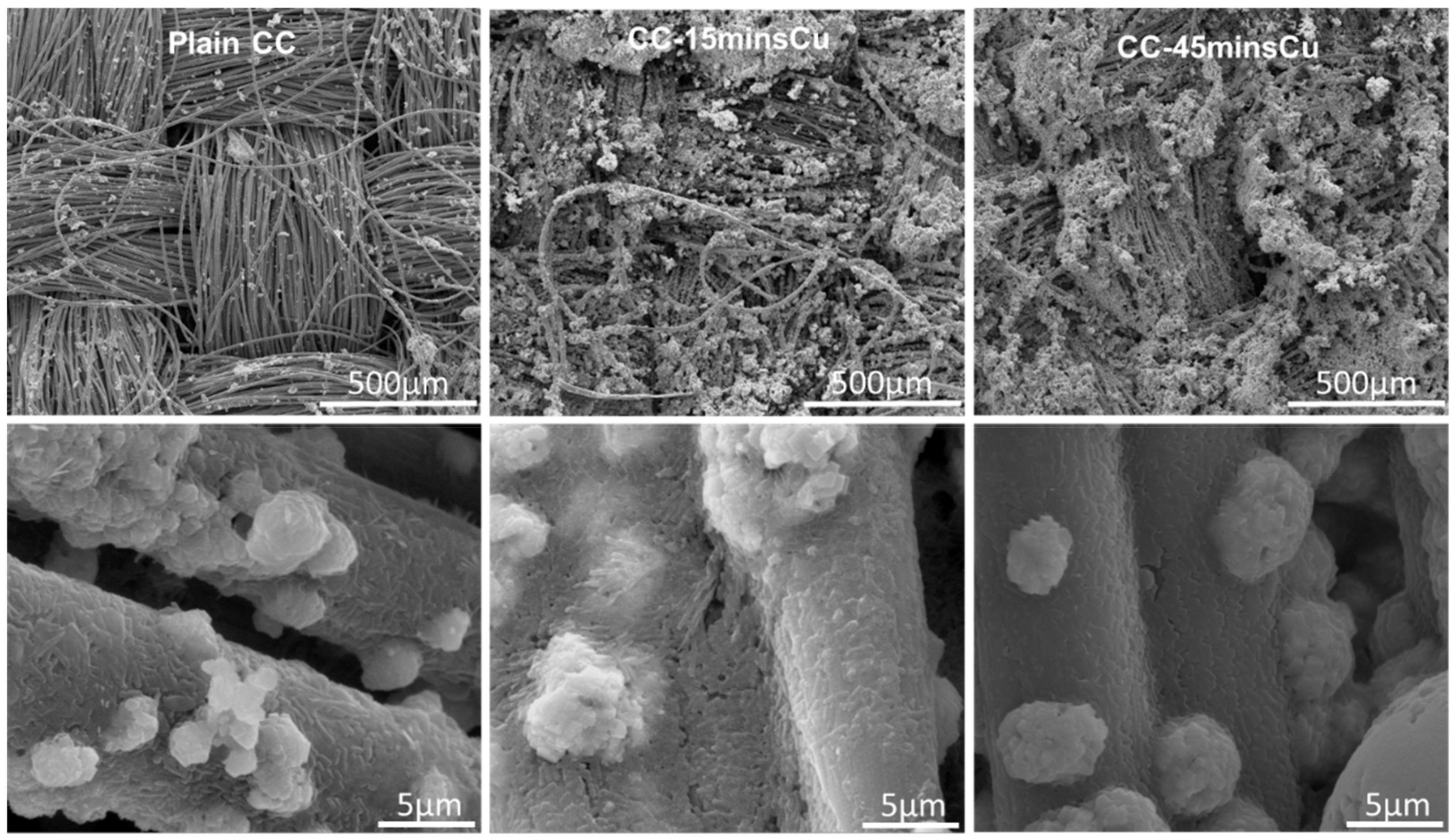
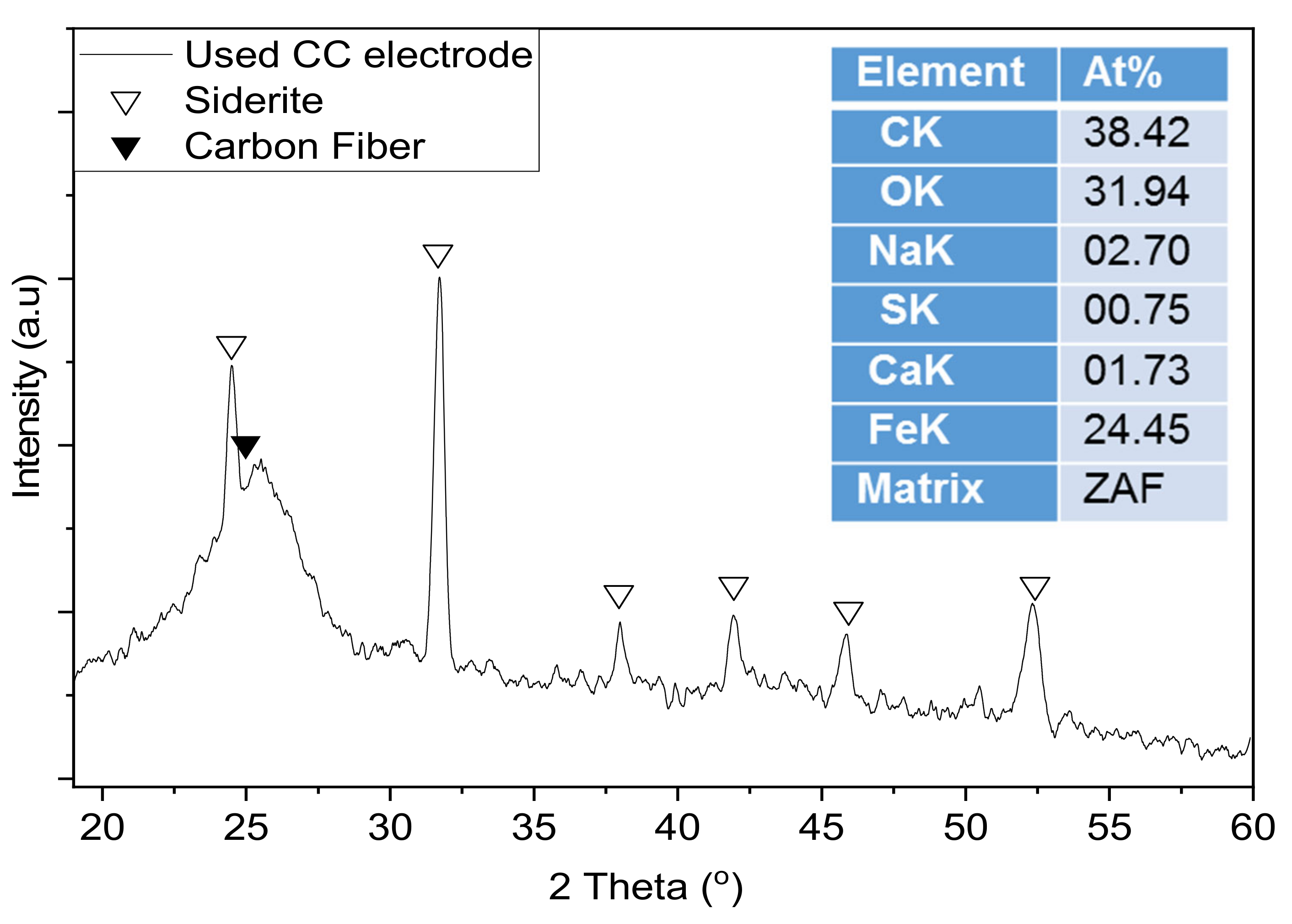
3.1. CC Electrodes Functionalized with Cu NPs
3.2. MES System Performance
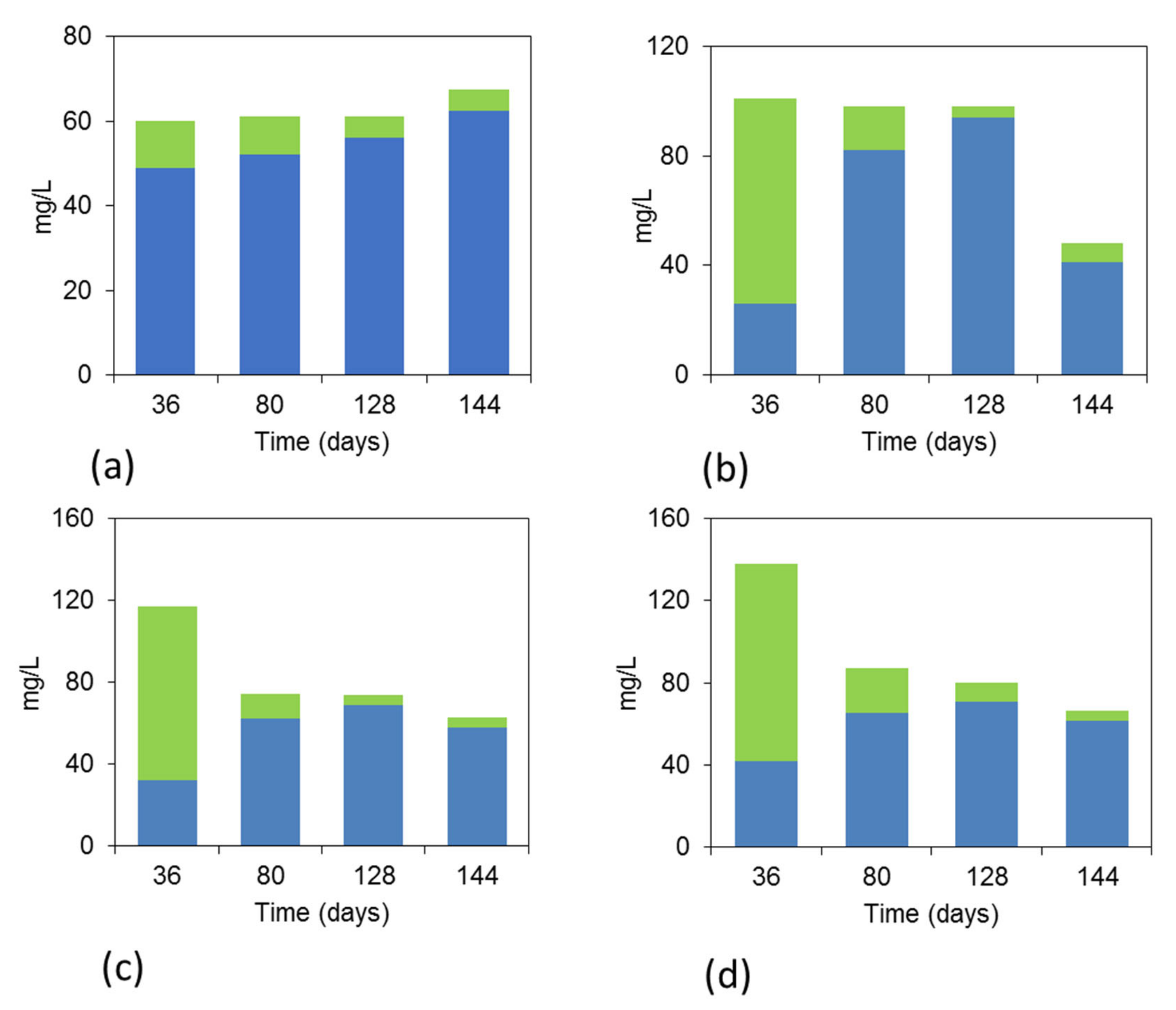
3.3. VFAs
3.4. Energy Efficiency
3.5. Microbial Profile Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Daneshvar, E.; Wicker, R.J.; Show, P.L.; Bhatnagar, A. Biologically-mediated carbon capture and utilization by microalgae towards sustainable CO2 biofixation and biomass valorization—A review. Chem. Eng. J. 2022, 427, 130884. [Google Scholar] [CrossRef]
- Vyrides, I.; Andronikou, M.; Kyprianou, A.; Modic, A.; Filippeti, A.; Yiakoumis, C.; Samanides, C.G. CO2 conversion to CH4 using Zero Valent Iron (ZVI) and anaerobic granular sludge: Optimum batch conditions and microbial pathways. J. CO2 Util. 2018, 27, 415–422. [Google Scholar] [CrossRef]
- Rabaey, K.; Rozendal, R.A. Microbial electrosynthesis—Revisiting the electrical route for microbial production. Nat. Rev. Microbiol. 2010, 8, 706–716. [Google Scholar] [CrossRef]
- Villano, M.; Aulenta, F.; Ciucci, C.; Ferri, T.; Giuliano, A.; Majone, M. Bioelectrochemical reduction of CO2 to CH4 via direct and indirect extracellular electron transfer by a hydrogenophilic methanogenic culture. Bioresour. Technol. 2010, 101, 3085–3090. [Google Scholar] [CrossRef]
- Tharak, A.; Venkata Mohan, S. Electrotrophy of biocathodes regulates microbial-electro-catalyzation of CO2 to fatty acids in single chambered system. Bioresour. Technol. 2021, 320, 124272. [Google Scholar] [CrossRef]
- Zhang, Z.; Song, Y.; Zheng, S.; Zhen, G.; Lu, X.; Takuro, K.; Xu, K.; Bakonyi, P. Electro-conversion of carbon dioxide (CO2) to low-carbon methane by bioelectromethanogenesis process in microbial electrolysis cells: The current status and future perspective. Bioresour. Technol. 2019, 279, 339–349. [Google Scholar] [CrossRef]
- Zhang, S.; Jiang, J.; Wang, H.; Li, F.; Hua, T.; Wang, W. A review of microbial electrosynthesis applied to carbon dioxide capture and conversion: The basic principles, electrode materials, and bioproducts. J. CO2 Util. 2021, 51, 101640. [Google Scholar] [CrossRef]
- Giang, H.; Zhang, J.; Zhu, Z.; Suni, I.I.; Liang, Y. Single-chamber microbial electrochemical cell for CH4 production from CO2 utilizing a microbial consortium. Int. J. Energy Res. 2018, 42, 1308–1315. [Google Scholar] [CrossRef]
- Giddings, C.G.S.; Nevin, K.P.; Woodward, T.; Lovley, D.R.; Butler, C.S. Simplifying microbial electrosynthesis reactor design. Front. Microbiol. 2015, 6, 468. [Google Scholar] [CrossRef] [Green Version]
- Jadhav, D.A.; Park, S.G.; Pandit, S.; Yang, E.; Ali Abdelkareem, M.; Jang, J.K.; Chae, K.J. Scalability of microbial electrochemical technologies: Applications and challenges. Bioresour. Technol. 2022, 345, 126498. [Google Scholar] [CrossRef]
- Yu, H.; Song, Y.C.; Bae, B.U.; Li, J.; Jang, S.H. Electrostatic Fields Promote Methanogenesis More than Polarized Bioelectrodes in Anaerobic Reactors with Conductive Materials. ACS Omega 2021, 6, 29703–29712. [Google Scholar] [CrossRef]
- Kim, K.R.; Kang, J.; Chae, K.J. Improvement in methanogenesis by incorporating transition metal nanoparticles and granular activated carbon composites in microbial electrolysis cells. Int. J. Hydrogen Energy 2017, 42, 27623–27629. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Y.; Quan, X.; Chen, S.; Afzal, S. Enhanced anaerobic digestion of organic contaminants containing diverse microbial population by combined microbial electrolysis cell (MEC) and anaerobic reactor under Fe(III) reducing conditions. Bioresour. Technol. 2013, 136, 273–280. [Google Scholar] [CrossRef]
- Selembo, P.A.; Merrill, M.D.; Logan, B.E. Hydrogen production with nickel powder cathode catalysts in microbial electrolysis cells. Int. J. Hydrogen Energy 2010, 35, 428–437. [Google Scholar] [CrossRef]
- Chatzipanagiotou, K.R.; Jourdin, L.; Bitter, J.H.; Strik, D.P. Concentration-dependent effects of nickel doping on activated carbon biocathodes. Catal. Sci. Technol. 2022, 12, 2500–2518. [Google Scholar] [CrossRef]
- Park, S.G.; Rajesh, P.P.; Sim, Y.U.; Jadhav, D.A.; Noori, M.T.; Kim, D.H.; Al-Qaradawi, S.Y.; Yang, E.; Jang, J.K.; Chae, K.J. Addressing scale-up challenges and enhancement in performance of hydrogen-producing microbial electrolysis cell through electrode modifications. Energy Reports 2022, 8, 2726–2746. [Google Scholar] [CrossRef]
- Wei, J.; Liang, P.; Huang, X. Recent progress in electrodes for microbial fuel cells. Bioresour. Technol. 2011, 102, 9335–9344. [Google Scholar] [CrossRef]
- Fan, Y.; Xu, S.; Schaller, R.; Jiao, J.; Chaplen, F.; Liu, H. Nanoparticle decorated anodes for enhanced current generation in microbial electrochemical cells. Biosens. Bioelectron. 2011, 26, 1908–1912. [Google Scholar] [CrossRef]
- Oyama, M. Recent nanoarchitectures in metal nanoparticle-modified electrodes for electroanalysis. Anal. Sci. 2010, 26, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Siegert, M.; Yates, M.D.; Call, D.F.; Zhu, X.; Spormann, A.; Logan, B.E. Comparison of nonprecious metal cathode materials for methane production by electromethanogenesis. ACS Sustain. Chem. Eng. 2014, 2, 910–917. [Google Scholar] [CrossRef] [Green Version]
- Teran-Salgado, E.; Bahena-Uribe, D.; Márquez-Aguilar, P.A.; Reyes-Rodriguez, J.L.; Cruz-Silva, R.; Solorza-Feria, O. Platinum nanoparticles supported on electrochemically oxidized and exfoliated graphite for the oxygen reduction reaction. Electrochim. Acta 2019, 298, 172–185. [Google Scholar] [CrossRef]
- Aryal, N.; Wan, L.; Overgaard, M.H.; Stoot, A.C.; Chen, Y.; Tremblay, P.L.; Zhang, T. Increased carbon dioxide reduction to acetate in a microbial electrosynthesis reactor with a reduced graphene oxide-coated copper foam composite cathode. Bioelectrochemistry 2019, 128, 83–93. [Google Scholar] [CrossRef]
- Thatikayala, D.; Min, B. Copper ferrite supported reduced graphene oxide as cathode materials to enhance microbial electrosynthesis of volatile fatty acids from CO2. Sci. Total Environ. 2021, 768, 144477. [Google Scholar] [CrossRef]
- Tartakovsky, B.; Mehta, P.; Bourque, J.S.; Guiot, S.R. Electrolysis-enhanced anaerobic digestion of wastewater. Bioresour. Technol. 2011, 102, 5685–5691. [Google Scholar] [CrossRef] [Green Version]
- Kato, M.T.; Field, J.A.; Lettinga, G. Methanogenesis in granular sludge exposed to oxygen. FEMS Microbiol. Lett. 1993, 114, 317–323. [Google Scholar] [CrossRef]
- Kato, M.T.; Field, J.A.; Lettinga, G. High tolerance of methanogens in granular sludge to oxygen. Biotechnol. Bioeng. 1993, 42, 1360–1366. [Google Scholar] [CrossRef]
- Charalambous, P.; Vyrides, I. In situ biogas upgrading and enhancement of anaerobic digestion of cheese whey by addition of scrap or powder zero-valent iron (ZVI). J. Environ. Manag. 2021, 280, 111651. [Google Scholar] [CrossRef]
- Constantinou, M.; Nikolaou, P.; Koutsokeras, L.; Avgeropoulos, A.; Moschovas, D.; Varotsis, C.; Patsalas, P.; Kelires, P.; Constantinides, G. Metal (Ag/Ti)-containing hydrogenated amorphous carbon nanocomposite films with enhanced nanoscratch resistance: Hybrid PECVD/PVD system and microstructural characteristics. Nanomaterials 2018, 8, 209. [Google Scholar] [CrossRef] [Green Version]
- Owen, W.F.; Stuckey, D.C.; Healy, J.B.; Young, L.Y.; McCarty, P.L. Bioassay for monitoring biochemical methane potential and anaerobic toxicity. Water Res. 1979, 13, 485–492. [Google Scholar] [CrossRef]
- Vardanyan, A.; Kafa, N.; Konstantinidis, V.; Shin, S.G.; Vyrides, I. Phosphorus dissolution from dewatered anaerobic sludge: Effect of pHs, microorganisms, and sequential extraction. Bioresour. Technol. 2018, 249, 464–472. [Google Scholar] [CrossRef]
- Vyrides, I.; Stuckey, D.C. Effect of fluctuations in salinity on anaerobic biomass and production of soluble microbial products (SMPs). Biodegradation 2009, 20, 165–175. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Y.; Du, H.; Zhu, J.; Peng, L.; Yang, C.; Luo, F. Exploring the effect of voltage on biogas production performance and the methanogenic pathway of microbial electrosynthesis. Biochem. Eng. J. 2021, 171, 108028. [Google Scholar] [CrossRef]
- Gatidou, G.; Samanides, C.G.; Fountoulakis, M.S.; Vyrides, I. Microbial electrolysis cell coupled with anaerobic granular sludge: A novel technology for real bilge water treatment. Chemosphere 2022, 296, 133988. [Google Scholar] [CrossRef]
- Feng, D.; Xia, A.; Liao, Q.; Nizami, A.S.; Sun, C.; Huang, Y.; Zhu, X.; Zhu, X. Carbon cloth facilitates semi-continuous anaerobic digestion of organic wastewater rich in volatile fatty acids from dark fermentation. Environ. Pollut. 2021, 272, 116030. [Google Scholar] [CrossRef]
- Yuan, Y.; Cheng, H.; Chen, F.; Zhang, Y.; Xu, X.; Huang, C.; Chen, C.; Liu, W.; Ding, C.; Li, Z.; et al. Enhanced methane production by alleviating sulfide inhibition with a microbial electrolysis coupled anaerobic digestion reactor. Environ. Int. 2020, 136, 105503. [Google Scholar] [CrossRef]
- Siegert, M.; Li, X.F.; Yates, M.D.; Logan, B.E. The presence of hydrogenotrophic methanogens in the inoculum improves methane gas production in microbial electrolysis cells. Front. Microbiol. 2014, 5, 778. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Chen, Q.; Dong, H.; Lu, J.; Sun, D.; Wei, Y.; He, H.; Tang, R.; Li, Y.; Dang, Y. Effect of applying potentials on anaerobic digestion of high salinity organic wastewater. Sci. Total Environ. 2022, 822, 153416. [Google Scholar] [CrossRef]
- Andronikou, M.; Adamou, V.; Koutsokeras, L.; Constantinides, G.; Vyrides, I. Magnesium ribbon and anaerobic granular sludge for conversion of CO2 to CH4 or biogas upgrading. Chem. Eng. J. 2022, 435, 134888. [Google Scholar] [CrossRef]
- Cotterill, S.E.; Dolfing, J.; Curtis, T.P.; Heidrich, E.S. Community assembly in wastewater-fed pilot-scale microbial electrolysis cells. Front. Energy Res. 2018, 6, 1–12. [Google Scholar] [CrossRef]
- Carmona-Martínez, A.A.; Trably, E.; Milferstedt, K.; Lacroix, R.; Etcheverry, L.; Bernet, N. Long-term continuous production of H2 in a microbial electrolysis cell (MEC) treating saline wastewater. Water Res. 2015, 81, 149–156. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Zeng, S.; Du, H.; Xie, H.; Igarashi, Y.; Luo, F. The changes of microbial abundance and functional genes in bioelectrochemistry at 15 °C. J. Environ. Chem. Eng. 2022, 10, 106996. [Google Scholar] [CrossRef]




| CODE | Serum Bottle |
|---|---|
| CC-45minCu-Volts | Developed electrode that copper nanoparticles were deposited for 45 min. A voltage was applied. |
| CC-15minCu-Volts | Developed electrode that copper nanoparticles were deposited for 15 min. A voltage was applied. |
| CC-Volts | Commercial carbon cloth |
| CC-45minCu | Developed electrodes used in serum bottle without applied external potential |
| CC-15minCu | Developed electrodes used in serum bottle without applied external potential |
| CC | Developed electrodes used in serum bottle without applied external potential |
| Control | Only anaerobic granular sludge no electrodes |
| Cycle | Days | Volts * | Carbon Source |
|---|---|---|---|
| 1st cycle | 1–10 | 1 | CO2 + 10 g NaHCO3/L |
| 2nd cycle | 10–36 | 1 | CO2 + 10 g NaHCO3/L |
| 3rd cycle | 36–86 | 1 | CO2 + 10 g NaHCO3/L |
| 4th cycle | 86–95 | 1 | CO2 + 10 g NaHCO3/L |
| 5th cycle | 95–128 | 2 | CO2 + 10 g NaHCO3/L |
| 6th cycle | 128–144 | 2 | CO2 + 10 g NaHCO3/L |
| Average Current Density (mA) | ||||||
|---|---|---|---|---|---|---|
| Cycle | 1st | 2nd | 3rd | 4th | 5th | 6th |
| CC-45minCu-Volt | 5.2 | 5.1 | 1.8 | 5.5 | 3.3 | 4.9 |
| CC-15minCu-Volt | 6.4 | 7.5 | 2.1 | 4.1 | 3.1 | 11.2 |
| CC-Volt | 13.5 | 20.2 | 4.2 | 6 | 7.5 | 11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Georgiou, S.; Koutsokeras, L.; Constantinou, M.; Majzer, R.; Markiewicz, J.; Siedlecki, M.; Vyrides, I.; Constantinides, G. Microbial Electrosynthesis Inoculated with Anaerobic Granular Sludge and Carbon Cloth Electrodes Functionalized with Copper Nanoparticles for Conversion of CO2 to CH4. Nanomaterials 2022, 12, 2472. https://doi.org/10.3390/nano12142472
Georgiou S, Koutsokeras L, Constantinou M, Majzer R, Markiewicz J, Siedlecki M, Vyrides I, Constantinides G. Microbial Electrosynthesis Inoculated with Anaerobic Granular Sludge and Carbon Cloth Electrodes Functionalized with Copper Nanoparticles for Conversion of CO2 to CH4. Nanomaterials. 2022; 12(14):2472. https://doi.org/10.3390/nano12142472
Chicago/Turabian StyleGeorgiou, Sofia, Loukas Koutsokeras, Marios Constantinou, Rafał Majzer, Justyna Markiewicz, Marcin Siedlecki, Ioannis Vyrides, and Georgios Constantinides. 2022. "Microbial Electrosynthesis Inoculated with Anaerobic Granular Sludge and Carbon Cloth Electrodes Functionalized with Copper Nanoparticles for Conversion of CO2 to CH4" Nanomaterials 12, no. 14: 2472. https://doi.org/10.3390/nano12142472
APA StyleGeorgiou, S., Koutsokeras, L., Constantinou, M., Majzer, R., Markiewicz, J., Siedlecki, M., Vyrides, I., & Constantinides, G. (2022). Microbial Electrosynthesis Inoculated with Anaerobic Granular Sludge and Carbon Cloth Electrodes Functionalized with Copper Nanoparticles for Conversion of CO2 to CH4. Nanomaterials, 12(14), 2472. https://doi.org/10.3390/nano12142472








