Feasible Structure Manipulation of Vanadium Selenide into VSe2 on Au(111)
Abstract
:1. Introduction
2. Materials and Methods
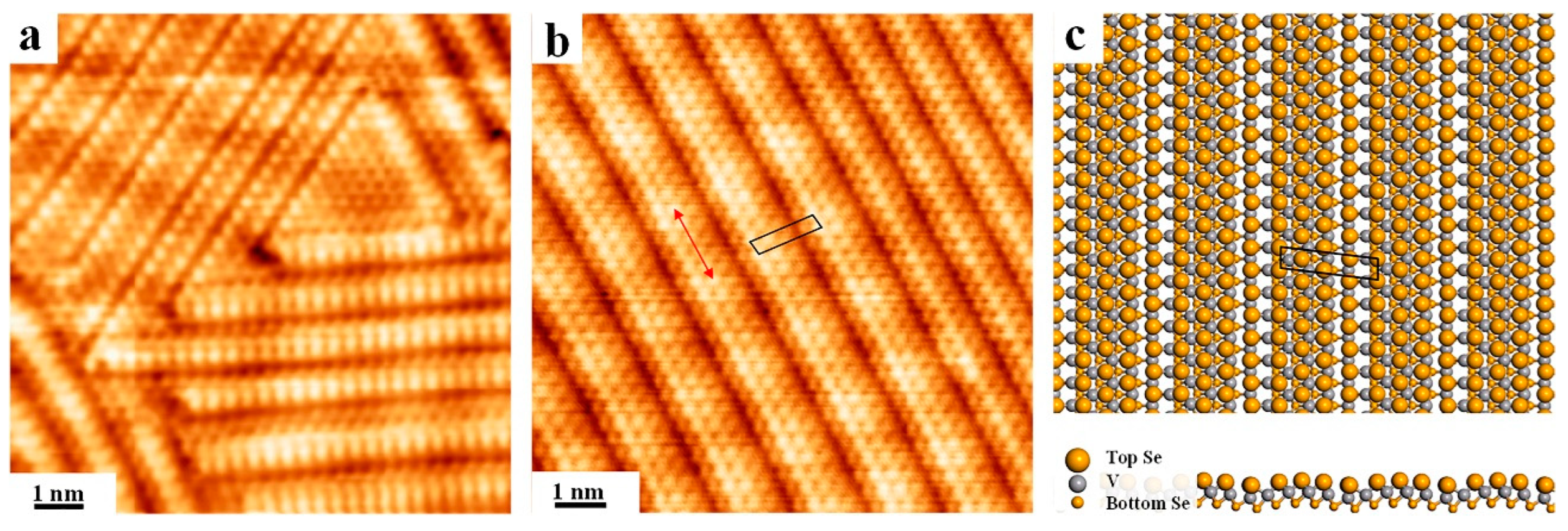
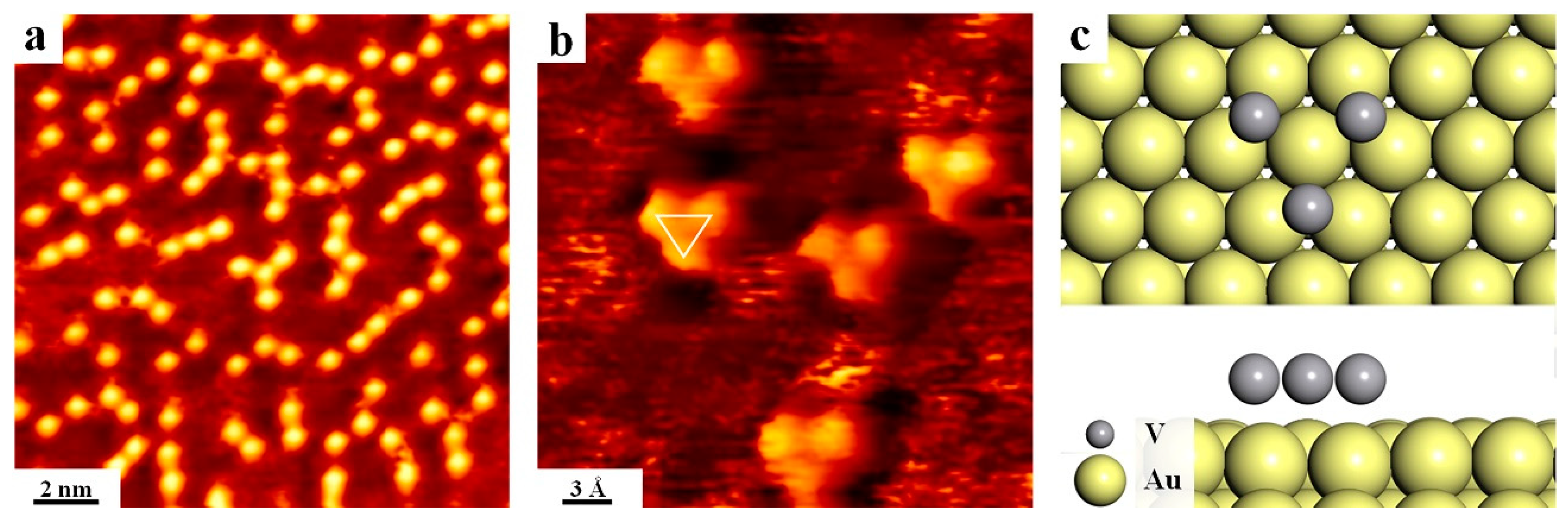
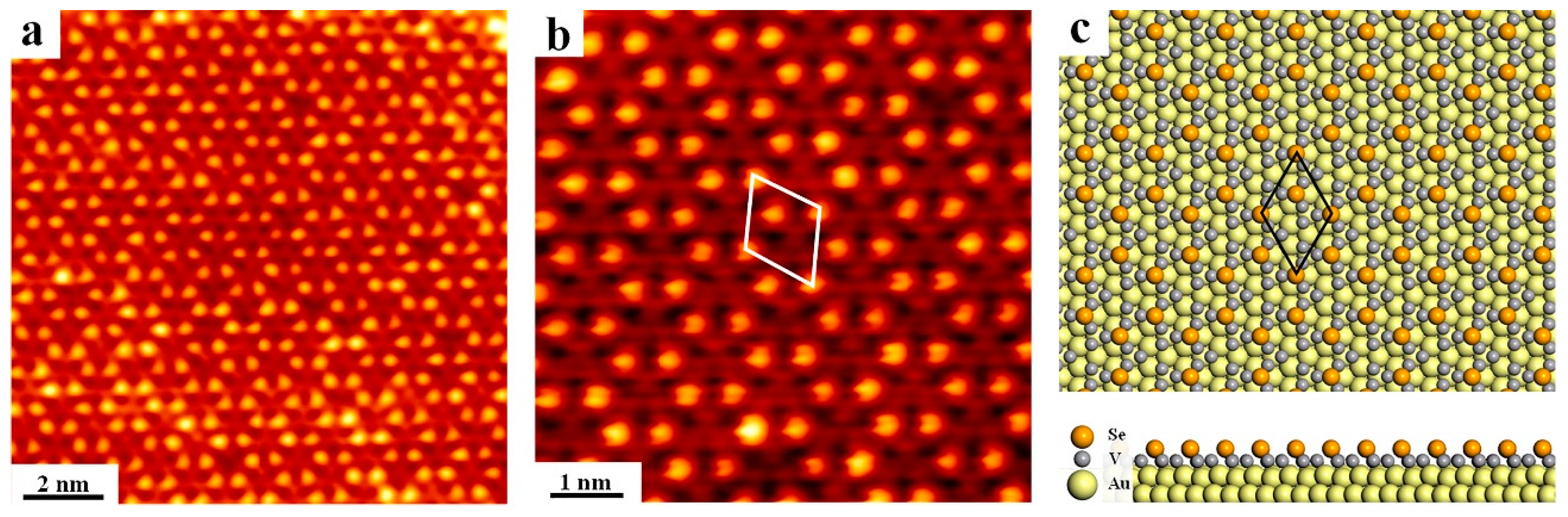
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chhowalla, M.; Shin, H.S.; Eda, G.; Li, L.; Loh, K.P.; Zhang, H. The chemistry of two-dimensional layered transition metal dichalcogenide nanosheets. Nat. Chem. 2013, 5, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Li, B.; Xia, C.; Cui, Y.; He, J.; Xia, J.-B.; Li, J. Various Structures of 2D Transition-Metal Dichalcogenides and Their Applications. Small Methods 2018, 2, 1800094. [Google Scholar] [CrossRef]
- Ramakrishna, M.H.S.S.; Gomathi, A.; Manna, A.K.; Late, D.J.; Datta, R.; Pati, S.K.; Rao, C.N.R. MoS2 and WS2 analogues of graphene. Angew. Chem. Int. Ed. 2010, 49, 4059–4062. [Google Scholar] [CrossRef]
- Eda, G.; Yamaguchi, H.; Voiry, D.; Fujita, T.; Chen, M.; Chhowalla, M. Photoluminescence from chemically exfoliated MoS2. Nano Lett. 2011, 11, 5111–5116. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, H.R.; Perea-Lopez, N.; Elias, A.L.; Berkdemir, A.; Wang, B.; Lv, R.; Lopez-Urias, F.; Crespi, V.H.; Terrones, H.; Terrones, M. Extraordinary Room-Temperature Photoluminescence in Triangular WS2 Monolayers. Nano Lett. 2013, 13, 3447–3454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Q.H.; Kalantar-Zadeh, K.; Kis, A.; Coleman, J.N.; Strano, M.S. Electronics and optoelectronics of two-dimensional transition metal dichalcogenides. Nat. Nanotechnol. 2012, 7, 699–712. [Google Scholar] [CrossRef]
- Ma, Y.; Dai, Y.; Guo, M.; Niu, C.; Zhu, Y.; Huang, B. Evidence of the existence of magnetism in pristine VX monolayers (X = S, Se) and their strain-induced tunable magnetic properties. ACS Nano 2012, 6, 1695–1701. [Google Scholar] [CrossRef]
- Whitwick, M.B.; Kis, A.; Radisavljevic, B. Integrated Circuits and Logic Operations Based on Single-Layer MoS2. ACS Nano 2011, 5, 9934–9938. [Google Scholar]
- Lee, H.S.; Min, S.W.; Chang, Y.G.; Park, M.K.; Nam, T.; Kim, H.; Kim, J.H.; Ryu, S.; Im, S. MoS2 Nanosheet Phototransistors with Thickness-Modulated Optical Energy Gap. Nano Lett. 2012, 12, 3695–3700. [Google Scholar] [CrossRef]
- Feng, W.; Pang, W.; Xu, Y.; Guo, A.; Gao, X. Transition Metal Selenides for Electrocatalytic Hydrogen Evolution Reaction. ChemElectroChem 2019, 7, 31–54. [Google Scholar] [CrossRef]
- Lauritsen, J.V.; Nyberg, M.; Nørskov, J.K.; Clausen, B.S.; Topsøe, H.; Lægsgaard, E.L.; Besenbacher, F. Hydrodesulfurization reaction pathways on MoS2 nanoclusters revealed by scanning tunneling microscopy. J. Catal. 2004, 224, 94–106. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, B.; Wang, Y.; Zhao, L.; Zhang, J.; Zong, Q.; Gao, J.; Xu, C. The effect of cobalt promoter on the CO methanation reaction over MoS2 catalyst: A density functional study. RSC Adv. 2017, 7, 11862–11871. [Google Scholar] [CrossRef] [Green Version]
- Hinnemann, B.; Moses, P.G.; Bonde, J.; Jørgensen, K.P.; Nielsen, J.H.; Horch, S.; Chorkendorff, I.; Nørskov, J.K. Biomimetic Hydrogen Evolution: MoS2 Nanoparticles as Catalyst for Hydrogen Evolution. J. Am. Chem. Soc. 2005, 127, 5308–5309. [Google Scholar] [CrossRef] [PubMed]
- Jaramillo, T.F.; Jorgensen, K.P.; Bonde, J.; Nielsen, J.H.; Horch, S.; Chorkendorff, I. Identification of Active Edge Sites for Electrochemical H2 Evolution from MoS2 Nanocatalysts. Science 2007, 317, 100–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hai, L.; Yin, Z.; He, Q.; Li, H.; Huang, X.; Lu, G.; Fam, D.W.H.; Tok, A.I.Y.; Zhang, Q.; Zhang, H. Fabrication of single- and multilayer MoS2 film-based field-effect transistors for sensing NO at room temperature. Small 2012, 8, 63–67. [Google Scholar]
- Haering, R.R.; Stiles, J.A.R.; Brandt, K. Lithium Molybdenum Disulphide Battery Cathode. U.S. Patent 4,224,390, 23 September 1980. [Google Scholar]
- Bhandavat, R.; David, L.; Singh, G. Synthesis of Surface-Functionalized WS2 Nanosheets and Performance as Li-Ion Battery Anodes. J. Phys. Chem. Lett. 2012, 3, 1523–1530. [Google Scholar] [CrossRef]
- Manzeli, S.; Ovchinnikov, D.; Pasquier, D.; Yazyev, O.V.; Kis, A. 2D transition metal dichalcogenides. Nat. Rev. Mater. 2017, 2, 17033. [Google Scholar] [CrossRef]
- Xu, K.; Chen, P.; Li, X.; Wu, C.; Guo, Y.; Zhao, J.; Wu, X.; Xie, Y. Ultrathin Nanosheets of Vanadium Diselenide: A Metallic Two-Dimensional Material with Ferromagnetic Charge-Density-Wave Behavior. Angew. Chem. Int. Ed. 2013, 52, 10477–10481. [Google Scholar] [CrossRef]
- Barua, S.; Hatnean, M.C.; Lees, M.R.; Balakrishnan, G. Signatures of the Kondo effect in VSe2. Sci. Rep. 2017, 7, 10964. [Google Scholar] [CrossRef] [Green Version]
- Bonilla, M.; Kolekar, S.; Ma, Y.; Diaz, H.C.; Kalappattil, V.; Das, R.; Eggers, T.; Gutierrez, H.R.; Phan, M.-H.; Batzill, M. Strong room-temperature ferromagnetism in VSe2 monolayers on van der Waals substrates. Nat. Nanotechnol. 2018, 13, 289–293. [Google Scholar] [CrossRef]
- Wong, P.K.J.; Zhang, W.; Bussolotti, F.; Yin, X.; Herng, T.S.; Zhang, L.; Huang, Y.L.; Vinai, G.; Krishnamurthi, S.; Bukhvalov, D.W.; et al. Evidence of Spin Frustration in a Vanadium Diselenide Monolayer Magnet. Adv. Mater. 2019, 31, e1901185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, W.; Li, J.; Herng, T.S.; Wang, Z.; Zhao, X.; Chi, X.; Fu, W.; Abdelwahab, I.; Zhou, J.; Dan, J.; et al. Chemically Exfoliated VSe2 Monolayers with Room-Temperature Ferromagnetism. Adv. Mater. 2019, 31, e1903779. [Google Scholar] [CrossRef] [PubMed]
- Bruggen, C.; Haas, C.; Wiegers, G.A. Charge-density waves and electron localization in vanadium chalcogenides. J. Solid State Chem. 1979, 27, 9–18. [Google Scholar] [CrossRef]
- Duvjir, G.; Choi, B.K.; Jang, I.; Ulstrup, S.; Kang, S.; Ly, T.T.; Kim, S.; Choi, Y.H.; Jozwiak, C.; Bostwick, A.; et al. Emergence of a Metal-Insulator Transition and High-Temperature Charge-Density Waves in VSe2 at the Monolayer Limit. Nano Lett. 2018, 18, 5432–5438. [Google Scholar] [CrossRef] [Green Version]
- Jang, I.; Duvjir, G.; Choi, B.K.; Kim, J.; Chang, Y.J.; Kim, K.-S. Universal renormalization group flow toward perfect Fermi-surface nesting driven by enhanced electron-electron correlations in monolayer vanadium diselenide. Phys. Rev. B 2019, 99, 014106. [Google Scholar] [CrossRef] [Green Version]
- Zhao, W.; Dong, B.; Guo, Z.; Su, G.; Gao, R.; Wang, W.; Cao, L. Colloidal synthesis of VSe2 single-layer nanosheets as novel electrocatalysts for the hydrogen evolution reaction. Chem. Commun. 2016, 52, 9228–9231. [Google Scholar] [CrossRef]
- Ulusoy, G.T.G.; Patil, B.; Karadas, F.; Okyay, A.K.; Yilmaz, E. Catalytic Properties of Vanadium Diselenide: A Comprehensive Study on Its Electrocatalytic Performance in Alkaline, Neutral, and Acidic Media. ACS Omega 2017, 2, 8319–8329. [Google Scholar] [CrossRef] [Green Version]
- Mahler, B.; Hoepfher, V.; Liao, K.; Ozin, G.A. Colloidal synthesis of 1T-WS2 and 2H-WS2 nanosheets: Applications for photocatalytic hydrogen evolution. J. Am. Chem. Soc. 2014, 136, 14121–14127. [Google Scholar] [CrossRef]
- Ji, Q.; Zhang, Y.; Zhang, Y.; Liu, Z. Chemical vapour deposition of group-VIB metal dichalcogenide monolayers: Engineered substrates from amorphous to single crystalline. Chem. Soc. Rev. 2015, 44, 2587–2602. [Google Scholar] [CrossRef]
- Boscher, N.D.; Blackman, C.S.; Carmalt, C.J.; Parkin, I.P.; Prieto, A.G. Atmospheric pressure chemical vapour deposition of vanadium diselenide thin films. Appl. Surf. Sci. 2007, 253, 6041–6046. [Google Scholar] [CrossRef]
- Liu, H.; Bao, L.; Zhou, Z.; Che, B.; Zhang, R.; Bian, C.; Ma, R.; Wu, L.; Yang, H.; Li, J.; et al. Quasi-2D Transport and Weak Antilocalization Effect in Few-layered VSe2. Nano Lett. 2019, 19, 4551–4559. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Niu, J.; Yang, P.; Gong, Y.; Ji, Q.; Shi, J.; Fang, Q.; Jiang, S.; Li, H.; Zhou, X.; et al. Van der Waals Epitaxial Growth of 2D Metallic Vanadium Diselenide Single Crystals and their Extra-High Electrical Conductivity. Adv. Mater. 2017, 29, 1702359. [Google Scholar] [CrossRef] [PubMed]
- Ji, Q.; Zhang, Y.; Shi, J.; Sun, J.; Zhang, Y.; Liu, Z. Morphological Engineering of CVD-Grown Transition Metal Dichalcogenides for Efficient Electrochemical Hydrogen Evolution. Adv. Mater. 2016, 28, 6207–6212. [Google Scholar] [CrossRef] [PubMed]
- Coleman, J.N.; Lotya, M.; O’Neill, A.; Bergin, S.D.; King, P.J.; Khan, U.; Young, K.; Gaucher, A.; De, S.; Smith, R.J.; et al. Two-Dimensional Nanosheets Produced by Liquid Exfoliation of Layered Materials. Science 2011, 331, 568–571. [Google Scholar] [CrossRef] [Green Version]
- Cunningham, G.; Lotya, M.; Cucinotta, C.S.; Sanvito, S.; Bergin, S.D.; Menzel, R.; Shaffer, M.S.P.; Coleman, J.N. Solvent Exfoliation of Transition Metal Dichalcogenides: Dispersibility of Exfoliated Nanosheets Varies Only Weakly between Compounds. ACS Nano 2012, 6, 3468–3480. [Google Scholar] [CrossRef]
- Liu, Z.L.; Wu, X.; Shao, Y.; Qi, J.; Cao, Y.; Huang, L.; Liu, C.; Wang, J.-O.; Zheng, Q.; Zhu, Z.-L.; et al. Epitaxially grown monolayer VSe2: An air-stable magnetic two-dimensional material with low work function at edges. Sci. Bull. 2018, 63, 419–425. [Google Scholar] [CrossRef] [Green Version]
- Chua, R.; Yang, J.; He, X.; Yu, X.; Yu, W.; Bussolotti, F.; Wong, P.K.J.; Loh, K.P.; Breese, M.B.H.; Goh, K.E.J.; et al. Can Reconstructed Se-Deficient Line Defects in Monolayer VSe2 Induce Magnetism? Adv. Mater. 2020, 32, e2000693. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, T.; Zhang, W.; Qi, D.; He, X.; Xing, K.; Wong, P.K.J.; Feng, Y.P.; Wee, A.T.S. Bi-stable electronic states of cobalt phthalocyanine molecules on two-dimensional vanadium diselenide. Appl. Mater. Today 2020, 18, 100535. [Google Scholar] [CrossRef]
- Luo, R.; Xu, W.W.; Zhang, Y.; Wang, Z.; Wang, X.; Gao, Y.; Liu, P.; Chen, M. Van der Waals interfacial reconstruction in monolayer transition-metal dichalcogenides and gold heterojunctions. Nat. Commun. 2020, 11, 1011. [Google Scholar] [CrossRef] [Green Version]
- Sun, H.; Liang, Z.; Shen, K.; Luo, M.; Hu, J.; Huang, H.; Zhu, Z.; Li, Z.; Jiang, Z.; Song, F. Fabrication of NiSe2 by direct selenylation of a nickel surface. Appl. Surf. Sci. 2018, 428, 623–629. [Google Scholar] [CrossRef]
- Horcas, I.; Fernandez, R.; Gomez-Rodriguez, J.M.; Colchero, J.; Gómez-Herrero, J.; Baro, A.M. WSXM: A software for scanning probe microscopy and a tool for nanotechnology. Rev. Sci. Instrum. 2007, 78, 013705. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Hu, J.; Zhang, Z.; Shen, K.; Liang, Z.; Zhang, H.; Tian, Q.; Wang, P.; Jiang, Z.; Huang, H.; et al. Ullmann coupling of 2,7-dibromopyrene on Au(1 1 1) assisted by surface adatoms. Appl. Surf. Sci. 2020, 513, 145797. [Google Scholar] [CrossRef]
- Zhang, H.; Xie, L.; Huang, C.; Ren, Z.; Wang, H.; Hu, J.; Zhang, H.; Jiang, Z.; Song, F. Exploring the CO2 reduction reaction mechanism on Pt/TiO2 with the ambient-pressure X-ray photoelectron spectroscopy. Appl. Surf. Sci. 2021, 568, 150933. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef] [Green Version]
- Perdew, J.P.; Chevary, J.A.; Vosko, S.H.; Jackson, K.A.; Pederson, M.R.; Singh, D.J.; Fiolhais, C. Atoms, molecules, solids, and surfaces: Applications of the generalized gradient approximation for exchange and correlation. Phys. Rev. B 1992, 46, 6671–6687. [Google Scholar] [CrossRef]
- Stefan, G.; Jens, A.; Stephan, E.; Helge, K. A Consistent and Accurate Ab Initio Parametrization of Density Functional Dis-persion Correction (DFT-D) for the 94 Elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar]
- Liang, Z.; Wang, Y.; Hua, C.; Xiao, C.; Chen, M.; Jiang, Z.; Tai, R.; Lu, Y.; Song, F. Electronic structures of ultra-thin tellurium nanoribbons. Nanoscale 2019, 11, 14134–14140. [Google Scholar] [CrossRef]
- Miwa, J.A.; Ulstrup, S.; Sorensen, S.G.; Dendzik, M.; Čabo, A.G.; Bianchi, M.; Lauritsen, J.V.; Hofmann, P. Electronic structure of epitaxial single-layer MoS2. Phys. Rev. Lett. 2015, 114, 046802. [Google Scholar] [CrossRef] [Green Version]
- Dubout, Q.; Calleja, F.; Sclauzero, G.; Etzkorn, M.; Lehnert, A.; Claude, L.; Papagno, M.; Natterer, F.D.; Patthey, F.; Rusponi, S.; et al. Giant apparent lattice distortions in STM images of corrugated sp2-hybridised monolayers. New J. Phys. 2016, 18, 103027. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Lin, C.; Fu, R.; Wang, S.; Pan, R.; Chen, G.; Shen, Q.; Liu, C.; Guo, X.; Wang, Y.; et al. A simple method to tune graphene growth between monolayer and bilayer. AIP Adv. 2016, 6, 025026. [Google Scholar]
- Zhao, X.; Chen, H.; Wu, H.; Wang, R.; Cui, Y.; Fu, Q.; Yang, F.; Bao, X. Growth of Ordered ZnO Structures on Au(111) and Cu(111). Acta Phys.-Chim. Sin. 2018, 34, 1373–1380. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, L.; Dai, Y.; Tong, C.; Han, X. Tunable electronic and magnetic properties from structure phase transition of layered vanadium diselenide. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2017, 32, 574–578. [Google Scholar] [CrossRef]
- Schwedhelm, R.; Schlomka, J.P.; Woedtke, S.; Adelung, R.; Kipp, L.; Tolan, M.; Press, W.; Skibowski, M. Epitaxial thin-film growth of C60 on VSe2 studied with scanning tunneling microscopy and x-ray diffraction. Phys. Rev. B 1999, 59, 13394. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Wang, X.; Kan, C.M.; He, D.; Li, Z.; Hao, Q.; Zhao, H.; Wu, C.; Jin, C.; Cui, X. Structural Phase Transition of Multilayer VSe2. ACS Appl. Mater. Interfaces 2020, 12, 25143–25149. [Google Scholar] [CrossRef] [PubMed]
- Krane, N.; Lotze, C.; Franke, K.J. Moiré structure of MoS2 on Au(111): Local structural and electronic properties. Surf. Sci. 2018, 678, 136–142. [Google Scholar] [CrossRef] [Green Version]
- Cheng, F.; Hu, Z.; Xu, H.; Shao, Y.; Su, J.; Chen, Z.; Ji, W.; Loh, K.P. Interface Engineering of Au(111) for the Growth of 1T’-MoSe2. ACS Nano 2019, 13, 2316–2323. [Google Scholar] [CrossRef]
- Sørensen, S.G.; Füchtbauer, H.G.; Tuxen, A.K.; Walton, A.S.; Lauritsen, J.V. Structure and Electronic Properties of In Situ Synthesized Single-Layer MoS2 on a Gold Surface. ACS Nano 2014, 8, 6788–6796. [Google Scholar] [CrossRef]
- Liu, Z.L.; Lei, B.; Zhu, Z.L.; Tao, L.; Qi, J.; Bao, D.-L.; Wu, X.; Huang, L.; Zhang, Y.-Y.; Lin, X.; et al. Spontaneous Formation of 1D Pattern in Monolayer VSe2 with Dispersive Adsorption of Pt Atoms for HER Catalysis. Nano Lett. 2019, 19, 4897–4903. [Google Scholar] [CrossRef]
- Arnold, F.; Stan, R.M.; Mahatha, S.K.; Lund, H.E.; Curcio, D.; Dendzik, M.; Bana, H.; Travaglia, E.; Bignardi, L.; Lacovig, P.; et al. Novel single-layer vanadium sulphide phases. 2D Mater. 2018, 5, 045009. [Google Scholar] [CrossRef] [Green Version]
- Guimond, S.; Abu, H.M.; Kaya, S.; Lu, J.; Weissenrieder, J.; Shaikhutdinov, S.; Kuhlenbeck, H.; Freund, H.-J.; Döbler, J.; Sauer, J. Vanadium oxide surfaces and supported vanadium oxide nanoparticles. Top. Catal. 2006, 38, 117–125. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, C.; Xie, L.; Zhang, H.; Wang, H.; Hu, J.; Liang, Z.; Jiang, Z.; Song, F. Feasible Structure Manipulation of Vanadium Selenide into VSe2 on Au(111). Nanomaterials 2022, 12, 2518. https://doi.org/10.3390/nano12152518
Huang C, Xie L, Zhang H, Wang H, Hu J, Liang Z, Jiang Z, Song F. Feasible Structure Manipulation of Vanadium Selenide into VSe2 on Au(111). Nanomaterials. 2022; 12(15):2518. https://doi.org/10.3390/nano12152518
Chicago/Turabian StyleHuang, Chaoqin, Lei Xie, Huan Zhang, Hongbing Wang, Jinping Hu, Zhaofeng Liang, Zheng Jiang, and Fei Song. 2022. "Feasible Structure Manipulation of Vanadium Selenide into VSe2 on Au(111)" Nanomaterials 12, no. 15: 2518. https://doi.org/10.3390/nano12152518





