Systematic Microwave-Assisted Postsynthesis of Mn-Doped Cesium Lead Halide Perovskites with Improved Color-Tunable Luminescence and Stability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Preparation of the CsPbBr3 Precursor
2.3. Postsynthesis of Mn2+ Doped Perovskites
2.4. Characterizations
3. Results and Discussion
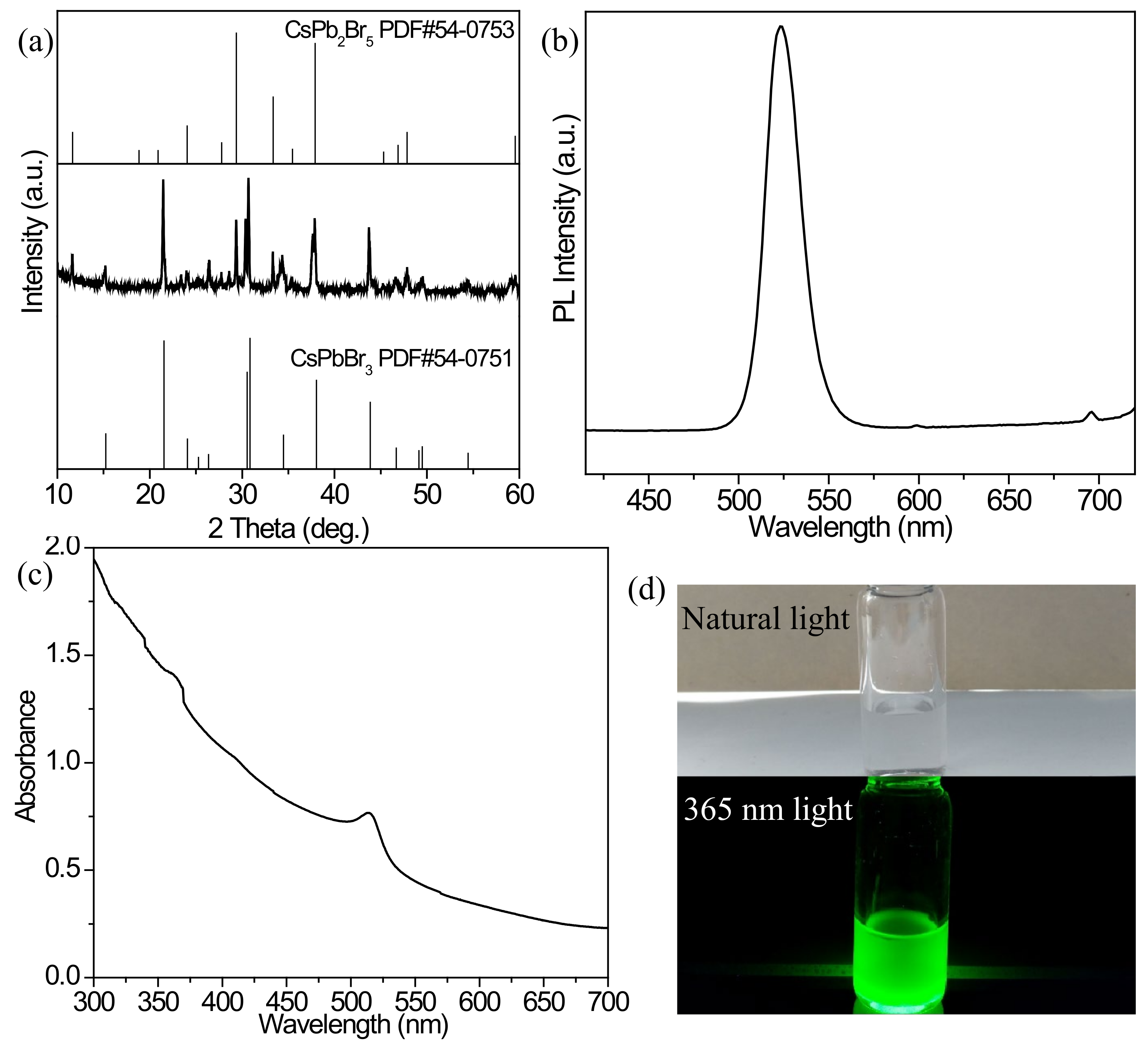
3.1. Optimization of the Synthesis of the CsPbBr3 Precursor
3.2. Postsynthesis of the Mn2+ Doped Products
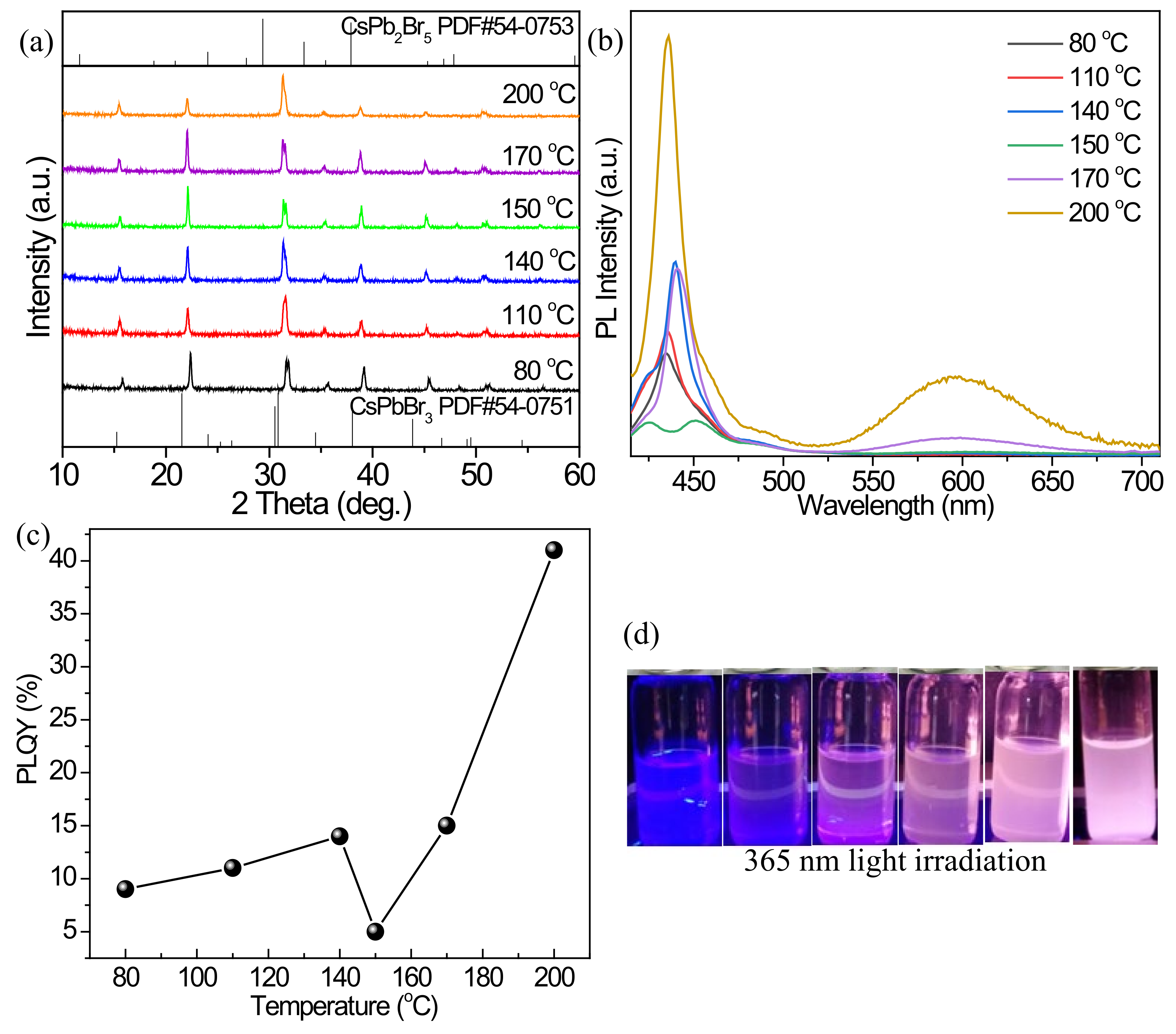
3.2.1. Effect of Reaction Temperature
3.2.2. Effect of Reaction Time
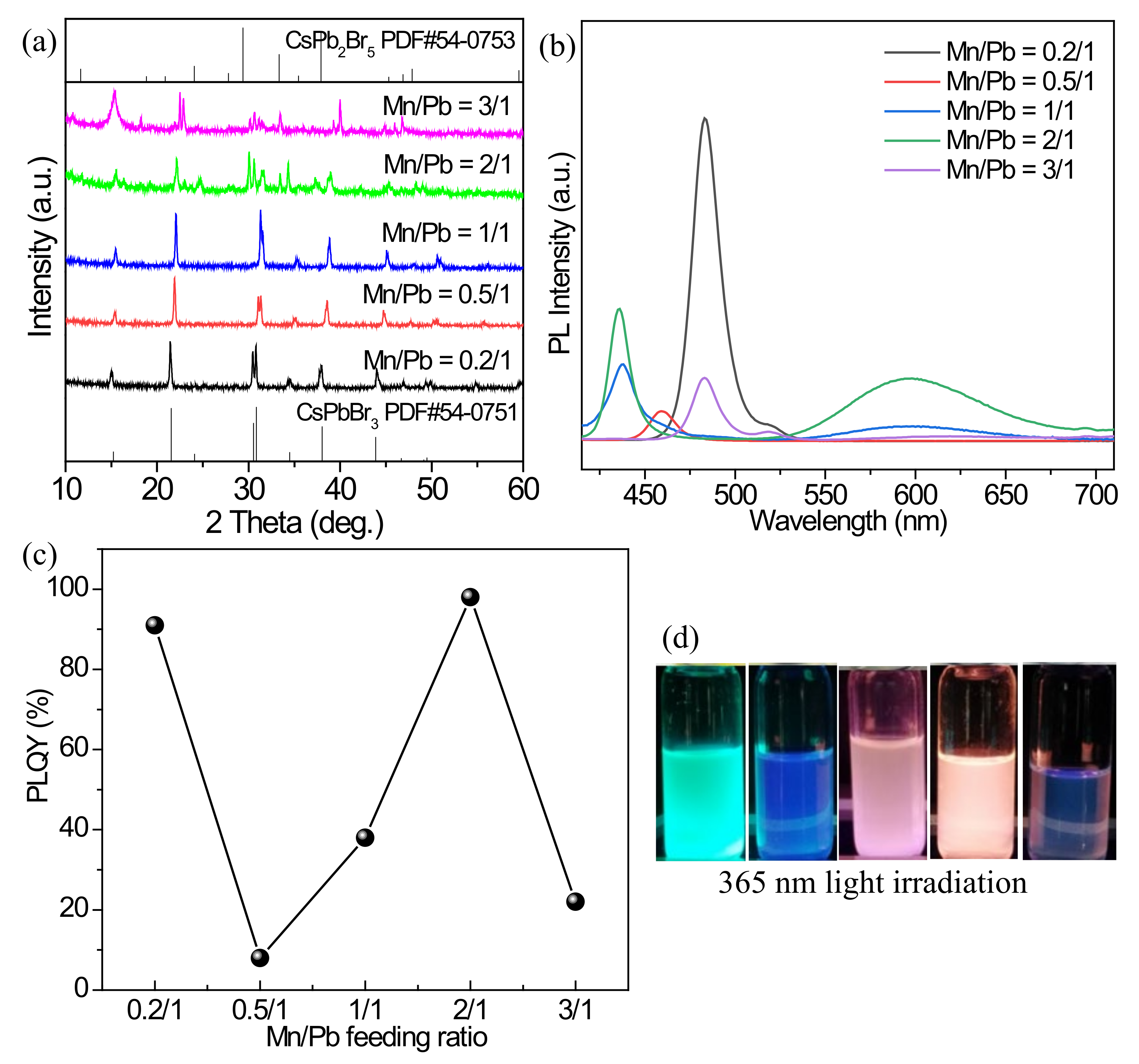
3.2.3. Effect of Mn2+ and Pb2+ Feeding Ratio
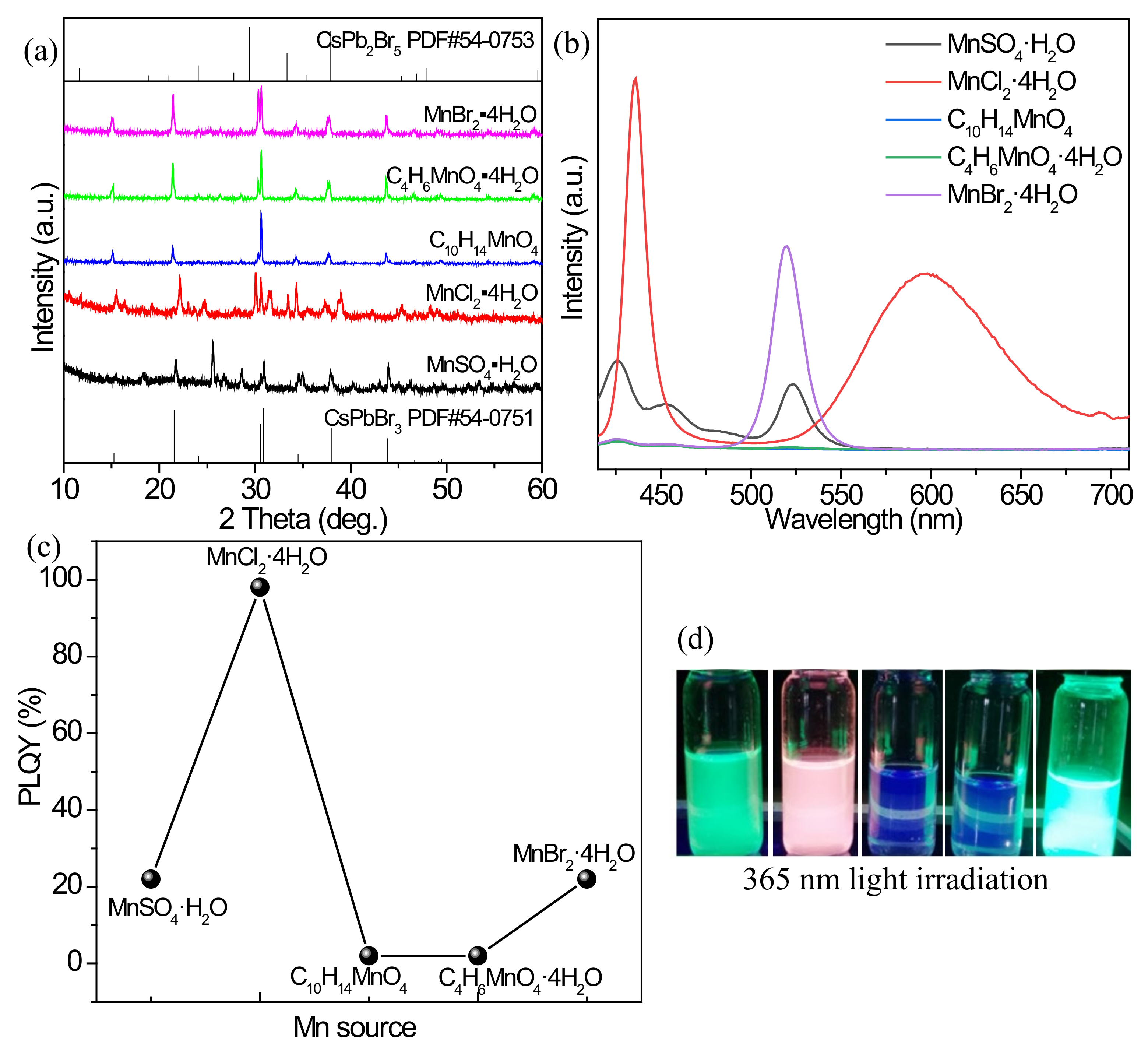
3.2.4. Effect of Mn2+ Source
3.2.5. Effect of ODE/DGBE Ratio
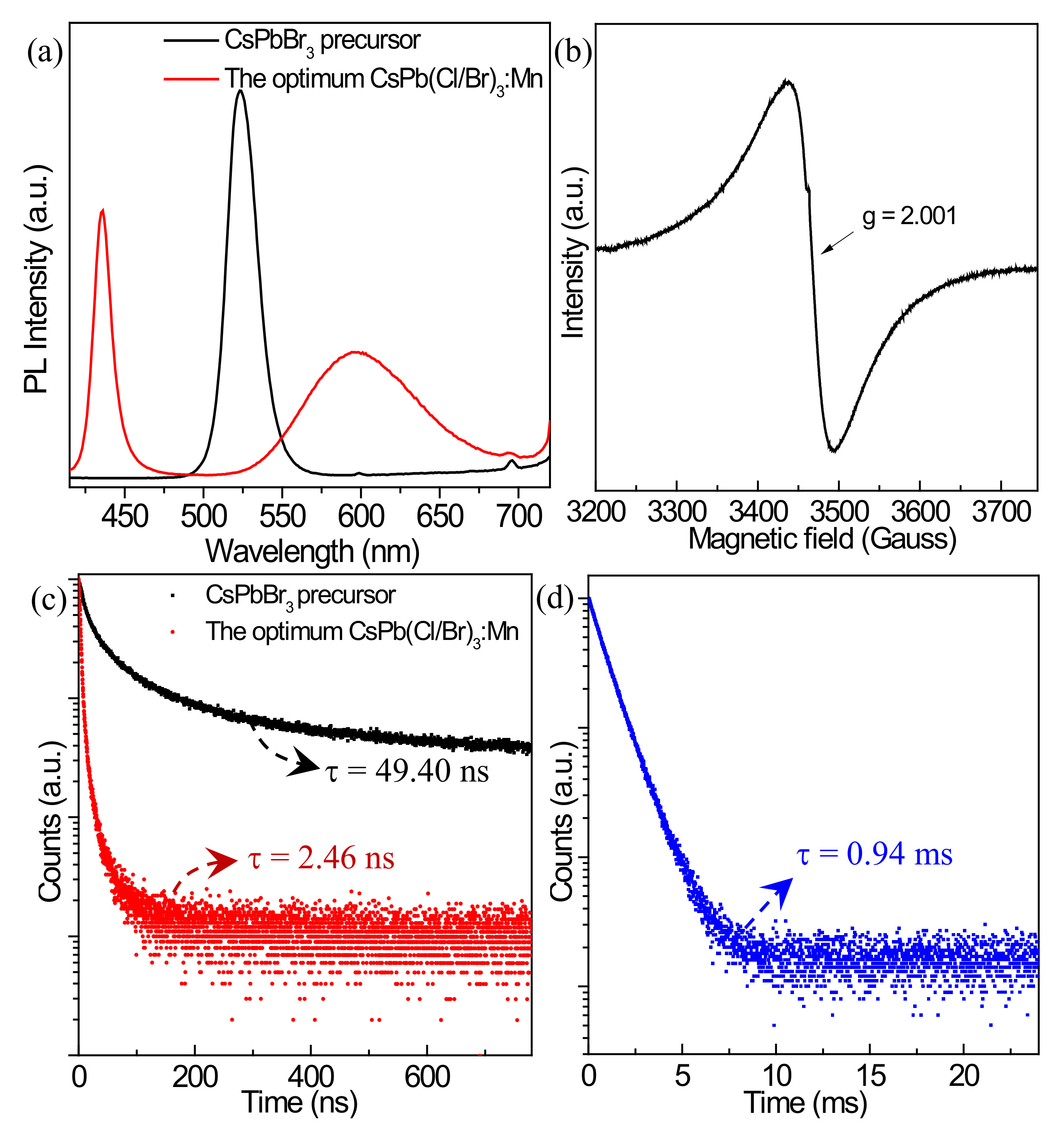
3.3. Optical Properties before and after Mn2+ Doping
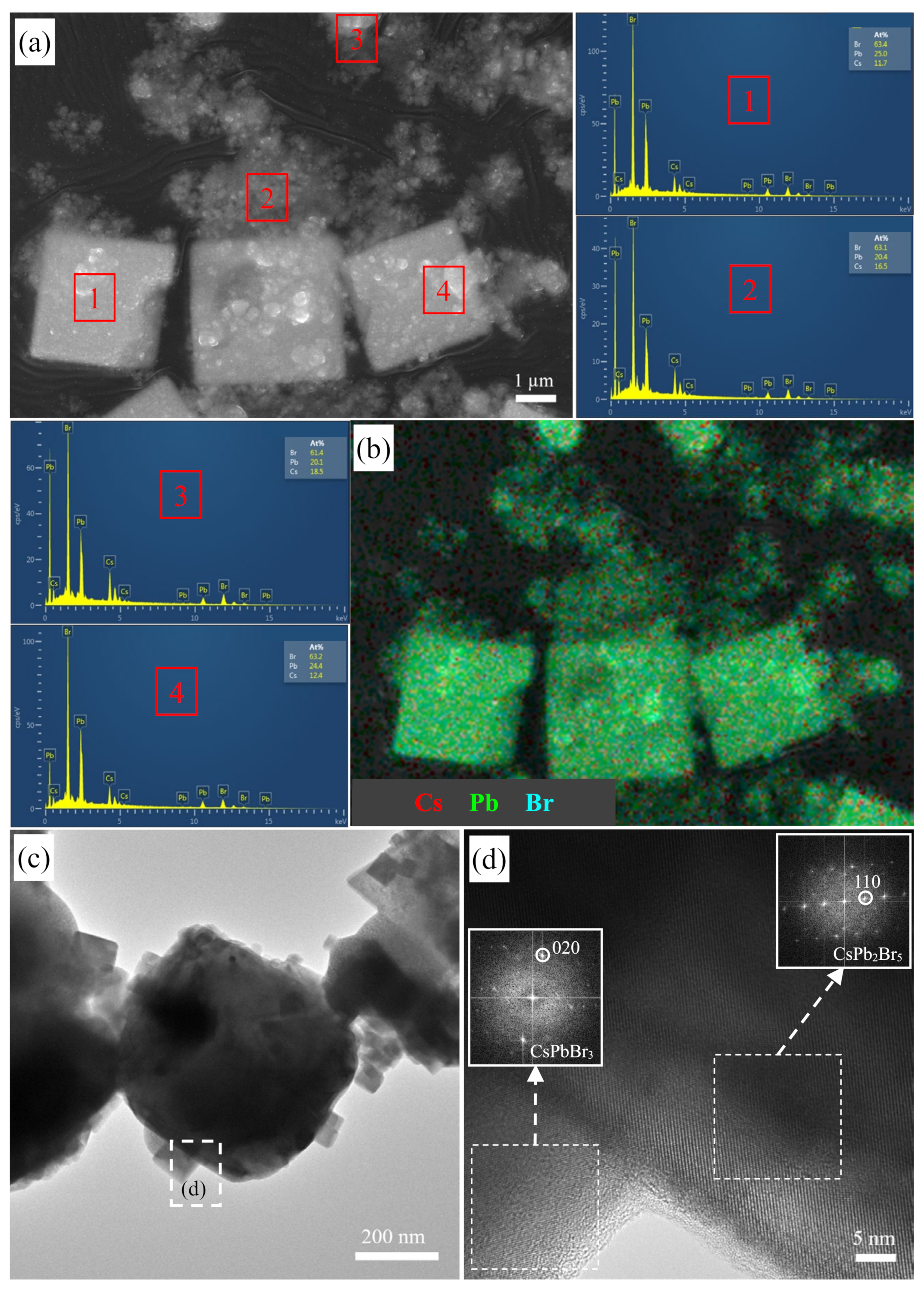
3.4. Morphology Evolution before and after Mn2+ Doping
3.5. XPS Analysis before and after Mn2+ Doping
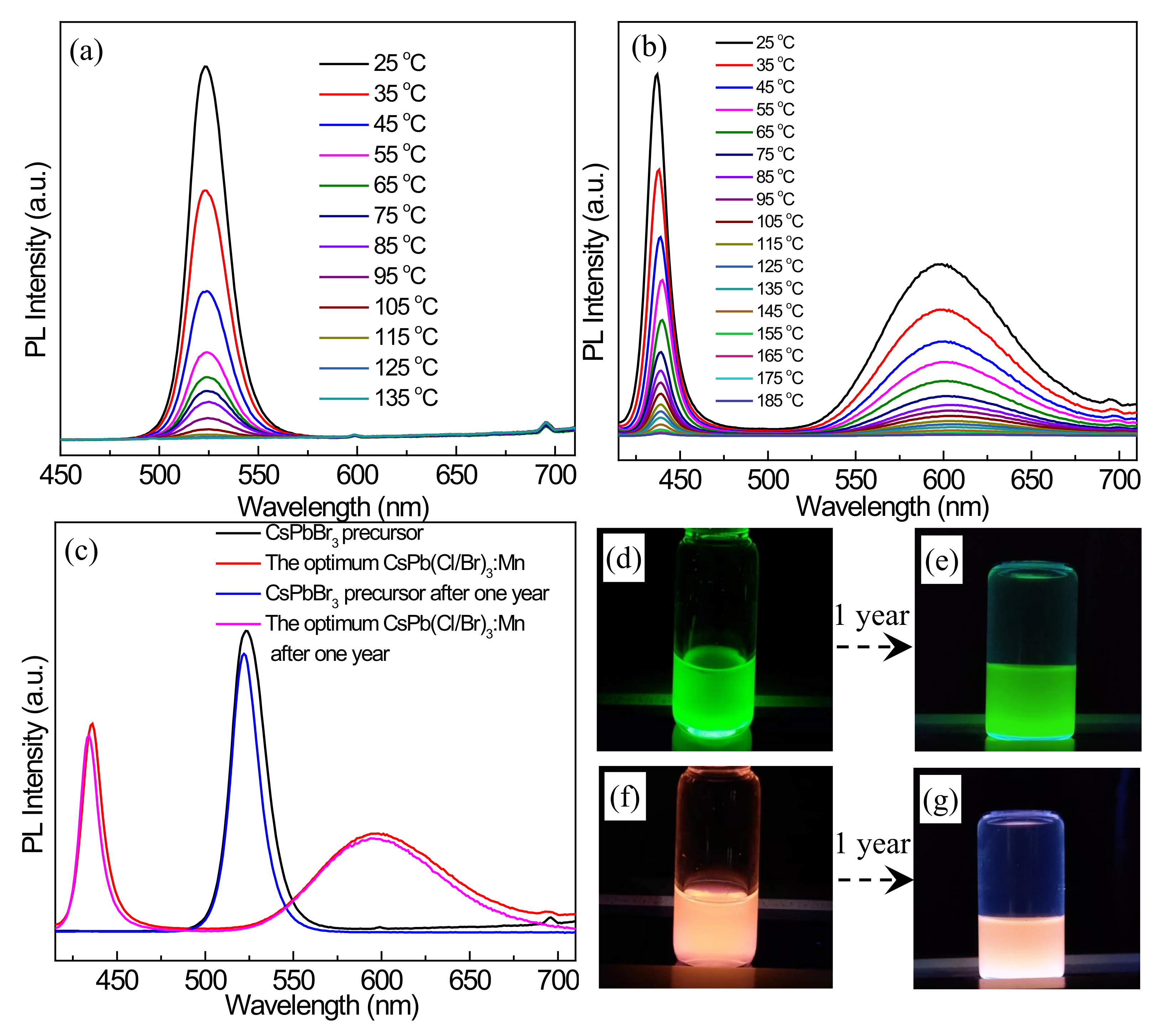
3.6. Stability before and after Mn2+ Doping
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stranks, S.D.; Snaith, H.J. Metal-halide perovskites for photovoltaic and light-emitting devices. Nat. Nanotechnol. 2015, 10, 391–402. [Google Scholar] [CrossRef] [PubMed]
- Park, N.-G.; Grätzel, M.; Miyasaka, T.; Zhu, K.; Emery, K. Towards stable and commercially available perovskite solar cells. Nat. Energy 2016, 1, 16152. [Google Scholar] [CrossRef]
- Protesescu, L.; Yakunin, S.; Bodnarchuk, M.I.; Krieg, F.; Caputo, R.; Hendon, C.H.; Yang, R.X.; Walsh, A.; Kovalenko, M.V. Nanocrystals of cesium lead halide perovskites (CsPbX3, X = Cl, Br, and I): Novel optoelectronic materials showing bright emission with wide color gamut. Nano Lett. 2015, 15, 3692–3696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nedelcu, G.; Protesescu, L.; Yakunin, S.; Bodnarchuk, M.I.; Grotevent, M.J.; Kovalenko, M.V. Fast anion-exchange in highly luminescent nanocrystals of cesium lead halide perovskites (CsPbX3, X = Cl, Br, I). Nano Lett. 2015, 15, 5635–5640. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Wang, S.; Zhang, Y.; Zhang, K.; Yi, L. The development of all-inorganic CsPbX3 perovskite solar cells. J. Mater. Sci. 2019, 55, 464–479. [Google Scholar] [CrossRef]
- He, S.; Han, Y.; Guo, J.; Wu, K. Long-lived delayed emission from CsPbBr3 perovskite nanocrystals for enhanced photochemical reactivity. ACS Energy Lett. 2021, 6, 2786–2791. [Google Scholar] [CrossRef]
- Li, X.; Wu, Y.; Zhang, S.; Cai, B.; Gu, Y.; Song, J.; Zeng, H. CsPbX3 quantum dots for lighting and displays: Room-temperature synthesis, photoluminescence superiorities, underlying origins and white light-emitting diodes. Adv. Funct. Mater. 2016, 26, 2435–2445. [Google Scholar] [CrossRef]
- Yang, Y.; You, J. Make perovskite solar cells stable. Nature 2017, 544, 155–156. [Google Scholar] [CrossRef]
- Huang, S.; Wang, B.; Zhang, Q.; Li, Z.; Shan, A.; Li, L. Postsynthesis potassium-modification method to improve stability of CsPbBr3 perovskite nanocrystals. Adv. Optical Mater. 2018, 6, 1701106. [Google Scholar] [CrossRef]
- He, X.; Qiu, Y.; Yang, S. Fully-inorganic trihalide perovskite nanocrystals: A new research frontier of optoelectronic materials. Adv. Mater. 2017, 29, 1700775. [Google Scholar] [CrossRef]
- Swarnkar, A.; Mir, W.J.; Nag, A. Can B-site doping or alloying improve thermal- and phase-stability of all-inorganic CsPbX3 (X = Cl, Br, I) perovskites? ACS Energy Lett. 2018, 3, 286–289. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Lin, Q.; Li, H.; Wu, K.; Robel, I.; Pietryga, J.M.; Klimov, V.I. Mn2+-doped lead halide perovskite nanocrystals with dual-color emission controlled by halide content. J. Am. Chem. Soc. 2016, 138, 14954–14961. [Google Scholar] [CrossRef]
- Yao, Z.; Zhao, W.; Chen, S.; Jin, Z.; Liu, S.F. Mn doping of CsPbI3 film towards high-efficiency solar cell. ACS Appl. Energy Mater. 2020, 3, 5190–5197. [Google Scholar] [CrossRef]
- Liu, W.; Zheng, J.; Cao, S.; Wang, L.; Gao, F.; Chou, K.-C.; Hou, X.; Yang, W. Mass production of Mn2+-doped CsPbCl3 perovskite nanocrystals with high quality and enhanced optical performance. Inorg. Chem. Front. 2018, 5, 2641–2647. [Google Scholar] [CrossRef]
- Dong, L.; Chen, Z.; Ye, L.; Yu, Y.; Zhang, J.; Liu, H.; Zang, J. Gram-scale synthesis of all-inorganic perovskite quantum dots with high Mn substitution ratio and enhanced dual-color emission. Nano Res. 2019, 12, 1733–1738. [Google Scholar] [CrossRef]
- Zhao, Y.; Xie, C.; Zhang, X.; Matras-Postolek, K.; Yang, P. Mn:CsPbBr3 nanoplatelets for bright white-emitting displays. ACS Appl. Nano Mater. 2021, 4, 6223–6230. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, J.; Wang, F.; Yin, Y.; Jin, Y.; Wang, J.; Peng, X.; Han, R.; Zhang, C.; Kong, J.; et al. Direct fabrication of CsPbxMn1-x(Br,Cl)3 thin film by a facile solution spraying approach. Nanomaterials 2021, 11, 3242. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Zhou, S.; Tian, F.; Ke, H.; Jiang, N.; Wang, S.; Peng, Y.; Liu, Y. Halogen-hot-injection synthesis of Mn-doped CsPb(Cl/Br)3 nanocrystals with blue/orange dual-color luminescence and high photoluminescence quantum yield. Adv. Opt. Mater. 2019, 7, 1901082. [Google Scholar] [CrossRef]
- Mir, W.J.; Mahor, Y.; Lohar, A.; Jagadeeswararao, M.; Das, S.; Mahamuni, S.; Nag, A. Postsynthesis doping of Mn and Yb into CsPbX3 (X = Cl, Br, or I) perovskite nanocrystals for downconversion emission. Chem. Mater. 2018, 30, 8170–8178. [Google Scholar] [CrossRef] [Green Version]
- Parobek, D.; Roman, B.J.; Dong, Y.; Jin, H.; Lee, E.; Sheldon, M.; Son, D.H. Exciton-to-dopant energy transfer in Mn-doped cesium lead halide perovskite nanocrystals. Nano Lett. 2016, 16, 7376–7380. [Google Scholar] [CrossRef] [PubMed]
- Zou, S.; Liu, Y.; Li, J.; Liu, C.; Feng, R.; Jiang, F.; Li, Y.; Song, J.; Zeng, H.; Hong, M.; et al. Stabilizing cesium lead halide perovskite lattice through Mn(II) substitution for air-stable light-emitting diodes. J. Am. Chem. Soc. 2017, 139, 11443–11450. [Google Scholar] [CrossRef]
- Cheng, J.; Li, Y.; Qu, W.; Sun, M.; Liu, Y.; Shi, W.; Du, W.; Zhang, Y. Mechanochemical synthesis and characterization of Mn-doped CsPbCl3 perovskite nanocrystals. J. Alloys Compd. 2020, 822, 1901082. [Google Scholar] [CrossRef]
- Begum, R.; Parida, M.R.; Abdelhady, A.L.; Murali, B.; Alyami, N.M.; Ahmed, G.H.; Hedhili, M.N.; Bakr, O.M.; Mohammed, O.F. Engineering Interfacial Charge Transfer in CsPbBr3 Perovskite Nanocrystals by Heterovalent Doping. J. Am. Chem. Soc. 2017, 139, 731–737. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wu, Z.; Shao, J.; Yao, D.; Gao, H.; Liu, Y.; Yu, W.; Zhang, H.; Yang, B. CsPbxMn1-xCl3 perovskite quantum dots with high Mn substitution ratio. ACS Nano 2017, 11, 2239–2247. [Google Scholar] [CrossRef]
- Yuan, X.; Ji, S.; De Siena, M.C.; Fei, L.; Zhao, Z.; Wang, Y.; Li, H.; Zhao, J.; Gamelin, D.R. Photoluminescence temperature dependence, dynamics, and quantum efficiencies in Mn2+-doped CsPbCl3 perovskite nanocrystals with varied dopant concentration. Chem. Mater. 2017, 29, 8003–8011. [Google Scholar] [CrossRef]
- Liu, H.; Wu, Z.; Gao, H.; Shao, J.; Zou, H.; Yao, D.; Liu, Y.; Zhang, H.; Yang, B. One-step preparation of cesium lead halide CsPbX3 (X = Cl, Br, and I) perovskite nanocrystals by microwave irradiation. ACS Appl. Mater. Interfaces 2017, 9, 42919–42927. [Google Scholar] [CrossRef]
- Hu, Q.; Li, Z.; Tan, Z.; Song, H.; Ge, C.; Niu, G.; Han, J.; Tang, J. Rare earth ion-doped CsPbBr3 nanocrystals. Adv. Opt. Mater. 2018, 6, 1700864. [Google Scholar] [CrossRef]
- Mir, W.J.; Jagadeeswararao, M.; Das, S.; Nag, A. Colloidal Mn-doped cesium lead halide perovskite nanoplatelets. ACS Energy Lett. 2017, 2, 537–543. [Google Scholar] [CrossRef]
- Zhu, J.; Yang, X.; Zhu, Y.; Wang, Y.; Cai, J.; Shen, J.; Sun, L.; Li, C. Room-temperature synthesis of Mn-doped cesium lead halide quantum dots with high Mn substitution ratio. J. Phys. Chem. Lett. 2017, 8, 4167–4171. [Google Scholar] [CrossRef]
- Guria, A.K.; Dutta, S.K.; Adhikari, S.D.; Pradhan, N. Doping Mn2+ in lead halide perovskite nanocrystals: Successes and challenges. ACS Energy Lett. 2017, 2, 1014–1021. [Google Scholar] [CrossRef] [Green Version]
- Van der Stam, W.; Geuchies, J.J.; Altantzis, T.; van den Bos, K.H.; Meeldijk, J.D.; Van Aert, S.; Bals, S.; Vanmaekelbergh, D.; de Mello Donega, C. Highly emissive divalent-ion-doped colloidal CsPb1-xMxBr3 perovskite nanocrystals through cation exchange. J. Am. Chem. Soc. 2017, 139, 4087–4097. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Wang, C.; Xu, S.; Zong, S.; Lu, J.; Wang, Z.; Lu, C.; Cui, Y. Postsynthetic doping of MnCl2 molecules into preformed CsPbBr3 perovskite nanocrystals via a halide exchange-driven cation exchange. Adv. Mater. 2017, 29, 1700095. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Qiao, B.; Xu, Z.; Song, D.; Song, P.; Liang, Z.; Shen, Z.; Cao, J.; Zhang, J.; Zhao, S. Postsynthetic, Reversible Cation Exchange between Pb2+ and Mn2+ in Cesium Lead Chloride Perovskite Nanocrystals. J. Phys. Chem. C 2017, 121, 20387–20395. [Google Scholar] [CrossRef]
- Li, F.; Xia, Z.; Gong, Y.; Gu, L.; Liu, Q. Optical properties of Mn2+ doped cesium lead halide perovskite nanocrystals via a cation-anion co-substitution exchange reaction. J. Mater. Chem. C 2017, 5, 9281–9287. [Google Scholar] [CrossRef]
- Yang, Y.; Li, Q.; Liu, Y.; Cong, R.; Sun, Y.; Hou, J.; Ge, M.; Shi, J.; Zhang, F.; Zhao, G.; et al. Magenta-emitting cesium lead halide nanocrystals encapsulated in dimethicone for white light-emitting diodes. ACS Appl. Nano Mater. 2020, 3, 4886–4892. [Google Scholar] [CrossRef]
- Xu, K.; Vickers, E.T.; Luo, B.; Allen, A.C.; Chen, E.; Roseman, G.; Wang, Q.; Kliger, D.S.; Millhauser, G.L.; Yang, W.; et al. First synthesis of Mn-doped cesium lead bromide perovskite magic sized clusters at room temperature. J. Phys. Chem. Lett. 2020, 11, 1162–1169. [Google Scholar] [CrossRef]
- Almutlaq, J.; Mir, W.J.; Gutiérrez-Arzaluz, L.; Yin, J.; Vasylevskyi, S.; Maity, P.; Liu, J.; Naphade, R.; Mohammed, O.F.; Bakr, O.M. CsMnBr3: Lead-Free Nanocrystals with High Photoluminescence Quantum Yield and Picosecond Radiative Lifetime. ACS Materials Lett. 2021, 3, 290–297. [Google Scholar] [CrossRef]
- De, A.; Mondal, N.; Samanta, A. Luminescence tuning and exciton dynamics of Mn-doped CsPbCl3 nanocrystals. Nanoscale 2017, 9, 16722–16727. [Google Scholar] [CrossRef] [PubMed]
- Hills-Kimball, K.; Pérez, M.J.; Nagaoka, Y.; Cai, T.; Yang, H.; Davis, A.H.; Zheng, W.; Chen, O. Ligand engineering for Mn2+ doping control in CsPbCl3 perovskite nanocrystals via a quasi-solid–solid cation exchange reaction. Chem. Mater. 2020, 32, 2489–2500. [Google Scholar] [CrossRef]
- Li, X.; Cai, W.; Guan, H.; Zhao, S.; Cao, S.; Chen, C.; Liu, M.; Zang, Z. Highly stable CsPbBr3 quantum dots by silica-coating and ligand modification for white light-emitting diodes and visible light communication. Chem. Eng. J. 2021, 419, 129551. [Google Scholar] [CrossRef]
- Chen, W.; Shi, T.; Du, J.; Zang, Z.; Yao, Z.; Li, M.; Sun, K.; Hu, W.; Leng, Y.; Tang, X. Highly Stable Silica-Wrapped Mn-Doped CsPbCl3 Quantum Dots for Bright White Light-Emitting Devices. ACS Appl. Mater. Interfaces 2018, 10, 43978–43986. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Fan, C.; Tang, J.; Huang, G.; Qiang, X.; Fu, Y.; Zhou, W.; Wu, J.; Huang, S. Systematic Microwave-Assisted Postsynthesis of Mn-Doped Cesium Lead Halide Perovskites with Improved Color-Tunable Luminescence and Stability. Nanomaterials 2022, 12, 2535. https://doi.org/10.3390/nano12152535
Zhang Y, Fan C, Tang J, Huang G, Qiang X, Fu Y, Zhou W, Wu J, Huang S. Systematic Microwave-Assisted Postsynthesis of Mn-Doped Cesium Lead Halide Perovskites with Improved Color-Tunable Luminescence and Stability. Nanomaterials. 2022; 12(15):2535. https://doi.org/10.3390/nano12152535
Chicago/Turabian StyleZhang, Yaheng, Chao Fan, Jianghong Tang, Gaoming Huang, Xinfa Qiang, Yu Fu, Wenjuan Zhou, Juan Wu, and Shouqiang Huang. 2022. "Systematic Microwave-Assisted Postsynthesis of Mn-Doped Cesium Lead Halide Perovskites with Improved Color-Tunable Luminescence and Stability" Nanomaterials 12, no. 15: 2535. https://doi.org/10.3390/nano12152535
APA StyleZhang, Y., Fan, C., Tang, J., Huang, G., Qiang, X., Fu, Y., Zhou, W., Wu, J., & Huang, S. (2022). Systematic Microwave-Assisted Postsynthesis of Mn-Doped Cesium Lead Halide Perovskites with Improved Color-Tunable Luminescence and Stability. Nanomaterials, 12(15), 2535. https://doi.org/10.3390/nano12152535






