Antibacterial Bio-Nanocomposite Textile Material Produced from Natural Resources
Abstract
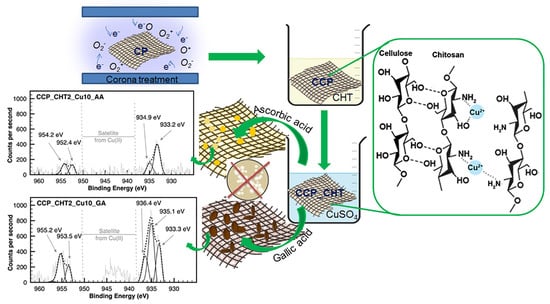
:1. Introduction
2. Materials and Methods
2.1. Production of Peat-Based Material
2.2. Corona Treatment
2.3. Preparation of Bio-Nanocomposites
2.3.1. Coating of CCP Samples with Biopolymer Chitosan
2.3.2. Synthesis of Cu-Based Nanostructures
2.4. FTIR Analysis
2.5. FESEM and NPs Size Analysis
2.6. XPS Analysis
2.7. AAS Analysis
2.8. Antibacterial Test
2.9. Quantification of Cu2+-Ions Release
2.10. Biodegradation Test
3. Results and Discussion
3.1. Chemical and Morphological Characterization of the Samples
3.2. Antimicrobial Activity
3.3. Release of Cu2+-Ions
3.4. Biodegradation Behavior of the Samples in Soil
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tavares, T.D.; Antunes, J.C.; Ferreira, F.; Felgueiras, H.P. Biofunctionalization of Natural Fiber-Reinforced Biocomposites for Biomedical Applications. Biomolecules 2020, 10, 148. [Google Scholar] [CrossRef] [Green Version]
- Sarasini, F.; Fiore, V. A systematic literature review on less common natural fibres and their biocomposites. J. Clean. Prod. 2018, 195, 240–267. [Google Scholar] [CrossRef]
- Mikučioniene, D.; Čepukone, L.; Milašiene, D. Investigation on mechanical and thermal properties of knits from peat fibers and their combination with other natural fibers. Text. Res. J. 2018, 88, 1660–1670. [Google Scholar] [CrossRef]
- Cerqueira, S.C.A.; Romao, L.P.C.; Lucas, S.C.O.; Fraga, L.E.; Simoes, M.L.; Hammer, P.; Lead, J.R.; Mangoni, A.P.; Mangrich, A.S. Spectroscopic characterization of the reduction and removal of chromium (VI) by tropical peat and humin. Fuel 2012, 91, 141–146. [Google Scholar] [CrossRef]
- Mikučioniene, D.; Čepukone, L.; Salmeia, K.A.; Gaan, S. Comparative analysis of peat fibre properties and peat fibre-based flammability. Autex Res. J. 2019, 19, 157–164. [Google Scholar] [CrossRef] [Green Version]
- Xiao, Y.; Shen, G.; Zheng, W.; Fu, J.; Fu, F.; Hu, X.; Jin, Z.; Liu, X. Remarkable durability of the antibacterial function achieved via a coordination effect of Cu(II) ion and chitosan grafted on cotton fibers. Cellulose 2022, 29, 1003–1015. [Google Scholar] [CrossRef]
- Radetić, M. Functionalization of textile materials with Ag nanoparticles. J. Mater. Sci. 2013, 48, 95–107. [Google Scholar] [CrossRef]
- Ahmed, T.; Ogulata, R.T. A Review on silver nanoparticles-green synthesis, antimicrobial action and application in textiles. J. Nat. Fibers 2021. [Google Scholar] [CrossRef]
- Marković, D.; Radetić, M. Nano-finishing of cellulose textile materials with copper and copper oxide nanoparticles. Cellulose 2019, 26, 8971–8991. [Google Scholar] [CrossRef]
- Petkova, P.; Francesko, A.; Perelshtein, I.; Gedanken, A.; Tzanov, T. Simultaneous sonochemical-enzymatic coating of medical textiles with antibacterial ZnO nanoparticles. Ultrason. Sonochem. 2016, 29, 244–250. [Google Scholar] [CrossRef]
- Fouda, A.; EL-Din Hassan, S.; Salem, S.S.; Shaheen, T.I. In-Vitro cytotoxicity, antibacterial, and UV protection properties of the biosynthesized zinc oxide nanoparticles for medical textile applications. Microb. Pathog. 2018, 125, 252–261. [Google Scholar] [CrossRef]
- D’Água, R.B.; Branquinho, R.; Duarte, M.P.; Maurício, E.; Fernando, A.L.; Martins, R.; Fortunato, E. Efficient coverage of ZnO nanoparticles on cotton fibres for antibacterial finishing using a rapid and low cost in situ synthesis. New J. Chem. 2018, 42, 1052–1060. [Google Scholar] [CrossRef]
- Morent, R.; de Geyter, N.; Leys, C.; Gengembre, L.; Payen, E. Comparison between XPS- and FTIR-analysis of plasma-treated polypropylene film surfaces. Surf. Interfaces Anal. 2008, 40, 597–600. [Google Scholar] [CrossRef]
- Niu, Y.; Ying, D.; Li, K.; Wang, Y.; Jia, J. Fast removal of copper ions from aqueous solution using an ecoefriendly fibrous adsorbent. Chemosphere 2016, 161, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Socrates, G. Infrared Characteristic Group Frequencies. Tables and Charts; John Wiley & Sons Ltd.: Chichester, UK, 2001. [Google Scholar] [CrossRef]
- Verasoundarapandian, G.; Zakaria, N.N.; Shaharuddin, N.A.; Khalil, K.A.; Puasa, N.A.; Azmi, A.A.; Gomez-Fuentes, C.; Zulkharnain, A.; Wong, C.Y.; Rahman, M.F.; et al. Coco Peat as Agricultural Waste Sorbent for Sustainable Diesel-Filter System. Plants 2021, 10, 2468. [Google Scholar] [CrossRef] [PubMed]
- Krkobabić, A.; Marković, D.; Kovačević, A.; Tadić, V.; Radoičić, M.; Tomic-Ilic, T.; Barudžija, T.; Radetić, M. In situ green synthesis of Cu-based nanoparticles on oxidized cotton fabric using bearberry leaves extract. Fibers Polym. 2022, 23, 954–966. [Google Scholar] [CrossRef]
- Marković, D.; Korica, M.; Kostić, M.; Radovanović, Ž.; Šaponjić, Z.; Mitrić, M.; Radetić, M. In situ synthesis of Cu/Cu2O nanoparticles on the TEMPO oxidized cotton fabrics. Cellulose 2018, 25, 829–841. [Google Scholar] [CrossRef]
- Marković, D.; Deeks, C.; Nunney, T.; Radovanović, Ž.; Radoičić, M.; Šaponjić, Z.; Radetić, M. Antibacterial activity of Cu-based nanoparticles synthesized on the cotton fabrics previously modified with polycarboxylic acids. Carbohydr. Polym. 2018, 200, 173–183. [Google Scholar] [CrossRef]
- Ogawa, K.; Oka, K.; Yui, T. X-ray study of chitosan-transition metal complexes. Chem. Mater. 1993, 5, 726–728. [Google Scholar] [CrossRef]
- Domard, A. pH and c.d. measurements on a fully deacetylated chitosan: Application to CuII-polymer interactions. Int. J. Biol. Macromol. 1987, 9, 98–104. [Google Scholar] [CrossRef]
- Gomes, J.R.B.; Jorge, M.; Gomes, P. Interaction of chitosan and chitin with Ni, Cu and Zn ions: A computational study. J. Chem. Thermodynam. 2014, 73, 121–129. [Google Scholar] [CrossRef] [Green Version]
- Schlick, S. Binding sites of Cu2+ in chitin and chitosan. An electron spin resonance study. Macromolecules 1986, 19, 192–195. [Google Scholar] [CrossRef]
- Mahdieh, Z.M.; Shekarriz, S.; Taromi, F.A. The effect of silver concentration on Ag-TiO2 nanoparticles coated polyester/cellulose fabric by in situ and ex situ photo-reduction method—A comparative study. Fibers Polym. 2021, 22, 87–96. [Google Scholar] [CrossRef]
- Oudrhiri Hassani, F.; Merbahi, N.; Oushabi, A.; Elfadili, M.H.; Kammouni, A.; Oueldna, N. Effects of corona discharge treatment on surface and mechanical properties of Aloe Vera fibers. Mater. Today Proc. 2020, 24, 46–51. [Google Scholar] [CrossRef]
- Islam, S.; Bhuiyan, M.A.R.; Islam, M.N. Chitin and Chitosan: Structure, Properties and Applications in Biomedical Engineering. J. Polym. Environ. 2016, 25, 854–866. [Google Scholar] [CrossRef]
- Bastidas, J.; Venditti, R.; Pawlak, J.; Gilbert, R.; Zauscher, S.; Kadla, J. Chemical force microscopy of cellulosic fibers. Carbohydr. Polym. 2005, 62, 369–378. [Google Scholar] [CrossRef]
- Ly, B.; Thielemans, W.; Dufresne, A.; Chaussy, D.; Belgacem, M.N. Surface functionalization of cellulose fibres and their incorporation in renewable polymeric matrices. Compos. Sci. Technol. 2008, 68, 3193–3201. [Google Scholar] [CrossRef]
- Belgacem, M.N.; Czeremuszkin, G.; Sapieha, S.; Gandini, A. Surface characterization of cellulose fibres by XPS and inverse gas chromatography. Cellulose 1995, 2, 145–157. [Google Scholar] [CrossRef]
- Fuentes, C.A.; Tran, L.Q.N.; Dupont-Gillain, C.; Vanderlinden, W.; De Feyter, S.; Van Vuure, A.W.; Verpoest, I. Wetting behaviour and surface properties of technical bamboo fibres. Colloids Surf. A 2011, 380, 89–99. [Google Scholar] [CrossRef]
- Li, P.C.; Liao, G.M.; Kumar, S.R.; Shih, C.M.; Yang, C.C.; Wang, D.M.; Lue, S.J. Fabrication and characterization of chitosan nanoparticle-incorporated quaternized poly(vinyl alcohol) composite membranes as solid electrolytes for direct methanol alkaline fuel cells. Electrochim. Acta 2016, 187, 616–628. [Google Scholar] [CrossRef]
- Zhenhuan, Z.; Yujun, W.; Gan, W.; Qiang, A.A.W.; Yandao, G.; Xiufang, Z. Surface properties of chitosan films modified with polycations and their effects on the behavior of PC12 cells. J. Bioact. Compat. Polym. 2009, 24, 63–82. [Google Scholar] [CrossRef]
- Gieroba, B.; Sroka-Bartnicka, A.; Kazimierczak, P.; Kalisz, G.; Lewalska-Graczyk, A.; Vivcharenko, V.; Nowakowski, R.; Pieta, I.S.; Przekora, A. Spectroscopic studies on the temperature-dependent molecular arrangements in hybrid chitosan/1,3-β-D-glucan polymeric matrices. Int. J. Biol. Macromol. 2020, 159, 911–921. [Google Scholar] [CrossRef] [PubMed]
- Hamad, H.; Bailón-García, E.; Morales-Torres, S.; Carrasco-Marín, F.; Pérez-Cadenas, A.F.; Maldonado-Hódar, F.J. Functionalized cellulose for the controlled synthesis of novel carbon–Ti nanocomposites: Physicochemical and photocatalytic properties. Nanomaterials 2020, 10, 729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, F.D.; Yu, Y.L.; Zhang, Y.M.; Yu, W.J.; Gao, J.M. Surface chemical composition analysis of heat-treated bamboo. Appl. Surf. Sci. 2016, 371, 383–390. [Google Scholar] [CrossRef]
- Sitthichai, S.; Pilapong, C.; Thongtem, T.; Thongtem, S. CMC-coated Fe3O4 nanoparticles as new MRI probes for hepatocellular carcinoma. Appl. Surf. Sci. 2015, 356, 972–977. [Google Scholar] [CrossRef]
- Chen, R.; Li, L.; Liu, Z.; Lu, M.; Wang, C.; Li, H.; Ma, W.; Wang, S. Preparation and characterization of activated carbons from tobacco stem by chemical activation. J. Air Waste Manag. 2017, 67, 713–724. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.C.; Hsu, Y.K.; Lin, Y.G.; Lin, Y.K.; Horng, Y.Y.; Chen, L.C.; Chen, K.H. Highly flexible supercapacitors with manganese oxide nanosheet/carbon cloth electrode. Electrochim. Acta 2011, 56, 7124–7130. [Google Scholar] [CrossRef]
- Zuo, P.P.; Feng, H.F.; Xu, Z.Z.; Zhang, L.F.; Zhang, Y.L.; Xia, W.; Zhang, W.Q. Fabrication of biocompatible and mechanically reinforced graphene oxide-chitosan nanocomposite films. Chem. Cent. J. 2013, 7, 39. [Google Scholar] [CrossRef] [Green Version]
- Godoi, F.C.; Rodriguez-Castellon, E.; Guibal, E.; Beppu, M.M. An XPS study of chromate and vanadate sorption mechanism by chitosan membrane containing copper nanoparticles. Chem. Eng. J. 2013, 234, 423–429. [Google Scholar] [CrossRef]
- Liu, L.; Li, W.; Xiong, Z.; Xia, D.; Yang, C.; Wang, W.; Sun, Y. Synergistic effect of iron and copper oxides on the formation of persistent chlorinated aromatics in iron ore sintering based on in situ XPS analysis. J. Hazard. Mater. 2019, 366, 202–209. [Google Scholar] [CrossRef]
- Liu, X.; Chen, J.; Liu, P.; Zhang, H.; Li, G.; An, T.; Zhao, H. Controlled growth of CuO/Cu2O hollow microsphere composites as efficient visible-light-active photocatalysts. Appl. Catal. A-Gen. 2016, 521, 34–41. [Google Scholar] [CrossRef]
- Hayez, V.; Franquet, A.; Hubin, A.; Terryn, H. XPS study of the atmospheric corrosion of copper alloys of archaeological interest. Surf. Interface Anal. 2004, 36, 876–879. [Google Scholar] [CrossRef]
- Ruparelia, J.P.; Chatterjee, A.K.; Duttagupta, S.P.; Mukherji, S. Strain specificity in antimicrobial activity of silver and copper nanoparticles. Acta Biomater. 2008, 4, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Pang, H.; Gaob, F.; Lu, Q. Morphology effect on antibacterial activity of cuprous oxide. Chem. Commun. 2009, 9, 1076–1078. [Google Scholar] [CrossRef]
- Hong, R.; Kang, T.Y.; Michels, C.A.; Gaduraa, N. Membrane lipid peroxidation in copper alloy-mediated contact killing of Escherichia coli. Appl. Environ. Microbiol. 2012, 78, 1776–1784. [Google Scholar] [CrossRef] [Green Version]
- Chatterjee, A.K.; Chakraborty, R.; Basu, T. Mechanism of antibacterial activity of copper nanoparticles. Nanotechnology 2014, 25, 135101. [Google Scholar] [CrossRef]
- Errokh, A.; Ferraria, A.M.; Conceição, D.S.; Ferreira, L.F.V.; Rego, A.M.B.; Vilar, M.R.; Boufi, S. Controlled growth of Cu2O nanoparticles bound to cotton fibres. Carbohydr. Polym. 2016, 141, 229–237. [Google Scholar] [CrossRef]
- Marković, D.; Thseng, H.H.; Nunney, T.; Radoičić, M.; Ilic-Tomic, T.; Radetić, M. Novel antimicrobial nanocomposite based on polypropylene non-woven fabric, biopolymer alginate and copper oxides nanoparticles. Appl. Surf. Sci. 2020, 527, 146829. [Google Scholar] [CrossRef]
- Wongrakpanich, A.; Mudunkotuwa, I.A.; Geary, S.M.; Morris, A.M.; Mapuskar, K.A.; Spitz, D.R.; Grassiand, V.H.; Salem, A.K. Size-dependent cytotoxicity of copper oxide nanoparticles in lung epithelial cells. Environ. Sci. Nano 2016, 3, 365–374. [Google Scholar] [CrossRef] [Green Version]
- Naz, S.; Gul, A.; Zia, M. Toxicity of copper oxide nanoparticles: A review study. IET Nanobiotechnol. 2020, 14, 1–13. [Google Scholar] [CrossRef]
- Alarifi, S.; Ali, D.; Verma, A.; Alakhtani, S.; Ali, B.A. Cytotoxicity and genotoxicity of copper oxide nanoparticles in human skin keratinocytes cells. Int. J. Toxicol. 2013, 32, 296–307. [Google Scholar] [CrossRef]
- Li, H.; Toh, P.Z.; Tan, J.Y.; Zin, M.T.; Lee, C.Y.; Li, B.; Leolukman, M.; Bao, H.; Kang, L. Selected biomarkers revealed potential skin toxicity caused by certain copper compounds. Sci. Rep. 2016, 28, 37664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, C.; Li, Y.; Yang, L.; Zheng, Y.; Long, J.; Jia, J.; Xiao, S.; Liu, J. Activation of Erk and p53 regulates copper oxide nanoparticle-induced cytotoxicity in keratinocytes and fibroblasts. Int. J. Nanomed. 2014, 9, 4763–4772. [Google Scholar] [CrossRef] [Green Version]
- Kubo, A.L.; Vasiliev, G.; Vija, H.; Krishtal, J.; Tõugu, V.; Visnapuu, M.; Kisand, V.V.; Kahru, A.; Bondarenko, O.M. Surface carboxylation or PEGylation decreases CuO nanoparticles’ cytotoxicity to human cells in vitro without compromising their antibacterial properties. Arch. Toxicol. 2020, 94, 1561–1573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dharmalingam, K.; Bordoloi, D.; Kunnumakkara, A.B.; Anandalakshmi, R. Preparation and characterization of cellulose-based nanocomposite hydrogel films containing CuO/Cu2O/Cu with antibacterial activity. J. Appl. Polym. Sci. 2020, 137, 49216. [Google Scholar] [CrossRef]
- Klemenčič, D.; Simončič, B.; Tomšič, B.; Orel, B. Biodegradation of silver functionalized cellulose fibres. Carbohydr. Polym. 2010, 80, 426–435. [Google Scholar] [CrossRef]
- Tomšič, B.; Klemenčič, D.; Simončič, B.; Orel, B. Influence of antimicrobial finishes on the biodeterioration of cotton and cotton/polyester fabrics: Leaching versus bio-barrier formation. Polym. Degrad. Stabil. 2011, 96, 1286–1296. [Google Scholar] [CrossRef]
- Tomšič, B.; Marković, D.; Janković, V.; Simončič, B.; Nikodinovic-Runic, J.; Ilic-Tomic, T.; Radetić, M. Biodegradation of cellulose fibers functionalized with CuO/Cu2O nanoparticles in combination with polycarboxylic acids. Cellulose 2022, 29, 287–302. [Google Scholar] [CrossRef]












| Sample | C (µmol/g) | |
|---|---|---|
| Reduced with GA | Reduced with AA | |
| CCP-CHT1-Cu1 | 28 ± 1.7 | 24 ± 0.3 |
| CCP-CHT2-Cu1 | 32 ± 2.3 | 25 ± 0.8 |
| CCP-CHT1-Cu5 | 48 ± 0.2 | 48 ± 0.7 |
| CCP-CHT2-Cu5 | 48 ± 0.2 | 48 ± 1.0 |
| CCP-CHT1-Cu10 | 51 ± 2.8 | 50 ± 2.9 |
| CCP-CHT2-Cu10 | 54 ± 2.8 | 55 ± 3.9 |
| Samples | C 1s (at.%) | O 1s (at.%) | N 1s (at.%) | Cu 2p (at.%) | O/C Ratio | N/C Ratio |
|---|---|---|---|---|---|---|
| CP | 85.86 ± 0.41 | 12.11 ± 0.25 | 1.62 ± 0.32 | - | 0.14 | 0.02 |
| CCP-CHT2 | 67.62 ± 0.34 | 27.68 ± 0.29 | 4.39 ± 0.24 | - | 0.41 | 0.06 |
| CCP-CHT2-Cu10-AA | 65.34 ± 0.45 | 30.62 ± 0.40 | 2.85 ± 0.26 | 1.20 ± 0.17 | 0.47 | 0.04 |
| CCP-CHT2-Cu10-GA | 67.95 ± 0.38 | 27.13 ± 0.33 | 3.64 ± 0.24 | 1.28 ± 0.11 | 0.40 | 0.05 |
| Samples | Cu2O | CuO | CuSO4 | Cu2O | Cu2O | CuO | CuO |
|---|---|---|---|---|---|---|---|
| 933.2 eV | 935.0 eV | 936.4 eV | 952.4 eV | 953.5 eV | 954.2 eV | 955.2 eV | |
| CCP-CHT2-Cu10-AA | 46.2% | 17.9% | - | 18.9% | - | 17.1% | - |
| CCP-CHT2-Cu10-GA | 25.1% | 32.7% | 14.0% | - | 12.6% | - | 15.6% |
| Sample | Microorganisms (CFU/mL) | |
|---|---|---|
| E. coli | S. aureus | |
| Inoculum | 5.6 × 106 | 2.5 × 106 |
| CP | 4.1 × 106 | 9.0 × 105 |
| CCP-CHT1 | 7.0 × 106 | 3.0 × 105 |
| CCP-CHT1-GA | 3.5 × 106 | 2.5 × 105 |
| CCP-CHT1-AA | 4.2 × 106 | 3.4 × 105 |
| CCP-CHT2 | 1.2 × 106 | 3.0 × 105 |
| CCP-CHT2-GA | 1.3 × 106 | 3.1 × 105 |
| CCP-CHT2-AA | 2.8 × 106 | 8.0 × 105 |
| CCP-CHT1-Cu1-GA | <10 | <10 |
| CCP-CHT2-Cu1-GA | <10 | <10 |
| CCP-CHT1-Cu5-GA | <10 | <10 |
| CCP-CHT2-Cu5-GA | <10 | <10 |
| CCP-CHT1-Cu10-GA | 10 | <10 |
| CCP-CHT2-Cu10-GA | <10 | <10 |
| CCP-CHT1-Cu1-AA | 5.4 × 105 | 10 |
| CCP-CHT2-Cu1-AA | 4.2 × 105 | 200 |
| CCP-CHT1-Cu5-AA | 110 | <10 |
| CCP-CHT2-Cu5-AA | 20 | <10 |
| CCP-CHT1-Cu10-AA | <10 | <10 |
| CCP-CHT2-Cu10-AA | <10 | <10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marković, D.; Zille, A.; Ribeiro, A.I.; Mikučioniene, D.; Simončič, B.; Tomšič, B.; Radetić, M. Antibacterial Bio-Nanocomposite Textile Material Produced from Natural Resources. Nanomaterials 2022, 12, 2539. https://doi.org/10.3390/nano12152539
Marković D, Zille A, Ribeiro AI, Mikučioniene D, Simončič B, Tomšič B, Radetić M. Antibacterial Bio-Nanocomposite Textile Material Produced from Natural Resources. Nanomaterials. 2022; 12(15):2539. https://doi.org/10.3390/nano12152539
Chicago/Turabian StyleMarković, Darka, Andrea Zille, Ana Isabel Ribeiro, Daiva Mikučioniene, Barbara Simončič, Brigita Tomšič, and Maja Radetić. 2022. "Antibacterial Bio-Nanocomposite Textile Material Produced from Natural Resources" Nanomaterials 12, no. 15: 2539. https://doi.org/10.3390/nano12152539
APA StyleMarković, D., Zille, A., Ribeiro, A. I., Mikučioniene, D., Simončič, B., Tomšič, B., & Radetić, M. (2022). Antibacterial Bio-Nanocomposite Textile Material Produced from Natural Resources. Nanomaterials, 12(15), 2539. https://doi.org/10.3390/nano12152539